Abstract
Background
Low-value clinical practices are common in healthcare, yet the optimal approach to de-adopting these practices is unknown. The objective of this study was to systematically review the literature on de-adoption, document current terminology and frameworks, map the literature to a proposed framework, identify gaps in our understanding of de-adoption, and identify opportunities for additional research.
Methods
MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Cochrane Database of Abstracts and Reviews of Effects, and CINAHL Plus were searched from 1 January 1990 to 5 March 2014. Additional citations were identified from bibliographies of included citations, relevant websites, the PubMed ‘related articles’ function, and contacting experts in implementation science. English-language citations that referred to de-adoption of clinical practices in adults with medical, surgical, or psychiatric illnesses were included. Citation selection and data extraction were performed independently and in duplicate.
Results
From 26,608 citations, 109 were included in the final review. Most citations (65 %) were original research with the majority (59 %) published since 2010. There were 43 unique terms referring to the process of de-adoption—the most frequently cited was “disinvest” (39 % of citations). The focus of most citations was evaluating the outcomes of de-adoption (50 %), followed by identifying low-value practices (47 %), and/or facilitating de-adoption (40 %). The prevalence of low-value practices ranged from 16 % to 46 %, with two studies each identifying more than 100 low-value practices. Most articles cited randomized clinical trials (41 %) that demonstrate harm (73 %) and/or lack of efficacy (63 %) as the reason to de-adopt an existing clinical practice. Eleven citations described 13 frameworks to guide the de-adoption process, from which we developed a model for facilitating de-adoption. Active change interventions were associated with the greatest likelihood of de-adoption.
Conclusions
This review identified a large body of literature that describes current approaches and challenges to de-adoption of low-value clinical practices. Additional research is needed to determine an ideal strategy for identifying low-value practices, and facilitating and sustaining de-adoption. In the meantime, this study proposes a model that providers and decision-makers can use to guide efforts to de-adopt ineffective and harmful practices.
Electronic supplementary material
The online version of this article (doi:10.1186/s12916-015-0488-z) contains supplementary material, which is available to authorized users.
Keywords: Abandon, Contradict, De-adoption, De-implementation, Disinvestment, Low-value, Medical reversal, Obsolete, Reassess, Withdrawal
Background
Clinical practice evolves in response to scientific evidence through a process of discovery (novel practice introduced into clinical practice, e.g., systemic thrombolysis for acute ST-elevation myocardial infarction (STEMI) [1]), replacement (newer, more effective practice supplants current practice, e.g. tenecteplase superior to alteplase among patients with STEMI [2]), or reversal (current practice shown to be ineffective or harmful, e.g., suppression of ventricular ectopy after a myocardial infarction using encainide, flecainide, or moricizine [3]) [4]. Discovery and replacement introduce novel, beneficial therapies into clinical practice, while reversal implies that patients receive no benefit and may be at risk of harm [5]. The adoption of clinical practices that are later de-adopted imposes substantial inefficiencies on the healthcare system wherein resources that could have been dedicated to other purposes are instead devoted to a practice that was ineffective or harmful (e.g., self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin) [6].
Practice reversal is common [5, 7, 8]. A recent review of articles published in a major general medical journal between 2001 and 2010 found that 27 % of original articles re-examined the efficacy of an established practice, among which 40 % found evidence for practice reversal [7]. In another review, commissioned by the Australian government’s Comprehensive Management Framework for managing their Medical Benefits Schedule, Elshaug and colleagues triangulated data from searches of the peer-reviewed literature, targeted health technology databases, and opportunistic sampling of stakeholder groups to identify 156 potentially unsafe and/or ineffective practices [8].
Medical reversal may be an unavoidable consequence of evidence-based medicine and/or early technology adoption; however, it is important that its incidence remain low given the threat that it poses to providing high-quality healthcare. It is equally important that any intervention with evidence for medical reversal be rapidly de-adopted. We were unable to identify any knowledge synthesis that systematically examined the de-adoption of established clinical practices. We conducted this scoping review to describe the literature on de-adoption, document current terminology and frameworks, map the literature to a proposed conceptual framework (Table 1), identify gaps in the understanding of this important concept, and identify opportunities for more detailed evidence syntheses and/or empirical research.
Table 1.
Proposed framework for conceptualizing de-adoption
| Phase of de-adoption | Operational definition |
|---|---|
| Identify low-value clinical practices | Ascertain which clinical practices are of low value |
| Facilitate the de-adoption process | Reduce the use of low-value clinical practices |
| Evaluate de-adoption outcomes | Evaluate the outcomes of a strategy of de-adoption |
| Sustain de-adoption | Prevent resurgence in use of low-value practices after their initial de-adoption |
Methods
We developed a conceptual framework for this work that employed the key features of Everett Rogers’ Innovation-Decision model to conceptualize de-adoption (Table 1) [9]. De-adoption was defined as the discontinuation of a clinical practice after it was previously adopted [9]. We followed established scoping review methodology [10, 11], and used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to report the methods and results [12].
Eligibility criteria
We included English-language citations that referred to the de-adoption of any clinical practice in adults (mean age ≥ 18 years) with medical, surgical, or psychiatric illnesses. All original and non-original quantitative and qualitative research citations were eligible; however, we excluded citations that exclusively described the adoption of practices or appropriateness of resource use (e.g., selected use of antimicrobials, appropriate use of surgical procedures, appropriate use of lumbar spine radiography among patients with lower back pain). Although de-adoption is a component within the larger issue of resource optimization, the “appropriateness” of a clinical practice embodies more than simply discontinuing its use. Therefore, we excluded citations primarily focused on clinical practice appropriateness.
Search strategy and data sources
With the help of a medical librarian, we searched the following electronic databases from 1 January 1990 to 5 March 2014: Ovid MEDLINE, Ovid EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews, the Cochrane Database of Abstracts and Reviews of Effects, and CINAHL Plus. Pilot searches in MEDLINE suggested that none of the currently available Medical Subject Heading (MeSH) terms were specific to articles reporting de-adoption. Therefore, the MEDLINE search was confined to use of text words that included combinations and synonyms of de-adoption and healthcare technologies (Additional file 1: Appendix). Search terms were combined using the appropriate Boolean logic, and included wildcards to account for plural words and variations in spelling. The search strategy included similar combinations of terms within the other databases. To ensure reproducibility, the MEDLINE search strategy was peer reviewed by a second medical librarian using the Peer Review of Electronic Search Strategies (PRESS) checklist [13].
To increase the sensitivity of the search strategy, we also searched the gray literature according to recommendations from the Canadian Agency for Drugs and Technologies in Health [14]. Relevant websites included The Canadian Agency for Drugs and Technologies in Health, Program for Assessment of Technology in Health, Australian Government Medical Services Advisory Committee, Austrian Institute of Technology Assessment, National Institute for Health and Care Excellence, Agency for Healthcare Research and Quality, Blue Cross & Blue Shield Association, Choosingwisely.org and Choosingwiselycanada.org, and the Trip Database. Additional citations were identified by (1) contacting experts in implementation science; (2) using the PubMed “related articles” function; and (3) hand-searching bibliographies from important implementation science/adoption of innovations textbooks [9, 15, 16], and reference lists of included citations. Reference management was performed in EndNote (version X7, Thomson Reuters).
Citation selection
Prior to the screening of titles and abstracts, the citation screening form was calibrated by three team members (DJN, KJM, JKH) independently with a random sample of 50 citations. Once consistent citation selection was achieved (kappa ≥ 0.8) [17], all citations were screened for inclusion independently and in duplicate by three reviewers through a two-stage process. During level-one screening, titles and abstracts were reviewed to determine citations that met the inclusion/exclusion criteria. The full text of any citation classified as “include” or “unclear” was reviewed to determine whether it met study inclusion criteria (level-two screening). Eligibility disagreements were resolved by consensus, or arbitration by a third reviewer. Agreement between reviewers at all stages of citation selection was quantified using the kappa statistic [17].
Data extraction and synthesis
Three reviewers independently extracted data from all included citations using a pre-designed electronic form that was pilot tested using a random sample of 10 citations. Once data were consistently abstracted (kappa ≥ 0.8) [17], reviewers proceeded with full data extraction. Extracted data pertained to (1) the citation (e.g., original research, non-original research, website); (2) the term(s) used to refer to de-adoption (e.g., discontinuance, medical reversal, rejection); (3) characteristics of the target condition(s) or clinical practice(s) (e.g., use of nesiritide in acute decompensated heart failure [18]); (4) characteristics of evidence suggesting de-adoption (e.g., original research versus non-original research); (5) whether barriers and facilitators to de-adoption were reported; and (6) whether conceptual frameworks to promote low-value practice de-adoption were used/cited.
Independently, and in duplicate, reviewers mapped the abstracted data onto the proposed conceptual framework. Articles were summarized using counts, proportions, mean (standard deviation), or median (inter-quartile range, IQR) where appropriate. Data were managed and analyzed using Stata version 13.1 (Stata Corp, College Station, TX, USA).
Results
The electronic database and gray literature searches identified 26,557 unique citations (Fig. 1) that were screened for inclusion, from which 110 full text citations were retrieved for further assessment. An additional 51 articles were identified through review of bibliographies, and consultation with knowledge translation experts. From these 161 full text citations, 109 were included in the final review. The most common reason citations were excluded after full text review was owing to an explicit focus on the adoption and/or appropriateness of clinical practices (n = 25).
Fig. 1.
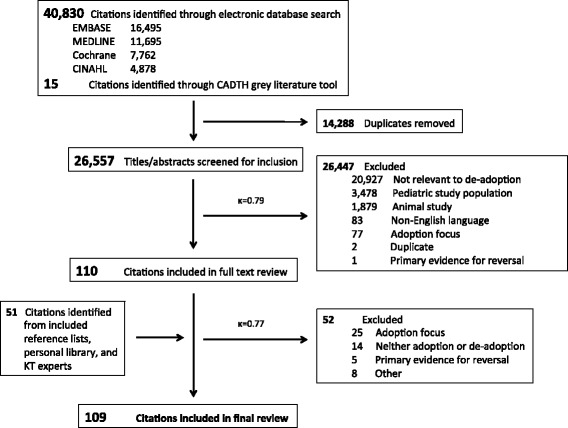
Details of the article selection process. CADTH Canadian Agency for Drugs and Technologies in Health, KT Knowledge Translation
Description of the included citations
A description of the included citations is provided in Table 2. Most citations were original research studies (65 %), with the majority being either quasi-experimental (28 %) or cohort studies (14 %). Among the non-original citations, most were editorials or letters to the editor (19 %), or narrative reviews (15 %). Most articles originated in North America (60 %) with the USA representing the most common country (47 % of all articles). The majority of articles were published from 2010 onwards (59 %), with very few published prior to 2000 (3 %). Most articles described the de-adoption of therapeutic interventions (62 %), with comparatively fewer describing the de-adoption of diagnostic interventions (30 %). The randomized clinical trial was most frequently cited (41 %) as the level of evidence that should trigger de-adoption, and most articles cited risk of harm (73 %), and/or lack of efficacy (63 %) as the reason practices should be de-adopted. Among the articles that reported the original reason for clinical practice adoption (n = 16, 15 %), most (n = 10, 63 %) cited observational research (case series and cohort studies) as the evidence that shaped adoption. A detailed, referenced bibliography of the citations is provided in Additional file 1: Table S1.
Table 2.
Characteristics of included citations
| Characteristic | Number (%) of 109 citations |
|---|---|
| Year of publication | |
| 1990–1999 | 3 (3) |
| 2000–2009 | 42 (38) |
| 2010–current | 64 (59) |
| Country of origina | |
| North America | 65 (60) |
| Europe | 30 (28) |
| Australasia | 22 (20) |
| Type of article | |
| Original research | 71 (65) |
| Quasi-experimentalc | 30 (28) |
| Cohort studyb | 15 (14) |
| Mixed methods | 8 (7) |
| Qualitative | 4 (4) |
| Predictive modeling | 3 (3) |
| Knowledge synthesis | 3 (3) |
| Consensus method | 3 (3) |
| Randomized clinical trial | 1 (1) |
| Otherd | 6 (6) |
| Non-original research | 38 (35) |
| Editorial, letter to the editor, news item, other | 21 (19) |
| Narrative review | 16 (15) |
| Guideline | 1 (1) |
| Focus of article | |
| Identify low-value practices | 51 (47) |
| Facilitate the de-adoption process | 44 (40) |
| Evaluate de-adoption outcomes | 54 (50) |
| Sustain de-adoption | 2 (2) |
| Type of interventione | |
| Therapeutic | 68 (62) |
| Drug | 34 (31) |
| Device or surgical procedure | 16 (15) |
| Drugs and devices/procedures | 16 (15) |
| Other | 3 (3) |
| Diagnostic | 27 (30) |
| Laboratory | 7 (8) |
| Physiologic measurement | 4 (4) |
| Diagnostic imaging | 3 (3) |
| Screening program | 1 (1) |
| Diagnostic tests not otherwise specified | 12 (13) |
| Evidence to promote de-adoptionf | |
| Randomized clinical trial | 45 (41) |
| Knowledge synthesis | 14 (13) |
| Clinical practice guideline | 6 (5) |
| Cohort study | 4 (4) |
| Quasi-experimentalc | 2 (2) |
| Expert consensus | 2 (2) |
| Reasons for de-adoptiong | |
| Harm | 80 (73) |
| Lack of efficacy | 69 (63) |
| Not cost-effective | 37 (34) |
Percentages within each characteristic may not always total to 100 due to rounding error, and/or redundancy within citations (e.g., a citation may have more than one country of origin)
aNorth American countries: Canada, USA; European countries: UK, Belgium, Denmark, France, Greece, Italy, Netherlands, Spain; Australian countries: Australia, New Zealand
bIncludes six studies wherein the study population was a cohort of articles identified through searches of the electronic literature
cIncludes interrupted time series, and before-and-after studies
dIncludes two surveys, one report on stakeholder engagement, one simulation
eType of intervention not reported in 32 studies
fNot reported in 46 studies (52 %)
gNot reported in 11 studies
De-adoption terminology
We identified 43 unique terms representative of the process of de-adoption (Table 3). The majority of citations (65 %) referred to de-adoption using more than one term, and among these the median (IQR) number of terms per citation was 3 (2–3). Disinvest* was the most frequently cited term (39 % of included citations). Other commonly cited terms included decrease use (24 %), discontinu* (16 %), abandon* (16 %), reassess* (14 %), obsole* (12 %), medical reversal (11 %), and contradict* (10 %). Terms such as de-implement* and de-adopt* were infrequently cited (4 % and 3 %, respectively). A term representative of the process of de-adoption was found in the title or abstract of 86 % of citations and most frequently included disinvest* (31 %), decrease use (12 %), reassess* (7 %), withdraw* (7 %), medical reversal (6 %), discontinu* (6 %), and obsole* (6 %). Each of the 43 unique terms was mapped onto our conceptual framework. The majority of terms (n = 22, 51 %) referred to facilitating the de-adoption process. Seventeen terms (40 %) mapped to more than one category within the conceptual framework, with the most common cross-classification being facilitate de-adoption and sustain de-adoption (13/17, 76 %).
Table 3.
De-adoption terms (n = 43) and frequency of their use within included citations
| Terma | Number (%) of 109 citationsb | Number (%) of citations with term listed in title or abstract | Relationship to the proposed conceptual framework | References |
|---|---|---|---|---|
| Disinvest* | 42 (39) | 34 (31) | Facilitate de-adoption | [7, 8, 19, 20, 23, 42, 46–81] |
| Sustain de-adoption | ||||
| Decrease use | 26 (24) | 13 (12) | Facilitate de-adoption | [8, 26–28, 31, 33, 35, 37, 80, 82–99] |
| Evaluate de-adoption outcomes | ||||
| Discontinu* | 17 (16) | 7 (6) | Facilitate de-adoption | [8, 18, 20, 23, 25, 28, 51, 56, 61, 63, 91, 94, 96, 100–103] |
| Evaluate de-adoption outcomes | ||||
| Abandon* | 17 (16) | 4 (4) | Sustain de-adoption | [31, 54, 58, 63, 65, 84, 86, 97, 98, 100, 101, 103–108] |
| Reassess* | 15 (14) | 8 (7) | Identify low-value practices | [8, 18, 23, 46, 52, 58, 59, 68, 71, 80, 102, 108–111] |
| Obsole* | 13 (12) | 6 (6) | Identify low-value practices | [19, 20, 49, 55, 57, 58, 68, 73, 76, 80, 108, 109, 112] |
| Medical reversal | 12 (11) | 7 (6) | Identify low-value practices | [4, 5, 7, 61, 83, 85, 107, 113–117] |
| Contradict | 11 (10) | 3 (3) | Identify low-value practices | [5, 7, 24, 54, 65, 68, 85, 86, 104, 118, 119] |
| Re-invest | 9 (8) | 0 (0) | Sustain de-adoption | [8, 52, 54, 55, 68, 71, 73, 78, 80] |
| Withdraw* | 8 (7) | 8 (7) | Facilitate de-adoption | [29–36] |
| Sustain de-adoption | ||||
| Reduc* | 8 (7) | 1 (1) | Evaluate de-adoption outcomes | [31, 32, 34–36, 120, 121] |
| Decline in use | 7 (6) | 0 (0) | Evaluate de-adoption outcomes | [96, 98, 99, 103, 120, 122, 123] |
| Health technology reassessment | 5 (5) | 4 (4) | Identify low-value practices | [52, 58, 59, 71, 110] |
| Change in use | 4 (4) | 2 (2) | Evaluate de-adoption outcome | [32, 118, 121, 124] |
| De-implement* | 4 (4) | 2 (2) | Facilitate de-adoption | [50, 65, 125, 126] |
| De-list | 4 (4) | 0 (0) | Facilitate de-adoption | [57, 68, 80, 109] |
| Sustain de-adoption | ||||
| Low value practice/intervention | 4 (4) | 2 (2) | Identify low-value practices | [7, 70, 78, 127] |
| Change in practice | 3 (3) | 1 (1) | Evaluate de-adoption outcome | [36, 103, 118] |
| De-adopt* | 3 (3) | 2 (2) | Facilitate de-adoption | [18, 100, 128] |
| Evaluate de-adoption outcomes | ||||
| De-commission | 3 (3) | 1 (1) | Facilitate de-adoption | [68, 72, 80] |
| Sustain de-adoption | ||||
| Do not do | 3 (3) | 1 (1) | Facilitate de-adoption | [21, 22, 56] |
| Reallocation | 3 (3) | 0 (0) | Sustain de-adoption | [58, 73, 109] |
| Remov* | 3 (3) | 0 (0) | Facilitate de-adoption | [29, 33, 37] |
| Sustain de-adoption | ||||
| Replace | 3 (3) | 0 (0) | Facilitate de-adoption | [4, 111, 114] |
| Sustain de-adoption | ||||
| Refute | 3 (3) | 1 (1) | Identify low-value practices | [24, 83, 104] |
| Over use | 3 (3) | 0 (0) | Identify low-value practices | [37, 127, 129] |
| Stop* | 3 (3) | 1 (1) | Facilitate de-adoption | [35, 77, 124] |
| Inappropriate use | 2 (2) | 1 (1) | Identify low-value practices | [112, 129] |
| Relinquish* | 2 (2) | 1 (1) | Facilitate de-adoption | [97, 98] |
| Sustain de-adoption | ||||
| Ineffective | 2 (2) | 1 (1) | Identify low-value practices | [19, 29] |
| Misuse | 1 (1) | 0 (0) | Identify low-value practices | [127] |
| Re-appraisal | 1 (1) | 0 (0) | Identify low-value practices | [79] |
| Re-prioritization | 1 (1) | 0 (0) | Sustain de-adoption | [79] |
| Substitutional re-investment | 1 (1) | 0 (0) | Facilitate de-adoption | [79] |
| Sustain de-adoption | ||||
| Evidence-based reassessment | 1 (1) | 0 (0) | Identify low-value practices | [79] |
| Clinical redesign | 1 (1) | 0 (0) | Facilitate de-adoption | [79] |
| Disadoption | 1 (1) | 0 (0) | Facilitate de-adoption | [101] |
| Defunding | 1 (1) | 0 (0) | Facilitate de-adoption | [57] |
| Sustain de-adoption | ||||
| Resource release | 1 (1) | 0 (0) | Facilitate de-adoption | [57] |
| Sustain de-adoption | ||||
| Withdrawing from a service and redeploying resources | 1 (1) | 0 (0) | Facilitate de-adoption | [57] |
| Sustain de-adoption | ||||
| Redeploy | 1 (1) | 1 (1) | Facilitate de-adoption | [68] |
| Sustain de-adoption | ||||
| Reversal | 1 (1) | 0 (0) | Identify low-value practices | [96] |
| Facilitate de-adoption | ||||
| Sustain de-adoption | ||||
| Drop in use | 1 (1) | 0 (0) | Facilitate de-adoption | [31] |
| Evaluate de-adoption |
a*wildcard notation denotes multiple endings for a given term
bPercentages do not total 100 owing to the appearance of multiple terms within individual citations
Barriers and facilitators to de-adoption
Barriers and facilitators to de-adoption were cited within 51 and 48 of the included citations respectively. The bulk of articles citing barriers to or facilitators of de-adoption were original research (Fig. 2).
Fig. 2.
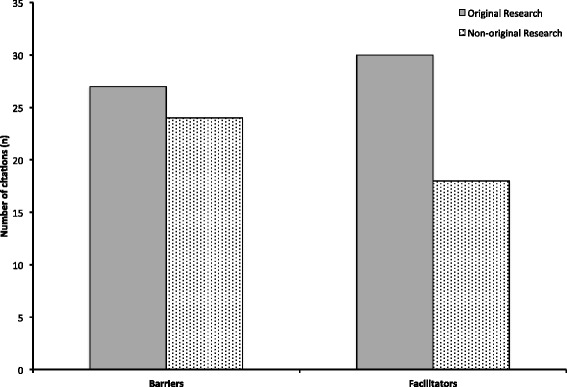
Distribution of articles citing barriers to and facilitators of de-adoption according to type of research
Mapping citations to the de-adoption conceptual framework
Articles frequently mapped to more than one category within our conceptual framework (Fig. 3). The primary focus among included citations was evaluating de-adoption outcomes (50 %), identifying low-value practices (47 %), and facilitating the de-adoption process (40 %). Two articles (2 %) discussed sustaining de-adoption. Most articles whose focus was on evaluating de-adoption outcomes were original research (80 %), whereas the majority of articles that discussed identifying low-value practices were non-original research (63 %).
Fig. 3.
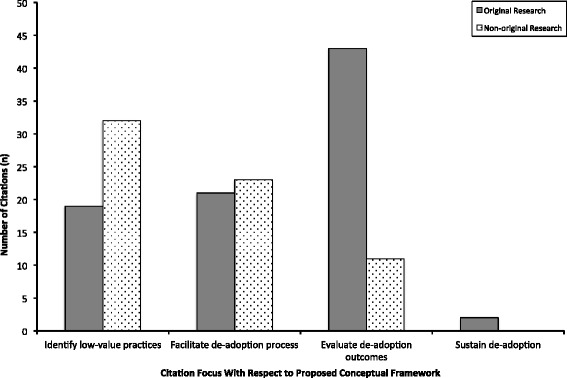
Distribution of articles according to classification within the conceptual framework and type of research
Frameworks for the de-adoption of low-value clinical practices were provided in 11 citations (Table 4), of which half were derived from original research (n = 5, 45 %). Two citations documented clinical application of their framework [19, 20]. Seven citations described frameworks for identifying and prioritizing candidate low-value practices, and nine citations described frameworks for facilitating the de-adoption process. Among citations that described a framework for identifying low-value practices, common mechanisms included consultation with clinical stakeholders, monitoring for new scientific evidence, examining for practices with large between-provider variation, and/or embedding the notion of health technology reassessment within the life cycle of any given practice. Commonly proposed criteria for prioritizing the de-adoption of low-value practices included the availability of evidence that a candidate practice is ineffective or harmful, the safety of the low-value practice (i.e., harmful practices prioritized ahead of those that are simply ineffective), potential health and cost impact of de-adoption, and availability of alternative practices. Among citations that described frameworks for facilitating the de-adoption process, common mechanisms included restructuring of funding associated with the given practice, changes to local and/or regional policies, and more consistent integration of health technology reassessment within existing health technology assessment programs.
Table 4.
Frameworks proposed to guide the de-adoption of low-value practices
| Citation | Type of citation | Relationship to conceptual framework (Table 1) | Description | Documented clinical application |
|---|---|---|---|---|
| Elshaug et al. 2009 [109] | Discussion paper prepared by Canadian Agency for Drugs and Technologies in Health Health Technology Strategy Policy Forum | Identify low-value practices | Criteria for identifying existing, potentially non-cost-effective practices as candidates for assessment | No |
| Criteria to inform the prioritization of candidates for detailed review after identification | ||||
| Facilitate the de-adoption process | Funding approaches to facilitating reduction in non-cost-effective practices | No | ||
| Joshi et al. 2009 [80] | Narrative review | Identify low-value practices | HTR approach to identifying candidate technologies | No |
| Ibargoyen-Roteta et al. 2010 [55] | Guideline | Identify of low-value practices | GuNFT: Hospital and patient-level criteria for not funding technologies | No |
| Facilitate the de-adoption process | Barriers and mechanisms to remove funding from existing technologies | No | ||
| Mortimer 2010 [68] | Narrative review | Facilitate the de-adoption process | Proposed re-orientation of traditional PBMA model to target strategies of disinvestment | Not with the re-oriented PBMA model as outlined by the authors |
| Donaldson et al. 2010 [72] | Narrative review | Facilitate the de-adoption process | Describes the use of PBMA to promote rational disinvestment | Not according to the model outlined by the authors |
| Gerdvilaite and Nachtnebel 2011 [57] | Systematic review | Identify of low-value practices | Authors cite criteria proposed by Elshaug et al. [109], Joshi et al. [80], Ibargoyen-Roteta et al. [55], and criteria proposed by National Institute for Health and Care Excellence | No |
| Overlapping criteria include new evidence, cost effectiveness, safety, and available alternatives | ||||
| Facilitate the de-adoption process | As described above for Ibargoyen-Roteta et al. [55] | No | ||
| Levin 2011 [20] | Conference presentation | Identify of low-value practices | Ontario’s Evidence-based Analyses to Manage Technology Adoption and Obsolescence: Mega-analysis Evidence Based Analyses of technologies around disease conditions; prioritized by effectiveness and cost-effectiveness; criteria for identifying practices unclear | Yes |
| Facilitate the de-adoption process | Mechanism for facilitating de-adoption appears to be based on funding effective technologies, and not funding ineffective technologies | Yes | ||
| Leggett et al. 2012 [58] | Systematic review | Identify of low-value practices; Facilitate the de-adoption process | 1.GuNFT as outlined above | No |
| 2.5-steps for HTR should include: identification, prioritization, evaluation, implementation, and monitoring | ||||
| Watt et al. 2012 [46] | Mixed methods | Facilitate the de-adoption process | Two technologies (assisted reproductive technology and vitamin B12/folate pathology tests) used as case studies to test a three-level model to facilitate de-adoption including: | No (study ongoing) |
| 1. Evidence reports | ||||
| 2. Stakeholder engagement | ||||
| 3. Policy deliberation and analysis; Process evaluation | ||||
| Henshall and Schuller 2012 [52] | Qualitative | Identify of low-value practices | Identification and prioritization approaches include clinical stakeholder involvement, monitoring new evidence, use of data to identify practices with high variability and/or cost, inclusion of HTR within life-cycle of any technology | No |
| Polisena et al. 2013 [19] | Systematic review | Facilitate the de-adoption process | Three different models to facilitate disinvestment decisions: | Yes; varied by included study |
| 1. Health technology assessment framework | ||||
| 2. Program budgeting and marginal analysis | ||||
| 3. Accountability for reasonableness and quality improvement theory |
GuNFT Guideline for Not Funding Health Technology, HTR health technology reassessment, PBMA program budgeting and marginal analysis
Lists of low-value practices were provided by eight citations (Table 5). Searches of the published literature were the most frequently employed means of identifying low-value practices (n = 7 citations, 88 %); however, the sources searched and the approach to defining a low-value practice varied by citation. Evidence was combined with stakeholder engagement to identify low-value practices in three citations [8, 21, 22], and one citation identified low-value practices as those shown to have high variability in rates of use between providers [23]. Among the seven citations that used the published literature to identify low-value practices, the prevalence of low-value practices ranged from 16 % [24] to 46 % [5], with two studies each identifying more than 100 low-value practices [7, 8].
Table 5.
Original research citations that identified lists of low-value clinical practices
| Citation | Stakeholder engagement | Single clinical area of focus | Methodology | Results |
|---|---|---|---|---|
| Ioannidis 2005 [24] | No | No | Broad literature search (1990–2003) for highly cited clinical research studies published in three major clinical journalsa or medical specialty journals with an impact factor >7.0 | 7 of 45 (16 %) highly cited studies claiming effectiveness eventually contradicted by replication research |
| Supplemental, tailored searches to determine if each highly cited study had been replicated | 7 other replication studies (16 %) found effect size not as large as in original study | |||
| Comparison of direction of results between replicated and original highly cited study | ||||
| Prasad et al. 2011 [5] | No | No | Review of all “original articles” published in New England Journal of Medicine in 2009 | 35 of 124 (28 %) articles examined an existing medical practice |
| Articles classified according to whether the practice examined was new or already in place, and whether the results were positive or negative for the primary endpoint | 16 of 35 (46 %) articles examining an existing practice demonstrated medical reversalb | |||
| Elshaug et al. 2012 [8] | Comprehensive Management Framework for Australia’s Medicare Benefits Schedule | No | Environmental scanning approach triangulating data from broad PubMed search (2000–2010), targeted searches within select databases (e.g., Cochrane library), and opportunistic sampling among clinical and non-clinical stakeholders | 156 potentially ineffective or unsafe practices identified from 5,209 screened articles |
| Excluded pharmaceuticals | ||||
| Choosing Wisely 2012 [21] | Yes | Yes, specialty specific recommendations | Varied by specialty society but generally included one or more of literature search, expert opinion, and/or a modified Delphi process | 67 specialty specific Top 5 ‘do not do’ lists |
| Garner et al. 2013 [70] | NICE | No | Present results from the first 6 months of the Cochrane Quality and Productivity project to identify low-value practices | 28 of 65 (43 %) reviews published over a 6-month period identified potentially low-value practices |
| Routine scanning of “implications for practice” section in new or updated Cochrane reviews to identify those wherein the author concluded an intervention is ineffective/harmful or should be confined to use within a research context | Most reviews cited a lack of randomized evidence of effectiveness, rather than robust evidence of lack of effectiveness | |||
| Each review is examined to ensure it meets Cochrane Quality and Productivity criteria (potential impact on quality, safety, patient/provider experience, and potential for cash-releasing savings) for recommendation as a potential “disinvestment” candidate | To date the NICE Health Technology Appraisal Program has generated 1,347 ‘do not do’ recommendations [130] | |||
| Hollingworth et al. 2013 [23] | No | Yes, interventional procedures | Used UK Hospital Episode Statistics to identify inpatient interventional procedures with high variation in rates of use between PCTs in England | Substantial inter-procedure, inter-PCT variation in procedure rates |
| Procedures with high variation not listedc | ||||
| Prasad et al. 2013 [7] | No | No | Review of all original research articles published in New England Journal of Medicine from 2001 to 2010 | 363 of 1,344 (27 %) articles re-examined an established practice |
| Articles classified according to whether the practice examined was new or already in place, and whether the results were positive or negative for the primary endpoint | 146 of 363 (40 %) articles re-examining an existing practice demonstrated evidence of reversal | |||
| Articles further classified as replacement, back to the drawing board, reversal, or reaffirmation d | ||||
| Choosing Wisely Canada 2014 [22] | Yes | Yes, specialty specific recommendations | Varied by specialty society but generally included one or more of literature search, expert opinion, and/or a modified Delphi process | 61 recommendations across 18 medical and surgical specialties |
aMajor clinical journals included New England Journal of Medicine, Journal of the American Medical Association, and The Lancet
bMedical reversal occurs when a new study—superior to predecessors because of better design, increased power, or more appropriate controls—contradicts current clinical practice [5]
cConference abstract limited availability of data from this study
dReplacement = new practice surpasses older standard of care; back to the drawing board = new practice fails to surpass standard of care; reversal = current practice inferior to a lesser or prior standard; reaffirmation = existing practice superior to a lesser or prior standard
NICE National Institute for Health and Care Excellence, PCT, Primary Care Trusts
The impact of de-adoption efforts was evaluated and reported in 39 original research citations (Table 6). Most studies used interrupted time series methodology (n = 21, 54 %) and obtained data from large administrative databases or clinical registries (n = 30, 76 %). The most common target conditions were cardiovascular disease (n = 11, 28 %), arthritides (n = 8, 21 %), and menopause (n = 7, 18 %). All but one of the practices (pulmonary artery catheter) examined were therapeutic interventions. The most frequently examined therapies included cyclo-oxygenase-2 (COX-2) inhibitors and other non-steroidal anti-inflammatory drugs (NSAIDs) (n = 8, 21 %), hormone replacement therapy (n = 7, 18 %), and percutaneous coronary intervention (n = 3, 8 %). Thirteen studies reported on de-adoption efforts that followed an active change intervention, all of which demonstrated reductions in the target low-value practice [25–37]. The most common intervention was withdrawal of a low-value drug from the market (n = 9, 23 %). Other active change interventions commonly included an education component targeted at patients and/or providers. Of the 26 studies that did not report on the effects of an active change intervention, 23 (88 %) demonstrated reductions in the target practice. Of the 27 and 11 studies that examined de-adoption efforts for harmful or ineffective practices, respectively, 25 (92 %) and 9 (81 %) demonstrated reductions in the target practice.
Table 6.
Original research citations that evaluated the de-adoption of low-value clinical practicesa
| Citation | Study design | Target condition | Low-value practice | Evidence guiding de-adoption | Reason practice considered low-value | Reduction in use of low-value practice | Other notable results |
|---|---|---|---|---|---|---|---|
| Active change intervention facilitated de-adoption b | |||||||
| Ross-Degnan et al. 1993 [29] | Interrupted time series | Arthritides | NSAIDs, Zomepirac | Case series | Harmful | Yes | Increased prescription of other NSAIDs |
| Williams et al. 2006 [30] | Interrupted time series | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Safety concerns for rofecoxib interpreted as class effect |
| Thiebaud et al. 2006 [31] | Cohort study | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Greater decrease in COX-2 inhibitor use among patients with greater number of cardiovascular comorbidities |
| Barozzi and Tett 2007 [32] | Interrupted time series | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Safety concerns for rofecoxib interpreted as class effect; prescription of non-selective NSAIDs increased |
| Sun et al. 2007 [33] | Interrupted time series | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Significant increases in non-selective NSAID use after withdrawal of rofecoxib and valdecoxib |
| Setakis et al. 2008 [34] | Before-and-after | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | After withdrawal of rofecoxib, remaining use of COX-2 inhibitors did not concentrate in patients with high gastrointestinal risk and low cardiovascular risk |
| Sukel et al. 2008 [35] | Before-and-after | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Safety concerns for rofecoxib interpreted as class effect |
| Hsiao et al. 2009 [36] | Cohort | Arthritides | COX-2 inhibitors | RCT | Harmful | Yes | Safety concerns for rofecoxib interpreted as class effect |
| Stafford and Radley 2003 [37] | Interrupted time series | Obesity | Fenfluramine and dexfenfluramine | Case–control study | Harmful | Yes | No change in practice after reports of adverse events. Market withdrawal of drug required to change practice |
| Krol et al. 2004 [27] | Cluster RCT | PPI use | PPIs in those without indications for their continued use | Clinical practice guideline | Not reported | Yes | No recrudescence of symptomatology associated with original PPI prescription after its discontinuation |
| Roumie et al. 2004 [25] | Interrupted time series | Post-menopausal women | HRT | RCT | Harmful | Yes | Greater rate of discontinuation of HRT after tailored de-adoption intervention compared to media release of results of WHI study |
| Kulawik et al. 2009 [28] | Before-and-after | End-stage renal disease | Use of tunnelled hemodialysis catheters in patients with end-stage renal disease | Cohort, quasi-experimental, and clinical practice guideline | Harmful, not cost effective | Yes | Involvement of medical leader improved rate of reduction in catheter use |
| Sindby et al. 2011 [26] | Before-and-after | Coronary artery bypass surgery | Blood transfusions | Not reported | Not reported | Yes | Not reported (conference abstract) |
| No intervention used to facilitate de-adoption c | |||||||
| Austin et al. 2003 [99] | Interrupted time series | Post menopausal women | HRT | RCT | Harmful | Yes | Unable to determine if decline in HRT use patient or physician-initiated |
| Lawton et al. 2003 [124] | Survey | Post menopausal women | HRT | RCT | Harmful | Yes | Factors associated with stopping HRT included older age, use of combined HRT, longer duration of HRT |
| Haas et al. 2004 [118] | Interrupted time series | Post menopausal women | HRT | RCT | Harmful | Yes | Greater decrease in HRT use after WHI study compared to Heart and Estrogen/progestin Replacement Study |
| Hersh et al. 2004 [103] | Interrupted time series | Post menopausal women | HRT | RCT | Harmful | Yes | Response to publication of WHI study was rapid |
| Majumdar et al. 2004 [98] | Interrupted time series | Post menopausal women | HRT | RCT | Harmful | Yes | Substantial decline in promotional spending for HRT after publication of WHI study |
| Huang et al. 2007 [122] | Cohort | Post menopausal women | HRT | RCT | Harmful | Yes | Factors associated with reduction in use of HRT included higher patient education, and care at an academic institution |
| Majumdar et al. 2001 [97] | Before-and-after | Acute coronary syndrome | Calcium channel blockers Lidocaine | Case–control study; Systematic review | Harmful | Yes | No difference in calcium channel blocker discontinuation according to physician specialty |
| Brunt et al. 2003 [120] | Interrupted time series | Hypertension | Short acting calcium channel blockers | Case–control study | Harmful | Yes | Proportionate increase in other anti-hypertensive medication paralleled discontinuation of calcium channel blockers |
| Stafford et al. 2004 [96] | Interrupted time series | Hypertension | Alpha-blockers | RCT | Harmful | Yes | Substantial decrease in office promotion expenditures for alpha-blockers following publication of ALLHAT trial |
| Xie et al. 2005 [95] | Interrupted time series | Hypertension | Alpha-blockers | RCT | Harmful | Yes | Decrease in alpha-blockers associated with increase in other anti-hypertensive medications |
| Hauptman et al. 2006 [18] | Interrupted time series | Congestive heart failure | Nesiritide | Systematic review | Harmful | Yes | Decrease in nesiritide use associated with increased use of inotropes |
| Atwater et al. 2009 [90] | Before-and-after | Coronary artery disease | PCI | RCT | Lack of efficacy | Yes | Decrease in PCI and increase in medical therapy following COURAGE trial |
| Bonakdar tehrani and Howard 2011 [84] | Before-and-after | Coronary artery disease | PCI | RCT | Lack of efficacy | Yes | PCI use decreased after COURAGE trial, however considerable number of patients with stable angina continued to receive PCI |
| Deyell et al. 2011 [89] | Interrupted time series | Coronary artery disease | PCI | RCT | Lack of efficacy | No | No change in PCI after OAT trial or guideline revisions |
| Ahmed et al. 2011 [93] | Interrupted time series | Coronary artery disease | PCI | RCT | Lack of efficacy | Yes | Decrease in PCI use was sustained up to 2 years after publication of COURAGE trial |
| Wiener and Welch 2007 [91] | Interrupted time series | Critical illness | PAC | RCT; Systematic review | Lack of efficacy | Yes | PAC use began to decline after publication of large observational study (before publication of any RCTs) |
| Koo et al. 2011 [87] | Interrupted time series | Critical illness | PAC | RCT; Systematic review | Lack of efficacy | Yes | Examined patient, physician, and unit-level predictors of PAC use |
| Gershengorn and Wunsch 2013 [128] | Cohort | Critical illness | PAC | RCT; Systematic review | Lack of efficacy | Yes | Surgical patients continue to have high likelihood of PAC use |
| Murphy et al. 2013 [92] | Cohort | Critical illness | Blood transfusions | RCT | Harmful | Yes (higher volume hospitals only) | Likelihood of receiving blood transfusion after publication of TRICC trial dependent on annualized intensive care unit patient volume |
| Duffy and Farley 1992 [105] | Cohort | Chronic obstructive pulmonary disease | IPPB | RCT | Lack of efficacy | Yes | Hospital-level traits and models of funding technologies were associated with discontinuing IPPB |
| Smalley et al. 2000 [121] | Before-and-after | Gastric motility disorders | Cisapride | Case series; Warning letter from Food and Drug Administration | Harmful | No | Cisapride use not effected by black-box US Food and Drug Administration warning regarding harmful effects |
| Howard et al. 2011 [101] | Interrupted time series | Breast cancer | High dose chemotherapy/Hematopoietic cell transplants | RCT | Lack of efficacy and harmful | Yes | No association between hospital teaching status and participation in clinical trials, and decline in use of the low-value practice |
| Chamberlain et al. 2013 [56] | Interrupted time series | (1) Pregnant women with hepatitis | (1) Caesarean section | Clinical practice guideline (NICE ‘do not do’ recommendation) | Lack of efficacy and harmful | No | “Do not do” recommendation reminders had no association with changes in clinical practice |
| (2) Infertile men and women | (2) Fertility procedures | ||||||
| Kowalczyk et al. 2012 [82] | Cohort | Prostate cancer | RRP | Cohort study | Not reported | Yes | Decrease in RRP was associated with an increase in RRP-related complications |
| Luetmer and Kallmes 2011 [88] | Before-and-after | Vertebral fracture | Vertebroplasty | RCT | Lack of efficacy | Yes | Referrals for vertebroplasty decreased, however proportion of referrals undergoing the procedure increased |
| Ehrenstein et al. 2013 [94] | Interrupted time series | Diabetes mellitus | Rosiglitazone | Systematic review Cohort study | Harmful | Yes | No significant change in markers of glycemic control after discontinuation of rosiglitazone |
aFive citations excluded from this table discussed, but did not actually evaluate the outcome of a de-adoption process [52, 59, 106, 123, 125]
bCitations that employed a de-adoption intervention included:
- Ross-Degnan et al. [29]: Market withdrawal of Zomepirac
- Williams et al. [30], Thiebud et al. [31], Barozzi and Tett [32], Sun et al. [33], Setakis et al. [34], Sukel et al. [35], Hsiao et al. [36]: Market withdrawal of rofecoxib
- Stafford and Radley [37]: Market withdrawal of fenfluramine and dexfenfluramine
- Krol et al. [27]: Information leaflet with recommendations for reducing inappropriate PPI use sent to patients from general practice clinics
- Roumie et al. [25]: Three-part intervention consisting of patient and provider education component and provider care component
- Kulawik et al.,[28]: Catheter reduction toolkit (education on types of vascular access) employed in facilities with high catheter utilization rates
- Sindby et al. [26]: Provider education, audit and feedback, and hospital-level guideline changes
cAny observed de-adoption reflects the effect of passive diffusion of evidence of a practice’s ineffectiveness or harm
COX-2 cyclo-oxygenase-2, HRT hormone replacement therapy, IPPB intermittent positive pressure breathing, NSAIDs non-steroidal anti-inflammatory drugs, PAC pulmonary artery catheter, PCI percutaneous coronary intervention, PPIs proton pump inhibitors, RRP retropubic radical prostatectomy, WHI Women’s Health Initiative
Discussion
De-adoption of low-value clinical practices is essential to improve healthcare quality and create a sustainable healthcare system. To our knowledge this is the first knowledge synthesis to comprehensively examine the de-adoption of low-value clinical practices. We identified 109 citations, most of which were published within the last five years, and concentrated on evaluating changes in practice that occurred following the publication of evidence for medical reversal. We identified 43 terms used to refer to the process of de-adoption, with disinvest being the most frequently cited term. We also identified 13 frameworks that conceptualize individual components of the de-adoption process, and from these frameworks propose a model for de-adoption (Fig. 4). These results provide foundations for guiding the de-adoption of ineffective and harmful clinical practices from patient care as well as directing future research.
Fig. 4.
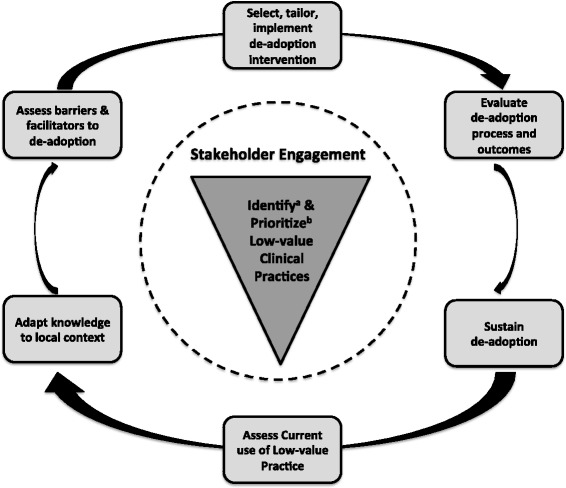
Synthesis model for the process of de-adoption. a Identification of low-value practices includes the process of reviewing and selecting de-adoption knowledge. b Current literature suggests prioritizing based on safety of the low-value practice (i.e., harmful practices eliminated first), potential health and cost impact of de-adoption, and availability of alternative practices
The first major finding from our study pertains to the diverse list of terms used to refer to de-adoption with no clearly established taxonomy. The implication of this is that communication is impaired, which may impact “branding” of de-adoption and efficient searching for relevant literature. Furthermore, it is unclear how different concepts and initiatives such as “less is more” [38], reducing research waste [39], and Choosing Wisely [40] are related. Conversely, knowledge translation and implementation science are increasingly recognized terms in healthcare research, facilitating understanding and communication of the related concepts. Terms such as de-adoption and de-implementation that have a more general connotation, and are natural antonyms of adoption and implementation, ought to be used as terms that brand the process of reducing or removing low-value clinical practices. Other terms, such as disinvest, describe specific elements of the de-adoption process and are not ideal candidates to brand this process. Interestingly, de-adoption and de-implementation were infrequently cited within the included citations, whereas disinvest was the most commonly cited term. Given this lack of clarity with regard to de-adoption terminology, there is an urgent need to develop a taxonomy of terms.
Using the proposed conceptual framework (Table 1), themes common to the frameworks identified in the scoping review (Table 4), and the Knowledge-to-Action framework [41], we derived the second major result from this study, a synthesis framework for facilitating de-adoption (Fig. 4). At the heart of this framework is the identification and prioritization of low-value practices. The identification process involves determining the low-value practice(s) and selection of the knowledge unit that defines a practice as low-value (i.e., randomized clinical trial, systematic review, and/or clinical practice guideline). With regard to prioritization when there is more than one low-value practice identified, current literature suggests prioritizing based on strength of evidence supporting lack of efficacy, safety of the low-value practice (i.e., harmful practices eliminated first), potential health and cost impact of de-adoption, and availability of alternative practices. To permit more of an integrated de-adoption process, and thus improve the probability of success, we suggest stakeholder engagement take place concomitant with practice identification and prioritization. The de-adoption process is then envisioned to follow a similar action cycle as in the original Knowledge-to-Action cycle [41]. However, given the anticipated challenges associated with discontinuing established clinical practices [42], the analysis of barriers and facilitators will require a greater in-depth exploration of both scientific (e.g., presence and quality of evidence supporting de-adoption) and non-scientific (e.g., historical, political, social, and economic factors) barriers to de-adoption [43]. In addition, the intervention that guides de-adoption will likely need to be more closely integrated into clinical care pathways compared to that for adoption, with policy changes and/or changes to funding models predicted to have the greatest likelihood of facilitating de-adoption. Implementation of the intervention will need to be evaluated, and outcomes such as low-value practice use, costs, and potential harms assessed. Finally, any de-adoption intervention should include a sustainability plan; else it is highly likely that healthcare providers will (knowingly or unknowingly) revert to using the practice to which they have become habituated [44].
The third important result from this review is the identification of key questions that require additional research to advance the science of de-adoption. For example, there are multiple factors that likely determine when a practice should be de-adopted (e.g., nature of the intervention, lack of effectiveness or degree of harm, nature of the evidence) but the role of each factor and the interplay among them that ultimately determines when to de-adopt is not clear. In addition, what do we do with clinical practices that are ineffective for a broad population, but may be effective in a small subgroup that is difficult to study? To answer these and other questions we need additional knowledge syntheses that establish a taxonomy of de-adoption terminology, summarize barriers and facilitators to de-adoption, and quantify the impact of past examples of de-adoption. We also need empirical research to examine optimal strategies for identifying candidate low-value practices, and to determine which de-adoption strategies are likely to have the greatest impact. Furthermore, given existing fiscal climates with limited resources, we also need to balance the need to refine and prioritize the science of de-adoption with the need to do the same for adopting new practices.
While we await this additional research, what can healthcare decision-makers practically do with the existing knowledge base? First, this review highlights that de-adoption requires a multi-dimensional construct that is far more complex than simply ceasing to provide a given practice. Second, several studies have demonstrated that de-adoption does occur in response to publication of new evidence (Table 6), with the most consistent de-adoption occurring in response to an active change intervention. The intervention with the greatest likelihood of de-adoption is market withdrawal of a harmful drug. However, the real challenge lies in how to actively facilitate de-adoption when market withdrawal is not possible (e.g., insulin [45]), or not clearly indicated (e.g., practices that are simply ineffective). Interventions cited as having the greatest likelihood of effecting de-adoption include changes to policies, and/or restructuring of funding associated with the low-value practice, the latter through strategies of disinvestment, reinvestment, or defunding. However, this scoping review did not identify any studies that applied a strategy of disinvestment in response to evidence for medical reversal. At this point, pending further research, we suggest use of our proposed synthesis model (Fig. 4) as a starting point for anyone interested in promoting the de-adoption of low-value practices.
There are limitations to this review. First, our search may have missed relevant articles due to the lack of indexing terminology specific to de-adoption that for practical reasons forced us to restrict the search to English language articles published from 1990 onwards. However, the majority of included citations were published after 1999, and originated in high-income countries, therefore it is unlikely that we missed any broad concepts related to de-adoption. Second, grouping articles and de-adoption terminology according to the main categories in the conceptual framework, even though completed in duplicate by independent reviewers, is partly subjective. Finally, we elected to conduct a scoping review in order to provide an inclusive and broad description of what is known about de-adoption and therefore are limited in our ability to present granular details. Our work identifies opportunity for future systematic reviews.
Conclusions
De-adoption of low-value clinical practices is essential to improve healthcare quality and create a sustainable healthcare system. We identified a large body of literature that describes current approaches, and challenges to the de-adoption of low-value clinical practices. Our results should promote future research in at least two areas. First, knowledge syntheses are required to explore areas wherein there is an abundance of literature, such as establishing a taxonomy of de-adoption terminology, summarizing barriers and facilitators to de-adoption, and quantifying the impact of past examples of de-adoption. Second, empirical research is required to examine optimal strategies for identifying candidate low-value practices, and to determine which de-adoption strategies are likely to have the greatest impact. In the meantime, we have developed a conceptual model that providers and decision-makers can use to guide efforts to de-adopt ineffective and harmful practices and describe examples of successful de-adoption that can be used to inform efforts.
Acknowledgements
We would like to acknowledge Diane Lorenzetti, MLS (University of Calgary) for assistance with the literature search strategy, and Laure Perrier, MSc (University of Toronto) for peer review of the literature search strategy. We also acknowledge Andrea Patey, MSc (School of Health Sciences, City University London), and Dr Sumit Majumdar, MD MSc FRCPC (University of Alberta) for identifying additional relevant citations.
Funding
DJN is funded through a Clinician Fellowship Award from Alberta Innovates – Health Solutions, and a Knowledge Translation Canada Student Fellowship and Training Program grant. HTS is supported by a Population Health Investigator Award from Alberta Innovates – Health Solutions. SES is funded by a Tier 1 Canada Research Chair. BRH is supported by the Roy and Vi Baay Chair in Kidney Research. The funding agencies did not contribute to design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the final manuscript.
Abbreviations
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CENTRAL
Cochrane Central Register of Controlled Trials
- COX-2
cyclo-oxygenase-2
- GuNFT
Guideline for Not Funding Health Technology
- HERS
Heart and Estrogen/progestin Replacement Study
- HRT
hormone replacement therapy
- HTR
health technology reassessment
- IPPB
intermittent positive pressure breathing
- IQR
inter-quartile range
- MeSH
Medical Subject Heading
- NICE
National Institute for Health and Care Excellence
- NSAIDs
non-steroidal anti-inflammatory drugs
- PAC
pulmonary artery catheter
- PBMA
program budgeting and marginal analysis
- PCI
percutaneous coronary intervention
- PCT
Primary Care Trusts
- PPI
proton pump inhibitor
- PRESS
Peer Review of Electronic Search Strategies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCT
randomized controlled trial
- RRP
retropubic radical prostatectomy
- STEMI
ST-elevation myocardial infarction
- WHI
Women’s Health Initiative
Additional file
Table S1. Description of articles included in the scoping review. Appendix. MEDLINE search. (DOCX 201 kb)
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
DJN designed the study, screened citations for inclusion, extracted data from included citations, performed and interpreted all analyses, and drafted the final manuscript. KJM and JKH screened citations for inclusion, extracted data from included citations, interpreted analyses, and revised the manuscript for important intellectual content. BRH and LPJ assisted with study design, interpreted analyses, and revised the manuscript for important intellectual content. SES and HTS supervised the conduct of the study, and as such were involved with study design, interpretation of analyses, and contributed key revisions to the intellectual content of the manuscript. All authors agree to be accountable for all aspects of the work and approved the final manuscript for submission.
Authors' information
Not applicable.
Contributor Information
Daniel J. Niven, Email: Daniel.niven@albertahealthservices.ca
Kelly J. Mrklas, Email: Kelly.mrklas@albertahealthservices.ca
Jessalyn K. Holodinsky, Email: jkholodi@ucalgary.ca
Sharon E. Straus, Email: Sharon.straus@utoronto.ca
Brenda R. Hemmelgarn, Email: Brenda.hemmelgarn@albertahealthservices.ca
Lianne P. Jeffs, Email: jeffsl@smh.ca
Henry Thomas Stelfox, Email: tstelfox@ucalgary.ca.
References
- 1.Second International Study of Infarct Survival (ISIS-2) Collaborative Group Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;332:349–360. doi: 10.1016/S0140-6736(88)92833-4. [DOI] [PubMed] [Google Scholar]
- 2.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/S0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 3.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 4.Prasad V, Cifu A. Medical reversal: why we must raise the bar before adopting new technologies. Yale J Biol Med. 2011;84:471–478. [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad V, Gall V, Cifu A. The frequency of medical reversal. Arch Intern Med. 2011;171:1675–1676. doi: 10.1001/archinternmed.2011.295. [DOI] [PubMed] [Google Scholar]
- 6.Cameron C, Coyle D, Ur E, Klarenbach S. Cost-effectiveness of self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin. CMAJ. 2010;182:28–34. doi: 10.1503/cmaj.090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad V, Vandross A, Toomey C, Cheung M, Rho J, Quinn S, et al. A decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clinic Proc. 2013;88:790–798. doi: 10.1016/j.mayocp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Elshaug AG, Watt AM, Mundy L, Willis CD. Over 150 potentially low-value health care practices: an Australian study. Med J Austr. 2012;197:556–560. doi: 10.5694/mja12.11083. [DOI] [PubMed] [Google Scholar]
- 9.Rogers EM. Diffusion of Innovations. 5. New York, New York: Free Press; 2003. The innovation-decision process; pp. 168–218. [Google Scholar]
- 10.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 11.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62:944–952. doi: 10.1016/j.jclinepi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Grey Matters: a practical search tool for evidence-based medicine. Canadian Agency for Drugs and Technologies in Health. 2013. http://www.cadth.ca/en/resources/grey-matters. Accessed 1 Mar 2014.
- 15.Straus SE, Tetroe J, Graham ID. Knowledge translation in health care: moving from evidence to practice. 2. Oxford, UK: John Wiley & Sons, Ltd.; 2013. [Google Scholar]
- 16.Greenhalgh T, Robert G, Bate P, Macfarlane F, Kyriakidou O. Diffusion of innovations in health service organisations: a systematic literature review. Malden, MA: Blackwell Publishing Ltd.; 2005. [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 18.Hauptman PJ, Schnitzler MA, Swindle J, Burroughs TE. Use of nesiritide before and after publications suggesting drug-related risks in patients with acute decompensated heart failure. JAMA. 2006;296:1877–1884. doi: 10.1001/jama.296.15.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polisena J, Clifford T, Elshaug AG, Mitton C, Russell E, Skidmore B. Case studies that illustrate disinvestment and resource allocation decision-making processes in health care: a systematic review. Int J Technol Assess Health Care. 2013;29:174–184. doi: 10.1017/S0266462313000068. [DOI] [PubMed] [Google Scholar]
- 20.Levin L. Disinvestment strategies based on evidence guided adoption and obsolescence of technologies: the Ontario experience. Canadian Foundation for Healthcare Improvement. 2011. http://www.cfhi-fcass.ca/Libraries/CEO_Forum_files/LevinENG.sflb.ashx. Accessed 4 Mar 2014.
- 21.Choosing Wisely: Five Things Physicians and Patients Should Question. American Board of Internal Medicine Foundation. 2012. http://www.choosingwisely.org/clinician-lists/. Accessed 4 Mar 2014.
- 22.Choosing Wisely Canada. Canadian Medical Association. 2014. http://www.choosingwiselycanada.org. Accessed 1 May 2014.
- 23.Hollingworth W, Busby J, Jones H, Sterne J. Can variation in hospital procedure rates identify candidates for health technology reassessment and disinvestment? Value Health. 2013;16:A470. doi: 10.1016/j.jval.2013.08.853. [DOI] [Google Scholar]
- 24.Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 25.Roumie CL, Grogan EL, Falbe W, Awad J, Speroff T, Dittus RS, et al. A three-part intervention to change the use of hormone replacement therapy in response to new evidence. Ann Intern Med. 2004;141:118–125. doi: 10.7326/0003-4819-141-2-200407200-00010. [DOI] [PubMed] [Google Scholar]
- 26.Sindby JE, Brocki BC, Rasmussen BS, Gorst-Rasmussen A, Andreasen JJ. Efforts to change transfusion practice behaviour and reduce transfusion rates are effective in coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2011;1:S46. doi: 10.1053/j.jvca.2011.03.122. [DOI] [PubMed] [Google Scholar]
- 27.Krol N, Wensing M, Haaijer-Ruskamp F, Muris JWM, Numans ME, Schattenberg G, et al. Patient-directed strategy to reduce prescribing for patients with dyspepsia in general practice: a randomized trial. Aliment Pharmacol Ther. 2004;19:917–922. doi: 10.1111/j.1365-2036.2004.01928.x. [DOI] [PubMed] [Google Scholar]
- 28.Kulawik D, Sands JJ, Mayo K, Fenderson M, Hutchinson J, Woodward C, et al. Focused vascular access education to reduce the use of chronic tunneled hemodialysis catheters: results of a network quality improvement initiative. Semin Dial. 2009;22:692–697. doi: 10.1111/j.1525-139X.2009.00647.x. [DOI] [PubMed] [Google Scholar]
- 29.Ross-Degnan D, Soumerai SB, Fortess EE, Gurwitz JH. Examining product risk in context. Market withdrawal of zomepirac as a case study. JAMA. 1993;270:1937–1942. doi: 10.1001/jama.1993.03510160055029. [DOI] [PubMed] [Google Scholar]
- 30.Williams D, Singh M, Hind C. The effect of the withdrawal of rofecoxib on prescribing patterns of COX-2 inhibitors in Scotland. Br J Clin Pharmacol. 2006;62:366–368. doi: 10.1111/j.1365-2125.2006.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiebaud P, Patel BV, Nichol MB. Impact of rofecoxib withdrawal on cyclooxygenase-2 utilization among patients with and without cardiovascular risk. Value Health. 2006;9:361–368. doi: 10.1111/j.1524-4733.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 32.Barozzi N, Tett SE. What happened to the prescribing of other COX-2 inhibitors, paracetamol and non-steroidal anti-inflammatory drugs when rofecoxib was withdrawn in Australia? Pharmacoepidemiol Drug Saf. 2007;16:1184–1191. doi: 10.1002/pds.1451. [DOI] [PubMed] [Google Scholar]
- 33.Sun SX, Lee KY, Bertram CT, Goldstein JL. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin. 2007;23:1859–1866. doi: 10.1185/030079907X210561. [DOI] [PubMed] [Google Scholar]
- 34.Setakis E, Leufkens HG, van Staa TP. Changes in the characteristics of patients prescribed selective cyclooxygenase 2 inhibitors after the 2004 withdrawal of rofecoxib. Arthritis Rheum. 2008;59:1105–1111. doi: 10.1002/art.23925. [DOI] [PubMed] [Google Scholar]
- 35.Sukel MP, van der Linden MW, Chen C, Erkens JA, Herings RM. Large-scale stopping and switching treatment with COX-2 inhibitors after the rofecoxib withdrawal. Pharmacoepidemiol Drug Saf. 2008;17:9–19. doi: 10.1002/pds.1508. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao FY, Tsai YW, Huang WF. Changes in physicians’ practice of prescribing cyclooxygenase-2 inhibitor after market withdrawal of rofecoxib: a retrospective study of physician-patient pairs in Taiwan. Clin Ther. 2009;31:2618–2627. doi: 10.1016/j.clinthera.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Stafford RS, Radley DC. National trends in antiobesity medication use. Arch Intern Med. 2003;163:1046–1050. doi: 10.1001/archinte.163.9.1046. [DOI] [PubMed] [Google Scholar]
- 38.Grady D, Redberg RF. Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750. doi: 10.1001/archinternmed.2010.90. [DOI] [PubMed] [Google Scholar]
- 39.Macleod MR, Michie S, Roberts I, Dirnagl U, Chalmers I, Ioannidis JP, et al. Biomedical research: increasing value, reducing waste. Lancet. 2014;383:101–104. doi: 10.1016/S0140-6736(13)62329-6. [DOI] [PubMed] [Google Scholar]
- 40.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- 41.Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13–24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]
- 42.Haas M, Hall J, Viney R, Gallego G. Breaking up is hard to do: why disinvestment in medical technology is harder than investment. Aust Health Rev. 2012;36:148–152. doi: 10.1071/AH11032. [DOI] [PubMed] [Google Scholar]
- 43.Montini T, Graham ID. “Entrenched practices and other biases”: unpacking the historical, economic, professional, and social resistance to de-implementation. Implement Sci. 2015;10:24. doi: 10.1186/s13012-015-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duhigg C. The power of habit: why we do what we do in life and business. New York: Random House Trade Paperbacks; 2012. [Google Scholar]
- 45.Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175:801–809. doi: 10.1001/jamainternmed.2015.0157. [DOI] [PubMed] [Google Scholar]
- 46.Watt AM, Hiller JE, Braunack-Mayer AJ, Moss JR, Buchan H, Wale J, et al. The ASTUTE Health study protocol: deliberative stakeholder engagements to inform implementation approaches to healthcare disinvestment. Implement Sci. 2012;7:101. doi: 10.1186/1748-5908-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watt AM, Willis CD, Hodgetts K, Elshaug AG, Hiller JE. Engaging clinicians in evidence-based disinvestment: role and perceptions of evidence. Int J Technol Assess Health Care. 2012;28:211–219. doi: 10.1017/S0266462312000402. [DOI] [PubMed] [Google Scholar]
- 48.Elshaug AG, Moss JR, Littlejohns P, Karnon J, Merlin TL, Hiller JE. Identifying existing health care services that do not provide value for money. Med J Austr. 2009;190:269–273. doi: 10.5694/j.1326-5377.2009.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 49.Elshaug AG, Hiller JE, Moss JR. Exploring policy-makers’ perspectives on disinvestment from ineffective healthcare practices. Int J Technol Assess Health Care. 2008;24:1–9. doi: 10.1017/S0266462307080014. [DOI] [PubMed] [Google Scholar]
- 50.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34:1262–1269. doi: 10.1093/eurheartj/ehs481. [DOI] [PubMed] [Google Scholar]
- 51.Garner S, Littlejohns P. Disinvestment from low value clinical interventions: NICEly done? BMJ. 2011;343:d4519. doi: 10.1136/bmj.d4519. [DOI] [PubMed] [Google Scholar]
- 52.Henshall C, Schuller T, Mardhani-Bayne L. Using health technology assessment to support optimal use of technologies in current practice: the challenge of “disinvestment”. Int J Technol Assess Health Care. 2012;28:203–210. doi: 10.1017/S0266462312000372. [DOI] [PubMed] [Google Scholar]
- 53.Moynihan RN. A healthy dose of disinvestment. Med J Austr. 2012;196:158–8. doi: 10.5694/mja12.10011. [DOI] [PubMed] [Google Scholar]
- 54.Ibargoyen-Roteta N, Gutierrez-Ibarluzea I, Asua J, Benguria-Arrate G, Galnares-Cordero L. Scanning the horizon of obsolete technologies: possible sources for their identification. Int J Technol Assess Health Care. 2009;25:249–254. doi: 10.1017/S0266462309990249. [DOI] [PubMed] [Google Scholar]
- 55.Ibargoyen-Roteta N, Gutierrez-Ibarluzea I, Asua J. Guiding the process of health technology disinvestment. Health Policy. 2010;98:218–226. doi: 10.1016/j.healthpol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Chamberlain CA, Martin RM, Busby J, Gilbert R, Cahill DJ, Hollingworth W. Trends in procedures for infertility and caesarean sections: was NICE disinvestment guidance implemented? NICE recommendation reminders. BMC Public Health. 2013;13:112. doi: 10.1186/1471-2458-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerdvilaite J, Nachtnebel A. Disinvestment: overview of disinvestment experiences and challenges in selected countries. Ludwig Boltzman Institut fur Health Technology Assessment. 2011. http://eprints.hta.lbg.ac.at/926/##. Accessed 4 Mar 2014.
- 58.Leggett L, Noseworthy TW, Zarrabi M, Lorenzetti D, Sutherland LR, Clement FM. Health technology reassessment of non-drug technologies: current practices. Int J Technol Assess Health Care. 2012;28:220–227. doi: 10.1017/S0266462312000438. [DOI] [PubMed] [Google Scholar]
- 59.Leggett LE, Mackean G, Noseworthy TW, Sutherland L, Clement F. Current status of health technology reassessment of non-drug technologies: survey and key informant interviews. Health Res Policy Syst. 2012;10:38. doi: 10.1186/1478-4505-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper C, Starkey K. Disinvestment in health care. BMJ. 2010;340:c1413–3. doi: 10.1136/bmj.c1413. [DOI] [Google Scholar]
- 61.Karnon J, Carlton J, Czoski-Murray C, Smith K. Informing disinvestment through cost-effectiveness modelling: is lack of data a surmountable barrier? Appl Health Econ Health Policy. 2009;7:1–9. doi: 10.1007/BF03256137. [DOI] [PubMed] [Google Scholar]
- 62.Hodgetts K, Elshaug AG, Hiller JE. What counts and how to count it: physicians constructions of evidence in a disinvestment context. Soc Sci Med. 2012;75:2191–2199. doi: 10.1016/j.socscimed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Hughes DA, Ferner RE. New drugs for old: disinvestment and NICE. BMJ. 2010;340:c572. doi: 10.1136/bmj.c572. [DOI] [PubMed] [Google Scholar]
- 64.Street JM, Hennessy SE, Watt AM, Hiller JE, Elshaug AG. News and social media: windows into community perspectives on disinvestment. Int J Technol Assess Health Care. 2011;27:376–383. doi: 10.1017/S026646231100033X. [DOI] [PubMed] [Google Scholar]
- 65.Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. doi: 10.1186/1748-5908-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elshaug AG, Hiller JE, Tunis SR, Moss JR. Challenges in Australian policy processes for disinvestment from existing, ineffective health care practices. Aust New Zealand Health Policy. 2007;4:23. doi: 10.1186/1743-8462-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams IP, Bryan S. Cost-effectiveness analysis and formulary decision making in England: findings from research. Soc Sci Med. 2007;65:2116–2129. doi: 10.1016/j.socscimed.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Mortimer D. Reorienting programme budgeting and marginal analysis (PBMA) towards disinvestment. BMC Health Serv Res. 2010;10:288. doi: 10.1186/1472-6963-10-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nuti S, Vainieri M, Bonini A. Disinvestment for re-allocation: a process to identify priorities in healthcare. Health Policy. 2010;95:137–143. doi: 10.1016/j.healthpol.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Garner S, Docherty M, Somner J, Sharma T, Choudhury M, Clarke M, et al. Reducing ineffective practice: challenges in identifying low-value health care using Cochrane systematic reviews. J Health Serv Res Policy. 2013;18:6–12. doi: 10.1258/jhsrp.2012.012044. [DOI] [PubMed] [Google Scholar]
- 71.Noseworthy T, Clement F. Health technology reassessment: scope, methodology, & language. Int J Technol Assess Health Care. 2012;28:201–202. doi: 10.1017/S0266462312000359. [DOI] [PubMed] [Google Scholar]
- 72.Donaldson C, Bate A, Mitton C, Dionne F, Ruta D. Rational disinvestment. QJM. 2010;103:801–807. doi: 10.1093/qjmed/hcq086. [DOI] [PubMed] [Google Scholar]
- 73.Haines T, O'Brien L, McDermott F, Markham D, Mitchell D, Watterson D, et al. A novel research design can aid disinvestment from existing health technologies with uncertain effectiveness, cost-effectiveness, and/or safety. J Clin Epidemiol. 2014;67:144–151. doi: 10.1016/j.jclinepi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Paulden M. Investment and disinvestment of health technologies: The need for two cost-effectiveness thresholds. Value Health. 2012;15:A33. doi: 10.1016/j.jval.2012.03.188. [DOI] [Google Scholar]
- 75.Pearson S, Littlejohns P. Reallocating resources: how should the National Institute for Health and Clinical Excellence guide disinvestment efforts in the National Health Service? J Health Serv Res Policy. 2007;12:160–165. doi: 10.1258/135581907781542987. [DOI] [PubMed] [Google Scholar]
- 76.Hislop JM. Societal preferences for health technology disinvestment policy: Views of Scottish taxpayers - a qualitative study. Value Health. 2011;14:A356–A357. doi: 10.1016/j.jval.2011.08.680. [DOI] [Google Scholar]
- 77.Kelly M. Public health programmes and interventions and disinvestment. National Institute for Health and Care Excellence. 2006. https://www.nice.org.uk/proxy/?sourceUrl=http%3A%2F%2Fwww.nice.org.uk%2FniceMedia%2Fpdf%2Fsmt%2F040406item5.pdf . Accessed 4 Mar 2014.
- 78.Garner S. Disinvestment: the UK experience. International Society for Pharmacoeconomics and Outcomes Research, 15th Annual European Congress. 2012. http://www.ispor.org/congresses/berlin1112/presentations/W10_Garner.pdf. Accessed 4 Mar 2014.
- 79.Harris E, Mundy L, Hewson K, Jacobsen N. Disinvestment in Australia and New Zealand. HealthPACT. 2013. http://www.health.qld.gov.au/healthpact. Accessed 4 Mar 2014.
- 80.Joshi NP, Stahnisch FW, Noseworthy TW. Reassessment of health technologies: obsolescence and waste. Canadian Agency for Drugs & Technologies in Health. 2009. https://www.cadth.ca/reassessment-health-technologies-obsolescence-and-waste. Accessed 22 October 2013.
- 81.Leng G. Introduction of new disinvestment programmes. National Institute for Health & Care Excellence. 2006. https://www.nice.org.uk/proxy/?sourceUrl=http%3A%2F%2Fwww.nice.org.uk%2FniceMedia%2Fpdf%2Fsmt%2F210206item3.pdf. Accessed 4 Mar 2014.
- 82.Kowalczyk KJ, Levy JM, Caplan CF, Lipsitz SR, Yu H-y. Gu X, et al. Temporal national trends of minimally invasive and retropubic radical prostatectomy outcomes from 2003 to 2007: results from the 100 % Medicare sample. Eur Urol. 2012;61:803–809. doi: 10.1016/j.eururo.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Ioannidis JP. In reply II-reversal of medical practices. Mayo Clinic Proc. 2013;88:1184. doi: 10.1016/j.mayocp.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Bonakdar tehrani A, Howard D. Potential cost savings from comparative effectiveness research: lessons from COURAGE study. Value Health. 2011;14:A2. doi: 10.1016/j.jval.2011.02.013. [DOI] [Google Scholar]
- 85.Prasad V, Cifu A. In reply I-reversal of medical practices. Mayo Clinic Proc. 2013;88:1183–1184. doi: 10.1016/j.mayocp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Tatsioni A, Siontis GCM, Ioannidis JPA. Partisan perspectives in the medical literature: a study of high frequency editorialists favoring hormone replacement therapy. J Gen Intern Med. 2010;25:914–919. doi: 10.1007/s11606-010-1360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koo KK, Sun JC, Zhou Q, Guyatt G, Cook DJ, Walter SD, et al. Pulmonary artery catheters: evolving rates and reasons for use. Crit Care Med. 2011;39:1613–1618. doi: 10.1097/CCM.0b013e318218a045. [DOI] [PubMed] [Google Scholar]
- 88.Luetmer MT, Kallmes DF. Have referral patterns for vertebroplasty changed since publication of the placebo-controlled trials? Am J Neuroradiol. 2011;32:647–648. doi: 10.3174/ajnr.A2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deyell MW, Buller CE, Miller LH, Wang TY, Dai D, Lamas GA, et al. Impact of National Clinical Guideline recommendations for revascularization of persistently occluded infarct-related arteries on clinical practice in the United States. Arch Int Med. 2011;171:1636–1643. doi: 10.1001/archinternmed.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atwater BD, Oujiri J, Wolff MR. The immediate impact of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial on the management of stable angina. Clin Cardiol. 2009;32:E1–E3. doi: 10.1002/clc.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 92.Murphy DJ, Needham DM, Netzer G, Zeger SL, Colantuoni E, Ness P, et al. RBC transfusion practices among critically ill patients. Crit Care Med. 2013;41:2344–2353. doi: 10.1097/CCM.0b013e31828e9a49. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed B, Dauerman HL, Piper WD, Robb JF, Verlee MP, Ryan TJ, Jr, et al. Recent changes in practice of elective percutaneous coronary intervention for stable angina. Circ Cardiovasc Qual Outcomes. 2011;4:300–305. doi: 10.1161/CIRCOUTCOMES.110.957175. [DOI] [PubMed] [Google Scholar]
- 94.Ehrenstein V, Hernandez RK, Ulrichsen SP, Rungby J, Lash TL, Riis AH, et al. Rosiglitazone use and post-discontinuation glycaemic control in two European countries, 2000–2010. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie F, Petitti DB, Chen W. Prescribing patterns for antihypertensive drugs after the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial: report of experience in a health maintenance organization. Am J Hypertens. 2005;18:464–469. doi: 10.1016/j.amjhyper.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Stafford RS, Furberg CD, Finkelstein SN, Cockburn IM, Alehegn T, Ma J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996–2002. JAMA. 2004;291:54–62. doi: 10.1001/jama.291.1.54. [DOI] [PubMed] [Google Scholar]
- 97.Majumdar SR, Inui TS, Gurwitz JH, Gillman MW, McLaughlin TJ, Soumerai SB. Influence of physician specialty on adoption and relinquishment of calcium channel blockers and other treatments for myocardial infarction. J Gen Intern Med. 2001;16:351–359. doi: 10.1046/j.1525-1497.2001.016006351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA. 2004;292:1983–1988. doi: 10.1001/jama.292.16.1983. [DOI] [PubMed] [Google Scholar]
- 99.Austin PC, Mamdani MM, Tu K, Jaakkimainen L. Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women’s Health Initiative Study. JAMA. 2003;289:3241–3242. doi: 10.1001/jama.289.24.3241. [DOI] [PubMed] [Google Scholar]
- 100.Massatti RR, Sweeney HA, Panzano PC, Roth D. The de-adoption of innovative mental health practices (IMHP): why organizations choose not to sustain an IMHP. Adm Policy Mental Health. 2008;35:50–65. doi: 10.1007/s10488-007-0141-z. [DOI] [PubMed] [Google Scholar]
- 101.Howard DH, Kenline C, Lazarus HM, Lemaistre CF, Maziarz RT, McCarthy PL, Jr, et al. Abandonment of high-dose chemotherapy/hematopoietic cell transplants for breast cancer following negative trial results. Health Serv Res. 2011;46:1762–1777. doi: 10.1111/j.1475-6773.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azermai M, Vander Stichele RR, Van Bortel LM, Elseviers MM. Barriers to antipsychotic discontinuation in nursing homes: an exploratory study. Aging Ment Health. 2014;18:346–353. doi: 10.1080/13607863.2013.832732. [DOI] [PubMed] [Google Scholar]
- 103.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 104.Tatsioni A, Bonitsis NG, Ioannidis JPA. Persistence of contradicted claims in the literature. JAMA. 2007;298:2517–2526. doi: 10.1001/jama.298.21.2517. [DOI] [PubMed] [Google Scholar]
- 105.Duffy SQ, Farley DE. The protracted demise of medical technology. The case of intermittent positive pressure breathing. Med Care. 1992;30:718–736. doi: 10.1097/00005650-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 106.Howard DH, Shen YC. Comparative effectiveness research, technological abandonment, and health care spending. Adv Health Econ Health Serv Res. 2012;23:103–121. doi: 10.1108/S0731-2199(2012)0000023007. [DOI] [PubMed] [Google Scholar]
- 107.Prasad V, Cifu A, Ioannidis JPA. Reversals of established medical practices: evidence to abandon ship. JAMA. 2012;307:37–38. doi: 10.1001/jama.2011.1960. [DOI] [PubMed] [Google Scholar]
- 108.Banta HD, Thacker SB. The case for reassessment of health care technology. Once is not enough. JAMA. 1990;264:235–240. doi: 10.1001/jama.1990.03450020087032. [DOI] [PubMed] [Google Scholar]
- 109.Elshaug AG, Watt AM, Moss JR, Hiller JE. Policy perspectives on the obsolescence of health technologies in Canada. Canadian Agency for Drugs and Technologies in Health. 2009. https://www.cadth.ca/media/pdf/Obsolescence%20of%20Health%20Technologies%20in%20Canada_Policy_Forum_e.pdf. Accessed 21 Oct 2013.
- 110.MacKean G, Noseworthy T, Elshaug AG, Leggett L, Littlejohns P, Berezanski J, et al. Health technology reassessment: the art of the possible. Int J Technol Assess Health Care. 2013;29:418–423. doi: 10.1017/S0266462313000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Towards better patient care: drugs to avoid. Prescrire Int. 2013, 22:108–111. [PubMed]
- 112.Kiechle FL, Arcenas RC, Rogers LC. Establishing benchmarks and metrics for disruptive technologies, inappropriate and obsolete tests in the clinical laboratory. Clin Chim Acta. 2014;427:131–136. doi: 10.1016/j.cca.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad V, Vandross A. Cardiovascular primary prevention: how high should we set the bar? Arch Intern Med. 2012;172:656–659. doi: 10.1001/archinternmed.2012.812. [DOI] [PubMed] [Google Scholar]
- 114.Warner JL, Yang P, Alterovitz G. Reversal of medical practices. Mayo Clinic Proc. 2013;88:1182–1183. doi: 10.1016/j.mayocp.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Medical Reversal. Life in the FastLane. 2014. http://lifeinthefastlane.com/education/ccc/medical-reversal. Accessed 3 Jun 2014.
- 116.Moscucci M. Medical reversal, clinical trials, and the “late” open artery hypothesis in acute myocardial infarction. Arch Intern Med. 2011;171:1643–1644. doi: 10.1001/archinternmed.2011.299. [DOI] [PubMed] [Google Scholar]
- 117.Fatovich DM. Medical reversal: what are you doing wrong for your patient today? Emerg Med Australas. 2013;25:1–3. doi: 10.1111/1742-6723.12044. [DOI] [PubMed] [Google Scholar]
- 118.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 119.Siontis GCM, Tatsioni A, Katritsis DG, Ioannidis JPA. Persistent reservations against contradicted percutaneous coronary intervention indications: citation content analysis. Am Heart J. 2009;157:695–701. doi: 10.1016/j.ahj.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 120.Brunt ME, Murray MD, Hui SL, Kesterson J, Perkins AJ, Tierney WM. Mass media release of medical research results: an analysis of antihypertensive drug prescribing in the aftermath of the calcium channel blocker scare of March 1995. J Gen Intern Med. 2003;18:84–94. doi: 10.1046/j.1525-1497.2003.20502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smalley W, Shatin D, Wysowski DK, Gurwitz J, Andrade SE, Goodman M, et al. Contraindicated use of cisapride: impact of food and drug administration regulatory action. JAMA. 2000;284:3036–3039. doi: 10.1001/jama.284.23.3036. [DOI] [PubMed] [Google Scholar]
- 122.Huang WF, Tsai YW, Hsiao FY, Liu WC. Changes of the prescription of hormone therapy in menopausal women: an observational study in Taiwan. BMC Public Health. 2007;7:56. doi: 10.1186/1471-2458-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Naylor CD. The complex world of prescribing behavior. JAMA. 2004;291:104–106. doi: 10.1001/jama.291.1.104. [DOI] [PubMed] [Google Scholar]
- 124.Lawton B, Rose S, McLeod D, Dowell A. Changes in use of hormone replacement therapy after the report from the Women’s Health Initiative: cross sectional survey of users. BMJ. 2003;327:845–846. doi: 10.1136/bmj.327.7419.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Voorn VM, de Mheen PJ. M-v, So-Osman C, Vlieland TP, Koopman-van Gemert AW, Nelissen RG, et al. Designing a strategy to implement cost-effective blood transfusion management in elective hip and knee arthroplasties: a study protocol. Implement Sci. 2012;7:58. doi: 10.1186/1748-5908-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Atkins D. Connecting research and patient care: lessons from the VA’s Quality Enhancement Research Initiative. J General Intern Med. 2009;25:1–2. doi: 10.1007/s11606-009-1149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elshaug AG, McWilliams JM, Landon BE. The value of low-value lists. JAMA. 2013;309:775–776. doi: 10.1001/jama.2013.828. [DOI] [PubMed] [Google Scholar]
- 128.Gershengorn HB, Wunsch H. Understanding changes in established practice: pulmonary artery catheter use in critically ill patients. Crit Care Med. 2013;41:2667–2676. doi: 10.1097/CCM.0b013e318298a41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colmenares P. Proposal for a state health technology assessment program. WMJ. 2012;111:176–182. [PubMed] [Google Scholar]
- 130.‘Do not do’ recommendations. National Institute for Health and Care Excellence. 2014 http://www.nice.org.uk/proxy/?sourceUrl=http%3a%2f%2fwww.nice.org.uk%2fusingguidance%2fdonotdorecommendations%2fsearch.jsp. Accessed 3 Jun 2014.


