Abstract
Matrix metalloproteinases (MMPs) are crucial extracellular matrices degrading enzymes that have important roles in metastasis of cancer progression as well as other significant conditions such as oxidative stress and hepatic fibrosis. Marine plants are on the rise for their potential to provide natural products that exhibit remarkable health benefits. In this context, brown algae species have been of much interest in the pharmaceutical field with reported instances of isolation of bioactive compounds against tumor growth and MMP activity. In this study, eight different brown algae species were harvested, and their extracts were compared in regard to their anti-MMP effects. According to gelatin zymography results, Ecklonia cava, Ecklonia bicyclis, and Ishige okamurae showed higher inhibitory effects than the other samples on MMP-2 and -9 activity at the concentrations of 10, 50, and 100 μg/mL. However, only I. okamurae was able to regulate the MMP activity through the expression of MMP and tissue inhibitor of MMP observed by mRNA levels. Overall, brown algae species showed to be good sources for anti-MMP agents, while I. okamurae needs to be further studied for its potential to yield pharmaceutical molecules that can regulate MMP-activity through cellular pathways as well as enzymatic inhibition.
Keywords: brown algae, matrix metalloproteinase (MMP)-9, MMP-2, tissue inhibitor of MMP (TIMP)-1, TIMP-2
INTRODUCTION
Ongoing cancer research has acknowledged the matrix metalloproteinases (MMPs) as significant enzymes that are involved in several steps of cancer-related conditions (1). Especially in terms of invasive tumor cells, MMP expression and activitiy are deteriorated and closely linked to cancer onset (2,3). MMPs are zinc-dependent endo-peptidases responsible for extracellular matrix degradation that play crucial roles in the progression of various disorders including inflammation, arthritis, cardiovascular diseases, pulmonary diseases, and cancer (4,5). Different types of MMPs have different roles depending on which tissue they are produced. The main duty of MMPs is in extracellular matrix degradation, cell surface receptor cleave, apoptotic ligand release, and activation/inactivation of several cytokines that are processed by the involvement of MMPs. Regarding their function in different tissues, different classifications occur. For instance, collagenases, responsible for collagen degradation, are mainly present in bone-related tissues while gelatinases are expressed mainly by fibroblasts and are responsible for gelatin cleavage (6). Among all different types of MMPs, MMP-2 (gelatinase A, 72 kDa), and MMP-9 (gelatinase B, 92 kDa) are suggested to play pivotal roles in tumor invasion and metastasis. Both of these MMPs are abundantly produced in various malignant tumors (7). Notably, increased MMP-2 and -9 expression levels are suggested to result in markedly enhanced metastasis (8). Hence, MMPs are considered to be essential in tumor cells due to their ability to degrade the extracellular matrix. Therefore, much attention has focused on the development of MMP inhibitors as a new class of cancer therapeutic targets of current interest. Regulation of MMP-2 and -9 activities are suggested to possess a therapeutic potential against cancer (5). The pathways of MMP-2 and -9 activation is regulated by tissue inhibitors of MMP (TIMP) through a negative feedback mechanism. All MMPs, except gelatinases, are inhibited by TIMP expression while gelatinases are expressed to form complexes with TIMPs and further activated by a cell surface MMP complex called MT1-MMP (MMP-14). In case of malignant tumors, the normal action of TIMPs ought to be deteriorated to further facilitate the activation of elevated MMP expression coupled with MT1-MMP (9).
Although there are important achievements in synthetic drug development, natural products and compounds derived from natural origins are still of high interest due to various health beneficial properties with high potential to be utilized as nutraceuticals. In terms of bioactive natural products, algae have been intensively studied and several bioactivities have already been reported promoting marine plants as important sources for pharmaceuticals (6,7). In addition, marine algae are credited to be a healthy source for consumption due to their low content in harmful lipids and high content in polysaccharides, unsaturated fatty acids, vitamins, and minerals as well as reported bioactive compounds such as sulfated polysaccharides, phlorotannins and glycoproteins (8–10). Among algae, the division Phaeophyta, also known as brown seaweeds, includes some of the most studied species of marine plants, namely Ecklonia, Laminaria, Undaria, and Himanthalia. In addition to contain high nutritious value, other important contents found in the aforementioned brown seaweeds include phenolic compounds, sulfated polysaccharides, quinones, and several secondary metabolites that are studied for their activities against numerous diseases and conditions (11–13). To date, marine plants have been studied to develop anticancer materials such as MMP inhibitors. Brown algae species also were found to produce MMP inhibitors, although any antitumor compound that is able to reach clinical trials has not been reported. Therefore, on the way to develop natural anticancer compounds, specifically MMP inhibitors, this study aims to provide insights on the potential of brown algae as a MMP inhibitor source. Hence, eight different kinds of brown algae from Korean shores were compared in regard to their anti-metastatic properties through MMP-activity based assays. Following a literature survey, Sargassum siliquastrum, Ecklonia cava, Sargassum thunbergii, Ecklonia bicyclis, Hizikia fusiformis, Ishige okamurae, Sargassum horneri, and Laminaria japonica were chosen to be screened as potential MMP-inhibitors due to their availability in the daily Korean diet, as well as reported bioactive potential as sources of compounds with notable health effects (10,11).
MATERIALS AND METHODS
Plant materials and extract preparation
Brown algae samples were purchased from different locations as follows: Sargassum siliquastrum, Ecklonia cava, Sargassum thunbergii, Ishige okamurae, and Sargassum horneri from Parajeju (Jeju, Korea). Hizikia fusiformis and Laminaria japonica from Jeonbok Maeul (Mokpo, Korea), and Ecklonia bicyclis from Ulleungdomall (Ulleung, Korea). The samples were air-dried under shade and ground to powder. Next, 250 g of powdered sample was extracted with 10-fold (w/v, 2,500 mL) of 80% ethanol for 4 h by stirring at 80°C. The extract was then filtered with a Whatman® qualitative filter paper (Sigma-Aldrich, St. Louis, MO, USA) and dried to powder. The obtained extract powder was extracted once more with 10-fold (w/v, 2,500 mL) of 80% ethanol for 4 h by stirring at 80°C and filtered again. Finally, the extract was concentrated using a vacuum rotary evaporator (RV 10 Series, IKA, Wilmington, NC, USA) followed by dissolution in dimethyl sulfoxide (DMSO).
Cell culture and cytotoxicity determination
Human fibrosarcoma HT1080 cells (Korean Cell Line Bank, Seoul, Korea) were grown as monolayers in T-75 tissue culture flasks (Nunc A/S, Roskilde, Denmark) at 5% CO2 and 37°C humidified atmosphere using Dulbecco’s modified eagle medium (DMEM, Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum, 2 mM glutamine, and 100 μg/mL penicillin-streptomycin (Gibco-BRL). The medium was changed twice or three times each week.
In order to determine non-toxic concentrations of brown algae samples, the cytotoxic effects were evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay in HT1080 cells. The cells were grown in 96-well plates at a density of 5×103 cells/well. After 24 h, the cells were washed with fresh medium and were treated with control medium or the medium supplemented with brown algae samples. After incubation for 24 and 48 h, cells were rewashed, and 100 μL of MTT solution (1 mg/mL) was added and incubated for 4 h. Finally, 100 μL of DMSO was added to solubilize the formed formazan crystals, and the amount of formazan crystal was determined by measuring the absorbance at 540 nm using a GENios® microplate reader (Tecan Austria GmbH, Grödig, Austria). Relative cell viability was determined by the amount of MTT converted into formazan crystal. Viability of cells was quantified as a percentage compared to the control, and dose response curves were developed.
Cell migration assay
Cells were plated on a 12-well culture dish with 90% confluence, and an injury line with a width of 2 mm was made by scraping across the cell monolayer with a sterile scraper. After floating cell debris was removed by washing with PBS, cell medium was replaced with serum-free medium, and cells were treated with 10, 50, and 100 μg/mL brown algae samples. Cell migration in the presence of samples was monitored under an inverted microscope (Nikon Eclipse TS100, Nikon Inc., Melville, NY, USA) and photographed at incubation starting time and after 24 h incubation.
Determination of MMP activity by gelatin zymography
Activities of MMP-2 and MMP-9 in HT1080 cells treated with samples were determined by gelatin zymography. HT1080 cells were seeded in 24-well plates at a density of 2×105 cells/well and incubated for 24 h to reach 80% confluence. After the incubation, cell culture media was changed to serum-free media, and MMP expression was stimulated by introduction of phorbol 12-myristate 13-acetate (PMA, 10 ng/mL) to the wells. At this stage, different concentrations of the sample were treated, and cells were incubated for another 24 h. Total protein contents were normalized by the Bradford protein determination method. Cell conditioned medium was subjected to substrate gel electrophoresis. A same amount of protein containing conditioned media was applied under non-reducing conditions on 10% polyacrylamide gels containing 1.5 mg/mL gelatin. After electrophoresis, polyacrylamide gels were washed with 50 mM Tris-HCl (pH 7.5) containing 2.5% Triton X-100 at room temperature to remove sodium dodecyl sulfate. Gels were then incubated for 48 h at 37°C in a developing buffer containing 10 mM CaCl2, 50 mM Tris-HCl, and 150 mM NaCl to digest gelatin by MMP. Areas of gelatin hydrolyzed by MMP were visualized as clear zones against blue background by Coomassie Blue staining, and the intensities of the bands were estimated by densitometry (Multi Gauge V3.0 software, Fujifilm Life Science, Tokyo, Japan).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total cellular RNA was extracted from sample-treated cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Changes in the steady-state concentration of mRNA for MMP-2 and MMP-9 were assessed by RT-PCR. Briefly, total RNA (2 μg) was converted to single stranded cDNA using a reverse transcription system (Promega, Madison, WI, USA). The target cDNA was amplified using the following primers: forward 5′-TGA AGG TCG GTG TGA ACG GA-3′ and reverse 5′-CAT GTA GCC ATG AGG TCC ACC AC-3′ for MMP-2; forward 5′-CAC TGT CCA CCC CTC AGA GC-3′ and reverse 5′-CAC TTG TCG GCG ATA AGG-3′ for MMP-9; forward 5′-AAT TCC GAC CTC GTC ATC AG-3′ and reverse 5′-TGC AGT TTT CCA GCA ATG AG-3′ for TIMP-1; forward 5′-TGA TCC ACA CAC GTT GGT CT-3′ and reverse 5′-TTT GAG TTG CTT GCA GGA TG-3′ for TIMP-2; forward 5′-GCC ACC CAG AAG ACT GTG GAT-3′ and reverse 5′-TGG TCC AGG GTT TCT TAC TCC-3′ for β-actin. The amplification cycles were 95°C for 45 s, 60°C for 1 min, and 72°C for 45 s. After 30 cycles, the PCR products were separated by electrophoresis on 1.5% agarose gel for 30 min at 100 V. Gels were then stained with 1 mg/mL EtBr visualized by Davinch-Chemi CAS-400SM Imager (Davinch-k, Seoul, Korea) and AlphaEase® gel image analysis software (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
The data were presented as means±SD (n=3). Differences between the means of the individual groups were assessed by one-way ANOVA followed by Duncan’s multiple range tests. Differences were considered significant at P<0.05. The statistical software package, SAS v9.1 (SAS Institute Inc., Cary, NC, USA), was used for these analyses.
RESULTS AND DISCUSSION
MMPs are known to be a part of different important pathways including metastasis, oxidative stress, and fibrosis (14,15). Hence, potent MMP-inhibitors are of high interest in several fields of pharma and nutraceutical studies. On the way to obtain natural MMP-inhibiting compounds, marine organisms hold a great deal of potential because they are present in a unique and challenging environment. Various organisms, especially marine algae, and metabolites have been identified as potential MMP-inhibitors, and possible mechanisms of action for isolated compounds have been suggested (16, 17). In order to provide valuable insights on that matter, eight different brown algae species from the shores of Korea, namely S. siliquastrum, E. cava, S. thunbergii, E. bicyclis, H. fusiformis, I. okamurae, S. horneri, and L. japonica, were compared in regard to their MMP-inhibition efficiency which will help the future utilization of marine sources as nutraceuticals.
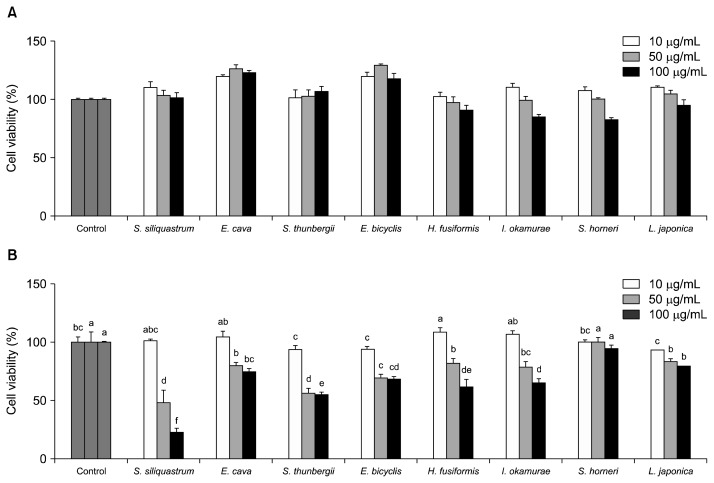
First, the extracts were tested for their cytotoxic presence in human fibrosarcoma cell line HT1080 for 24 and 48 h at three different concentrations (10, 50, and 100 μg/mL) (Fig. 1). The cytotoxicity test revealed that following 24 h incubation these concentrations were cyto-compatible for all tested brown algae extracts and possible inhibition of MMP-2 and MMP-9 was not due to cytotoxic influence. At 48 h incubation, some significant differences were observed between the different concentrations of samples. Therefore, all assays were carried out with 24 h sample treatments.
Fig. 1.
Cytotoxicity of eight brown algae extract treatments for 24 h (A) and 48 h (B) on human fibrosarcoma cell line HT1080 assessed by the MTT-formazan assay at the concentrations of 10, 50, and 100 μg/mL. Cell viability was indicated as percentage of untreated control cells. Means with different letters (a–f) are significantly different (P<0.05) among same concentration by Duncan’s multiple range test.
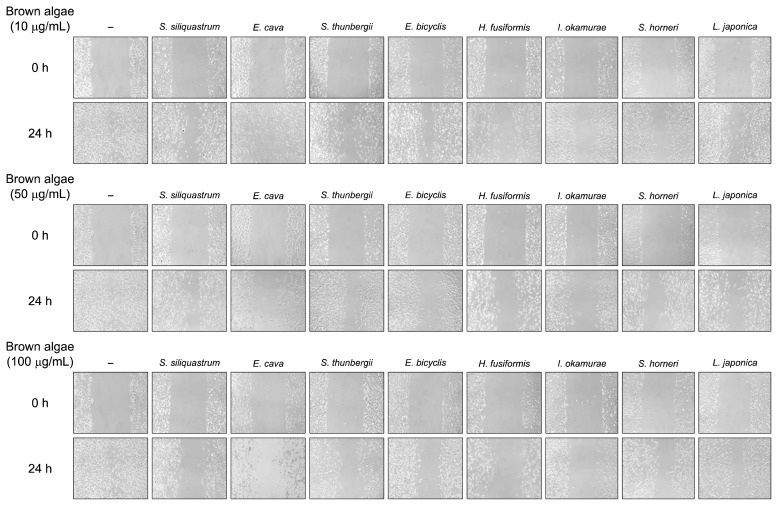
Effects of samples on the invasive properties of HT1080 cells were observed by migration assay. Presence of samples halted the migration of cells shown by microscopic images (Fig. 2). All of the samples were able to prevent cell migration in a dose-dependent manner following 24-h incubation. Among all tested samples, I. okamurae and E. cava were the most effective samples inhibiting cell invasion of HT1080 cells. Inhibited cell migration of invasive cancerous cells indicated a possible anti-MMP activity for tested samples as the MMP activity is the crucial regulator for successful migration of tumor cells into unoccupied extracellular space. Screening anti-invasive effect of samples paved the way for further experiments in order to elucidate the mechanism behind the migration inhibitory effect.
Fig. 2.
Effect of brown algae extracts on the migration of HT1080 cells after 24 h incubation. HT1080 cells were grown to 80% confluence prior to the application of 2 mm wide injury line. Pictures of cell migrating towards the injury line were taken at the start and at 24 h incubation with samples.
Inhibitory effect on MMP-2 and MMP-9 activities by gelatin zymography
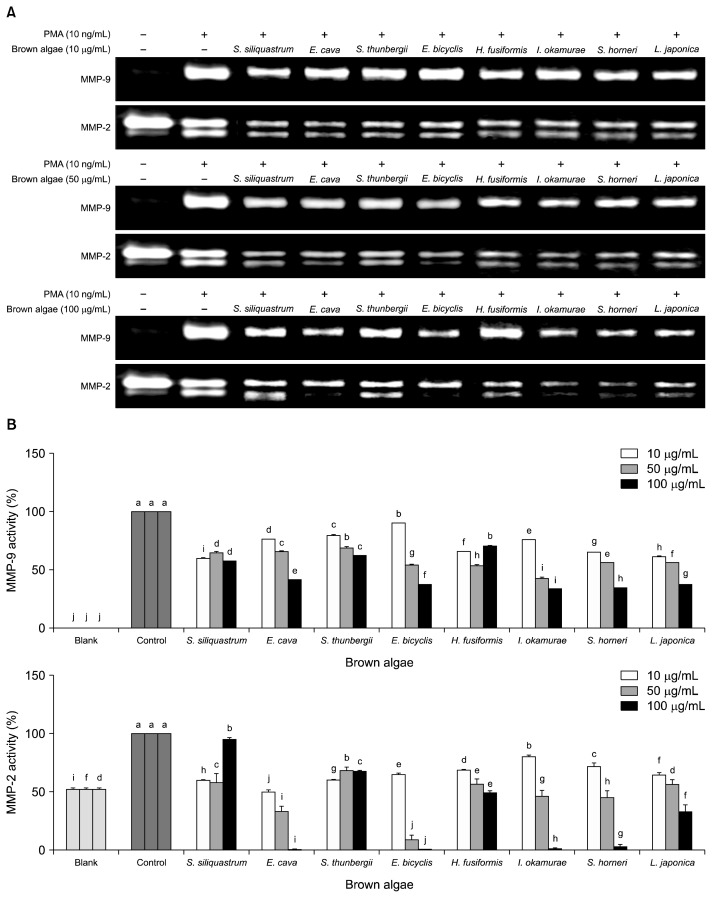
To investigate the effect of brown algae on MMP enzymatic activity, brown algae species were tested for their efficiency to inhibit MMP-2 and -9 activities following a PMA stimulation. Gelatinolytic activities of MMP-2 and -9 secreted from fibrosarcoma cell line HT1080 were evaluated with gelatin zymography which was carried out with PMA stimulated conditioned medium of brown algae-extract treated cells (Fig. 3). Administration of PMA (10 ng/mL) enhanced the expression of MMP-2 and -9 hence the elevated gelatinolytic activity in gelatin zymography. All treated cells showed a decreased activityies for both MMP-2 and -9. There was no consistent inhibitory activity for treated concentrations and brown algae. In the case of MMP-2 activity, S. siliquastrum was the most active one at the concentration of 10 μg/mL, while I. okamurae showed the most active inhibition at the concentration of 50 μg/mL. On the other hand, E. cava and E. bicyclis were observed to have the highest inhibition ratio for 10 and 50 μg/mL, respectively for MMP-9 activity. Nonetheless, all tested brown algae showed an inhibitory effect to a level against both MMP-2 and -9. Brown algae are known for their bioactive phenolic compounds mostly, but they also have sulfated polysaccharides and derivatives which are very prominent health beneficial compounds (18). Accordingly, S. siliquastrum was reported to contain a very active metabolite known as fucoxanthin, which is also a MMP-inhibitor (19). I. okamurae is known for glycolipids and polysaccharides that are known to inhibit MMP as well (20). Considering the efficiency of these isolated compounds, the observed effects and unresponsiveness to increased doses of these species can be credited to the presence of these compounds along other non-specific MMP-inhibitors of brown algae such as phlorotannins and fucoidan. However, at the concentration of 100 μg/mL, S. siliquastrum showed an unexpected failure to further inhibit MMP-2 activity in a dose-dependent manner. At lower concentrations, dose-dependency was relatively small in case of S. siliquastrum, suggesting that at 100 μg/mL a saturation effect occurred with the increasing dose of polyphenols, probably the effective ingredient fucoxanthin. E. bicyclis contains different polyphenol derivatives and sulfated polysaccharides in high amounts and reported to have glycolipidic bioactive compounds. Considering the superior inhibitory effect of E. bicyclis only at the concentration of 50 μg/mL compared to other brown algae species, presence of high concentrations of phlorotannins were responsible for dose-dependent elevation in MMP-inhibitory activity. In that manner, different compounds that are present in E. bicyclis might prevent the higher inhibitory effect, and this lesser efficiency was overcomed by higher concentration treatments.
Fig. 3.
Effect of brown algae extracts on the gelatinolytic activities of MMP-2 and MMP-9 in PMA-stimulated (10 ng/mL) HT1080 cells determined by gelatin zymography (A). Gelatinolytic activities of MMP-2 and MMP-9 in conditioned media were detected by SDS-PAGE electrophoresis on gelatin containing 10% polyacrylamide gel and band sizes of multiple assays (n=3) were compared (B). Means with different letters (a–j) are significantly different (P<0.05) among same concentration by Duncan’s multiple range test. Blank: unstimulated, untreated; Control: stimulated, untreated.
Effect on expressions of MMPs and TIMPs
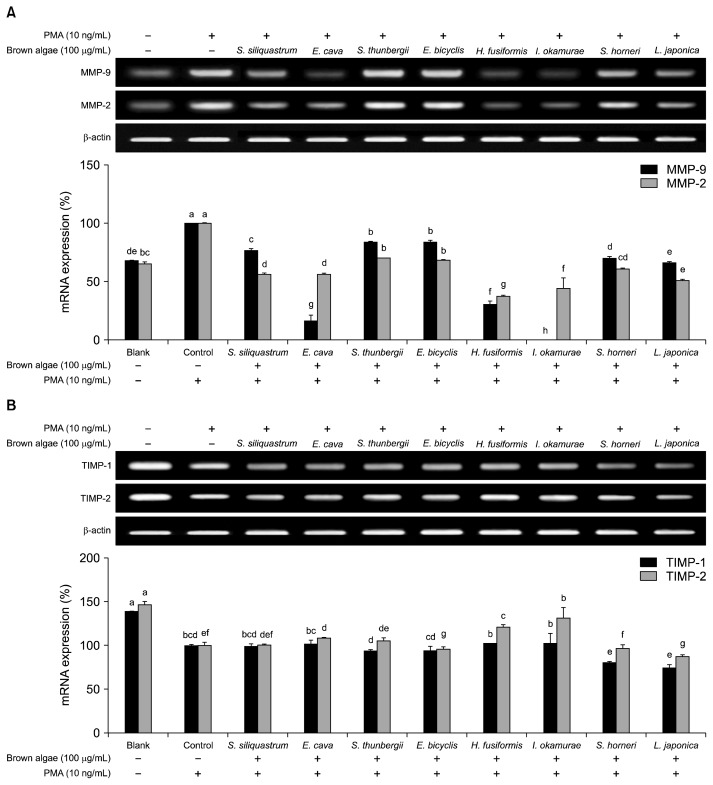
In order to examine the mechanism of brown algae extracts acting on MMP activity, gene expressions of MMP-2 and -9 were evaluated by RT-PCR along the levels of TIMP-1 and -2. TIMPs are known inhibitors of MMPs that are also known to elevate the activity of MMP-2 in some occasions (21). RT-PCR results indicated the presence of all eight brown algae extracts was able to suppress the expression of MMP-2 and -9 in mRNA levels. Presence of TIMPs is expected to be an indicator for inhibited MMP activity as a part of the cellular response to extracellular stimuli (22). Hence, PMA stimulation caused TIMP levels to decrease and MMP expression to increase. However, treatment with brown algae extracts was observed to suppress TIMP levels further following PMA stimuli. Expected results were to suppress MMP expression while regulating TIMP expression in order to balance the extracellular matrix degradation. Some brown algae species such as E. bicyclis and S. thunbergii did not to affect MMP-2 and -9 expressions in an unexpected manner. In addition, these two species inhibited both TIMP-1 and TIMP-2 expression compared to un-stimulated and PMA-stimulated controls indicating negative effects against MMP activity. However, I. okamurae extract was able to suppress MMP-9 expression by a remarkable 98% of PMA-stimulated control and a notable 64% for MMP-2 (Fig. 4A). Unlike all of the other brown algae species, I. okamurae was able to increase TIMP-1 and TIMP-2 expressions which were suppressed following PMA-stimulation (Fig. 4B). The observed inhibitory effect of I. okamurae against both MMP activity and expression coupled with the regulated TIMP expression suggests that I. okamurae also acted on cellular expression of MMP-related pathways as well as enzymatic inhibition. On the other hand, failure to regulate the MMP-related gene expression pathway by treated brown algae species, except I. okamurae, indicated that in general brown algae extracts did not exhibit their MMP-inhibitory effect through intracellular signaling pathways as expected. Considering that both MMP and TIMP mRNA were suppressed by treatment, it might be suggested that brown algae extracts inhibit overall MMP activity through a direct enzyme-bound inhibition rather than regulation of the expression of MMPs or TIMPs. Presence of compounds such as fucoidan, fucoxanthin, ishigoside, dieckol, and related derivatives that can stimulate the TIMP-MMP binding, hence the MMP inhibition, are reported for most of the brown algae species (23). However, current results indicated that extracts of brown algae were unable to regulate the TIMP expression as expected in order to regulate a successful MMP activity to prevent metastatic and unwanted fibrotic degradation. Under these conditions, utilization of crude extracts of brown algae might be limited to support nutrition rather than as potential nutraceutical as they only inhibit MMPs enzymatically and probably not with specific binding considering the different types of metabolites present. Moreover, compared to the other brown algae species tested, I. okamurae was suggested to contain higher concentrations of anti-MMP compounds that can act both on MMPs and their expression pathways. All tested brown algae species contained phytochemicals as their major bioactive constituents with some specific variations unique to each species. It was expected that all samples showed a recognizable effect on MMP expression and activity due to these phytochemicals. However, the notable difference of I. okamurae hints to inclusion of slightly more effective compounds and/or total contents of phlorotannins and glycolipids. In this context, the total polyphenol contents of all tested samples were calculated using the Folin-Ciocalteau assay and presented as mg gallic acid equivalents per 100 g dry weight of extracts (Table 1). As expected, three of the most effective samples were shown to contain significantly higher amount of polyphenols than the remaining. Specifically, the polyphenol contents of E. cava, E. bicyclis and I. okamurae were 135, 115, and 70 mg GAE/100 g dry weight, respectively. However, I. okamurae showed higher inhibitory potential than E. cava and E. bicyclis despite containing slightly less polyphenols. I. okamurae is reported to contain ishigoside which other species do not produce unlike fucoxanthin and fucoidan most brown algae contain. In this context, different derivatives of ishigoside-related compounds might be the reason behind the higher efficiency of I. okamurae on MMP expression and activity compared to the other tested brown algae species. E. cava and E. bicyclis are also known to possess notably high amounts of polyphenols, mainly tannin derivatives. Tannin derivatives inhibit MMP-related pathways as well as MMP enzymatic activity (10). Nonetheless, brown algae species can inhibit MMP action regardless of the difference in species and concentration of different bioactive molecules which make them good sources for further studies to isolate and elucidate lead compounds that can be further utilized in pharmaceutical and nutraceutical fields and MMP inhibitors. Further, I. okamurae stands out from the rest of the brown algae species assayed for its ability to inhibit MMP activity and regulate deteriorated MMP and TIMP expression.
Fig. 4.
Effect of brown algae extracts on mRNA expression levels of MMP-2, -9 (A) and TIMP-1, -2 (B) in HT1080 cells stimulated with PMA (10 ng/mL). Cells were treated with different concentrations of sample and PMA (10 ng/mL) for 24 h. The expression levels of these genes were detected using RT-PCR analysis and band sizes of multiple assays (n=3) were compared. β-actin was used as an internal standard. Means with different letters (a–h) at each gene are significantly different (P<0.05) among same enzymes by Duncan’s multiple range test. Blank: unstimulated, untreated; Control: stimulated, untreated.
Table 1.
Polyphenol contents of eight brown algae as milligrams of gallic acid equivalents (mg GAE) per 100 g of dry extract weight (unit: mg GAE/100 g)
| Sample | Polyphenol content |
|---|---|
| Sargassum siliquastrum | 6 |
| Ecklonia cava | 135 |
| Sargassum thunbergii | 16 |
| Ecklonia bicyclis | 115 |
| Hizikia fusiformis | 5 |
| Ishige okamurae | 70 |
| Sargassum horneri | 19 |
| Laminaria japonica | 4 |
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2059310).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Jones JL, Walker RA. Control of matrix metalloproteinase activity in cancer. J Pathol. 1997;183:377–379. doi: 10.1002/(SICI)1096-9896(199712)183:4<377::AID-PATH951>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 6.Ibañez E, Cifuentes A. Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric. 2013;93:703–709. doi: 10.1002/jsfa.6023. [DOI] [PubMed] [Google Scholar]
- 7.Yu P, Gu H. Bioactive substances from marine fishes, shrimps, and algae and their functions: present and future. Crit Rev Food Sci Nutr. 2015;55:1114–1136. doi: 10.1080/10408398.2012.686933. [DOI] [PubMed] [Google Scholar]
- 8.Patarra RF, Paiva L, Neto AI, Lima E, Baptista J. Nutritional value of selected macroalgae. J Appl Phycol. 2011;23:205–208. doi: 10.1007/s10811-010-9556-0. [DOI] [Google Scholar]
- 9.Matanjun P, Mohamed S, Mustapha NM, Muhammad K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol. 2009;21:75–80. doi: 10.1007/s10811-008-9326-4. [DOI] [Google Scholar]
- 10.Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- 11.Plaza M, Santoyo S, Jaime L, García-Blairsy Reina G, Herrero M, Señoráns FJ, Ibáñez E. Screening for bioactive compounds from algae. J Pharm Biomed Anal. 2010;51:450–455. doi: 10.1016/j.jpba.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NV, Kim SK. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ Toxicol Pharmacol. 2011;32:325–335. doi: 10.1016/j.etap.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 13.de la Mare JA, Lawson JC, Chiwakata MT, Beukes DR, Edkins AL, Blatch GL. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro. Invest New Drugs. 2012;30:2187–2200. doi: 10.1007/s10637-011-9788-0. [DOI] [PubMed] [Google Scholar]
- 14.Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223–229. doi: 10.1097/MOG.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatnagar I, Kim SK. Marine antitumor drugs: status, shortfalls and strategies. Mar Drugs. 2010;8:2702–2720. doi: 10.3390/md8102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu G, Xu W, Huang H, Li S. Progress in the development of matrix metalloproteinase inhibitors. Curr Med Chem. 2008;15:1388–1395. doi: 10.2174/092986708784567680. [DOI] [PubMed] [Google Scholar]
- 18.Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen VT, Qian ZJ, Lee B, Heo SJ, Kim KN, Jeon YJ, Park WS, Choi IW, Jang CH, Ko SC, Park SJ, Kim YT, Kim GH, Lee DS, Yim MJ, Je JY, Jung WK. Fucoxanthin derivatives from Sargassum siliquastrum inhibit matrix metalloproteinases by suppressing NF-κB and MAPKs in human fibrosarcoma cells. ALGAE. 2014;29:355–366. doi: 10.4490/algae.2014.29.4.355. [DOI] [Google Scholar]
- 20.Kim MM, Rajapakse N, Kim SK. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-κB transcription factor in RAW 264.7 cells. Phytother Res. 2009;23:628–634. doi: 10.1002/ptr.2674. [DOI] [PubMed] [Google Scholar]
- 21.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Kim SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: the current situation and future prospects. Mar Drugs. 2009;7:71–84. doi: 10.3390/md7020071. [DOI] [PMC free article] [PubMed] [Google Scholar]