Abstract
Abalone protein was hydrolyzed by enzymatic hydrolysis and the optimal enzyme/substrate (E/S) ratios were determined. Abalone protein hydrolysates (APH) produced by Protamex at E/S ratio of 1:100 showed angiotensin I converting enzyme inhibitory activity with IC50 of 0.46 mg/mL, and APH obtained by Flavourzyme at E/S ratio of 1:100 possessed the oxygen radical absorbance capacity value of 457.6 μM trolox equivalent/mg sample. Flavourzyme abalone protein hydrolysates (FAPH) also exhibited H2O2 scavenging activity with IC50 of 0.48 mg/mL and Fe2+ chelating activity with IC50 of 2.26 mg/mL as well as high reducing power. FAPH significantly (P<0.05) protected H2O2-induced hepatic cell damage in cultured hepatocytes, and the cell viability was restored to 90.27% in the presence of FAPH. FAPH exhibited 46.20% intracellular ROS scavenging activity and 57.89% lipid peroxidation inhibition activity in cultured hepatocytes. Overall, APH may be useful as an ingredient for functional foods.
Keywords: abalone, angiotensin I converting enzyme, antioxidant, enzymatic hydrolysis
INTRODUCTION
Fish and shellfish protein hydrolysates have been extensively studied due to their potential health benefits such as antioxidant (1,2), antihypertensive (3), anti-inflammatory (4), anti-cancer (5), and enzyme inhibition (2). These bioactive protein hydrolysates are specific protein fragments with the ability to impact body functions. Some of these protein hydrolysates may exhibit multifunctional properties, thereby indicating that they may be useful as ingredients for functional foods. Enzymatic hydrolysis is generally considered one of the best techniques to produce bioactive protein hydrolysates without nutrient loss. The bioactivities of produced protein hydrolysates are strongly dependent on amino acid composition, peptide size and sequence as well as proteases for production of protein hydrolysates (1).
One of the most valuable marine gastropods in Korea is abalone (Haliotis discus hannai). Abalone has been used in traditional medicine and several researches reported that abalone exhibited anti-fatigue and detoxification properties (6,7). More than 90% of the world abalone production is based on farming, and abalone mariculture has been increasing due to its nutritive and pharmaceutical values (8). In our previous research, we attempted to produce protein hydrolysates with multifunctional bioactivities from abalone viscera, which is normally considered as an inedible part by producers and customers. The produced protein hydrolysates exhibited strong antioxidant and cytoprotective activity in a model cell line (1). However, scanty information for protein hydrolysates with bioactivities from abalone muscle by enzymatic hydrolysis has been reported. The objective of this study is to produce bioactive protein hydrolysates from abalone muscle by enzymatic hydrolysis and to determine multifunctional bioactivities including cellular antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities.
MATERIALS AND METHODS
Materials
Alcalase, Flavourzyme, Neutrase, and Protamex were purchased from Novozyme Nordisk (Bagsvaerd, Denmark). Fluorescein, trolox (6-hydroxy-2,5,7,8-tetrameth-ylchroman-2-carboxylic acid), 2,2-azobis(2-amidino-propane) dihydrochloride (AAPH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and ferrozine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Diphenyl-1-pyrenylphosphine (DPPP) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) were obtained from Molecular Probes Inc. (Eugene, OR, USA).
Preparation of abalone protein hydrolysates
Before digestion, the abalone was washed using tap water, and then freeze dried. The abalone protein hydrolysates (APH) were obtained by enzymatic hydrolysis with Alcalase, Flavourzyme, Neutrase, and Protamax at pH 7.0 and 50°C according to the manufacturer’s instructions. Briefly, the enzyme was mixed with a 12% substrate solution at a ratio of 1:25 (w/w), and then hydrolysis was performed with simple agitation for 8 h. The mixture was then boiled for 10 min to inactivate the protease. After selecting the optimal enzyme based on bioactivities [ACE inhibitory and oxygen radical absorbance capacity (ORAC) assays], new digestions using enzyme/substrate (E/S) ratios of 1:50 and 1:100 were performed to determine the optimal E/S ratio for this enzyme. Unhydrolyzed protein was removed using a filter cloth, and the supernatant was lyophilized and stored at −20°C until use.
ACE inhibitory assay
ACE inhibitory activity was measured according to the method of Cushman and Cheung (9) with slight modifications. The mixture of APH solution (50 μL) and 50 μL of ACE (25 mU/mL) was pre-incubated at 37°C for 10 min, after which the mixture was re-incubated with 150 μL of substrate (8.3 mM HHL in 50 mM sodium borate buffer containing 0.5 M NaCl, at pH 8.3) for 30 min at the same temperature. The reaction was terminated by adding 250 μL of 1.0 M HCl. The resulting hippuric acid was extracted with 0.5 mL of ethyl acetate. After centrifugation (800 g, 15 min), 0.2 mL of the upper layer was transferred into a test tube and evaporated at room temperature for 2 h in a vacuum. The hippuric acid was dissolved in 1.0 mL of distilled water, and the absorbance was measured at 228 nm using an UV-spectrophotometer (SpectraMax® M2/M2e, Molecular Devices, Sunnyvale, CA, USA). The IC50 value was defined as the concentration required for inhibiting 50% of ACE.
Determination of antioxidant activity
ORAC assay
The ORAC values of APH were measured using a previously described method with slight modifications (10). Briefly, APH (50 μL) or distilled water (control) was mixed with fluorescein (50 μL, 78 nM) and incubated at 37°C for 15 min, followed by addition of AAPH (25 μL, 221 mM). The fluorescence intensity was measured every 5 min for 60 min at Ex 485 nm/Em 535 nm (SpectraMax® M2/M2e). The ORAC values were expressed as μM trolox equivalent (TE)/mg APH using a trolox standard curve.
H2O2 scavenging assay
The H2O2 scavenging activity of APH was measured using a previously described method (1). Briefly, APH (100 μL) was mixed with sodium phosphate buffer (100 μL, 0.1 M, pH 5.0) in a 96-well plate followed by addition of H2O2 (20 μL, 20 mM), and then the mixture was incubated at 37°C for 5 min. Thereafter, ABTS (30 μL, 1.25 mM) and peroxidase (30 μL, 1 U/mL) were added to the mixture followed by incubation at 37°C for 10 min. The absorbance values of APH or buffer were measured at 405 nm. The IC50 value was defined as the concentration required for scavenging 50% of H2O2.
Fe2+ chelating assay
The Fe2+ chelating activity of APH was measured using a previously described method (1). Briefly, APH (100 μL) or distilled water (control) was mixed with FeCl2 (100 μL, 0.1 mM) for 30 s, followed by addition of ferrozine (100 μL, 0.25 mM), and then allowed to equilibrate at room temperature for 10 min. The absorbance was measured at 562 nm. The IC50 value was defined as the concentration required for scavenging 50% of Fe2+.
Reducing power assay
The reducing power of APH was measured using a previously described method (11). Briefly, APH (200 μL) was mixed with sodium phosphate buffer (300 μL, 0.1 M, pH 6.6) and potassium ferricyanide (500 μL, 1%) followed by incubation at 50°C for 20 min. Thereafter, TCA (500 μL, 10%) was added to the mixture followed by centrifugation at 1,036 g for 10 min. Finally, 100 μL of the supernatant was mixed with 100 μL of distilled water and 20 μL of FeCl3 (0.1%, w/v), and the absorbance was measured at 700 nm.
Cellular antioxidant activity
MTT assay and cytoprotective activity of APH
Chang liver hepatocytes were purchased from ATCC (Rockville, MD, USA), maintained in an incubator at 37°C with a humidified atmosphere of 5% CO2, and cultured in DMEM containing 10% heat-inactivated fetal bovine serum (FBS), streptomycin (100 μg/mL) and penicillin (100 unit/mL).
The cytotoxicity of APH was determined using the MTT assay. Chang liver hepatocytes were seeded in a 96-well plate at a density of 1.0×105 cells/well. After 18 h, the hepatocytes were pretreated with various concentrations of APH for 1 h, and then washed with phosphate buffered saline (PBS). The cells were then exposed to 650 μM H2O2 to induce oxidative stress, followed by incubation for 24 h at 37°C. After the 24 h incubation, the MTT assay was performed.
Intracellular ROS scavenging activity
The intracellular ROS scavenging activity of APH was measured using a previously described method (12). Hepatocytes were seeded in a 96-well black plate at a density of 1.0×105 cells/well. Hepatocytes were labeled with 20 μM DCFH-DA in Hank’s balanced salt solution (HBSS) for 20 min, followed by treatment with APH for 1 h. Then 500 μM H2O2 in HBSS was added after washing the cells with PBS. The formation of 2′7′-dichlorofluorescin due to oxidation of DCFH in the presence of ROS was measured every 30 min at Ex 485 nm/Em 528 nm. The percentage of fluorescence intensity (ROS generation) was compared with that of the control cells without APH, which were arbitrarily assigned a value of 100%.
Lipid peroxidation inhibition activity
The lipid peroxidation inhibition activity of APH was measured using a previously described method (13). Hepatocytes were grown in culture dishes and washed with PBS, followed by staining of 13 μM DPPP (in DMSO) for 30 min at 37°C in the dark. The cells were then washed three times with PBS and seeded in a 96-well black plate at a density of 1.0×107 cells/mL using serum-free media. Following complete attachment, the cells were treated with the extracts for 1 h and then challenged with 3 mM AAPH in PBS to initiate cell membrane lipid peroxidation. DPPP oxide fluorescence intensity after 6 h was measured at Ex 361 nm/Em 380 nm. The percentage of fluorescence intensity (lipid peroxidation) was compared with that of the control cells without APH, which were arbitrarily assigned a value of 100%.
Statistical analysis
The data are presented as the mean±standard deviation (SD) of at least three independent experiments (n=3). Differences between means of each group were assessed by one-way analysis of variance followed by Duncan’s multiple range test using PASW Statistics 19.0 software (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
ACE inhibitory activity of APHs
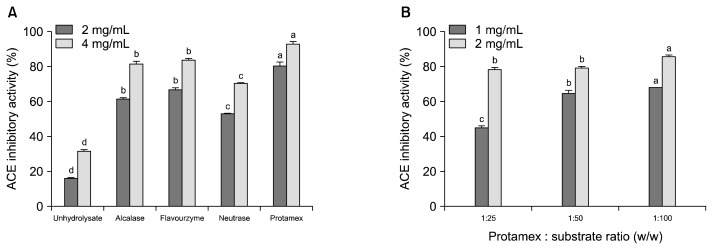
ACE is a zinc metallopeptidase important for blood pressure control by converting angiotensin I to angiotensin II, which constricts the vessels (14). ACE also inactivates bradykinin, which has a depressor action. Thus, the inhibition of ACE activity is thought to be a major target for antihypertension. Abalone protein was hydrolyzed using various proteases and the yields of un-hydrolyzed abalone protein and APHs were 11.84% (unhydrolysate), 62.40% (Alcalase), 47.24% (Flavourzyme), 44.88% (Neutrase), and 56.50% (Protamex), respectively. The ACE inhibitory activity of APHs produced by various proteases showed different inhibitory activities (Fig. 1A). At E/S ratio of 1:25, protamex abalone protein hydrolysates (PAPH) showed the highest ACE inhibitory activity with 92.69±1.08% (at 4 mg/mL) than that of the other protease hydrolysates. Thus, we conducted the enzymatic hydrolysis using protamex with different E/S ratios in order to determine the optimal E/S ratio that exhibited the best ACE inhibitory activity. As depicted in Fig. 1B, ACE inhibitory activities varied with the E/S ratios. At E/S ratio of 1:100, PAPH showed the highest ACE inhibitory activity with 85.93±0.81% (at 2 mg/mL) than that of the other E/S ratios, and the optimal E/S ratio was found to be 1:100. The calculated IC50 values of APHs are shown in Table 1, and the IC50 value of PAPH against ACE was found to be 0.46±0.01 mg/mL, which was lower than the 2.45 mg/mL of oyster sauce (15), 1.17 mg/mL of Atlantic salmon skin collagen peptide (16), and 0.79 mg/mL of Atlantic salmon hydrolysate (17), but much higher than the 0.27 mg/mL of sea cucumber digest (18), and 0.014 mg/mL of pentapeptide purified from a sea squirt (19). It is well known that bioactivities of protein hydrolysates are strongly dependent on the amino acid composition of the parent protein, the type of hydrolyzing enzymes, and the distribution of molecular weights of hydrolysates. However, the IC50 comparison indicates that PAPH may have potential ACE inhibitory peptides, thus purification of ACE inhibitory peptides is in progress.
Fig. 1.
(A) Angiotensin I converting enzyme (ACE) inhibitory activities of abalone protein hydrolysates (APHs) and (B) determination of optimal E/S ratio for production of ACE inhibitory APH by Protamex. Bars with different letters (a–d) indicate significant differences at the same concentration (P<0.05). Values are expressed as means±SD (n=3).
Table 1.
ACE inhibitory activities of abalone protein hydrolysates by various proteases (unit: mg/mL)
| IC50 values | |
|---|---|
| Unhydrolysate | >5.0 |
| Alcalase | 0.86±0.04 |
| Flavourzyme | 0.77±0.03 |
| Neutrase | 1.64±0.05 |
| Protamex | 0.46±0.01 |
Antioxidant activity of APHs
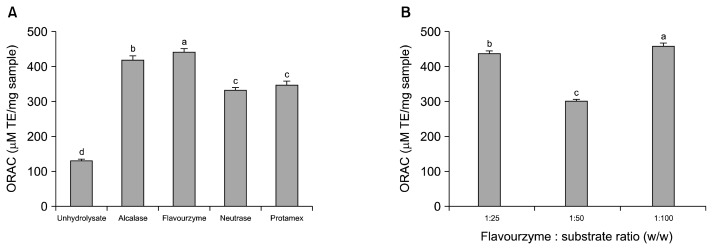
Antioxidant activity of APHs was determined by measuring the ORAC value and is depicted in Fig. 2. At E/S ratio of 1:25, all APHs exhibited better ORAC values than the unhydrolyzed abalone, and Flavourzyme abalone protein hydrolysates (FAPH) showed the highest ORAC value of 441.8±10.2 μM TE/mg FAPH. Thus, enzymatic hydrolysis with different E/S ratios was conducted to obtain FAPH with the highest ORAC value. As shown in Fig. 2B, the ORAC values varied with different E/S ratios, and FAPH exhibited the ORAC value of 457.6±7.8 μM TE/mg FAPH at E/S ratio of 1:100. The ORAC value observed in this study was higher than that of other reported ORAC values including abalone viscera protein hydrolysates (415.8 μM) (1), sea cucumber digests (4.4 μM) (18), catfish hydrolysates (3.5 μM) (20), and pacific hake hydrolysates (225 μM) (21). The ORAC assay evaluates the ability to scavenge peroxyl radicals, a common radical in human biology, and thus it is considered the most reliable method for evaluating the antioxidant capacity of food-derived antioxidants (22). In this study, we showed that FAPH possessed high ORAC value, indicating FH might be useful as an ingredient for dietary antioxidants.
Fig. 2.
(A) Oxygen radical absorbance capacity (ORAC) values of abalone protein hydrolysates (APHs) and (B) determination of optimal E/S ratio for production of APH with the highest ORAC value by Flavourzyme. Bars with different letters (a–d) indicate significant differences at the same concentration (P<0.05). Values are expressed as means±SD (n=3).
We also evaluated H2O2 scavenging activity, Fe2+ chelating activity, and reducing power of FAPH obtained at E/S ratio of 1:100 (Table 2). The IC50 values of FAPH against H2O2 scavenging and Fe2+ chelating were 0.48± 0.02 mg/mL and 2.26±0.03 mg/mL, respectively. The reducing power of FAPH was 0.83±0.01 at 2.0 mg/mL. In the body, highly reactive hydroxyl radicals can be generated by the Fenton reaction in the presence of H2O2 and Fe2+, which can damage virtually all types of macromolecules including carbohydrates, nucleic acids, lipids, and proteins (23). The use of antioxidants could prevent the generation of hydroxyl radicals through scavenging and/or chelating of H2O2 and Fe2+, thus antioxidants could reduce hydroxyl radical-induced damage in the body. Another method for measuring the antioxidant potential of FAPH is the ferric reducing antioxidant power. This assay is based on the donation of an electron or hydrogen and an increase in absorbance at 700 nm would indicate an increase in the reducing power. Our previous work reported that abalone viscera protein hydrolysates possessed reducing power with an absorbance of 0.98 at 2.0 mg/mL, which is higher than the absorbance value obtained in this work (1). However, the absorbance value of FAPH is higher than in smooth muscle protein hydrolysates (0.60 at 2.0 mg/mL) and yellow stripe trevally protein hydrolysates (0.52 at 3.6 mg/mL), respectively (24,25).
Table 2.
Antioxidant activities of abalone protein hydrolysates by Flavourzyme at E/S ratio of 1:100
| FAPH2) | |
|---|---|
| H2O2 scavenging (IC50, mg/mL) | 0.48±0.02 |
| Fe2+ chelating (IC50, mg/mL) | 2.26±0.03 |
| Reducing power1) (A700) | 0.83±0.01 |
Reducing power was evaluated at 2.0 mg/mL.
FAPH: Flavourzyme abalone protein hydrolysate.
Cellular antioxidant capacity of FAPH
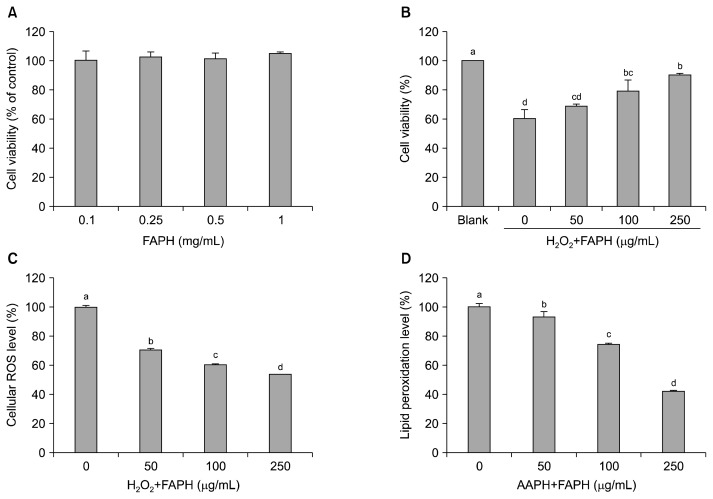
Since FAPH showed the highest antioxidant activity, we evaluated the cellular antioxidant activity of FAPH in cultured hepatocytes under oxidative stress. Prior to evaluating cellular antioxidant activity, cytotoxicity of FAPH in cultured hepatocytes was determined using the MTT assay. As depicted in Fig. 3A, FAPH did not show any cytotoxic effect against cultured hepatocytes in the tested concentrations. Further, we investigated whether FAPH could protect H2O2-induced hepatic damage in cultured hepatocytes. FAPH was added to cultured hepatocytes for 1 h, and washed with PBS, followed by exposure of 650 μM H2O2 to induce oxidative stress in cultured hepatocytes. In the absence of FAPH, hepatocytes were significantly (P<0.05) damaged by oxidative stress, and the cell viability was 60.16±5.96% compared to the blank group (without FAPH and H2O2). However, this decrease was significantly (P<0.05) restored by pretreatment with FAPH in a dose-dependent manner (Fig. 3B), and the cell viability was restored to 90.27± 1.00% at 250 μg/mL of FAPH. To verify the antioxidant effect of FAPH, cellular ROS scavenging activity was measured in cultured hepatocytes under oxidative stress conditions. As shown in Fig. 3C, FAPH significantly (P<0.05) scavenged intracellular ROS in a dose-dependent manner, and intracellular ROS levels were decreased by 53.80±0.04% compared to the blank (without FAPH and H2O2). To measure lipid peroxidation inhibition ability of FAPH, AAPH was exposed to cultured hepatocytes in order to cause lipid peroxidation in the presence or absence of FAPH. As depicted in Fig. 3D, lipid peroxidation in cultured hepatocytes was significantly (P<0.05) decreased by pretreatment with FAPH in a dose-dependent manner and the inhibition activity was 57.89±0.26% at 250 μg/mL of FAPH.
Fig. 3.
(A) Cytotoxicity of Flavourzyme abalone protein hydrolysates (FAPH) from abalone protein, (B) protective effect of FAPH against H2O2-induced hepatic cell damage in cultured hepatocytes, (C) intracellular ROS scavenging activity of FAPH in cultured hepatocytes, and (D) lipid peroxidation inhibition of FAPH in cultured hepatocytes. Bars with different letters (a–d) indicate significant differences (P<0.05). Values are expressed as means±SD (n=3).
Oxidative stress is thought to be involved in the development of human diseases such as cancer, atherosclerosis, Alzheimer’s, and heart failure (26). Therefore, preventing or delaying the pathogenesis of these chronic diseases is an essential strategy to promote health conditions. In the present study, H2O2 was used to induce oxidative cell injury, and the protective ability of FAPH was investigated. Previous reports had demonstrated that treatment with H2O2 induced cell death and this damage was suppressed by pretreatment with antioxidants (27–29). Our results also agreed with these observations. In addition, overproduction of ROS is involved in the pathogenesis of hepatic fibrosis through lipid peroxidation in hepatocytes (30). Previous studies revealed that marine-derived hydrolysates scavenged cellular ROS; however, FAPH showed comparable or even higher cellular ROS scavenging activity as well as inhibition of lipid peroxidation (29,31).
CONCLUSIONS
Abalone protein hydrolysates (APH) were produced by enzymatic hydrolysis, and the optimal E/S ratios for ACE inhibitory and cellular antioxidant activities were determined. APH produced by Protamex at E/S ratio of 1:100 showed the best ACE inhibitory activity, and APH produced by Flavourzyme at E/S ratio of 1:100 possessed the highest ORAC value. Furthermore, Flavourzyme abalone protein hydrolysates (FAPH) exhibited protective effects against oxidative stress-induced hepatic cell damage through cellular ROS scavenging and lipid peroxidation inhibition. Collectively, these results suggest that APH can be used as an ingredient for functional food applications.
ACKNOWLEDGEMENTS
This study was financially supported by Chonnam National University, 2012.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Je JY, Park SY, Hwang JY, Ahn CB. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J Funct Foods. 2015;16:94–103. doi: 10.1016/j.jff.2015.04.023. [DOI] [Google Scholar]
- 2.Choi JH, Kim KT, Kim SM. Biofunctional properties of enzymatic squid meat hydrolysate. Prev Nutr Food Sci. 2015;20:67–72. doi: 10.3746/pnf.2015.20.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Kim YS, Kim SE, Kim EK, Hwang JW, Park TK, Kim BK, Moon SH, Jeon BT, Jeon YJ, Ahn CB, Je JY, Park PJ. Purification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from an enzymatic hydrolysate of duck skin byproducts. J Agric Food Chem. 2012;60:10035–10040. doi: 10.1021/jf3023172. [DOI] [PubMed] [Google Scholar]
- 4.Ahn CB, Cho YS, Je JY. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015;168:151–156. doi: 10.1016/j.foodchem.2014.05.112. [DOI] [PubMed] [Google Scholar]
- 5.Chi CF, Hu FY, Wang B, Li T, Ding GF. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods. 2015;15:301–313. doi: 10.1016/j.jff.2015.03.045. [DOI] [Google Scholar]
- 6.Kim SK, Pallela R. Medicinal foods from marine animals: current status and prospects. Adv Food Nutr Res. 2012;65:1–9. doi: 10.1016/B978-0-12-416003-3.00001-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee KA, Shin ES, Lee HK, Kim MJ, Kim KBWR, Byun MW, Lee JW, Kim JH, Ahn DH, Lyu ES. Quality characteristics of abalone porridge with viscera. J Korean Soc Food Sci Nutr. 2008;37:103–108. doi: 10.3746/jkfn.2008.37.1.103. [DOI] [Google Scholar]
- 8.Cook PA, Roy Gordon H. World abalone supply, markets, and pricing. J Shellfish Res. 2010;29:569–571. doi: 10.2983/035.029.0303. [DOI] [Google Scholar]
- 9.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 10.Zuluet A, Esteve MJ, Frígola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–316. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]
- 11.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 12.Engelmann J, Volk J, Leyhausen G, Geurtsen W. ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res B Appl Biomater. 2005;75:272–276. doi: 10.1002/jbm.b.30360. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Shibata M, Niki E. Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radic Biol Med. 2001;31:164–174. doi: 10.1016/S0891-5849(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 14.Qian ZJ, Je JY, Kim SK. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of Bigeye tuna dark muscle, Thunnus obesus. J Agric Food Chem. 2007;55:8398–8403. doi: 10.1021/jf0710635. [DOI] [PubMed] [Google Scholar]
- 15.Je JY, Park JY, Jung WK, Park PJ, Kim SK. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005;90:809–814. doi: 10.1016/j.foodchem.2004.05.028. [DOI] [Google Scholar]
- 16.Gu RZ, Li CY, Liu WY, Yi WX, Cai MY. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res Int. 2011;44:1536–1540. doi: 10.1016/j.foodres.2011.04.006. [DOI] [Google Scholar]
- 17.Nakajima K, Yoshie-Stark Y, Ogushi M. Comparison of ACE inhibitory and DPPH radical scavenging activities of fish muscle hydrolysates. Food Chem. 2009;114:844–851. doi: 10.1016/j.foodchem.2008.10.083. [DOI] [Google Scholar]
- 18.Pérez-Vega JA, Olivera-Castillo L, Gómez-Ruiz JÁ, Hernán-dez-Ledesma B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus) J Funct Foods. 2013;5:869–877. doi: 10.1016/j.jff.2013.01.036. [DOI] [Google Scholar]
- 19.Ko SC, Kang MC, Lee JK, Byun HG, Kim SK, Lee SC, Jeon BT, Park PJ, Jung WK, Jeon YJ. Effect of angiotensin I-converting enzyme (ACE) inhibitory peptide purified from enzymatic hydrolysates of Styela plicata. Eur Food Res Technol. 2011;233:915–922. doi: 10.1007/s00217-011-1585-7. [DOI] [Google Scholar]
- 20.Samaranayaka AGP, Li-Chan ECY. Autolysis-assisted production of fish protein hydrolysates with antioxidant properties from Pacific hake (Merluccius productus) Food Chem. 2008;107:768–776. doi: 10.1016/j.foodchem.2007.08.076. [DOI] [Google Scholar]
- 21.Theodore AE, Raghavan S, Kristinsson HG. Anti-oxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J Agric Food Chem. 2008;56:7459–7466. doi: 10.1021/jf800185f. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Ahn CB, Je JY. Antioxidant and anti-inflammatory activities of protein hydrolysates from Mytilus edulis and ultrafiltration membrane fractions. J Food Biochem. 2014;38:460–468. doi: 10.1111/jfbc.12070. [DOI] [Google Scholar]
- 23.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- 25.Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- 26.Si H, Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J Nutr Biochem. 2014;25:581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He B, Tao HY, Liu SQ. Neuroprotective effects of carboxymethylated chitosan on hydrogen peroxide induced apoptosis in Schwann cells. Eur J Pharmacol. 2014;740:127–134. doi: 10.1016/j.ejphar.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Linden A, Gülden M, Martin HJ, Maser E, Seibert H. Peroxide-induced cell death and lipid peroxidation in C6 glioma cells. Toxicol In Vitro. 2008;22:1371–1376. doi: 10.1016/j.tiv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Wiriyaphan C, Xiao H, Decker EA, Yongsawatdigul J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015;167:7–15. doi: 10.1016/j.foodchem.2014.06.077. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- 31.Malaypally SP, Liceaga AM, Kim KH, Ferruzzi M, San Martin F, Goforth RR. Influence of molecular weight on intracellular antioxidant activity of invasive silver carp (Hypophthalmichthys molitrix) protein hydrolysates. J Funct Foods. 2014 doi: 10.1016/j.jff.2014.06.011. In Press. [DOI] [Google Scholar]