Abstract
The aim of this study was to investigate the acute and subacute oral toxicity of crude antifungal compounds produced by Lactobacillus plantarum HD1 in Sprague-Dawley rats. In the acute toxicity study, the crude antifungal compounds (0.625, 1.25, 2.5, and 5.0 g/kg) did not produce mortality, significant changes in general behavior, or changes in the gross appearance of the organs. In the subacute toxicity study, the crude antifungal compounds were administered orally to rats at doses of 0, 0.5, 1.0, and 2.0 g/kg daily for 28 days. There were no test article-related deaths, abnormal clinical signs, or body weight changes. The study also showed no significant differences between the control and treated groups in hematological and serum biochemical parameters, histopathological examination, or any other findings. These results suggest that acute or subacute oral administration of crude antifungal compounds from L. plantarum HD1 is not toxic in rats.
Keywords: Lactobacillus plantarum HD1, crude antifungal compounds, acute toxicity, subacute toxicity
INTRODUCTION
Filamentous molds and yeasts are important organisms in the spoilage of food and animal feed. Chemical fungicides and additives in food and feed have been widely used as preservatives (1). However, molds and yeasts are becoming resistant to chemical fungicides and additives (2). In recent years, health-conscious consumers have demanded the reduced use of chemical preservatives and additives. For centuries, lactic acid bacteria (LAB) have been used as natural preservatives in food and animal feed (3).
Many species of LAB are promising alternatives to chemical preservatives (4). The anti-microbial effects of LAB are mainly related to the production of organic acids such as lactic, acetic, and propionic acid. Acid production lowers the pH of food and helps inhibiting the growth of other microorganisms (5). LAB produces other compounds such as hydrogen peroxide, CO2, diacetyl, and bacteriocin that may also contribute to their preserving properties (6). Bacteriocin produced by LAB has been reported to have antibacterial and antifungal activity (7–9). LAB can produce many compounds with anti-fungal activity including organic acids, proteinaceous compounds, phenyllactic acid, reuterin, cyclic dipeptides, fatty acids, and their derivatives (10). Therefore, species of LAB that produce antifungal compounds may be useful as effective natural preservatives to replace chemical preservatives in food and feed. Two Lactobacillus planta-rum strains with antifungal activity have been isolated from kimchi (4,8). Active antifungal compounds from L. plantarum AF1 and L. plantarum HD1 were identified as 3,6-bis(2-methylpropyl)-2,5-piperazinedion (4), 5-oxododecanoic acid, 3-hydroxy decanoic acid, and 3-hydroxyl-5-decanoic acid (8). The isolates, L. plantarum AF1 (4) and L. plantarum HD1 (8), showed strong inhibitory activity against food- and feed-borne filamentous fungi and yeasts.
LAB is classified as substances generally recognized as safe and probiotics. However, the toxicological aspects of probiotic LAB and LAB end products have been neglected because they have been used as natural bio-preservatives for many years. Safety data on these products is limited. Therefore, toxicological studies should be conducted to determine potential toxicities even for substances that have been used as natural food and feed additives.
The novel probiotic, Propionibacterium jensenii 702, isolated from raw milk, was found to be comparatively non-toxic when the repeated-dose oral toxicity was examined in male rats (11). Shu et al. (12) also reported that pro-biotic LAB such as L. acidophilus HN017, L. rhamnosus HN001, and Bifidobacterium lactis HN019 had no adverse effects on the health of mice. These probiotics have been shown to have strong immune enhancing and anti-infective properties. No toxic effects were detected in rats following acute and subacute oral administration of L. plantarum AF1 (13,14). Furthermore, in another study, the acute and repeated-dose oral toxicity of crude anti-fungal compounds produced by L. plantarum AF1 in rodents showed these compounds were non-toxic (15,16). However, no toxicity studies have been performed on the crude antifungal compounds produced by L. plantarum HD1. Consequently, it was necessary to carry out a safety assessment for these crude antifungal compounds.
The aim of present study was to assess the acute and subacute oral toxicity of crude antifungal compounds produced by L. plantarum HD1 (8), a lactic acid bacterium isolated from kimchi, on Sprague-Dawley (SD) male and female mice in vivo. These compounds will be tested in SD female and male rats according to the Organization for Economic Cooperation and Development (OECD) guidelines (17,18) for their potential use in natural food and feed additives.
MATERIALS AND METHODS
Preparation of antifungal compounds from L. plantarum HD1
The test article for these studies is the crude preparation of antifungal compounds produced by L. plantarum HD1, a lactic acid bacterium isolated from Korean kimchi (8). The test article was obtained from Professor Hae-Choon Chang (Chosun University). To prepare the partially purified antifungal compounds from L. plantarum HD1, the supernatant from a culture of L. plantarum HD1 was fractionated on a solid phase extraction (SPE) column (Isolate, C18 EC cartridges, 10 g; International Sorbent Technology Ltd., Hengoed, UK). The SPE column was activated with 200 mL of methanol of 10 mM sodium acetate (pH 4.0). Following a 2.5 L sample loading per SPE column, the column was washed with 200 mL of 5% (v/v) aqueous acetonitrile and then eluted with 30 mL of 95% aqueous acetonitrile. The eluted sample was evaporated under a vacuum and prepared as a powder (4). All of the other chemicals used were of analytical grade.
Experimental animals and housing conditions
Specific pathogen free SD rats of both sexes (4 weeks old, weighing 105±6 g, male and female) were purchased from Central Lab Animal Inc. (Seoul, Korea). The animals were acclimated for at least one week before the start of the study. The rats were housed under standard conditions of a good laboratory practice-based facility and kept in a well-ventilated and specific pathogen-free room. During acclimation and experiment periods, the rats were housed in plastic cages (5 per cage, segregated by gender) in an environmentally controlled room at 23±2°C, relative humidity of 50±10%, air ventilation of 10 to 20 times/h, and 12-h light/dark cycle. Rats were allowed with free access to drinking water and standard diet. This study was approved by the Animal Care and Use Committee at Chosun University (approval no. CIACUC 2015-S0009).
Acute oral toxicity
Healthy SD rats were fasted overnight, with free access to water. According to the OECD guideline for testing of chemicals in foods using rodents, TG420 (17), rats were randomly divided into five groups of male rats and five groups of female rats. The control group received water. Experimental groups were orally treated with the anti-fungal compounds produced by L. plantarum HD1 in doses of 0.625, 1.25, 2.5, and 5.0 g/kg. The crude anti-fungal compounds were dissolved in sterile distilled water (10 mL/kg body weight) and were administered by oral gavage. General behavioral observations, body weight changes, signs of toxicity, and mortality were recorded at 1, 2, 4, and 6 h after treatment and once daily for 14 days (19). On day 15, all rats were fasted for 12~14 h, and then anesthetized with carbon dioxide and sacrificed. The lethal dose (LD50) was estimated according to the method described by Litchfield and Wilcoxon (20).
Subacute oral toxicity
This study was performed according to the OECD test guidelines with minor modifications (18). Healthy SD rats of either sex were housed in rectangular polycarbonate cages under the conditions described in previous section. The male and female rats were randomly assigned to four groups of five rats each. The crude antifungal compounds was dissolved in sterile distilled water (40 mg/mL) and orally administrated at doses of 0.5, 1.0, and 2.0 g/kg/d over a 4-week period. Body weights were measured weekly, and feed intake and water intake were monitored daily. During the treatment period, all rats were observed for clinical signs and mortality once a day. At the end of the period, all animals were fasted for 12~14 h and then anesthetized with diethyl ether. After anesthesia, the studies described below were conducted.
Hematological and biochemical assay
Hematological variables were measured in all rats at the end of the treatment period. Blood samples were collected from the descending aorta in heparinized vacutainers and analyzed for alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), glucose (GLU), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), total protein (TP), albumin, and creatinine (CRE) using a biochemical blood analyzer (Hitachi-747, Hitachi Medical Corporation, Tokyo, Japan). The blood was also analyzed for the red blood cell count (RBC), hematocrit (HCT), hemoglobin concentration (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet counts, reticulocytes, white blood cell count (WBC), neutrophils, eosinophils, basophils, lymphocytes, and monocytes using a blood cell counter (MEK-6318J/K, Nihon Kohden, Tokyo, Japan).
Gross necropsy and histopathological examination
After collecting the blood samples, animals were sacrificed for tissue studies. Organs (brain, lung, testes, kidney, heart, spleen, and liver) were removed and weighed. The liver and kidney were dissected out and fixed in 10% neutral buffered formalin. After 18 h of fixation, the tissue samples were embedded in paraffin, and 3~4 μm sections were prepared by routine histological methods (21). The liver was stained with hematoxylin and eosin, and the kidney was stained with periodic acid Schiff stain for evaluation by light microscopy (Olympus BX51, Olympus Optical Co., Ltd., Tokyo, Japan).
Statistical analysis
The statistical analyses were carried out using the Statistical Package for Social Science (version 12.0, SPSS Inc., Chicago, IL, USA). Results were expressed as mean± standard error (SE). A one-way analysis of variance was carried out to test the statistical significance on the mean difference between groups, and post-hoc tests were conducted by using Tukey’s test. Significance was set at the P<0.05 level.
RESULTS AND DISCUSSION
Acute toxicity of crude antifungal compounds
Acute treatment of rats with the crude antifungal compounds by the oral route at doses up to 5.0 g/kg did not cause death during the 14 days of observation. The animals did not show any clinical signs of toxicity, changes in general behavior, or changes in physical activity (e.g., hyperactivity, ataxia, tremors, convulsions, salvation, diarrhea, lethargy, sleep, or coma). In addition, there was no gender difference in terms of the acute toxicity of antifungal compounds. Pathological examination of the internal organs revealed no abnormalities (data not shown). There were no differences in the weight or appearance of the internal organs of rats in the control and treated groups.
In this study, the LD50 could not be estimated, and it is likely to be more than 5.0 g/kg. Chemical materials with a LD50 in the range of 5.0 g/kg by oral route were considered to have low-acute toxicity (22). Therefore, it was shown that the acute toxicity of crude antifungal compounds produced by L. plantarum HD1 is practically null via oral route. Similar results were reported by Son et al. (15) in mice using crude antifungal compounds produced by L. plantarum AF1, another LAB isolated from kimchi.
Subacute toxicity of body weight, feed intake, and water intake
All rats survived to the end of the 28-d period of oral dosing with crude antifungal compounds (0.5, 1.0, and 2.0 g/kg). There were no abnormal clinical observations. The effects of crude antifungal compounds produced by L. plantarum HD1 on the body weights, feed intake, and water intake of the experimental and control rats are presented in Tables 1, 2, and 3. The body weights of both genders that were administered the antifungal compounds (0.5, 1.0, and 2.0 g/kg/d) were slightly increased but were not significantly different when compared with the control group (Table 1).
Table 1.
Body weights of male and female Sprague-Dawley (SD) rats treated orally with crude antifungal compounds produced by Lactobacillus plantarum HD1 for 28 days
| Sex | Dose (g/kg/d) | Body weight (g)1) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Days after treatment | ||||||
|
| ||||||
| 0 | 7 | 14 | 21 | 28 | ||
| Male | 0 | 115.33±11.65NS2) | 147.77±11.74NS | 205.74±10.30NS | 255.11±10.78NS | 305.22±11.13NS |
| 0.5 | 116.72±12.68 | 151.68±10.86 | 210.26±14.24 | 262.34±7.68 | 303.10±11.39 | |
| 1.0 | 115.49±8.69 | 147.08±13.38 | 207.73±7.36 | 259.73±11.46 | 301.51±12.63 | |
| 2.0 | 116.86±7.56 | 149.63±12.25 | 210.49±8.66 | 259.16±12.91 | 300.92±10.67 | |
| Female | 0 | 99.01±7.86NS | 129.64±12.24NS | 154.14±8.67NS | 189.65±6.54NS | 220.42±6.76NS |
| 0.5 | 98.15±6.35 | 128.76±9.75 | 152.35±6.43 | 186.73±8.25 | 218.37±9.78 | |
| 1.0 | 98.85±7.31 | 128.58±12.22 | 153.26±10.11 | 188.46±9.86 | 217.08±7.98 | |
| 2.0 | 97.79±6.75 | 127.96±9.11 | 152.71±9.02 | 187.28±7.37 | 218.67±8.68 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
Table 2.
Daily feed consumption of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days
| Sex | Dose (g/kg/d) | Feed consumption (g/d/rat)1) | |||
|---|---|---|---|---|---|
|
| |||||
| Days after treatment | |||||
|
| |||||
| 7 | 14 | 21 | 28 | ||
| Male | 0 | 20.12±0.84NS2) | 22.94±3.67NS | 26.77±1.23NS | 29.03±2.32NS |
| 0.5 | 19.45±1.65 | 23.23±2.82 | 27.34±3.07 | 28.33±3.45 | |
| 1.0 | 18.69±2.93 | 21.92±4.51 | 26.61±2.02 | 27.65±3.28 | |
| 2.0 | 18.25±3.59 | 20.28±3.67 | 26.28±1.73 | 26.92±2.69 | |
| Female | 0 | 17.12±1.96NS | 19.35±1.09NS | 21.38±3.52NS | 23.17±2.34NS |
| 0.5 | 16.89±2.63 | 18.15±2.83 | 22.44±2.67 | 24.16±1.67 | |
| 1.0 | 15.73±2.43 | 17.89±4.36 | 21.97±3.15 | 22.62±2.53 | |
| 2.0 | 16.68±2.12 | 17.28±2.68 | 21.75±3.27 | 22.06±3.76 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
Table 3.
Daily water consumption of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days
| Sex | Dose (g/kg/d) | Water consumption (mL/d/rat)1) | |||
|---|---|---|---|---|---|
|
| |||||
| Days after treatment | |||||
|
| |||||
| 7 | 14 | 21 | 28 | ||
| Male | 0 | 29.45±1.53NS2) | 33.45±2.35NS | 39.36±3.54NS | 43.48±3.84NS |
| 0.5 | 30.32±2.56 | 35.76±3.85 | 39.37±3.49 | 44.35±2.75 | |
| 1.0 | 29.37±2.57 | 33.88±2.40 | 40.33±5.85 | 45.22±5.54 | |
| 2.0 | 29.25±3.45 | 34.83±3.56 | 40.72±4.56 | 45.73±4.82 | |
| Female | 0 | 24.38±3.45NS | 29.55±4.11NS | 34.18±3.47NS | 38.46±2.37NS |
| 0.5 | 24.58±5.38 | 30.45±3.45 | 35.54±4.51 | 39.46±3.33 | |
| 1.0 | 25.55±3.76 | 29.87±2.27 | 35.44±2.29 | 37.44±3.27 | |
| 2.0 | 25.74±4.45 | 30.35±3.23 | 35.75±3.55 | 38.35±4.14 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
The average body weight gain in males was higher compared to females. According to Waynforth and Flecknell (23), the rate of growth of female rats is usually lower than male rats at the same age. Feed and water consumption were similar for all study groups (Table 2 and 3). Body weight gain is an integral part of the conventional safety evaluation of a test article (1). According to Hilaly et al. (24), changes in body weight have been used as an indicator of adverse effects of a test material. Schiler et al. (1) also reported that significant body weight loss was considered as one of the most sensitive indicators of an animal’s deteriorating health status.
In the present study, no adverse clinical signs or mortality were observed in any of the rats during the period of experimentation. No significant differences were observed in feed intake, water intake, or weight gain in rats treated with different doses of crude antifungal compounds produced by L. plantarum HD1. These results were consistent with the results of Son et al. (15). From these results, it was concluded that the no-observed effect level of crude antifungal compounds produced by L. plantarum HD1 is greater than 2.0 g/kg/d in rats.
Subacute toxicity of hematological parameters
The effects of crude antifungal compounds produced by L. plantarum HD1 on the hematological parameters of the experimental and control rats are presented in Table 4. Hematological parameters are one of the most sensitive indicators of the effects of toxic substances and an important index of physiological and pathological status in humans and animals (25).
Table 4.
Hematology of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days
| Sex | Parameters | Dose (g/kg/d)1) | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 0.5 | 1.0 | 2.0 | ||
| Male | WBC (×103/μL) | 7.40±0.95NS2) | 7.41±0.53 | 7.43±0.38 | 7.45±0.84 |
| RBC (×103/μL) | 7.51±0.95NS | 7.53±0.49 | 7.55±0.82 | 7.56±0.67 | |
| Hb (%) | 15.56±4.60NS | 15.59±3.58 | 15.61±3.62 | 15.57±3.47 | |
| HCT (%) | 48.32±5.68NS | 48.35±6.40 | 48.46±6.51 | 48.57±5.23 | |
| MCV (fL) | 63.31±4.32NS | 63.65±6.12 | 63.84±6.47 | 63.67±6.59 | |
| MCH (pg) | 22.36±3.33NS | 23.87±3.64 | 23.74±3.48 | 23.61±3.42 | |
| MCHC (g/dL) | 35.12±8.32NS | 35.37±8.47 | 36.58±8.12 | 36.13±7.79 | |
| Platelets (×103/μL) | 915.25±74.13NS | 916.78±75.46 | 918.36±77.23 | 914.75±76.45 | |
| Reticulocytes (%) | 3.96±0.61NS | 4.02±0.57 | 4.12±0.58 | 3.87±0.43 | |
| Neutropils (%) | 7.93±0.84NS | 7.91±0.83 | 8.01±0.79 | 8.23±0.84 | |
| Eosinopils (%) | 0.94±0.68NS | 0.81±0.74 | 0.91±0.69 | 0.78±0.61 | |
| Basophils (%) | 1.01±0.68NS | 1.03±0.66 | 1.07±0.64 | 1.13±0.84 | |
| Lymphocytes (%) | 89.38±10.74NS | 90.12±10.85 | 88.54±9.98 | 93.68±9.32 | |
| Monocytes (%) | 1.29±0.11NS | 1.26±0.16 | 1.24±0.18 | 1.35±0.21 | |
| Female | WBC (×103/μL) | 6.75±0.41NS | 6.74±0.67 | 6.76±0.92 | 6.75±0.84 |
| RBC (×103/μL) | 6.51±0.68NS | 6.52±0.69 | 6.56±0.75 | 6.55±0.74 | |
| Hb (%) | 13.49±3.21NS | 13.51±3.41 | 13.53±2.68 | 13.55±2.43 | |
| HCT (%) | 43.69±4.72NS | 44.01±4.6 | 44.12±4.35 | 44.24±4.32 | |
| MCV (fL) | 60.36±3.58NS | 60.75±3.49 | 60.95±3.92 | 60.45±2.36 | |
| MCH (pg) | 20.21±2.49NS | 20.36±2.47 | 20.65±2.81 | 20.47±2.42 | |
| MCHC (g/dL) | 31.22±6.45NS | 31.69±6.48 | 31.25±6.41 | 32.97±6.25 | |
| Platelets (×103/μL) | 871.32±64.32NS | 869.38±63.23 | 870.46±65.23 | 871.59±66.42 | |
| Reticulocytes (%) | 2.91±0.36NS | 3.08±0.25 | 3.12±0.34 | 3.15±0.38 | |
| Neutropils (%) | 7.12±0.64NS | 7.31±0.59 | 7.46±0.58 | 7.15±0.38 | |
| Eosinopils (%) | 0.72±0.28NS | 0.76±0.36 | 0.62±0.47 | 0.63±0.51 | |
| Basophils (%) | 0.84±0.76NS | 0.86±0.72 | 0.92±0.63 | 0.96±0.33 | |
| Lymphocytes (%) | 88.36±8.65NS | 88.59±9.87 | 89.57±8.21 | 88.15±9.12 | |
| Monocytes (%) | 1.16±0.07NS | 1.17±0.09 | 1.29±0.08 | 1.16±0.09 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
Subacute oral administration of crude antifungal compounds produced by L. plantarum HD1 did not cause any significant changes in hematological variables including RBC, HCT, Hb, MCV, MCH, MCHC, platelets, reticulocytes, WBC, neutrophils, eosinophils, basophils, lymphocytes, and monocytes. The results of this study are in agreement with a study performed using crude antifungal compounds produced by L. plantarum AF1 (16). Neutrophils, lymphocytes, and monocytes in the male 2.0 g/kg/d group differed slightly from the control group, but these values were still within the reference range (26). These effects may therefore be due to variations in the animals. In the present study, it was shown that crude antifungal compounds produced by L. plantarum HD1 had no effects on the circulating blood cells or their production.
Subacute toxicity of serum biochemical parameters
Biochemical measurements in serum also serve as indicators of toxicity of a test material (1). According to Olson et al. (27), analysis of blood parameters is relevant for risk evaluation, i.e., changes in the hematological and biochemical parameters in animal studies have a high predictive value for human toxicity. The serum biochemistry parameters of rats administered the crude antifungal compounds are shown in Table 5. Subacute administration of crude antifungal compounds did not cause any significant changes in GLU, TC, TG, TP, and albumin levels. Hepatic toxicity was monitored by quantitative analysis of serum ALT, AST, and ALP activity. High serum levels of these enzymes are used as biochemical markers of liver damage in animals and human (24) because of increased membrane permeability or liver cell necrosis and cytosol leakage into the serum (28). Thus, increased levels of ALT, AST, and ALP activity in serum are associated with structural and functional damage to hepatocellular membranes (29). In this study, activities of serum enzymes (ALT, AST, and ALP) were not affected by repeated oral dosing of the crude antifungal compounds. No changes in ALT, AST, and ALP activity suggested that the subacute oral administration of crude antifungal compounds did not alter hepatocyte function or metabolism.
Table 5.
Levels of serum biochemical indices of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days
| Sex | Parameters | Dose (g/kg/d)1) | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 0.5 | 1.0 | 2.0 | ||
| Male | ALT (U/L) | 39.67±5.37NS2) | 39.21±4.59 | 40.13±5.85 | 40.78±4.98 |
| AST (U/L) | 161.01±23.36NS | 164.02±24.51 | 163.28±24.75 | 162.14±25.18 | |
| ALP (U/L) | 141.36±16.34NS | 142.36±15.54 | 144.31±16.53 | 145.27±16.50 | |
| GLU (mg/dL) | 113.58±17.19NS | 115.47±18.18 | 117.42±19.15 | 118.36±17.68 | |
| BUN (mg/dL) | 17.36±5.21NS | 18.65±5.13 | 19.37±5.41 | 17.18±5.68 | |
| TC (mg/dL) | 77.36±6.48NS | 82.87±8.34 | 76.74±7.49 | 76.61±7.43 | |
| TG (mg/dl) | 100.20±15.54NS | 94.48±16.13 | 95.85±17.15 | 96.75±16.69 | |
| TP (g/dL) | 8.48±1.58NS | 9.16±2.11 | 9.10±2.36 | 9.08±1.75 | |
| Albumin (mg/dL) | 4.35±0.33NS | 4.36±0.37 | 5.00±0.43 | 4.47±0.29 | |
| CRE (mg/dL) | 0.94±0.08NS | 0.75±0.09 | 0.69±0.11 | 0.84±0.11 | |
| Female | ALT (U/L) | 38.67±3.23NS | 38.89±3.65 | 39.13±3.54 | 39.53±3.41 |
| AST (U/L) | 153.37±23.40NS | 152.27±24.37 | 154.17±23.49 | 153.62±22.25 | |
| ALP (U/L) | 106.71±9.17NS | 108.42±10.12 | 109.42±9.62 | 111.34±9.68 | |
| GLU (mg/dL) | 95..64±13.36NS | 96.48±13.42 | 99.12±12.35 | 97.18±11.15 | |
| BUN (mg/dL) | 13.37±4.35NS | 14.26±4.41 | 15.38±5.10 | 16.56±4.75 | |
| TC (mg/dL) | 73.21±6.36NS | 79.36±6.25 | 77.65±6.49 | 75.47±6.07 | |
| TG (mg/dL) | 90.32±9.51NS | 85.34±10.62 | 86.36±10.85 | 84.78±9.76 | |
| TP (g/dL) | 7.31±1.34NS | 7.64±1.05 | 7.89±1.18 | 7.68±1.70 | |
| Albumin (mg/dL) | 3.48±0.19NS | 3.68±0.20 | 3.79±0.37 | 3.45±0.22 | |
| CRE (mg/dL) | 0.46±0.03NS | 0.49±0.04 | 0.51±0.04 | 0.37±0.01 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
High serum levels of BUN and CRE may indicate protein catabolism and/or renal dysfunction (28). Subacute oral administration of crude antifungal compounds did not cause any significant changes in serum BUN and CRE levels. Normal values for renal biochemical variables (BUN and CRE) suggested that crude antifungal compounds did not produce any disruption of renal function. These results indicated that crude antifungal compounds did not induce any damage to these organs as measured by biochemical tests of blood. These results agree with those reported by Son et al. (15). In addition, all biochemical parameters in this study remained in the reference range for the species (30).
Subacute toxicity study of organ weights and histopathology
Generally, a reduction in organ weights is a simple and sensitive index of test article-associated toxicities (31). Data for the absolute organ weights are shown in Table 6. In this study, the antifungal compounds did not produce any statistically significant differences among groups in internal organ weights. Gross examination of internal organs from all rats showed no detectable abnormalities.
Table 6.
Organ weights of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days
| Sex | Organs (g) | Dose (g/kg/d) | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 0.5 | 1.0 | 2.0 | ||
| Male | Brain | 1.98±0.18NS | 1.99±0.18 | 1.96±0.18 | 1.97±0.18 |
| Lungs | 1.38±0.65NS | 1.39±0.45 | 1.38±0.56 | 1.38±0.88 | |
| Testes | 2.91±0.34NS | 2.92±0.44 | 2.93±0.26 | 2.95±0.33 | |
| Kidney-Left | 1.20±0.65NS | 1.21±0.32 | 1.23±0.64 | 1.24±0.57 | |
| Kidney-Right | 1.21±0.60NS | 1.23±0.54 | 1.22±0.59 | 1.25±0.50 | |
| Heart | 1.01±0.22NS | 1.00±0.23 | 1.02±0.29 | 1.01±0.31 | |
| Spleen | 0.71±0.46NS | 0.72±0.47 | 0.72±0.50 | 0.73±0.53 | |
| Liver | 9.12±4.59NS | 9.05±5.74 | 9.06±5.68 | 9.07±5.63 | |
| Female | Brain | 1.66±0.18NS | 1.67±0.18 | 1.66±0.18 | 1.69±0.18 |
| Lungs | 1.11±0.51NS | 1.12±0.45 | 1.15±0.65 | 1.14±0.35 | |
| Ovary | 0.070±0.01NS | 0.071±0.01 | 0.069±0.01 | 0.072±0.02 | |
| Kidney-Left | 0.93±0.55NS | 0.95±0.54 | 0.95±0.68 | 0.94±0.61 | |
| Kidney-Right | 0.94±0.30NS | 0.95±0.24 | 0.96±0.28 | 0.95±0.37 | |
| Heart | 0.79±0.10NS | 0.80±0.13 | 0.81±0.18 | 0.80±0.19 | |
| Spleen | 0.60±0.23NS | 0.61±0.37 | 0.62±0.29 | 0.62±0.31 | |
| Liver | 6.71±3.39NS | 6.76±3.46 | 6.75±2.93 | 6.77±2.49 | |
Values are expressed as mean±SE (n=5).
NS: not significantly different from the other groups.
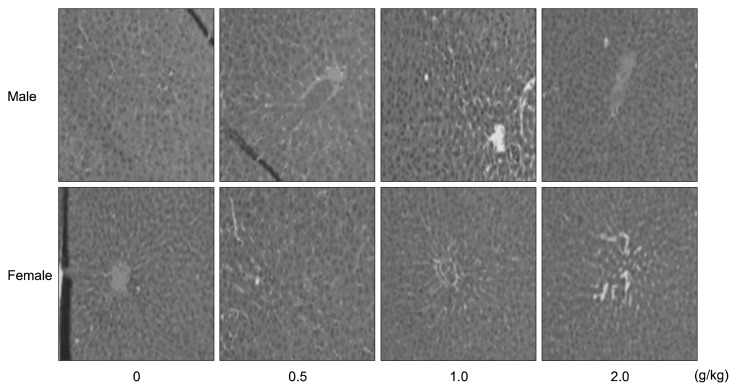
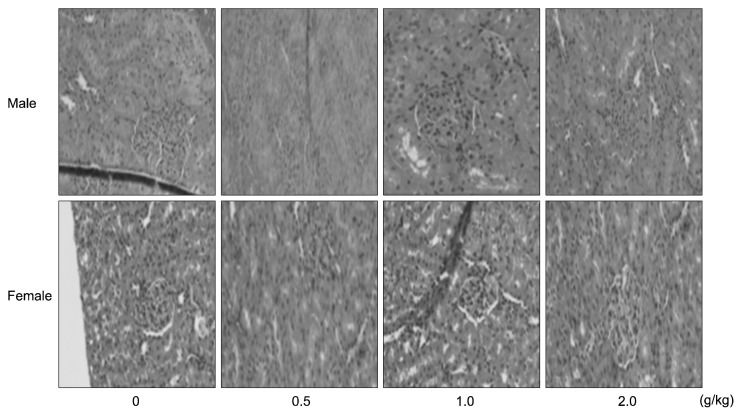
Representative microscopic findings in the livers and kidneys of rats after subacute oral treatments with the crude antifungal compounds are shown in Fig. 1 and 2, respectively. In the livers of the control and treated groups, the cross-section showed the normal appearance of liver. The central vein, sinusoids, and hepatocytes were clearly preserved (Fig. 1). Light microscopy revealed that the control and treated renal tissues showed normal structure of the renal glomerulus, tubule, and interstitium (Fig. 2). The histopathological study revealed normal architecture of livers and kidneys in rats treated with the crude antifungal compounds. These data are similar to a previous study which showed that crude antifungal compounds produced by L. plantarum AF1 were safe in subacute oral administration in rats (15).
Fig. 1.
Histopathological examinations of the liver of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days. Male and female SD rats were randomly assigned to four groups of five rats each. The sections of liver from control and treated rats revealed normal architecture and hepatic cells with granulated cytoplasm.
Fig. 2.
Histopathological examinations of the kidney of male and female SD rats treated orally with crude antifungal compounds produced by L. plantarum HD1 for 28 days. Male and female SD rats were randomly assigned to four groups of five rats each. The sections of kidney from control and treated rats showed glomeruli of normal size with normal tubules.
In conclusion, results of the present study demonstrated that the crude antifungal compounds from L. plantarum HD1, a LAB isolated from kimchi, were found to be non-toxic in oral acute toxicity (up to 5.0 g/kg/d) and subacute toxicity studies (up to 2.0 g/kg/d) in male and female SD rats. Chronic toxicity, mutagenicity, and carcinogenicity studies are needed to further support the safe use of these compounds.
ACKNOWLEDGEMENTS
This research was supported by the High Value-added Food Technology Development Program (Project No. 313027-3) in 2014, Ministry of Agriculture, Food and Rural Affairs, South Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R Natural Toxin Task Force of the European Branch of the International Life Sciences Institute. Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. Food Chem Toxicol. 2003;41:1625–1649. doi: 10.1016/S0278-6915(03)00221-7. [DOI] [PubMed] [Google Scholar]
- 2.Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria-potential for control of mould growth and mycotoxins: a review. Food Control. 2010;21:370–380. doi: 10.1016/j.foodcont.2009.07.011. [DOI] [Google Scholar]
- 3.Voulgari K, Hatzikamari M, Delepoglou A, Georgakopoulos P, Litopoulou-Tzanetaki E, Tzanetakis N. Antifungal activity of non-starter lactic acid bacteria isolates from dairy products. Food Control. 2010;21:136–142. doi: 10.1016/j.foodcont.2009.04.007. [DOI] [Google Scholar]
- 4.Yang EJ, Chang HC. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int J Food Microbiol. 2010;139:56–63. doi: 10.1016/j.ijfoodmicro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Brul S, Coote P. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50:1–17. doi: 10.1016/S0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 6.Stiles ME. Biopreservation by lactic acid bacteria. Antonie Van Leeuwenhoek. 1996;70:331–345. doi: 10.1007/BF00395940. [DOI] [PubMed] [Google Scholar]
- 7.Yang EJ, Chang HC. Antifungal activity of Lactobacillus plantarum isolated from kimchi. Kor J Microbiol Biotechnol. 2008;36:276–284. [Google Scholar]
- 8.Ryu EH, Yang EJ, Woo ER, Chang HC. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014;41:19–26. doi: 10.1016/j.fm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Cheong EYL, Sandhu A, Jayabalan J, Kieu Le TT, Nhiep NT, My Ho HT, Zwielehner J, Bansal N, Turner MS. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control. 2014;46:91–97. doi: 10.1016/j.foodcont.2014.05.011. [DOI] [Google Scholar]
- 10.Crowley S, Mahony J, van Sinderen D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci Technol. 2013;33:93–109. doi: 10.1016/j.tifs.2013.07.004. [DOI] [Google Scholar]
- 11.Huang Y, Kotula L, Adams MC. The in vivo assessment of safety and gastrointestinal survival of an orally administered novel probiotic, Propionibacterium jensenii 702, in a male Wistar rat model. Food Chem Toxicol. 2003;41:1781–1787. doi: 10.1016/S0278-6915(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 12.Shu Q, Zhou JS, Rutherfurd KJ, Birtles MJ, Prasad J, Gopal PK, Gill HS. Probiotic lactic acid bacteria (Lactobacillus acidophilus HN017, Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019) have no adverse effects on the health of mice. Int Dairy J. 1999;9:831–836. doi: 10.1016/S0958-6946(99)00154-5. [DOI] [Google Scholar]
- 13.Lee H, Lee JJ, Chang HC, Lee MY. Acute toxicity of Lactobacillus plantarum AF1 isolated from kimchi in mice. Korean J Food Preserv. 2012;19:315–321. doi: 10.11002/kjfp.2012.19.2.315. [DOI] [Google Scholar]
- 14.Lee JJ, Kim AR, Chang HC, Lee MY. Repeated-dose oral toxicity study of Lactobacillus plantarum AF1 isolated from kimchi in rats. J Korean Soc Food Sci Nutr. 2012;41:612–620. doi: 10.3746/jkfn.2012.41.5.612. [DOI] [Google Scholar]
- 15.Son HK, Lee MY, Chang HC, Lee JJ. Acute toxicity of crude anti-fungal compounds produced by Lactobacillus plantarum AF1. J Korean Soc Food Sci Nutr. 2013;42:892–897. doi: 10.3746/jkfn.2013.42.6.892. [DOI] [Google Scholar]
- 16.Lee H, Lee MY, Chang HC, Lee JJ. Repeated-dose oral toxicity study of crude antifungal compounds produced by Lactobacillus plantarum AF1 in rats. Korean J Food Preserv. 2013;20:394–403. doi: 10.11002/kjfp.2013.20.3.394. [DOI] [Google Scholar]
- 17.Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. OECD publishing; Paris, France: 2001. Test No. 420: Acute oral toxicity-fixed dose procedure. [Google Scholar]
- 18.Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. OECD publishing; Paris, France: 2001. Preliminary draft updated test guideline 407: repeated dose 28-day oral toxicity study in rodents; updated with parameters for endocrine effects. [Google Scholar]
- 19.Twaij HA, Kery A, Al-Khazraji NK. Some pharmacological, toxicological and phytochemical investigations on Centaurea phyllocephala. J Ethnopharmacol. 1983;9:299–314. doi: 10.1016/0378-8741(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 20.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 21.Singh A, Dubey SD, Patney S, Kumar V. Acute and subchronic toxicity study of calcium based Ayurvedic ‘Bhasmas’ and a ‘Pishti’ prepared from marine animals. J Herb Med Toxicol. 2010;4:35–47. [Google Scholar]
- 22.Kennedy GL, Jr, Ferenz RL, Burgess BA. Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. J Appl Toxicol. 1986;6:145–148. doi: 10.1002/jat.2550060302. [DOI] [PubMed] [Google Scholar]
- 23.Waynforth HB, Flecknell PA. Experimental and Surgical Technique in the Rat. 2nd ed. Academic Press; London, UK: 1980. Vital statistics and miscellaneous information: Organ weights; p. 346. [Google Scholar]
- 24.El Hilaly J, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Luo Y, Wang L, Li Y, Shi Y, Cui Y, Xue M. Acute and subacute toxicity of ethanol extracts from Salvia przewalskii Maxim in rodents. J Ethnopharmacol. 2010;131:110–115. doi: 10.1016/j.jep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Fedlman BV, Schalm OW, Zinkl JG, Jain NC. In: Schalm’s veterinary hematology. 5th ed. Feldman BV, Zinkl JG, Jain NC, editors. Blackwell Publishing Limited; Oxford, UK: 2000. pp. 1210–1218. [Google Scholar]
- 27.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Fattah SM, Sanad MI, Safaa MA, Ragaa FFG. The protective effect of white ginseng against biochemical and pathological changes induced by aflatoxins in rats. J Am Sci. 2010;6:461–472. [Google Scholar]
- 29.Bürger C, Fischer DR, Cordenunzzi DA, de Borba Batschauer AP, Filho VC, dos Santos Soares AR. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa (Acmela brasiliensis) (Asteraceae) in mice. J Pharm Pharmaceut Sci. 2005;8:370–373. [PubMed] [Google Scholar]
- 30.Levine BS. Animal clinical pathology. In: Derelanko MJ, Hollinger MA, editors. Handbook of Toxicology. 2nd ed. CRC Press Inc; New York, NY, USA: 1995. pp. 517–537. [Google Scholar]
- 31.Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm. 2002;70:135–145. [Google Scholar]