Abstract
Background
The exact role of shunting during carotid endarterectomy remains controversial and unclear. The aim of this experimental study was to investigate to what degree carotid clamping may induce changes in the cerebral oxidative status and to focus on the relation of these changes with shunt insertion.
Material/Methods
Forty New-Zealand rabbits were randomized into 4 groups: group 1 classifying animals with carotid shunt and patent contralateral carotid artery; group 2 shunt and occlusion of the contralateral carotid artery; group 3 no-shunt and patent contralateral carotid artery; and group 4 no-shunt and occlusion of the contralateral carotid artery. Blood samples were collected from the ipsilateral internal jugular vein, immediately after carotid clamping (time 0), and then at 5, 10, 15, 30, and 60 minutes afterwards. Evaluation of oxidative stress was accomplished by measuring the lag-time, representing the initial phase of oxidation, rate of accumulation (RA), showing concentration of free oxygen radical and total antioxidant status (TAS) representing antioxidant composition of serum.
Results
Lag-time was significantly different in time points 0, 30 and 60 minutes within each different group. TAS was significantly different in time points 0, 15 and 60 min and RA in time points 0, 5, 10 and 60 min within each different group. 60 minutes after carotid clamping, the rate of accumulation as well as lag-time and TAS were increased in all groups, independently of using or not shunting or the presence of contralateral occlusion. After comparing groups 1, 2 and 3 regarding lag-time, TAS and RA, we did not find statistical difference among the groups at any time point. On the contrary, groups 1, 2 and 3 did show significantly different values comparing to group 4 after 60 min of occlusion.
Conclusions
Our experimental work based on cerebral metabolism found a significantly higher oxidative stress in models with contralateral carotid occlusion. The use of shunt in all other models did not have any influence on oxidative response. Future human studies should focus on the relation of oxidative status and shunt insertion to determine the benefit of selective or routine shunting during CEA.
MeSH Keywords: Brain Ischemia; Endarterectomy, Carotid; Oxidative Stress
Background
Carotid endarterectomy (CEA) remains the recommended invasive treatment of carotid disease for specific groups of both asymptomatic and symptomatic patients with carotid stenosis of 70–99% and >50%, respectively [1]. CEA can be delivered under general or local anesthesia with similar results, with the latter anesthesia method reported to lead to decreased need for shunting [2,3]. Cerebral ischemia prevention during CEA can be avoided by the intraoperative applications of a shunt across the clamped section of the carotid artery. The exact role of shunting during CEA remains controversial and unclear [1,4]. A number of surgeons support routine shunting, while others prefer selective shunting or no shunting at all, even in the presence of contralateral internal carotid artery (ICA) occlusion [5,6]. The few published randomized trials have shown inconclusive results [7,8], while a recent Cochrane review concluded that the data available are too limited to either support or refute the use of routine or selective shunting in CEA [9].
The optimal modality for evaluating cerebral perfusion during CEA and determining the need for selective shunting has been also controversial. Patients undergoing CEA in the presence of an occluded contralateral carotid artery has increased perioperative and early postoperative risk and these patients might benefit from shunt insertion [10]. Many methods based on indirect measurement of cerebral perfusion have been performed, but none has gained wide acceptance or been proven to be superior when compared to others [11,12]. A recent review found no significant differences between the risk of ipsilateral stroke in participants selected for shunting with the combination of electroencephalographic and carotid pressure assessment compared with pressure assessment alone, although data were for afresh limited [9].
The pathophysiologic routes of cerebral ischemia in relation to shunting use have also been investigated. Several studies have attempted to quantify metabolic cerebral response to ischemia, showing increased levels of serum inflammatory markers in no-shunt patients [13]. Serum proteins excreted in brain damage, such as S-100 protein, neuron-specific enolase (NSE) and interleukin-6 (IL-6) seem to have a role [8]. Beyond hemodynamic parameters, identification of early changes in cerebral metabolism during carotid clamping might be a more specific alarming sign for ischemic injury. Cerebral ischemia is accompanied by production of free radicals that cannot be consumed by tissue antioxidants [14]. The balance between antioxidants and products of oxidative stress could potentially be the best approach to the indirect evaluation of oxidative stress and cerebral ischemia [15]. Serum oxidability and TAS have been used as markers of oxidative stress in aneurysmal and atherosclerotic diseases [16].
The aim of this experimental study was to investigate to what extent carotid clamping may induce changes in the cerebral oxidative status and to focus on the relation of these changes with shunt application.
Material and Methods
Experimental model
After receiving approval and authorization by the Animal Ethics Committee to proceed, 40 New-Zealand rabbits, weighting 3–3.5 kg were randomized into 4 groups:
Group 1: animals with carotid shunt and patent contralateral carotid artery (n=10);
Group 2: shunt and occlusion of the contralateral carotid artery (n=10);
Group 3: no-shunt and patent contralateral carotid artery (n=10);
Group 4: no-shunt and occlusion of the contralateral carotid artery (n=10).
Procedure
General anaesthesia induced by intravenous injection of 25 mg/kg pentobarbital sodium. Right common carotid artery was exposed in groups 1 and 3, while both common carotid arteries were exposed in groups 2 and 4. Heparin solution of 100 IU/ kg was administered intravenously before clamping. Occlusion of the contralateral carotid artery was achieved by carotid clamping. An 18–24 G catheter was used as a shunt and its patency was evaluated at 15-minute intervals during clamping. Carotid clamping lasted 60 min on average. After completion, all animals were sacrificed by anesthesia medication overdose.
Blood samples
Blood samples were collected from the internal jugular vein, ipsilateral to the site of carotid clamping at the following time intervals: immediately after carotid clamping (time 0), and 5, 10, 15, 30, and 60 min afterwards. Blood samples were immediately centrifuged at 15 000 rpm for 15 min and supernatant was collected.
Biochemical assays
Oxidative stress was evaluated by measuring the susceptibility of serum lipids to oxidation using spectrophotometric kinetic methods, and the total antioxidant capacity. Lag time (in seconds) is the time preceding oxidation when copper is introduced to serum, and represents serum antioxidative capacity. Maximal rate of accumulation of absorbing products of copper oxidation (RA), expressed in Optical density units/sec ×105 represents oxidizable serum products. TAS, expressed in mmol/L, is evaluated by 2, 2′-Azino-di-[3-ethylbenzthiazoline sulphonate] or ABTS, (ABTS® kit, RANDOX Laboratories Ltd., Ireland). The principle of the assay is to incubate ABTS with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS. Antioxidants present in the sample cause suppression of cation production proportional to their concentration.
Statistical analysis
Due to the small size of each group data are expressed as non-Gaussian parameters and are presented as median (range). Comparisons of continuous variables were made by the Mann-Whitney U test. All statistical tests were 2-sided and a p<0.05 was considered significant. All analyses were carried out with SPSS 20.0 statistical package for Windows (SPSS Inc., USA).
Results
Detailed data regarding lag time, TAS and RA are shown in Tables 1–3.
Table 1.
Total antioxidant status in all groups at different time points.
| Total antioxidant status | ||||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 30 | 60 | |
| Group 1 | 1.30 (1.14–1.49) | 1.16 (0.91–1.46) | 1.67 (1.18–2.1) | 1.33 (0.97–1.95) | 1.03 (0.78–1.48) | 1.38 (0.99–1.86) |
| Group 2 | 1.24 (1.05–1.43) | 1.09 (0.98–1.34) | 1.11 (1.05–1.2) | 0.89 (0.8–0.98) | 0.88 (0.68–1.1) | 0.86 (0.63–1.09) |
| Group 3 | 1.24 (1.2–1.28) | 1.11 (1.05–1.16) | 1.17 (1.15–1.19) | 0.96 (0.9–1) | 0.86 (0.83–0.92) | 0.95 (0.77–1.12) |
| Group 4 | 0.51 (0.3–0.8) | 0.9 (0.82–0.97) | 1.31 (0.74–1.94) | 0.79 (0.45–1.14) | 0.8 (0.45–1.15) | 0.61 (0.45–0.79) |
Table 2.
Lag time in all groups at different time points.
| Lag time | ||||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 30 | 60 | |
| Group 1 | 2660.1 (1427.9–4789.9) | 3155 (1503.8–5077.8) | 2116.1 (1183.1–2923.1) | 3396.9 (1953.6–5644.4) | 2843.4 (1265.1–4816.2) | 2182 (870.7–4417.7) |
| Group 2 | 2344.2 (1617.9–2962.9) | 3694.8 (2564.2–4958.2) | 4989.7 (3592.4–4988.1) | 3080.9 (2364.4–3797.4) | 3745.3 (2022.2–4959.7) | 4024.7 (3222.5–5077.8) |
| Group 3 | 2711.4 (1427.9–4789.9) | 4024.7 (322.5–5077.8) | 3840.8 (3138.1–4461.1) | 4122.6 (2626.3–5644.4) | 2855.2 (2559.2–3151.2) | 2672.9 (515.2–4417.7) |
| Group 4 | 1131 (890.4–1365.4) | 910.7 (554.2–1267.2) | 949.4 (664–1235.9) | 836.6 (457.7–1215.7) | 742.5 (150.8–1334.2) | 414.3 (405.2–423.1) |
Table 3.
Rate of accumulation in all groups at differents time points.
| Rate of accumulation | ||||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 30 | 60 | |
| Group 1 | 3.3 (1.16–6.7) | 2.8 (0.9–5) | 3.6 (1.4–7.1) | 3.4 (2.8–4.5) | 2.4 (2.3–2.5) | 5.7 (3.2–8.2) |
| Group 2 | 2.1 (1.7–2.8) | 1.4 (0.7–2.1) | 1.1 (0.4–1.8) | 3.0 (2.25–3.8) | 2.6 (2.5–2.7) | 1.17 (1.1–2.1) |
| Group 3 | 1.8 (0.4–3.5) | 1.9 (0.9–2.7) | 1.4 (0.8–2.2) | 2.6 (2.2–2.9) | 2.7 (2.5–2.9) | 3.4 (3.2–3.6) |
| Group 4 | 5.0 (4–5.8) | 6.5 (4.9–7.8) | 7.9 (7.1–8.7) | 4.7 (0.9–7.1) | 5.7 (1.8–8.8) | 6.8 (5.1–8.4) |
Within group analysis
Lag-time was significantly different in time points 0, 30 and 60 min within each different group. TAS was significantly different in time points 0, 15 and 60 min and RA in time points 0, 5, 10, and 60 min within each different group. 60 minutes after carotid clamping, the rate of accumulation as well as lag-time and TAS were increased in all groups, independently of using or not shunting or the presence of contralateral occlusion.
Between groups analysis
After comparing groups 1, 2 and 3 regarding lag-time, TAS and RA, we did not find any statistical difference among the different groups at any time point. However, groups 1, 2 and 3 showed significantly different values comparing to group 4 after 60 min of occlusion (Figure 1). Specifically, production of free radicals measured as rate of accumulation was: group 1 vs. group 4 at 60 min, 5.7 vs. 6.8 OD/ s×10−5 (p=0.050); group 2 vs. 4 at 60 min, 1.17 vs. 6.8 OD/s×10−5 (p=0.031); and group 3 vs. 4 at 60 min 3.42 vs. 6.8 OD/s×10−5 (p=0.046). Concentration of antioxidants was measured as lag-time: group 1 vs. 4 at 60 min, 2182 vs. 414 s (p=0.057); group 2 vs. 4 at 60 min, 4024 vs. 414 s (p=0.049); group 3 vs. 4 at 60 min 2672 vs. 414 s (p=0.048) and TAS: group 1 vs. 4 at 60 min, 1.38 vs. 0.61 mM (p=0.034), group 2 vs. 4 at 60 min, 0.85 vs. 0.61 mM (p=0.057); and group 3 vs. 4 at 60 min 0.95 vs. 0.61 mM (p=0.050).
Figure 1.
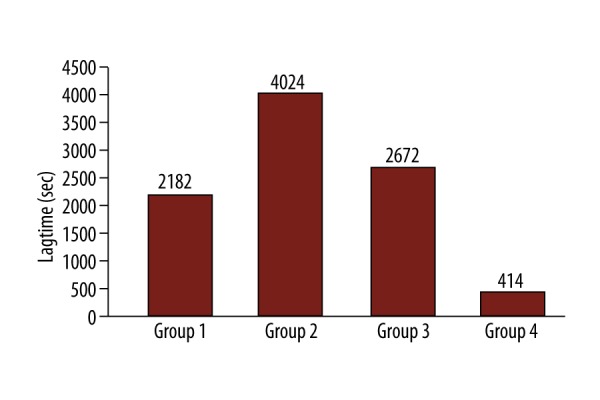
Lag-time 60 min after carotid clamping.
In group 4 (no shunt, clamped contralateral carotid), where practically both carotids are occluded, oxidative stress as measured by RA was statistically higher after 60 min of clamping, while TAS and lag-time (representing antioxidant capacity) were lower compared to other groups (Figures 1–3).
Figure 2.
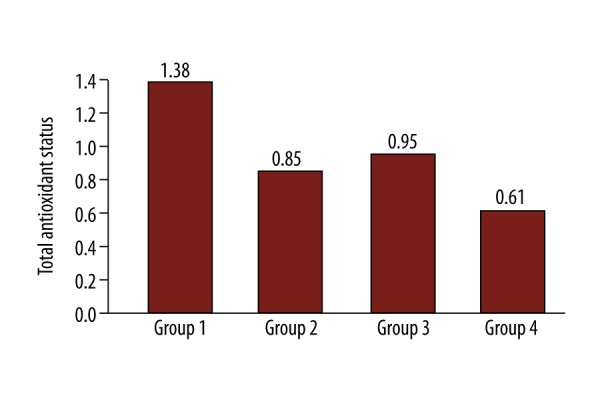
Total antioxidant status 60min after carotid clamping.
Figure 3.
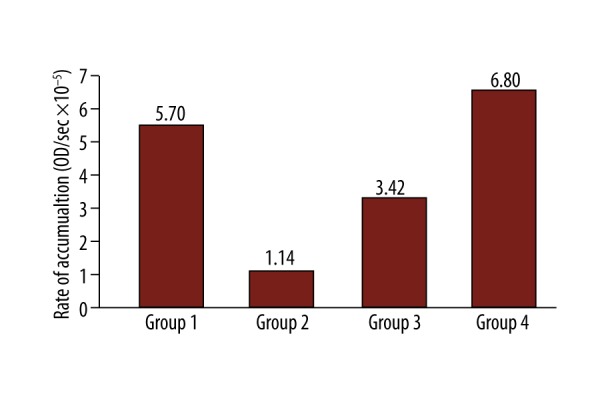
Rate of accumulation 60min after carotid clamping.
All shunts remained patent during clamping and no intraoperative thrombosis occurred.
Discussion
Cerebral ischemia during carotid clamping initiates a cascade of biochemical processes that produce oxidative radicals. Free radicals promote lipid peroxidation and fragmentation of proteins and nucleic acids. Under physiologic conditions free radicals are normally generated in cells, mitochondria and endoplasmic reticulum, and are neutralized by radical scavengers. When this equilibrium is lost, the process of lipid peroxidation results in damage of poly-unsaturated acids of cellular lipid membranes and production of malondialdehyde (MDA) and diene conjugates, which leads to loss of cellular integrity. Brain is prone to oxidative damage, as it is rich in poly-unsaturated fatty acids, a substrate of peroxidation and poor in antioxidant enzymes. Antioxidant systems include enzymes such as superoxide dismutase, catalase, and glutathione peroxidase; macromolecules such as albumin, ceruloplasmin, and ferritin; and an array of small molecules, including ascorbic acid, α-tocopherol, β-carotene, ubiquinol-10, reduced glutathione (GSH), methionine, uric acid and bilirubin [17].
In experimental evaluation of oxidative stress, lag-time represents the initial phase of oxidation where endogenous antioxidants found in plasma interfere with oxygen radicals, and slow-down oxidation. Lag-time is proportional to the concentration of antioxidants and represents the resistance of serum lipids to oxidation or antioxidative capacity of serum. Afterwards, the antioxidants are consumed linearly with time and rate-competing reaction slows-down. Consequently RA is relative to the concentration of free oxygen radical. TAS represents antioxidant composition of serum.
There are different methods in assessing oxidative capacity [18]. Studies in humans showed that oxidative reaction can be influenced by the presence of cerebral symptoms, collateral circulation, systematic blood pressure, surgical skills and incomplete circle of Willis, found in nearly 21% of patients in autopsy studies [19], making the interpretation of the studies’ results a difficult task. The animal model that has been used in our study eliminates the majority of these factors.
In humans, cerebral damage during carotid clamping may be related to microembolism or hypoperfusion, but in the animal model we have used, no atheromatous debris exists, therefore embolism is not possible. Reperfusion is also eliminated in our model, as animals are sacrificed at the end of the 60 minutes period. Some authors suggest that contralateral carotid occlusion and poor collateral flow exaggerates the severity of microvascular autoregulation, leading to hyperperfusion syndrome [20]. In our study, animals have never been exposed to chronic ischemia that would lead to consequent loss of vasoregulation.
In the present study, we evaluated three parameters of oxidative stress under different ischemia conditions and concluded that after 60 minutes, oxidative radical production and concentration of antioxidants in serum is increased independently of shunting or the presence of contralateral occlusion. Our findings suggest that after 60 minutes of occlusion of both carotid arteries (no shunt, clamped contralateral carotid), oxidative stress is more prominent when compared to the groups with at least 1 patent carotid artery (ipsilateral shunting or contralateral internal carotid patency). Low values of lag-time in group 4 signify lower antioxidative capacity and higher vulnerability to oxidation of serum lipids. The reduced antioxidative status can be attributed to the overconsumption of serum antioxidants by increased concentration of oxidative radicals (oxidative status). This is proven by increased rate of accumulation. Decreased lag-time might result from consumption of antioxidants by increased oxidative status.
Our study shows that oxidative stress reflects cerebral vulnerability to ischemia and contralateral carotid occlusion might be an indicator for the necessity of carotid shunting. This is in accordance with other non-experimental studies showing that patients with contralateral occlusion are considered being under higher risk for perioperative stroke [21–23]. There is a suggestion that contralateral occlusion predisposes cerebral ischemia and this is more evident in the presence of an inadequate circle of Willis [24].
Many studies have attempted to evaluate the need for carotid shunting by estimating intraoperative cerebral ischemia. It was suggested that patients with contralateral occlusion might need more often shunting. Nearly half of patients with carotid obstruction may need shunting according to EEG findings [25] or stump pressure measurements [26]. Pulli et al. [27] showed that the presence of contralateral occlusion increased the need for shunting as determined by somatosensory evoked potentials. However, in the present study, there was no difference in clinical outcome, implying that selective shunting may be more appropriate. Cinar et al. [28] suggested selective shunting during CEA with concomitant contralateral occlusion based on intra-operative assessing of neurological status and regional anaesthesia.
Our results should be interpreted in the light of certain limitations, including the relatively small number of animals and the species chosen as animal model. Different species show variable hypoxia tolerance and neural regeneration ability. Hypoxia tolerance, reaction to neuroprotective agents and metabolism, are different between humans and rats. [29]
Authors admit that an angiographic evaluation of circle of Willis was not performed either preoperatively or postoperatively, in order to provide information about cerebral collateral anastomotic status. Another limitation was the rapid occlusion of contralateral carotid artery by clamping does not mirror the natural progress of carotid artery disease in humans: usually slow and progressive stenosis leading to occlusion, with increasing symptoms of cerebral ischemia with severity depending on an ability of the brain to adapt to new hemodynamic conditions and perfusion. On the contrary, carotid clamping leads to sudden interruption of intracerebral flow, which usually leads to stroke. A third limitation was that animals of this study were healthy with “healthy” arteries, in contrast to carotid disease patients who are usually aged and present with multiple diseases and atheromatous arteries. Therefore, the brains of the animals were not adapted to chronic ischemia and underwent a rapid hypoperfusion, unlike the patients’ group undergoing CEA.
Conclusions
In conclusion, our experimental work based on cerebral metabolism found a significantly higher oxidative stress in models with contralateral carotid occlusion. The use of shunt in all other models did not have any influence on oxidative response. Primate animal models with complications would be more in line with the human clinical situation of carotid disease and a number of factors need to be simulated, including age, diabetes, hypertension, hyperlipidemia, and smoking.
Future human studies should focus on the relation of oxidative status and shunt insertion to determine the benefit of selective or routine shunting during CEA.
Footnotes
Source of support: Departmental sources
Conflict of interest
Nothing to report.
References
- 1.Liapis CD, Bell PR, Mikhailidis D, et al. Esvs guidelines. Invasive treatment for carotid stenosis: Indications, techniques. Eur J Vasc Endovasc Surg. 2009;37:1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 2.GALA Trial Collaborative Group. Lewis SC, Warlow CP, Bodenham AR, et al. General anaesthesia versus local anaesthesia for carotid surgery (gala): A multicentre, randomised controlled trial. Lancet. 2008;372:2132–42. doi: 10.1016/S0140-6736(08)61699-2. [DOI] [PubMed] [Google Scholar]
- 3.Guay J. The gala trial: Answers it gives, answers it does not. Lancet. 2008;372:2092–93. doi: 10.1016/S0140-6736(08)61700-6. [DOI] [PubMed] [Google Scholar]
- 4.Brott TG, Halperin JL, Abbara S, et al. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery. J Am Coll Cardiol. 2011;57:e16–94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Bydon A, Thomas AJ, Seyfried D, Malik G. Carotid endarterectomy in patients with contralateral internal carotid artery occlusion without intraoperative shunting. Surg Neurol. 2002;57:325–30. doi: 10.1016/s0090-3019(02)00678-x. discussion 31–32. [DOI] [PubMed] [Google Scholar]
- 6.Frawley JE, Hicks RG, Gray LJ, Niesche JW. Carotid endarterectomy without a shunt for symptomatic lesions associated with contralateral severe stenosis or occlusion. J Vasc Surg. 1996;23:421–27. doi: 10.1016/s0741-5214(96)80006-1. [DOI] [PubMed] [Google Scholar]
- 7.Gumerlock MK, Neuwelt EA. Carotid endarterectomy: To shunt or not to shunt. Stroke. 1988;19:1485–90. doi: 10.1161/01.str.19.12.1485. [DOI] [PubMed] [Google Scholar]
- 8.Palombo D, Lucertini G, Mambrini S, Zettin M. Subtle cerebral damage after shunting vs. non shunting during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007;34:546–51. doi: 10.1016/j.ejvs.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Chongruksut W, Vaniyapong T, Rerkasem K. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting) Cochrane Database Syst Rev. 2014;6:CD000190. doi: 10.1002/14651858.CD000190.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniou GA, Kuhan G, Sfyroeras SG, et al. Contralateral occlusion of the internal carotid artery increases the risk of patients undergoing carotid endarterectomy. J Vasc Surg. 2013;57:1134–45. doi: 10.1016/j.jvs.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Pennekamp CW, Bots ML, Kappelle LJ, et al. The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surge. 2009;38:539–45. doi: 10.1016/j.ejvs.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Anzola GP, Limoni P, Cavrini G. Predictors of carotid clamping intolerance during endarterectomy that would be wise to apply to stenting procedures. Cerebrovasc Dis. 2008;26:494–501. doi: 10.1159/000155987. [DOI] [PubMed] [Google Scholar]
- 13.Parsson HN, Lord RS, Scott K, Zemack G. Maintaining carotid flow by shunting during carotid endarterectomy diminishes the inflammatory response mediating ischaemic brain injury. Eur J Vasc Endovasc Surg. 2000;19:124–30. doi: 10.1053/ejvs.1999.0954. [DOI] [PubMed] [Google Scholar]
- 14.Hong JM, Bang OY, Chung CS, et al. Influence of recanalization on uric acid patterns in acute ischemic stroke. Cerebrovasc Dis. 2010;29:431–39. doi: 10.1159/000289346. [DOI] [PubMed] [Google Scholar]
- 15.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39:841–52. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Delimaris I, Georgopoulos S, Kroupis C, et al. Serum oxidizability, total antioxidant status and albumin serum levels in patients with aneurysmal or arterial occlusive disease. Clin Biochem. 2008;41:706–11. doi: 10.1016/j.clinbiochem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Bonfatti V, Albera A, Carnier P. Genetic associations between daily bw gain and live fleshiness of station-tested young bulls and carcass and meat quality traits of commercial intact males in piemontese cattle. J Anim Sci. 2013;91:2057–66. doi: 10.2527/jas.2012-5386. [DOI] [PubMed] [Google Scholar]
- 19.Manninen H, Makinen K, Vanninen R, et al. How often does an incomplete circle of willis predispose to cerebral ischemia during closure of carotid artery? Postmortem and clinical imaging studies. Acta neurochir. 2009;151:1099–105. doi: 10.1007/s00701-009-0468-1. [DOI] [PubMed] [Google Scholar]
- 20.Adhiyaman V, Alexander S. Cerebral hyperperfusion syndrome following carotid endarterectomy. QJM. 2007;100:239–44. doi: 10.1093/qjmed/hcm009. [DOI] [PubMed] [Google Scholar]
- 21.Bagaev E, Pichlmaier AM, Bisdas T, et al. Contralateral internal carotid artery occlusion impairs early but not 30-day stroke rate following carotid endarterectomy. Angiology. 2010;61:705–10. doi: 10.1177/0003319710369792. [DOI] [PubMed] [Google Scholar]
- 22.Menyhei G, Bjorck M, Beiles B, et al. Outcome following carotid endarterectomy: Lessons learned from a large international vascular registry. Eur J Vasc Endovasc Surg. 2011;41:735–40. doi: 10.1016/j.ejvs.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Maatz W, Kohler J, Botsios S, et al. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg. 2008;22:45–51. doi: 10.1016/j.avsg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Choi CG, Kim DK, et al. Relationship between circle of willis morphology on 3d time-of-flight mr angiograms and transient ischemia during vascular clamping of the internal carotid artery during carotid endarterectomy. Am J Neuroradiol. 2004;25:558–64. [PMC free article] [PubMed] [Google Scholar]
- 25.Tan TW, Garcia-Toca M, Marcaccio EJ, Jr, et al. Predictors of shunt during carotid endarterectomy with routine electroencephalography monitoring. J Vasc Surg. 2009;49:1374–78. doi: 10.1016/j.jvs.2009.02.206. [DOI] [PubMed] [Google Scholar]
- 26.AbuRahma AF, Mousa AY, Stone PA, et al. Correlation of intraoperative collateral perfusion pressure during carotid endarterectomy and status of the contralateral carotid artery and collateral cerebral blood flow. Ann Vasc Surg. 2011;25:830–36. doi: 10.1016/j.avsg.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Pulli R, Dorigo W, Barbanti E, et al. Carotid endarterectomy with contralateral carotid artery occlusion: Is this a higher risk subgroup? Eur J Vasc Endovasc Surg. 2002;24:63–68. doi: 10.1053/ejvs.2002.1612. [DOI] [PubMed] [Google Scholar]
- 28.Cinar B, Goksel OS, Karatepe C, et al. Is routine intravascular shunting necessary for carotid endarterectomy in patients with contralateral occlusion? A review of 5-year experience of carotid endarterectomy with local anaesthesia. Eur J Vasc Endovasc Surg. 2004;28:494–99. doi: 10.1016/j.ejvs.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Xu SY, Pan SY. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med Sci Monit Basic Res. 2013;19:37–45. doi: 10.12659/MSMBR.883750. [DOI] [PMC free article] [PubMed] [Google Scholar]


