Abstract
Background Context
This is the first study to systematically evaluate the value of a longer treatment period for massage. We provide a framework of how to conceptualize an optimal dose in this challenging setting of non-pharmacological treatments.
Purpose
To determine the optimal dose of massage for neck pain.
Study Design/Setting
Two-phase randomized trial for persons with chronic non-specific neck pain. Primary randomization to one of 5 groups receiving 4 weeks of massage (30 minutes 2×/ or 3×/week or 60 minutes 1×, 2×, or 3×/week). Booster randomization of participants to receive an additional 6 massages, 60 minute 1×/week, or no additional massage.
Patient Sample
179 participants from Group Health and the general population of Seattle, WA USA recruited between June 2010 and August 2011.
Outcome Measures
Primary outcomes self-reported neck-related dysfunction (Neck Disability Index) and pain (0–10 scale) were assessed at baseline, 12, and 26 weeks. Clinically meaningful improvement was defined as >5 point decrease in dysfunction and > 30% decrease in pain from baseline.
Methods
Clinically meaningful improvement for each primary outcome with both follow-up times was analyzed using adjusted modified Poisson generalized estimating equations. Secondary analyses for the continuous outcomes used linear generalized estimating equations. This study was funded the National Center for Complementary and Alternative Medicine, NIH, USA (R01 AT004411). The funders had no role in the interpretation or reporting of results.
Results
There were no observed differences by primary treatment group at 12 or 26 weeks. Those receiving booster dose had improvements in both dysfunction and pain at 12 weeks (dysfunction: RR=1.56(1.08–2.25), P=0.018; pain: RR=1.25(0.98–1.61); P=0.077), but those were non-significant at 26 weeks (dysfunction: RR=1.22(0.85–1.74); pain: RR=1.09(0.82–1.43)). Subgroup analysis by primary and booster treatments found the booster dose only effective amongst those initially randomized to one of the 60 minutes massage groups.
Conclusions
“Booster” doses for those initially receiving 60 minutes of massage should be incorporated into future trials of massage for chronic neck pain.
Keywords: chronic neck pain, dosing, massage, randomized clinical trial, complementary medicine, clinical trial methods
INTRODUCTION
One challenge in evaluating the efficacy of non-pharmacological treatments for spinal pain is the paucity of data available on the optimal dose of the treatment.[1] Without this information, researchers and clinicians cannot be sure that research findings capture the potential for the therapy to improve pain and function. In fact, several Cochrane reviews of massage for neck pain have noted that previous studies used such different types and doses of massage that the optimum dose for practice and clinical trials is unknown.[2, 3] These reviews called for studies to explicitly remedy this deficit. Moreover, for massage, there are a variety of elements that go into dosing, including the length of each treatment session, the weekly frequency of treatments and the number of weeks of treatment.
To address the lack of knowledge regarding the optimal dose of massage for chronic neck pain, we designed a study to look at the optimal combination of treatment frequency and session duration for massage over a 4 week period and to determine whether an additional 6 weeks of massage extended the benefits of the initial month of treatment. We have previously reported the outcomes of the initial 4-week treatment period [4] and in this manuscript, we report on the effects of an additional 6 weeks of treatment.
MATERIAL AND METHODS
Design
We conducted a two-phase individually randomized clinical trial to assess the optimal dose of massage for chronic neck pain that would be evaluated in future full-scale effectiveness studies. In the first phase (the “Primary treatment” period) participants were randomly assigned to receive 4-weeks of one of five different doses of massage or to a wait-list control group. Those receiving massage during the Primary treatment period were then randomized to receive an additional six weekly 60 minute massages (Booster treatment”) or to stop having massage.
The Group Health Research Institute (Seattle, WA) institutional review board approved the trial protocol and all study procedures. Prospective participants giving oral consent by telephone, were screened for eligibility and those found eligible were asked to provide written consent before their in-person examination and study enrollment. The published study protocol [5] is summarized below. Results from the Primary treatment period have been previously published.[4] This paper presents the effects of the 6-week Booster treatment after 12 and 26 weeks.
Study participants
We recruited study participants from Group Health, an integrated health care system serving about 600,000 members, and from the general population of Seattle. We recruited prospective participants between June 2010 and August 2011 using mailed invitations to Group Health members those with neck pain-related visits to primary care providers, advertisements in the health plan’s magazine, posters, a study website, neighborhood blogs, and direct-mail postcards. Persons ages 20 to 64 with chronic non-specific neck pain who were able and willing to attend treatments at our clinic and give informed consent were potentially eligible.
We excluded persons whose neck pain had a pathologically identifiable cause (e.g., vertebral fracture, metastatic cancer), was complex (e.g., cervical radiculopathy, recent automobile accident), or was too mild (<4 on an 11 point pain intensity scale and <5 on the 0 to 50 Neck Disability Index[6, 7]). We also excluded those with potential contraindications for massage (e.g., hypersensitivity to touch), any massage within the last 3 months, massage for neck pain within the last year, an inability to give informed consent or speak English, or with medico-legal issues related to neck or back pain.
Randomization
After completing the baseline interview, a research assistant randomized participants using a computer assisted telephone interviewing program, to one of the six groups in the Primary treatment randomization. Treatment assignments were generated by a statistician (AJC) using R (version 2.11.0), with random block sizes of 6 and 12 within two strata, based on Neck Disability Index scores (5 to 14 and 15+). One week after the end of the 4-week Primary treatment period, those who had received one of 5 massage treatments and completed the 5 week follow-up interview, and consented to Booster treatment were randomized to either the Booster treatment or no additional massage. This randomization used blocks of 2 or 4 and stratified within Primary treatment assignment.
Treatments
For the 4-week Primary treatment period, participants were randomized to a waitlist control group or one of five different dosing schedules of massage: 30 minute treatments 2 or 3 times per week or 60 minute treatments 1, 2, or 3 times per week. For the Booster treatment period, those eligible for randomization to the 6-week Booster treatments received either 60 minute treatments 1 time per week or no additional massage. We defined adherence as completion of at least 75% of the visits in each protocol.
Based on an earlier study[8], distinct treatment protocols were defined for both 30 and 60-minute treatments.[5] Therapists were given time limits for each part of the massage and permitted to use a broad range of massage techniques. No self-care recommendations were permitted. Eight licensed massage therapists with at least 5 years of experience were trained in the study protocol and provided massage treatments in the research clinic at Group Health.
Adverse Event Reporting
Participants were asked about adverse events during each clinic visit and during the 5 and 12 week telephone interviews. In addition, participants were invited to call our study phone number to let us know about any harms due to massage. We defined adverse events as any unfavorable and unintended sign, symptom or disease temporally associated with the use of the massage treatments that could reasonably be related to the procedure. Because massage has relatively short-term physiological effects, we will not report adverse events that begin more than two weeks after a participant’s final massage treatment (or more than 14 weeks after randomization for the usual care control group).
Outcomes and Follow-up
We assessed outcomes at baseline, and 5, 12, and 26 weeks after randomization using telephone interviewers who were unaware of treatment assignment. We attempted to obtain follow-up data from all trial participants. This paper will focus on the 12 and 26 week results since 5 weeks results have been previously published.[4] Neck pain related dysfunction and pain intensity were the pre-specified primary outcomes and clinically meaningful improvement was the pre-specified primary outcome measure. We measured neck dysfunction using the well-validated 10-item Neck Disability Index (NDI),[6, 7] which ranges from 0 to 50 points, with higher scores indicating worse disability. Clinically meaningful improvement in NDI was defined as a decrease of more than 5 points from baseline.[9] We measured neck pain intensity (NPI) using an 11 point numerical rating scale with higher scores indicating worse pain.[10] Clinically meaningful improvement in NPI was defined as a decrease of 30% or more from baseline.[11] Secondary outcomes included activity limitations[12], perceived stress[12], patient global rating of improvement and patient satisfaction[13].
Sample Size
We briefly summarize our sample size assumptions here, but for more details please refer to our published study protocol[5]. Our initial sample size of 228 participants was chosen to ensure adequate power to detect a meaningful difference across the six primary treatment groups for both outcomes NDI and NPI measured at 5 weeks (findings previously published[4]). To address the results that are being discussed in this manuscript, which was the second aim of the study, we conducted the following sample size calculations. We assumed that we would have an initial sample size of 190 participants that would be eligible for booster randomization (received some massage assignment during the primary treatment period). We originally assumed a 10% loss of follow-up that would then yield a sample size of 170 included in the analysis for this aim (Note that our sample size achieved was 173, see Results: Recruitment and follow-up).
To simplify the power calculations, we focused on the 12 week response outcome. For the outcome clinically meaningful improvement in NDI, the power was 81%, assuming that those that received the booster treatment had a 0.20 increase in proportion improvement relative to those not receiving the booster treatment, adjusting for primary treatment group. We further assumed that those whom did not receive additional booster treatment had the same improvement at 12 weeks as they observed at 5 weeks and that the average proportion with improved NDI at 5-weeks was 0.52. The power was 88% for the outcome clinically meaningful improvement in NPI, making similar assumptions as detailed for the outcome NDI except that the average 5 week proportion improvement across primary treatment groups for NPI was assumed to be 0.63.
Statistical Analysis
Summary statistics (frequencies, means, and standard deviations) for baseline characteristics of study participants by treatment groups during the Primary and Booster treatment periods are presented to identify any important baseline differences among groups. Following the a priori primary analysis plan, we first assessed the main effects of Primary and Booster treatments on the dual primary outcomes: meaningful improvement in the NDI and the NPI at 12 and 26 weeks. To estimate a relative risk (RR) and control for multiple outcomes on the same participant we used modified Poisson regression, fitting a Poisson log-link regression model with generalized estimating equations (GEE) and robust standard errors.[14] Specifically, we included an indicator variable for each Primary treatment group and an indicator for Booster treatment, but no interaction terms for effects of Primary and Booster treatment effects. Pre-specified exploratory analyses evaluating whether there is an interaction between Primary and Booster treatment assignments are also presented, but the study was not powered to test for such interaction effects. Secondary analyses for NDI and NPI continuous outcomes used linear regression models with GEE and robust standard errors to estimate differences in mean changes from baseline across Primary and Booster treatment groups for the 12- and 26-week time points.
To protect against multiple comparisons due to multiple Primary treatment groups, we used the Fisher protected least-significant difference approach.[15] This approach makes pairwise comparisons among the 5 treatment groups only if the overall omnibus Wald test statistic is significant.
We conducted both unadjusted and adjusted analyses, with the adjusted analyses specified a priori as primary. All adjusted models included baseline NDI and NPI, age, sex, neck pain >5 years in duration, use of medications for neck pain and race (White Non-Hispanic vs. other). Similar methods were used to analyze secondary outcomes except only baseline NDI and NPI were adjusted as covariates, since most of the secondary outcomes had low prevalence, allowing for fewer adjustment variables.
All analyses were conducted under the principal of intention-to-treat (i.e. comparing participants in the groups to which they were originally randomly assigned). Analyses were performed using R statistical software (version 2.15.1). All p-values are two-sided and Wald based with statistical significance at the P=0.05 level.
RESULTS
Recruitment and follow-up
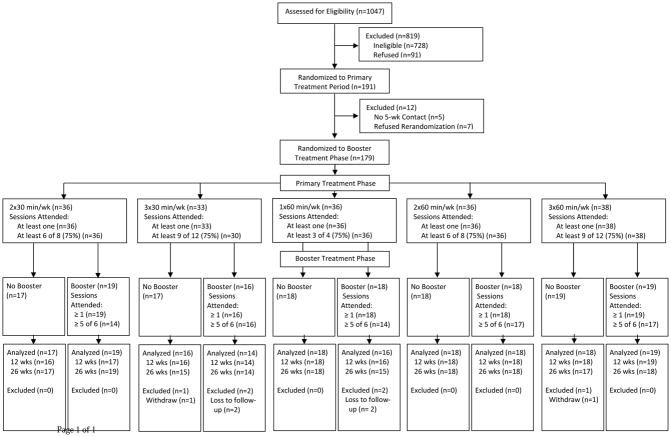
Among the 1027 people assessed for eligibility between July 2010 and August 2011, 728 were ineligible, 91 refused and 228 were randomized to one of 6 Primary treatments (Figure 1). Of the 191 randomized to one of the 5 active massage treatments during the Primary treatment period, 179 both completed the 5 week follow-up interview (N=5 did not complete) and agreed to participate in the Booster randomization (N=7 did not agree). Of these, 90 were randomized to Booster massage and 89 were randomized to no further treatment. Most (86%) participants were recruited from Group Health. Overall, 97% (N=173) participants completed at least one of the 12 or 26 week follow-up questionnaires. Primary treatment group specific follow-up rates ranged from 91% to 100% and Booster follow-up rates were 96% and 98%.
Figure 1.
Trial Flow
Baseline Characteristics
Baseline characteristics were well balanced across groups, except for race (Table 1). Study participants typically had moderately severe neck pain, but reported relatively few substantial activity limitations due to their pain.
Table 1.
Demographic and Neck Pain Characteristics by primary and booster treatment assignments
| Primary Treatment (Baseline to 4wk treatments) | Booster Treatment (6 to 12wk trts) | ||||||
|---|---|---|---|---|---|---|---|
| DEMOGRAPHIC, NECK PAIN, AND GENERAL HEALTH CHARACTERISTICS | 30min 2/wk n=36 |
30min 3/wk n=33 |
60min 1/wk n=36 |
60min 2/wk n=36 |
60min 3/wk n=38 |
No Booster n=89 |
60min 1/wk n=90 |
| DEMOGRAPHICS | |||||||
| Age in yrs, mean (SD) | 41.7 (11.3) | 45.9 (11.1) | 50.8 (10.8) | 48.0 (11.7) | 49.4 (9.7) | 47.4 (11.4) | 47.1 (11.1) |
| Female, n (%) | 26 (72.2) | 23 (69.7) | 28 (77.8) | 27 (75.0) | 27 (71.1) | 65 (73.0) | 66 (73.3) |
| College graduate, n (%) | 25 (69.4) | 24 (72.7) | 20 (55.6) | 24 (66.7) | 27 (71.1) | 60 (67.4) | 60 (66.7) |
| White Non-Hispanic, n (%) | 25 (69.4) | 18 (54.5) | 29 (80.6) | 30 (83.3) | 28 (73.7) | 60 (67.4) | 70 (77.8) |
| Household Income > $45,000/yr, n (%) | 20 (55.6) | 27 (81.8) | 23 (63.9) | 26 (72.2) | 22 (57.9) | 59 (66.3) | 59 (65.6) |
| Employment, n (%) | |||||||
| Unemployed | 5 (13.9) | 5 (15.2) | 9 (25.0) | 7 (19.4) | 7 (18.4) | 16 (18.0) | 17 (18.9) |
| Mainly sedentary (sitting) work | 10 (27.8) | 16 (48.5) | 15 (41.7) | 15 (41.7) | 19 (50.0) | 40 (44.9) | 35 (38.9) |
| Work that involves mainly standing and walking | 8 (22.2) | 5 (15.2) | 6 (16.7) | 7 (19.4) | 3 (7.9) | 17 (19.1) | 12 (13.3) |
| Work that requires any lifting and carrying | 13 (36.1) | 7 (21.2) | 6 (16.7) | 7 (19.4) | 9 (23.7) | 16 (18.0) | 26 (28.9) |
| NECK PAIN CHARACTERISTICS | |||||||
| Neck Disability Index (NDI), Mean (SD) | 13.1 (3.7) | 13.3 (5.7) | 14.0 (4.6) | 13.8 (5.1) | 14.3 (5.5) | 13.8 (5.1) | 13.7 (4.7) |
| Neck Pain Index (NPI), Mean (SD) | 5.8 (1.4) | 6.0 (1.4) | 5.9 (1.5) | 5.7 (1.1) | 5.7 (1.2) | 5.8 (1.4) | 5.8 (1.3) |
| > 7 days in past 4 wks that normal activities were cut by half a day or more because of NP, n (%) | 0 (0.0) | 4 (12.1) | 4 (11.1) | 2 (5.6) | 6 (15.8) | 8 (9.0) | 8 (8.9) |
| 1+ days in the past 4 wks that NP kept you in bed/lying down for all or most of the day, n (%) | 2 (5.6) | 4 (12.1) | 4 (11.1) | 0 (0.0) | 2 (5.3) | 5 (5.6) | 7 (7.8) |
| 1+ days in the past 4 wks that NP kept you out of work or school, n (%) | 1 (2.9) | 1 (3.3) | 3 (9.1) | 2 (5.9) | 3 (8.3) | 6 (7.4) | 4 (4.6) |
| Duration of NP > 5 years, n (%) | 18 (50.0) | 11 (33.3) | 15 (41.7) | 14 (38.9) | 16 (42.1) | 39 (43.8) | 35 (38.9) |
| >3 days of neck exercise in past week, n (%) | 7 (19.4) | 11 (33.3) | 13 (36.1) | 13 (36.1) | 14 (36.8) | 29 (32.6) | 29 (32.2) |
| Any medications used for NP in the past week, n (%) | 21 (58.3) | 15 (45.5) | 21 (58.3) | 23 (63.9) | 27 (71.1) | 52 (58.4) | 55 (61.1) |
| NSAID use for NP, n (%) | 16 (44.4) | 11 (33.3) | 14 (38.9) | 18 (50.0) | 23 (60.5) | 39 (43.8) | 43 (47.8) |
| Very satisfied with overall care for NP, n (%) | 4 (13.8) | 1 (3.3) | 6 (24.0) | 3 (11.5) | 3 (9.4) | 13 (18.3) | 4 (5.6) |
| GENERAL HEALTH AND STRESS | |||||||
| SF-36 General health very good or excellent, n (%) | 21 (58.3) | 21 (63.6) | 23 (63.9) | 24 (66.7) | 28 (73.7) | 62 (69.7) | 55 (61.1) |
| Perceived Stress Scale (PSS), Mean (SD) | 16.1 (5.7) | 16.6 (4.9) | 16.1 (7.3) | 16.9 (6.5) | 15.9 (6.9) | 16.9 (6.6) | 15.8 (5.9) |
NP=Neck pain
Study Treatment Adherence
Treatment adherence, defined as attending at least 75% of the assigned dose, was 100% for the Primary treatment period in four dosing groups and 90.9% in the 30 minute 3×/week. Those randomized to Booster had an 87% adherence rate.
Non-study Treatments
The use of medication as a non-study treatment varied across groups. Among those randomized to 60 minute 3×/week treatments, medication usage in the prior week dropped from 71.1% at baseline to 32.4% at 12 weeks and 48.6% at 26 weeks, while amongst those in the 30 minutes 3×/week group, medication usage stayed relatively flat from 45.5% at baseline to 46.7% at 12 weeks and 44.8% at 26 wks. In all other treatment groups, the absolute percentage change in medication varied between −21.9% and −2.4% at 12 weeks and −19.5% and −3.8% at 26 weeks. Amongst those that received booster massage treatments medication usage decreased from 61.1% at baseline to 39.3% at 12 weeks and 47.6% at 26 weeks. Amongst those that did not received booster treatment the reduction was less from 58.4% at baseline to 47.7% at 12 weeks and 48.4% at 26 weeks.
Primary Outcomes
There were no statistically significant differences between any of the Primary treatment period groups at 12 or 26 weeks for clinically meaningful improvement in NDI or NPI (Table 2). For NDI, relative risks which represent all 10 of the pairwise comparisons of any two primary treatment groups (primary treatment group with less improvement is always the reference) with each other ranged from 1.02 to 1.43 (=1.17/0.82) at 12 weeks and 1.02 to 1.31 (=1.05/0.80) at 26 weeks (Table 2). For NPI, relative risks ranged from 1.10 to 1.83 (=1.43/0.78) at 12 weeks and 1.10 (=0.75/0.68) to 1.66 (=1.13/0.68) at 26 weeks. At 12 weeks, those randomized to the Booster treatment were 56% more likely to have a clinically meaningful improvement in NDI compared to those in the no treatment group (Adjusted RR: 1.56 (1.08, 2.25)). At 26 weeks, this improvement was only 22% better and no longer statistically significant (Adjusted RR: 1.22 (0.85, 1.74)).
Table 2.
Proportion with clinically relevant improvement on the Neck Disability Index (5 points) and Neck Pain Intensity scale (30%) at 12 and 26 wks post-randomization. Main effects of primary and booster treatment assignments.
| Unadjusted* | Adjusted** | |||
|---|---|---|---|---|
| Massage Dose | RR (95% CI) | Omnibus P | RR (95% CI) | Omnibus P |
| NECK DISABILITY INDEX | ||||
|
| ||||
| 12 WEEK OUTCOME | ||||
| Primary Treatment | ||||
| 30min × 2/wk | 1.00 | 1.00 | ||
| 30min × 3/wk | 0.94 (0.47, 1.87) | 0.82 (0.42, 1.60) | ||
| 60min × 1/wk | 1.07 (0.58, 1.98) | 0.88 (0.48, 1.64) | ||
| 60min × 2/wk | 1.23 (0.69, 2.19) | 1.02 (0.57, 1.82) | ||
| 60min × 3/wk | 1.41 (0.83, 2.41) | 1.17 (0.67, 2.05) | ||
| Booster Treatment | ||||
| No Further Treatment | 1.00 | 1.00 | ||
| Booster Treatment | 1.62 (1.11, 2.35) | 0.012 | 1.56 (1.08, 2.25) | 0.018 |
| 26 WEEK OUTCOME | ||||
| 26 versus 12 week | 1.15 (0.77, 1.73) | 0.496 | 1.15 (0.76, 1.72) | 0.508 |
| Primary Treatment | ||||
| 30min × 2/wk | 1.00 | 1.00 | ||
| 30min × 3/wk | 1.06 (0.56, 2.02) | 0.90 (0.48, 1.68) | ||
| 60min × 1/wk | 1.28 (0.72, 2.26) | 1.05 (0.58, 1.88) | ||
| 60min × 2/wk | 1.24 (0.70, 2.19) | 1.02 (0.60, 1.76) | ||
| 60min × 3/wk | 0.95 (0.51, 1.80) | 0.80 (0.42, 1.52) | ||
| Booster Treatment | ||||
| No Further Treatment | 1.00 | 1.00 | ||
| Booster Treatment | 1.26 (0.87, 1.83) | 0.228 | 1.22 (0.85, 1.74) | 0.277 |
|
| ||||
| NECK PAIN INTENSITY | ||||
|
| ||||
| 12 WEEK OUTCOME | ||||
| Primary Treatment | ||||
| 30min × 2/wk | 1.00 | 1.00 | ||
| 30min × 3/wk | 1.32 (0.85, 2.06) | 1.21 (0.79, 1.87) | ||
| 60min × 1/wk | 0.86 (0.51, 1.45) | 0.78 (0.47, 1.31) | ||
| 60min × 2/wk | 1.21 (0.78, 1.87) | 1.10 (0.72, 1.68) | ||
| 60min × 3/wk | 1.56 (1.06, 2.29) | 1.43 (0.97, 2.11) | ||
| Booster Treatment | ||||
| No Further Treatment | 1.00 | 1.00 | ||
| Booster Treatment | 1.30 (1.01, 1.68) | 0.042 | 1.25 (0.98, 1.61) | 0.077 |
| 26 WEEK OUTCOME | ||||
| 26 versus 12 week | 1.24 (0.90, 1.71) | 0.189 | 1.24 (0.90, 1.72) | 0.189 |
| Primary Treatment | ||||
| 30min × 2/wk | 1.00 | 1.00 | ||
| 30min × 3/wk | 1.25 (0.85, 1.82) | 1.13 (0.78, 1.65) | ||
| 60min × 1/wk | 0.83 (0.52, 1.32) | 0.75 (0.47, 1.19) | ||
| 60min × 2/wk | 0.75 (0.46, 1.22) | 0.68 (0.43, 1.08) | ||
| 60min × 3/wk | 0.98 (0.64, 1.49) | 0.89 (0.57, 1.38) | ||
| Booster Treatment | ||||
| No Further Treatment | 1.00 | 1.00 | ||
| Booster Treatment | 1.13 (0.85, 1.50) | 0.398 | 1.09 (0.82, 1.43) | 0.558 |
Unadjusted model includes a main effect for each first randomization treatment assignment and a main effect for booster treatment assignment (i.e. No interaction included between first and booster treatment assignment)
Adjusted model further adjusts for baseline NDI and NPI, age, sex, duration of neck pain > 5 years, use of medications for NP, and race (White Non-hispanic vs. other).
Results from secondary analyses using the primary outcomes as continuous variables were similar to the analyses focused on clinically meaningful improvement: there was a main effect of the Booster treatment at 12 weeks (Table 3: Adjusted mean change difference (95% CI) for NDI of −2.87 (−4.37, −1.36) and NPI of −0.61(−1.17,−0.05)), but not at 26 weeks. No statistically significant effect of the Primary treatment group was found at 12 or 26 weeks.
Table 3.
Adjusted Mean Change from Baseline in Neck Disability Index and Neck Pain Intensity scale at 12 and 26 wks post-baseline. Main effects of primary and booster treatment assignments.
| Massage Dose | Mean Change (95% CI) | Mean Difference (95% CI) | P |
|---|---|---|---|
| NECK DISABILITY INDEX | |||
|
| |||
| 12 WEEK OUTCOME | |||
| Primary Treatment | |||
| 30min × 2/wk | −2.94 (−4.66, −1.23) | Ref | |
| 30min × 3/wk | −2.72 (−4.44, −0.99) | 0.23 (−2.16, 2.61) | 0.852 |
| 60min × 1/wk | −1.80 (−3.48, −0.11) | 1.15 (−1.33, 3.62) | 0.365 |
| 60min × 2/wk | −2.89 (−4.65, −1.13) | 0.05 (−2.41, 2.52) | 0.966 |
| 60min × 3/wk | −4.25 (−5.92, −2.59) | −1.31 (−3.73, 1.11) | 0.288 |
| Booster Treatment | |||
| No Further Treatment | −1.50 (−2.52, −0.48) | Ref | |
| Booster Treatment | −4.36 (−5.47, −3.25) | −2.87 (−4.37, −1.36) | <0.001 |
| 26 WEEK OUTCOME | |||
| 26 versus 12 week | NA | −0.59 (−2.37, 1.19) | 0.517 |
| Primary Treatment | |||
| 30min × 2/wk | −2.82 (−4.62, −1.01) | Ref | |
| 30min × 3/wk | −2.15 (−3.96, −0.35) | 0.66 (−1.92, 3.24) | 0.616 |
| 60min × 1/wk | −2.22 (−4.11, −0.33) | 0.60 (−2.04, 3.24) | 0.655 |
| 60min × 2/wk | −2.86 (−4.70, −1.02) | −0.04 (−2.58, 2.49) | 0.974 |
| 60min × 3/wk | −2.49 (−5.07, 0.09) | 0.32 (−2.83, 3.48) | 0.841 |
| Booster Treatment | |||
| No Further Treatment | −1.79 (−2.90, −0.68) | Ref | |
| Booster Treatment | −3.23 (−4.62, −1.84) | −1.44 (−3.21, 0.33) | 0.110 |
|
| |||
| NECK PAIN INDEX | |||
|
| |||
| 12 WEEK OUTCOME | |||
| Primary Treatment | |||
| 30min × 2/wk | −2.30 (−3.00, −1.59) | Ref | |
| 30min × 3/wk | −2.39 (−3.05, −1.72) | −0.09 (−1.05, 0.87) | 0.854 |
| 60min × 1/wk | −1.43 (−2.08, −0.77) | 0.87 (−0.09, 1.84) | 0.077 |
| 60min × 2/wk | −2.19 (−2.76, −1.62) | 0.11 (−0.81, 1.03) | 0.817 |
| 60min × 3/wk | −2.74 (−3.33, −2.15) | −0.45 (−1.38, 0.49) | 0.349 |
| Booster Treatment | |||
| No Further Treatment | −1.90 (−2.31, −1.50) | Ref | |
| Booster Treatment | −2.52 (−2.90, −2.13) | −0.61 (−1.17, −0.05) | 0.032 |
| 26 WEEK OUTCOME | |||
| 26 versus 12 week | NA | −0.31 (−0.98, 0.36) | 0.361 |
| Primary Treatment | |||
| 30min × 2/wk | −2.31 (−2.95, −1.66) | Ref | |
| 30min × 3/wk | −2.14 (−2.87, −1.40) | 0.17 (−0.79, 1.13) | 0.728 |
| 60min × 1/wk | −1.50 (−2.30, −0.70) | 0.81 (−0.24, 1.85) | 0.131 |
| 60min × 2/wk | −1.74 (−2.46, −1.02) | 0.56 (−0.41, 1.54) | 0.257 |
| 60min × 3/wk | −1.84 (−2.60, −1.07) | 0.47 (−0.54, 1.48) | 0.364 |
| Booster Treatment | |||
| No Further Treatment | −1.89 (−2.31, −1.48) | Ref | |
| Booster Treatment | −1.91 (−2.40, −1.41) | −0.01 (−0.66, 0.64) | 0.973 |
Linear regression model including a main effect for each primary treatment assignment and a main effect of booster (i.e. No interaction included between primary and booster treatment assignment) adjusting for baseline NDI and NPI, age, sex, duration of neck pain > 5 years, use of medications for NP, and race (White, Non-Hispanic vs other).
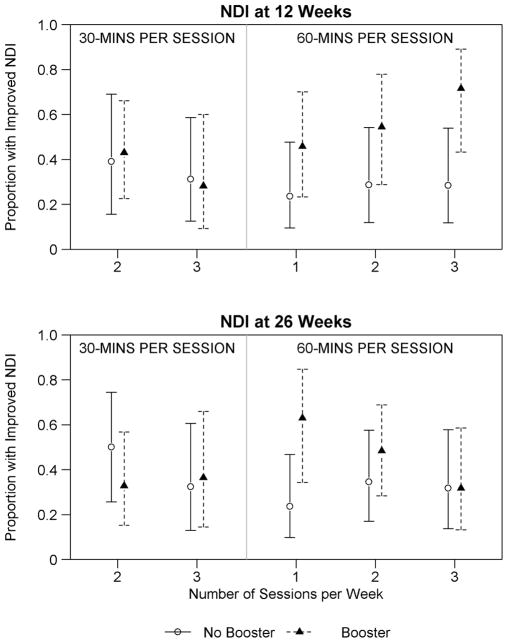
There was an indication of a differential Booster effect across Primary treatment groups at 12 weeks, although this study was underpowered for examining such an interaction. Benefits of the Booster dose were observed mainly for the NDI outcome and for those receiving 60 minute massage treatments during the Primary treatment period (Figure 2, NPI figure not shown). In these groups, those that received a Booster treatment had 1.92 (95%CI: 1.22, 3.04, p-value=0.005) times the likelihood of experiencing a clinically meaningful improvement in 12-week NDI compared to those with no further treatment. At this same time, the Booster effect was minimal for those receiving 30-minute Primary treatments (RR (95%CI): 1.07 (0.54, 2.13), p-value=0.841). There were no other indications of strong interactions between Booster and Primary treatment groups.
Figure 2.
Differential effect of improved NDI at 12 and 26 weeks by primary treatment assignment interacted with or without receiving booster treatment.
Secondary Outcomes
At 12 weeks, participants who had received Primary treatments of 60 minutes 3 times per week had reduced stress (PSS scale) compared to all other primary treatment groups (mean difference range −2.10 to −3.45; P<0.024 except when comparing to 30min 2/wk P=0.090; Table 4). Compared to all other treatment doses, the 60min 3/wk group was more than 2 times as likely to report their neck pain as better or completely gone (P<0.029 for all comparisons). These differences did not persist at 26 weeks. Those who received booster treatments were more likely to report their neck pain as much better or completely resolved at both 12 and 26 weeks (Table 4), but did not observe significant reductions in stress.
Table 4.
Secondary outcomes at 12 and 26-weeks.
| Primary Treatment (Baseline to 5wk trts) | Booster Treatment (6 to 12 wk trts) | |||||||
|---|---|---|---|---|---|---|---|---|
| Secondary Outcome | 30min 2/wk |
30min 3/wk |
60min 1/wk |
60min 2/wk |
60min 3/wk |
No Booster | 60 min 1/wk |
|
|
|
|
|||||||
| >7 days in past 4 wks that normal activities were cut by half a day or more due to NP, %* | 12 weeks | 0.0 | 13.3 | 11.8 | 5.6 | 16.2 | 9.3 | 9.5 |
| 26 weeks | 0.0 | 13.8 | 9.1 | 5.6 | 17.1 | 9.4 | 8.3 | |
| 1+ days in the past 4 wks that NP kept you in bed/lying down for all or most of the day, %* | 12 weeks | 6.1 | 13.3 | 11.8 | 0.0 | 5.4 | 5.8 | 8.3 |
| 26 weeks | 5.6 | 13.8 | 12.1 | 0.0 | 5.7 | 5.9 | 8.3 | |
| 1+ days in the past 4 wks that NP kept you out of work or school, %* | 12 weeks | 3.0 | 3.3 | 8.9 | 5.6 | 5.4 | 5.8 | 4.8 |
| 26 weeks | 2.8 | 3.5 | 6.1 | 5.6 | 5.7 | 5.9 | 3.6 | |
| Change in Perceived Stress Scale (PSS) from baseline, mean diff (95% CI)** | 12 weeks | Ref | −1.3 (−4.1, 1.4) | −1.1 (−3.4, 1.3) | −1.1 (−3.6, 1.3) | −3.5 (−5.9, −1.0) | Ref | −0.9 (−2.3, 0.6) |
| 26 weeks | Ref | −1.9 (−4.5, 0.8) | −1.9 (−4.5, 0.6) | −1.2 (−3.6, 1.2) | −0.8 (−3.4, 1.8) | Ref | −0.5 (−2.0, 1.1) | |
| NP is much better/completely gone compared to study start, RR (95% CI)*** | 12 weeks | 1.0 | 0.8 (0.3, 2.3) | 0.9 (0.3, 2.3) | 1.2 (0.5, 2.8) | 2.4 (1.1, 4.9) | 1.0 | 2.3 (1.3, 3.9) |
| 26 weeks | 1.0 | 1.3 (0.5, 3.2) | 1.3 (0.5, 3.2) | 1.0 (0.4, 2.6) | 1.2 (0.5, 3.0) | 1.0 | 1.9 (1.0, 3.4) | |
| Very satisfied with care for NP, RR (95% CI)*** | 12 weeks | 1.0 | 1.1 (0.7, 1.7) | 1.0 (0.6, 1.6) | 1.4 (0.9, 2.1) | 1.1 (0.7, 1.7) | 1.0 | 1.6 (1.2, 2.2) |
| Not asked at 26 weeks | ||||||||
NP=Neck pain
Unadjusted percents due to rare outcome and regression models had fitting problems
Linear regression model adjusted for baseline PSS, NDI, and NPI.
Modified Poisson regression model adjusted for baseline NDI and NPI.
Adverse Events
As previously detailed[4], during the primary treatment group period, 10 participants reported 14 adverse events (11 mild and 3 moderately severe) that at least possibly were related to massage. All these events were related to pain, primarily spine pain. Adverse events were similarly rare in participants attending 30 minute and 60 minute treatments (4% vs. 6%, respectively) and in those attending 1×, 2×, or 3×/week treatments (7.9% vs. 2.6% vs. 6.7%, respectively). During the additional 6 weeks during booster treatment, there were two adverse events reported, both mild and pain related.
DISCUSSION
Augmenting four weeks of massage treatments with six additional weeks provided short term reductions in neck dysfunction and pain. These benefits diminished and were no longer statistically significant several months after the treatments stopped. The benefits of additional massage were found for those who initially received 4-weeks of 60 minute massage treatments but not for those who had initially received 30 minute massages. There was some evidence that amongst those who had initially received the highest dose of massage (60 minutes 3/times per week) were more likely to report greater short-term reductions in their neck pain and stress levels compared to those receiving lower doses of massage.
Our findings suggest that there may be larger short term benefits to massage if those treatments are given for 10 weeks instead of 4 weeks, if 60 minute sessions of massage are administered instead of 30 minute sessions, and if more than one session of massage is given each week for the first 4 weeks. This would indicate for future studies of massage for neck pain, and possibly other musculoskeletal conditions, that researchers should consider more massage earlier in the treatment period, i.e. frontloading treatment, but continue treatment beyond 4 weeks. However, these treatment effects diminish after massage treatments are stopped.
The issue of diminished effects of massage and other non-pharmacological treatments over time has been previously observed. Specifically, in studies of person with chronic back pain, a typical pattern is that those who are treated show quicker improvement than those who are not, but that improvement may remain plateaued or attenuate slightly over a longer follow-up in the treatment group while those in the untreated group may improve over time.[16, 17] [18–21] In some cases the improvement in the control group completely eliminates the long term benefits of treatment. [16] Fewer studies of neck pain have been conducted, but the same pattern exists.[8, 22] Because this phase of our study lacked a control group without any massage, we cannot determine whether those receiving any of our massage treatments would benefit more than those not receiving any massage treatment.
This is the principal limitation of this dosing study, but another limitation is that because there were relatively few persons in each of the primary treatment groups (~30) it was difficult to assess the exact combination of primary treatment therapy and booster dose that was most effective. This indicates that for future larger studies it may be prudent to use both 60 minutes 2 and 3 times per week massage for the first four weeks and then an additional 6 weeks of treatment. Further since we only assessed for 60 minutes one time per week as our booster treatment potentially 2 and 3 times per week sessions as the booster may be more effective. Considerations of feasibility would need to be assessed to allow for such duration and quantity of massage.
The findings from this study are most generalizable to patients with chronic neck pain from a primary care population who are open to trying massage. As is true for all studies of chronic pain from a primary care population, we cannot fully tell how representative our findings are because we cannot identify those whom have chronic neck pain from our electronic medical record data. Among eligible participants, 71.5% (228/319) agreed to participate. While we know that massage is one of the most common forms of complementary and integrative medicine, we suspect it would not be attractive to all primary care patients. Based on our study participant’s demographics, we recruited a higher proportion whom were white non-hispanic (66%), college educated (67%), and with a household income above $45,000 (66%) compared to the average population in the US. However, our study population has similar demographics as those that have been shown to use complementary and integrative medicine in general[23]. We therefore suspect that our findings are generalizeable to primary care patients who are open to trying this therapy.
Our study strengths include a comprehensive range of early doses, excellent treatment adherence and follow-up rates and focus on outcomes important to patients.
CONCLUSION
Our findings suggest that future studies of therapeutic massage for neck pain should use multiple 60 minute treatments per week for the first 4 weeks and a booster dose of at least weekly 60 minute treatments for 6 weeks and include a comparison group of persons seeking conventional medical care to assess longer term effects of massage.
Acknowledgments
Funding:
Our study was funded by a grant R01 AT004411 from the National Center for Complementary and Alternative Medicine, NIH, USA. The funders had no role in the interpretation or reporting of results. The views expressed herein do not necessarily represent the views of the funders.
We thank our research team, including our project managers (Rene Hawkes, Beth Lapham), research assistants (Zoe Bermet, Kevin Filocamo, Melissa Parson, Kirsten Sullivan), massage therapists (Christine Chmielewski, Lesely Ernst, Tom Harvey, Michael Jacobus, Maureen McKelvey, Dawn Schmidt, Carol Tiebout), nurse practitioners (Wendy Robinson, David Diechert), programmers (Jane Grafton and DT Tran) and massage consultant Diana Thompson.
Footnotes
Trial Registration: NCT01122836 (ClinicalTrials.gov)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berman J, Chesney MA. Complementary and alternative medicine in 2006: optimising the dose of the intervention. Med J Aust. 2005;183(11–12):574–5. doi: 10.5694/j.1326-5377.2005.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 2.Haraldsson BG, et al. Massage for mechanical neck disorders. Cochrane Database Syst Rev. 2006;3:CD004871. doi: 10.1002/14651858.CD004871.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Patel KC, et al. Massage for mechanical neck disorders. Cochrane Database Syst Rev. 2012;9:CD004871. doi: 10.1002/14651858.CD004871.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Sherman KJ, et al. Five-week outcomes from a dosing trial of therapeutic massage for chronic neck pain. Ann Fam Med. 2014;12(2):112–20. doi: 10.1370/afm.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman KJ, et al. Dosing study of massage for chronic neck pain: protocol for the dose response evaluation and analysis of massage [DREAM] trial. BMC Complement Altern Med. 2012;12:158. doi: 10.1186/1472-6882-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–15. [PubMed] [Google Scholar]
- 7.Pietrobon R, et al. Standard scales for measurement of functional outcome for cervical pain or dysfunction: a systematic review. Spine. 2002;27(5):515–22. doi: 10.1097/00007632-200203010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sherman KJ, et al. Randomized trial of therapeutic massage for chronic neck pain. Clin J Pain. 2009;25(3):233–8. doi: 10.1097/AJP.0b013e31818b7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurwitz EL, et al. A randomized trial of chiropractic manipulation and mobilization for patients with neck pain: clinical outcomes from the UCLA neck-pain study. Am J Public Health. 2002;92(10):1634–41. doi: 10.2105/ajph.92.10.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25(24):3140–51. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Farrar JT, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 12.Reiss P. National Center for Health Statistics, editor. DHHS publication PHS 86-1584, 1986. National Center for Health Statistics; Hyattsville, MD: 1984. Current estimates from the national health interview survey: United States. [Google Scholar]
- 13.Cherkin DC, MacCornack FA. Patient evaluations of low back pain care from family physicians and chiropractors. West J Med. 1989;150(3):351–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.Levin J, Serlin R, Seaman M. A controlled, powerful multiple-comparison strategy for several situaitons. Psychological Bulletin. 1994;115:153–159. [Google Scholar]
- 16.Artus M, et al. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology (Oxford) 2010;49(12):2346–56. doi: 10.1093/rheumatology/keq245. [DOI] [PubMed] [Google Scholar]
- 17.Cherkin DC, et al. Randomized trial comparing traditional Chinese medical acupuncture, therapeutic massage, and self-care education for chronic low back pain. Arch Intern Med. 2001;161(8):1081–8. doi: 10.1001/archinte.161.8.1081. [DOI] [PubMed] [Google Scholar]
- 18.Sherman KJ, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143(12):849–56. doi: 10.7326/0003-4819-143-12-200512200-00003. [DOI] [PubMed] [Google Scholar]
- 19.Cherkin DC, et al. A randomized controlled trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch Intern Med. 2009;169(9):858–866. doi: 10.1001/archinternmed.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherkin D, et al. A Comparision of the Effects of 2 Types of Massage and Usual Care on Chronic Low Back Pain. Ann Intern Med. 2011;155:1–9. doi: 10.1059/0003-4819-155-1-201107050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman KJ, et al. A randomized trial comparing yoga, stretching, and a self-care book for chronic low back pain. Arch Intern Med. 2011;171(22):2019–26. doi: 10.1001/archinternmed.2011.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurwitz EL, et al. Treatment of neck pain: noninvasive interventions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S123–52. doi: 10.1097/BRS.0b013e3181644b1d. [DOI] [PubMed] [Google Scholar]
- 23.Clarke TC, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015;79:1–16. [PMC free article] [PubMed] [Google Scholar]