Abstract
Despite successful suppression of peripheral HIV-1 infection by combination antiretroviral therapy, immune activation by residual virus in the brain leads to HIV-associated neurocognitive disorders (HAND). In the brain, several types of cells, including microglia, perivascular macrophage, and astrocytes have been reported to be infected by HIV-1. Astrocytes, the most abundant cells in the brain, maintain homeostasis. The general consensus on HIV-1 infection in astrocytes is that it produces unproductive viral infection. HIV-1 enters astrocytes by pH-dependent endocytosis, leading to degradation of the virus in endosomes, but barely succeeds in infection. Here, we have discussed endocytosis-mediated HIV-1 entry and viral programming in astrocytes.
Keywords: HIV-1 brain, HIV-1 persistence, lysosomotropic drugs, chloroquine, proteasome inhibitors, LSP1, Rab, Rev, Tat
Introduction
In a significant number of HIV-1-infected patients undergoing suppressive antiretroviral therapy, residual viral activity in brain causes immune activation, which leads to HIV-associated neurocognitive disorders (HAND) (1,2). Astrocytes, the most abundant cells in brain, maintain homeostasis (3,4). In addition, in response to brain injury or viral infections such as HIV-1, astrocytes are activated to pathological state (reactive astrocytosis). Although HIV-1 in the brain productively infects myeloid lineage cells such as microglia and perivascular macrophages (5–12), only unproductive infection has been reported in astrocytes (13–24). Molecular investigations of HIV-1-infected brain tissues from post-mortem cases have demonstrated viral DNA in 3% to 19% of astrocytes (20,24–31). In vitro investigations of HIV-1-infected brain tissues and virus-infected astrocytes inferred unproductive HIV-1 infection from the presence of viral DNA and an absence of viral RNA and protein expression. However, limited HIV-1 infection in astrocytes has been reported and thought to occur because of intracellular restrictions (18,32). Several possibilities have been suggested for abortive viral infection in astrocytes; in particular, several intracellular host factors have been implicated in unproductive HIV-1 infection (33–38). However, several studies, including ours, have identified inefficient viral entry, which occurs because of the absence of CD4-receptor, as the major impediment to HIV-1 infection in astrocytes (19,39–45). The concept of inefficient viral entry is supported by the findings that use of vesicular stomatitis virus envelope (VSV)-pseudotyped HIV-1 or ectopic introduction of infectious viral DNA into astrocytes resulted in robust viral replication and release of infectious virus (39,42–44).
Viral entry into target cells occurs by viral envelope fusion at either the cell surface (plasma membrane fusion) or inside endosomes after endocytosis of viral particles (FAE) (46,47). Both of these fusion processes can be either pH-dependent or pH-independent. Viral entry into target cells occurs by several different endosomal pathways, such as clathrin-mediated endocytosis or caveolae-dependent endocytosis or macropinocytosis (48). In clathrin-mediated endocytosis, which is dependent on cytosolic GTPase dynamin, virus and its receptor are enclosed in clathrin-coated vesicles. Caveolae are invaginations in the plasma membrane that contain caeolin (49). In macropinocytosis, virus particles are internalized and transported to endosomes. In all of these processes, virus particles, once internalized, are routed to early and late endosomes and lysosomes (50). However, the endolysosomal path is destructive as well. HIV-1 infection in CD4+ lymphocytes uses both plasma membrane fusion and FEA (47,51). HIV-1 enters by endocytosis in epithelial and HeLa cells lacking CD4 receptor (52). HIV-1 entry into macrophages by macropinocytosis leads to degradation of virus in endolysosomal compartments, but allows a small number of virus particles to complete fusion. However, degradation efficiency is cell-type-specific. For example, VSV-envelope-pseudotyped HIV-1 (VSV-HIV-1), virus infection is least productive in macrophages (53), but produces extremely productive infection in astrocytes and other transformed cells (39,42,43). HIV-1 entry into astrocytes by endocytosis was proposed several years ago (23,54), but details of the mechanism by which this occurs have emerged only recently (43,45). Here, we have discussed the HIV-1 infection in astrocytes, in particular viral entry by endocytosis.
Natural endocytic entry of HIV-1 and viral infection in astrocytes
Lack of ample evidence on productive HIV-1 infection in astrocytes could be a result of the complexity of infection and failure to detect authentic viral infection. Although few studies have shown non-permissiveness of astrocytes to HIV-1 infection (23,55), several studies, including ours, have shown productive HIV-1 infection in astrocytes (32,41–44,56–60). Indeed, productive infection at the single-cell level was corroborated by viral p24 protein expression in HIV-1-infected astrocytes, even though viral activity was undetectable in culture supernatants after 10 days of infection (43,44). In particular, use of fluorescent HIV-1 reporter viruses unambiguously demonstrated barely productive infection by T- or M-tropic viruses, which was far below the limit of the viral p24 detection assay. Thus, irrespective of viral tropism, productive HIV-1 infection occurred in astrocytes. Minimal HIV-1 infection in astrocytes occurs by endocytosis-mediated viral entry and is pH dependent (43). Only a few studies of HIV-1 infection in astrocytes have elucidated the viral kinetics of the infection process. The most impressive data emerged with the use of live fluorescence microscopy which demonstrated HIV-1 infection in astrocytes on Day 4 after infection, which peaked between 12–15 days after infection (Fig. 1). The overall natural HIV-1 infection in astrocytes was less than 0.025% (43). However, earlier in-vitro studies on astrocytes found that HIV-1 infection was higher than 1% (24,41,61). By monitoring viral kinetics in HIV-1-infected astrocytes, a phenomenon of transient viral adsorption has been observed. Astrocytes have been shown to adsorb the majority of HIV-1 particles through incomplete plasma membrane fusion. These incompletely fused HIV-1 particles from the plasma membrane of astrocytes are released extracellularly within 10 days after infection without having actually entered astrocytes. (41,43,55). On follow-up of HIV-1 infection in astrocytes from day 0 to 21 post-infection, extracellular p24 levels peaked at day 3, declined by day 10 and became undetectable at 3 weeks. These findings are similar to those of other studies (39,41,55).
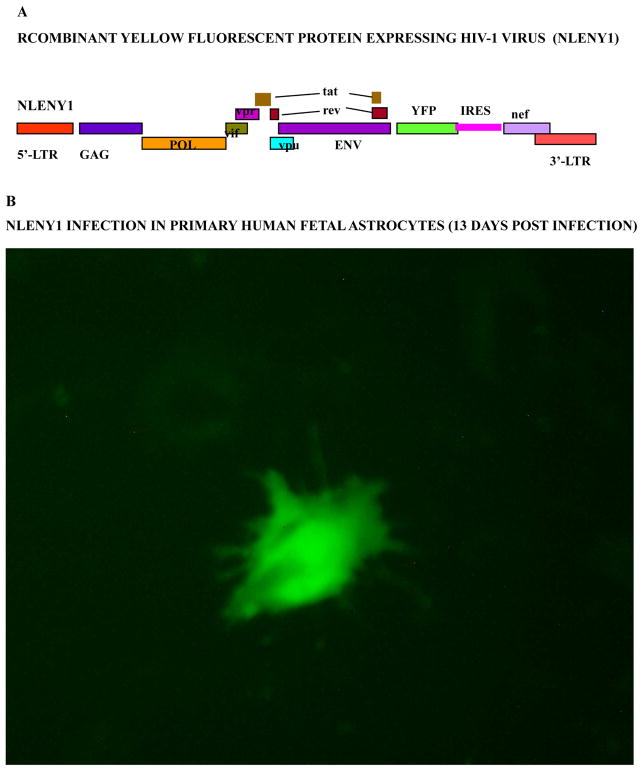
Figure 1. Natural HIV-1 infection in primary human fetal astrocytes.
(A): Diagrammatic view of recombinant HIV-1 infectious molecular clone, NLENY1 showing yellow fluorescent protein (YFP) gene insertion. (B): Six-week-old human fetal astrocytes (HFA) were seeded in six-well culture plates and, two days later, infected with NLENY1 (1.0 μg/mL p24) for 2 h. Infected HFA shows green fluorescence on day 13 post-infection.
Intriguingly, on monitoring the live fluorescence of recombinant yellow fluorescent reporter HIV-1 (NLENY1; Fig-1A) infected astrocytes, we found that during the first 10 days, intracellular viral infection did not correlate with extracellular viral p24 level. Authentic and pseudoviral activity were validated after 4 to 20 days of NLENY1 infection by green-positive astrocytes (Fig. 1B) in combination with viral p24 protein in culture supernatants (42,43). Similar results were obtained by infecting ectopically CD4-expressing human fetal astrocytes (HFA) or astrocytic reporter cells, recapitulating the scenario of HIV-1 infection in wild-type HFA; i.e., pseudoviral activity (residual). Thus, the presence of HIV-1 p24 protein in the culture supernatants of infected astrocytes between 3–10 days after infection is actually residual (pseudo) viral activity that did not correlate with viral replication (41,43,55). Accordingly, astrocytes transiently retain HIV-1. This suggested the presence of a major viral entry block, although one that was not complete for productive HIV-1 infection. Given the absence of HIV-1 classical receptor CD4 and co-receptors CCR5, but the presence of CXCR4 co-receptor in fetal astrocytes, virus infection is restricted at the viral entry level. Although few studies have claimed expression of CD4 or CCR5 in astrocytes (19,62,63), we and others could not confirm this in fetal astrocytes or transformed astrocytes (19,39–43,64–66).
Poor HIV-1 infection in astrocytes was observed with cell-free virus or transinfection studies. We (42,43) showed that this was because of the endocytic entry of the virus. However, earlier study suggested that cell-cell transinfection is efficient in astrocytes, but details on the mechanism of augmented infection were not provided (56). Interestingly, recent in-vitro study showed protrusions (synapse) from uninfected astrocytes acquiring virus from neighboring HIV-1-infected lymphocytes, but no details on efficiency of viral infection were provided (67). A few studies have suggested some HIV strain selectivity in infecting astrocytes (68–70). However, current knowledge does not explain strain selectivity beyond the major restriction at the viral entry level. In a recent study, it was concluded that HIV-1 infection in astrocytes is unproductive and nonpermissive, but that virus is retained after trypsinization of infected astrocytes (71). In this study, HIV-1 infected astrocytes were treated with trypsin to remove the attached virus after infection; this was followed by immediate co-culture with lymphocytes. After a few days of co-culture, transiently adsorbed virus particles on astrocytes (resistant to trypsin washing), transinfected lymphocytes in co-cultures (71). In general, residual virus activity is lost in 10 days by itself in-vitro cultures, as previously described (43,55). However, Chauhan et al, (43) have shown authentic, productive HIV-1 infection in astrocytes. In their study astrocytes were infected with recombinant yellow fluorescent protein expressing HIV-1 (NLENY1) and, 15 days after infection, co-cultured with uninfected lymphocytes. On follow-up of HIV-1 infection by live fluorescence microscopy, viral transinfection to lymphocytes was seen only from productively HIV-1-infected astrocytes. Transinfection or cell to cell infection from HIV-1-infected astrocytes or lymphocytes to uninfected astrocytes was minimal and similar to cell-free virus infection (43), suggesting that cell-free virus infection and cell-to-cell infection (transinfection) were equally restricted and pointing toward viral entry restriction. In both of these scenarios, restricted HIV-1 infection in astrocytes occurred by endocytosis.
Endocytic entry of HIV-1 into astrocytes came to light when treatment with lysosomotropic drugs (chloroquine and bafilomycin A), the drugs which localize to lysosomes and endosomes, resulted in several-fold increases in viral infection. This increase in HIV-1 infection occurred equally in both T- and M-tropic HIV-1 viruses. Further, it was anticipated that lysosomotropic drugs confer their effects through change in endosomal function, increasing lysosomal pH and inhibition of endolysosomal fusion which was corroborated in our earlier study (42). Further, lysosomotropic drug CQ behaved as an agonist on chronically HIV-1-infected astrocytes, ensuring that CQ also abrogates degradation of viral components, but was not involved in direct activation of HIV-1 LTR promoter (42,43). Overall, these observations suggested that in natural HIV-1 infection of astrocytes, adequate numbers of HIV-1 particles are entrapped within endosomes in astrocytes, but still might establish little productive infection owing to heightened degradation or limited escape of virus particles from endolysosomes.
Mechanism of HIV-1 endocytosis in astrocytes
HIV-1, an enveloped virus, enters cells by fusion either directly at the plasma membrane or at the endosomal membrane (47,53,72). Endosomal membrane fusion occurs after receptor interaction with virus at the surface of the target cell and successful infection depends on escape from degradation in endolysosomes (73). The status of the endolysosomal pathway, which depends on the type and activation of the cell, has been studied in HIV-1 infected macrophages. HIV enters by CD4 receptor and co-receptor either CCR5- or CXCR4-dependent fusion with the plasma membrane (74–77). Also, HIV-1 can enter by CD4-mediated, but co-receptor-independent endocytosis. Entry by CD4 receptors has been reported in primary CD4+ lymphocytes (78), while dynamin and clathrin-mediated endocytosis has been shown for CD4+ HeLa cells (79). These results were corroborated by blocking lysosomal degradation, leading to increased HIV-1 infection (51,80,81). More precisely, HIV-1 infection of target cells is a multistep process that begins with initial binding of the viral gp120 subunit of viral envelope glycoprotein to CD4 and co-receptor CCR5 or CXCR4. Its interactions with CD4 and a co-receptor trigger conformational changes in the transmembrane part of envelope gp41 and initiate membrane fusion (75–77,82). Several viruses enter target cells through intracellular compartments in which low pH and or cellular proteases activate the viral fusion proteins, permitting release of virus genome from intracellular compartments (reviewed in ref 83). Receptor and coreceptor-mediated HIV entry is pH-independent and does not rely on low (acidic) pH to infect host cells (84).
In general, the majority of HIV-1 enters through the classical pH-independent CD4-CXCR4 pathway in lymphocytes and is highly productive. However, limited pH-dependent viral endocytosis also seems to occur in lymphocytes (52,73,85). Another virus, VSV, enters target cells by pH-dependent endocytosis (46,86). VSV-envelope-pseudotyped HIV-1 (VSV-HIV-1) infection is highly productive in astrocytes (39,42–44). Interestingly, treatment with CQ or bafilomycin A severely impaired VSV-HIV-1 replication in astrocytes (43,44,51). Natural wild-type HIV-1 enters by pH-dependent endocytosis in CD4− astrocytes, but infection is barely productive. Treatment with CQ or bafilomycin A markedly increased wild-type HIV-1 infection in astrocytes (42,43). These differences between the VSV- and HIV-envelope seem to occur at the endolysosomal level, where the VSV envelope needs an acidic environment for activation, while the HIV-1 envelope remains inactivate in acidic environment.
Endocytosis is a complex cellular process involving endosomes and several proteins (reviewed in 87). Endosome functioning involves gaunosine triphosphatases (GTPases) of the Ras superfamily (Rab). Rab proteins (Ras-related proteins in brain) regulate specific steps of endocytosed vesicles from the plasma membrane to early endosomes (Rabs 4 and 5) (88), late endosomes and lysosomes (Rab7) (89), and vesicle-recycling endosomes (Rabs 4 and 11) (90,91). Chauhan et al (43) showed that HIV-1 infection in astrocytes via endocytosis involves early, late, and recycling endosomes. Using a molecular approach, RNAi-mediated ablation of Rab-5, -7, and -11 demonstrated their indispensible role in endocytosis-mediated HIV-1 infection in astrocytes. Leukocyte-specific protein 1 (LSP1), an F-actin binding protein, plays a role in endocytosis, as demonstrated by the finding that its depletion substantially decreases the rate of endocytosis (92). LSP1 has been shown directing HIV-1 particles to endosomes and proteasomes (93). Ablating LSP1 by siRNA, productive HIV-1 infection in astrocytes was severely impaired in the presence of lysosomotropic agents, indicating its intricate role in HIV-1 endocytosis (43). Despite the hostile environment in endosomes, productive HIV-1 infection in astrocytes succeeds, though to a minimum level. Minimum productive HIV-1 activity in astrocytes is a post-viral DNA-integration phenomenon, establishing the authentic replication from integrated viral DNA. Thus, although the endosomal route is indispensable for HIV-1 infection in astrocytes, it is, at the same time, detrimental, because endosomal internal machinery is least conducive to successful establishment of viral infection.
Perspective and conclusion
Normally, HIV-1 enters lymphocytes via a pH independent pathway (natural infection), using a classical receptor-coreceptor mechanism. CD4− astrocytes adsorb the majority of HIV-1 to the surface. In the absence of intracellular virus uptake, this partially fused virus at the cell surface is transiently retained, then released within 10 days. Few virus particles in astrocytes enter via pH-dependent endocytosis, resulting in barely productive HIV-1 infection (Fig. 1). Although most HIV-1 particles internalized by the vesicular pathway appear to be degraded in endolysosomes, a small number of virus particles escape the strongly acidic environment in endocytic vesicles to enter the cytoplasm. Inhibiting endosomal acidification or increasing pH by lysosomotropic agents such CQ or bafilomycin A, equally bolstered both M- and T-tropic virus infectivity irrespective of viral tropism (43). In addition to lysosomotropic drugs, proteasome inhibitor MG132 and an autophagy modulator slightly increased HIV-1 infection in astrocytes, but not to the extent that endosomal inhibitors did (43). None the less, inhibiting nucleases in astrocytes did not affect productive HIV-1 viral activity. However, ablating endosomal Rab proteins in astrocytes cripples productive HIV-1 infection, indicating viral entry by endocytosis. A few studies have suggested that there is some HIV-1 strain selectivity in infecting astrocytes efficiently (68,69). However, current knowledge does not explain why astrotropic strain selectivity will occur, given that the brain harbors HIV-1 permissive cells such as microglia and perivascular macrophages. The possibility that some strains may have overcome endolysosomal resistance and efficiently infect astrocytes needs further investigations. Chauhan et al. (43) have recently shown in co-cultures that HIV-1 infection does occur, but is not different from cell-free virus and was restricted, as is cell-free virus infection in astrocytes. In both of these HIV-1 infection scenarios, restricted HIV-1 infection in astrocytes occurs by endocytosis. Endocytic HIV-1 entry in astrocytes, albeit a fiery path on which the majority of viral particles are degraded, is the only natural way for HIV infection to occur in astrocytes and leads only to minimal survival of HIV-1.
Indeed, intracellular restrictions other than endosomal and proteasomal ones cannot be ruled out in non-CD4 astrocytes because HIV-1 infection could not exceed 0.5% after treatment with lysosomotropic drugs. However, bypassing natural viral entry by using VSV-HIV-1 or transfection of HIV-1 infectious DNA clone in astrocytes resulted in profound infection (39,42–44), ruling out any major intracellular restrictions for the virus. Mimicking natural receptor and co-receptor viral entry in astrocytes by ectopic expression of CD4 in human astrocytes, also resulted in profound HIV-1 infection, given that astrocytes naturally express CXCR4 viral co-receptor (43). Further, use of fluorescent reporter virus infection in astrocytes yielded other interesting observations, such as sustained production of the HIV-1 regulatory proteins Tat and Rev. These proteins regulate the viral life cycle at transcriptional and post-transcriptional levels. Alteration in their function cripples HIV-1 infection. Persistent production of Tat and Rev sustain chronic viral activity in limitedly dividing astrocytes. Corroborating this, Chauhan et al. (43) have demonstrated persistently productive HIV-1 infection in primary astrocytes for 160 days (43,44). Similarly, other studies have reported persistently productive HIV-1 infection in human astrocytes (39,94–96). Based on authors’ perspective and the evidences available, whatever little HIV-1 infection occurs in astrocytes, occurs exclusively via pH-dependent endocytosis. HIV-1 infection is impeded at the viral entry level because of an absence of CD4 receptor, as well restriction imposed intracellularly at the endo-lysosomal level. The contribution of this miniscule HIV-1 infection in astrocytes to overall viral load in the brain seems insignificant, even considering the abundance of this cell type in brain. However, this infection could be an elusive HIV-1 reservoir of productive infection and cause of immune activation in the brain. Using lysosomotropic drugs in HIV-1-infected patients may augment viral infection in cells lacking viral receptors. Although pH-dependent HIV-1 endocytosis is not an attractive target for therapy, it would be an impediment in efforts to purge virus from limitedly dividing astrocytes.
Highlights.
HIV-1 infection in astrocytes is impeded at the viral entry level
Astrocytes adsorb HIV-1 particles and release them within 10 days
HIV-1 infects astrocytes minimally by pH-dependent endocytosis
Inhibiting endosomal acidification or increasing endosomal pH by lysosomotropic agents, bolstered both M- and T-tropic HIV-1
Acknowledgments
Human fetal tissues at 10 to 12 weeks of gestational age were obtained following written approval from adult female patients undergoing therapeutic abortion at the University of Washington, Seattle. The work was supported by NIH grant RO1 NS0064 (AC) and internal funding from the University of South Carolina School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spudich SS. CROI 2014: neurologic complications of HIV infection. Top Antivir Med. 2014;22(2):594–601. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen MF, Gill AJ, Kolson DL. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS. 2014;9(6):559–64. doi: 10.1097/COH.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36:681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- 4.Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- 5.Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- 6.Gartner S, Markovits P, Markovitz DM, Betts RF, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986;256:2365–2371. [PubMed] [Google Scholar]
- 7.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 8.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. PNAS. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- 10.Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, et al. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- 11.Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Régulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 13.Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987;61:1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 15.Brack-Werner R, Kleinschmidt A, Ludvigsen A, Mellert W, Neumann M, Herrmann R, Khim MC, Burny A, Müller-Lantzsch N, Stavrou D, et al. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992;6(3):273–85. [PubMed] [Google Scholar]
- 16.Gorry PR, Howard JL, Churchill MJ, Anderson JL, Cunningham A, Adrian D, McPhee DA, Purcell DF. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32(2):253–67. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 18.Neumann M, Felber BK, Kleinschmidt A, Froese B, Erfle V, Pavlakis GN, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutet A, Salim H, Taoufik Y, Lledo PM, Vincent JD, Delfraissy JF, Tardieu M. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. [PubMed] [Google Scholar]
- 20.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Wesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–73. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 22.Petito CK. Human immunodeficiency virus type 1 compartmentalization in the central nervous system. J Neurovirol. 2004;10(Suppl 1):21–4. doi: 10.1080/753312748. [DOI] [PubMed] [Google Scholar]
- 23.Deiva K, Khiati A, Hery C, Salim H, Leclerc P, Horellou P, Tardieu M. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS Res Hum Retroviruses. 2006;22:1152–1161. doi: 10.1089/aid.2006.22.1152. [DOI] [PubMed] [Google Scholar]
- 24.Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 25.Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987;61:3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An SF, Groves M, Giometto B, Beckett AA, Scaravilli F. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 1999;98(5):481–7. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- 27.An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58(11):1156–62. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2013;13(1):95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 30.Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80(15):1415–23. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20(1):28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy M, He J, Wood C. HIV-1 strain-associated variability in infection of primary neuroglia. J Neurovirol. 1998;4(1):80–9. doi: 10.3109/13550289809113484. [DOI] [PubMed] [Google Scholar]
- 33.Reddy TR, Xu W, Mau JK, Goodwin CD, Suhasini M, et al. Inhibition of HIV replication by dominant negative mutants of Sam68, a functional homolog of HIV-1 Rev. Nature medicine. 1999;5:635–642. doi: 10.1038/9479. [DOI] [PubMed] [Google Scholar]
- 34.Gatignol A, Laine S, Clerzius G. Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology. 2005;2:65. doi: 10.1186/1742-4690-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong CL, Thorpe JC, Gorry PR, Bannwarth S, Jaworowski A, Howard JL, Chung S, Campbell S, Christensen HS, Clerzius G, Mouland AJ, Gatignol A, Purcell DF. Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response. J Virol. 2005;79(20):12763–72. doi: 10.1128/JVI.79.20.12763-12772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, et al. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Molecular and cellular biology. 2009;29:254–265. doi: 10.1128/MCB.01030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels SM, Melendez-Pena CE, Scarborough RJ, Daher A, Christensen HS, et al. Characterization of the TRBP domain required for dicer interaction and function in RNA interference. BMC molecular biology. 2009;10:38. doi: 10.1186/1471-2199-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanghvi VR, Steel LF. The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J Virol. 2011;85:12614–12621. doi: 10.1128/JVI.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;12:8, 31. doi: 10.1186/1471-2202-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijaykumar TS, Nath A, Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008;381:1–5. doi: 10.1016/j.virol.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauhan A, Mehla R, Theophilus-Sunder VK, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the HIV-1 in astrocytes. Virology. 2014;456–457C:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chauhan A. Unperturbed posttranscriptional regulatory Rev protein function and HIV-1 replication in astrocytes. PLoS ONE. 2014;9(9):e106910. doi: 10.1371/journal.pone.0106910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan A, Tikoo A, Patel J, Abdullah AM. HIV-1 endocytosis in astrocytes: A kiss of death or survival of the fittest? Neurosci Res. 2014 doi: 10.1016/j.neures.2014.08.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gobeil LA, Lodge R, Tremblay MJ. Macropinocytosis-like HIV-1 internalization in macrophages is CCR5 dependent and leads to efficient but delayed degradation in endosomal compartments. J Virol. 2013;87(2):735–45. doi: 10.1128/JVI.01802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–33. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 49.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 50.Weisz OA. Acidification and protein traffic. Int Rev Cytol. 2003;226:259–319. doi: 10.1016/s0074-7696(03)01005-2. [DOI] [PubMed] [Google Scholar]
- 51.Schaeffer E, Soros VB, Greene WC. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J Virol. 2004;78:1375–1383. doi: 10.1128/JVI.78.3.1375-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gobeil LA, Lodge R, Tremblay MJ. Differential HIV-1 endocytosis and susceptibility to virus infection in human macrophages correlate with cell activation status. J Virol. 2012;86(19):10399–407. doi: 10.1128/JVI.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao HN, Lyman WD. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain Res. 1999;823(1–2):24–32. doi: 10.1016/s0006-8993(98)01371-7. [DOI] [PubMed] [Google Scholar]
- 55.Clarke JN, Lake JA, Burrell CJ, Wesselingh SL, Gorry PR, Li P. Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology. 2006;348:141–155. doi: 10.1016/j.virol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropathol Exp Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Brengel-Pesce K, Innocenti-Francillard P, Morand P, Chanzy B, Seigneurin JM. Transient infection of astrocytes with HIV-1 primary isolates derived from patients with and without AIDS dementia complex. J Neurovirol. 1997;3(6):449–54. doi: 10.3109/13550289709031191. [DOI] [PubMed] [Google Scholar]
- 58.Wiley CA, Achim CL. No evidence of significant abortive HIV infection of the brain. AIDS. 1997;11:252. [PubMed] [Google Scholar]
- 59.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 60.Di Rienzo AM, Aloisi F, Santarcangelo AC, Palladino C, Olivetta E, Genovese D, Verani P, Levi G. Virological and molecular parameters of HIV-1 infection of human embryonic astrocytes. Arch Virol. 1998;143(8):1599–615. doi: 10.1007/s007050050401. [DOI] [PubMed] [Google Scholar]
- 61.Dewhurst S, Bresser J, Stevenson M, Sakai K, Evinger-Hodges MJ, Volsky DJ. Susceptibility of human glial cells to infection with human immunodeficiency virus (HIV) FEBS Lett. 1987;213(1):138–43. doi: 10.1016/0014-5793(87)81479-5. [DOI] [PubMed] [Google Scholar]
- 62.Simpson J, Rezaie P, Newcombe J, et al. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 63.Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. Journal of Neuroimmunology. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 64.Sabri F, Chiodi F, Fenyo EM. Lack of correlation between V3 amino acid sequence and syncytium-inducing capacity of some HIV type 1 isolates. AIDS Res Hum Retroviruses. 1996;12:855–858. doi: 10.1089/aid.1996.12.855. [DOI] [PubMed] [Google Scholar]
- 65.Schweighardt B, Shieh JT, Atwood WJ. CD4/CXCR4-independent infection of human astrocytes by a T-tropic strain of HIV-1. J Neurovirol. 2001;7:155–162. doi: 10.1080/13550280152058816. [DOI] [PubMed] [Google Scholar]
- 66.Willey SJ, Reeves JD, Hudson R, Miyake K, Dejucq N, Schols D, De Clercq E, Bell J, McKnight A, Clapham PR. Identification of a subset of human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus strains able to exploit an alternative coreceptor on untransformed human brain and lymphoid cells. J Virol. 2003;77(11):6138–52. doi: 10.1128/JVI.77.11.6138-6152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Do T, Murphy G, Earl LA, Del Prete GQ, Grandinetti G, Li G, Estes JD, Rao P, Trubey CM, Thomas J, Spector J, Bliss D, Nath A, Lifson JD, Subramaniam S. 3D imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. J Virol. 2014 doi: 10.1128/JVI.00788-14. JVI.00788–14. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy M, He J, Auger D, Geffin R, Woodson C, Hutto C, Wood C, Scott G. Cellular tropisms and co-receptor usage of HIV-1 isolates from vertically infected children with neurological abnormalities and rapid disease progression. J Med Virol. 2002;67(1):1–8. doi: 10.1002/jmv.2185. [DOI] [PubMed] [Google Scholar]
- 69.Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol. 2005;79(15):9618–24. doi: 10.1128/JVI.79.15.9618-9624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- 71.Gray LR, Turville SG, Hitchen TL, Cheng WJ, Ellett AM, Salimi H, Roche MJ, Wesselingh SL, Gorry PR, Churchill MJ. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS One. 2014;9(2):e90620. doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de la Vega M, Marin M, Kondo N, Miyauchi K, Kim Y, Epand RF, Epand RM, Melikyan GB. Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion. Retrovirology. 2011;8:99. doi: 10.1186/1742-4690-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Permanyer M, Ballana E, Este JA. Endocytosis of HIV: anything goes. Trends in microbiology. 2010;18:543–551. doi: 10.1016/j.tim.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Deng HR, Liu W, Ellmeier S, Choe D, Unutmaz M, Burkhart P, Di Marzio S, Marmon RE, Sutton CM, Hill, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 75.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardins E, Newman W, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 77.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 78.Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, Este JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem. 2004;279:51305–51314. doi: 10.1074/jbc.M408547200. [DOI] [PubMed] [Google Scholar]
- 79.Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin mediated endocytosis in human immunodeficiency virus type 1 entry. Journal of virology. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. Journal of virology. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei BL, Denton PW, O’Neill E, Luo T, Foster JL, Garcia JV. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J Virol. 2005;79:5705–5712. doi: 10.1128/JVI.79.9.5705-5712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stamatatos L, Cheng-Mayer C. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J Virol. 1993;67:5635–5639. doi: 10.1128/jvi.67.9.5635-5639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195(7):1071–82. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1998;7(2):513–8. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sloan RD, Kuhl BD, Mesplede T, Munch J, Donahue DA, et al. Productive Entry of HIV-1 during Cell-to-Cell Transmission via Dynamin-Dependent Endocytosis. Journal of virology. 2013;87:8110–8123. doi: 10.1128/JVI.00815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blumenthal R, Bali-Puri A, Walter A, Covell D, Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987;262(28):13614–13619. [PubMed] [Google Scholar]
- 87.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 88.Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 89.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- 92.Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 93.Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J, Nabel GJ. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204:421–430. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- 95.Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21(17):2271–81. doi: 10.1097/QAD.0b013e3282f12f27. [DOI] [PubMed] [Google Scholar]