Abstract
Purging HIV-1 to cure the infection in patients undergoing suppressive antiretroviral therapy requires targeting all possible viral reservoirs. Other than the memory CD4+ T cells, several other HIV-1 reservoirs have been identified. HIV-1 infection in the brain as a reservoir is well documented, but not fully characterized. There, microglia, perivascular macrophages, and astrocytes can be infected by HIV-1. HIV-1 infection in astrocytes has been described as a nonproductive and primarily a latent infection. Using primary human astrocytes, we investigated latent HIV-1 infection and tested phorbol 12-myristate 13-acetate (PMA), a protein kinase C agonist, as an HIV-1-latency- reversing agent in infected astrocytes. Chloroquine (CQ) was used to facilitate initial HIV-1 escape from endosomes in astrocytes. CQ significantly increased HIV-1 infection. But treatment with PMA or viral Tat protein was similar to untreated HIV-1-infected astrocytes. Long-term follow-up of VSV-envelope-pseudotyped HIV-1 infected astrocytes showed persistent infection for 110 days, indicating the active state of the virus.
Keywords: HIV-1 brain, chloroquine, HIV-1 latency, phorbol 12-myristate 13-acetate, DDX3, TRBP
1. Introduction
Human immunodeficiency virus -1 (HIV-1) infects the central nervous system and, in a significant number of patients, causes neuroinflammation, immune activation, and neurodegeneration, leading to HIV-associated neurocognitive disorders [1-3]. Astrocytes, the most abundant neuro-glial cells in the brain, are involved in brain plasticity and neuroprotection, and thus maintain homeostasis. Astrocytes are also important in HIV-1-mediated neuropathology, serving as inflammatory cells in response to viral and inflammatory products [4, 5]. The persistence of HIV-1, either as latent or active (productive) infection in patients in whom HIV is suppressed by combination antiretroviral therapy (cART) is currently the biggest hurdle in curing the viral infection. Currently, all efforts are focused on memory T-cells, which have been shown to harbor latent HIV-1 in cART-suppressed patients [6]. After interruption of suppressive cART, viral load rebounds in 2 weeks [7] and reseeding of the virus presumably is initiated by latently HIV-1-infected memory T-cells. HIV-1 reservoirs other than memory T-cells, including macrophages, microglia, astrocytes, and others, have been identified [8-10]. However, all treatment efforts aimed at curing HIV-1 infection have been mainly targeted against latently infected resting memory CD4+ lymphocytes. To cure HIV-1 infection, it is essential to consider all possible long-term HIV-1 reservoirs harboring either the latent or active form of the virus. Thus, we investigated astrocytes with regard to latent HIV-1 infection. Studies have shown that astrocytes, owing to the absence of a CD4 receptor, are infected limitedly by HIV-1 [11, 12]. A few studies have also reported that astrocytes are nonpermissive to HIV-1 infection [13, 14]. Molecular investigations of HIV-1-infected brain tissues from post-mortem cases have demonstrated that viral DNA in 3% to 19% of astrocytes is an unproductive infection [15-23]. Recently, immunohistochemical study of brain sections from individuals with HIV-associated neurocognitive disorders found that productive HIV-1 infection was undetectable [24]. Some studies, however, have shown that HIV-1 infection in astrocytes is moderately productive [12, 25-28]. Recently, we demonstrated barely productive HIV-1 infection (0.025%) in primary human fetal astrocytes [12, 28].
Earlier studies reported that astrocytes are latently infected by HIV-1 in-vivo and in-vitro [20, 29-33]. Other studies have reported epigenetic regulation of latent HIV-1 infection in astrocytes, which is modulated through the Wnt/β-catenin/TCF-4 signaling pathway [33, 34]. As yet, there is no other evidence from studies of the regulation of latent HIV-1 infection in astrocytes. So far in most of the studies, a latent viral state was assumed on the basis of findings after measuring HIV-1 DNA with absence of viral RNA and protein in infected astrocytes. Another observation on latent infection was the presence of viral protein p24 in culture supernatants from TNF-α- or IL-1β-treated HIV-1-infected astrocytes. Our findings were similar to those of earlier studies in that we noted the same type (residual) of viral kinetics in vitro after treatment with agents that do not reactivate latent HIV-1 infection. In our study and others [12, 14, 26], during the first 10 days of virus release from HIV-1-infected astrocytes, the virus released is actually a residual, not a replicated virus, although this was reported in earlier studies as latent virus infection.
In our earlier studies, barely detectable HIV-1 infection in primary human astrocytes was seen only by fluorescent microscopy but not by enzyme linked immunosorbent assay (ELISA) [12, 27]. Poor HIV-1 infection in astrocytes was equally observed with either cell-free virus or in transinfection studies. We [12, 28] showed that this was because of the endocytic entry of the virus. Given presence of barely productive HIV-1 infection in astrocytes and the inability of ELISA, a viral detection assay, to detect authentic viral infection in culture supernatants, we used fluorescence microscopy to pinpoint sporadically infected cells. Moreover, evidence is lacking that intracellular viral p24 is expressed after reactivation of latent HIV-1 in astrocytes. Thus, it was important to investigate whether latent HIV-1 infection in astrocytes really occurs as the main unproductive, but inducible (reactivation to productive viral state) event, which may have consequences with regard to HIV-eradication strategies.
2. Materials and Methods
2.1 Ethics Statement
Human fetal tissues at 10 to 12 weeks gestational age were obtained following written approval from adult female patients undergoing therapeutic abortion at the University of Washington, Seattle (IRB approval #11449) [5, 12]. The use of human fetal tissue was approved by the University of South Carolina (USCeRA#: HSA4636) and IRB (Institutional review board) exempt (45 CFR 46.102(d) [5, 12]. All cell-culture (primary human and cell lines), HIV-infection, and HIV-plasmid DNA studies were done according to University guidelines in a biocontainment facility approved by the Institutional Biosafety Committee of the University of South Carolina.
2.2 Primary human fetal brain and cell culture
Primary human fetal astrocytes (HFA) were cultured from human fetal tissues as described earlier [5]. HFA, SVGA, and 293T (HEK) cells were cultured. Human fetal brain specimens were obtained from the University of Washington, Seattle, where they were collected, following institutional guidelines, from fetuses at 8-12 weeks of gestational age. Briefly, the brain tissue was mechanically dissociated in Opti-MEM with 5% heat-inactivated fetal bovine serum (FBS) and antibiotic solution (penicillin G, 100 units/ml; streptomycin, 100 μg/ml; and amphotericin B, 25 μg/ml). The dissociated cells (without trypsin) were cultured in Dulbecco's modified Eagle medium (DMEM) with 10% FBS and antibiotics for at least one month before use. After 8 weeks in culture, HFA, after the 3rd or 4th passage, were used in experiments requiring a minimally non-dividing population of astrocytes and no minor contaminants (neurons and microglia). In some experiments, HFA cultures were further treated with 5 mM l-leucine methyl ester (Sigma) for 8 h to remove any contaminating microglia as described earlier [12]. The purity of cultures was verified using glial fibrillary- acidic-protein (GFAP), (microtubule-associated protein 2) MAP-2, and cluster of differentiation 68 markers; no microglia were found, but a few neurons were always observed. To reduce the chance of mycoplasma contamination in these cultures derived from aborted human fetuses, plasmocin, an antimycroplasma agent (Invitrogen, CA), was used during the initial culture passages. Cultures that tested negative for mycoplasma species on polymerase chain reaction were used.
SVGA (human astrocytic cells sub clone of SVG cells, a gift from Dr. Eugene Major, National Institutes of Health, NIH) [35] were maintained in DMEM supplemented with 2 mM L-glutamine, 10% FBS, and antibiotic solution. 293T cells used for viral packaging were grown in RPMI-1640 supplemented with 10% FBS and 10% human serum as described earlier [5].
2.3 Viral constructs, plasmids and transfection
The green fluorescent reporter HIV-1 infectious molecular clone NLENY1 was created by inserting the yellow fluorescent protein (YFP) gene between the viral envelope and nef genes without impairing the viral open-reading frames (Fig. 1A), as reported earlier [36]. NL4-3 infectious DNA clone was obtained from NIH. HIV-1 Tat, Rev, and DEAD (Asp-Glu-Ala-Asp) box helicase 3 (DDX3) were cloned into pCDNA-3 vector as described earlier [37, 38].
Figure 1.
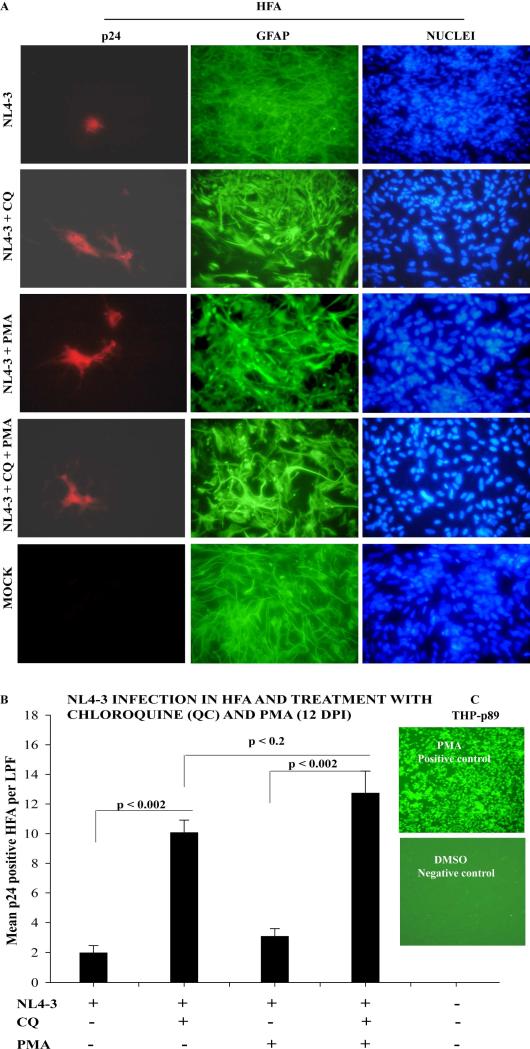
HIV-1 infection in primary human fetal astrocytes (HFA). (A) Diagram showing wild type T-tropic HIV-1 NL4-3 and recombinant YFP-expressing NL4-3 (NLENY1). (B) HFA seeded in 6-well culture plates were infected the next day with NL4-3 (0.1-1.0 μg/mL p24) or NLENY1 (0.1-1 μg/mL p24) for 2 h and washed two times with medium. After 16-18 h, HIV-1-infected cultures were washed and incubation was continued.HFA were then immunostained for viral capsid p24 protein and astrocytic marker GFAP. NLENY1-infected HFA were immunostained for GFAP. Controls were mock-infected HFA. Immunostaining was observed under a UV fluorescent microscope and photographed.
SVGA-VSVNL and 293T cells were transfected with HIV-1 infectious molecular clones or other plasmids expressing Tat, Rev, or DDX3, using Lipofectamine 2000 (Invitrogen). The transfection efficiency was ~80%. The plasmid concentrations used were from 0.5 to 17 μg, depending on the culture plates used.
2.4 Generation of persistently HIV-1 infected astrocytes
SVGA cells were infected with VSV-pseudotyped NLENY1 virus [12]. The persistently infected green fluorescent (GFP) cells (SVGA-VSVNL) were sorted by fluorescent-activated cell sorting (FACS) at the School of Public Health, Johns Hopkins University. Stable clones were obtained over 4 months by a multi-step cloning process that took advantage of GFP as a marker. After expansion in culture, the FACS-sorted cells were subcloned into bright or weak fluorescent and non-fluorescent clones. Weak green fluorescent cells were used in HIV-1 reactivation experiments.
2.5 Reverse genetics and VSV-G pseudotyping
HIV-1 NL4-3 and NLENY1 were packaged in 293T cells either as wild-type or pseudotyped with VSV-G envelope. All plasmids were prepared endotoxin-free, using a maxiprep kit (Qiagen). Viral packaging was done with 17 μg of each HIV-1 infectious DNA clone alone or with 3-4 μg of VSV-G plasmid DNA, using Lipofecamine-2000 (Invitrogen) as described earlier [12]. After 16 h of transfection, cells in 100-mm dishes were washed twice with fresh medium and incubated in RPMI 1640 containing 10% FBS and 1% each streptomycin, penicillin, and amphotericin B (Invitrogen). The culture supernatants were harvested 72-80 h after transfection, centrifuged at 1,200 rpm for 15 min, and filtered through a 0.20 μm filter. The filtrate, after the addition of MgCl2 (4 mM), was digested with 10-50 units of RNase-free DNase (Invitrogen) per 1 μg of plasmid DNA for 30 min at 37°C, then aliquoted and stored at −80°C. The viral stocks were quantified for p24 antigen by ELISA (ZeptoMetrix). An NL4-3 viral inoculum of 1.0 μg/mL and VSV-NLENY1 of 100 ng/mL of p24 was used to infect HFA and SVGA.
2.6. Virus infection and immunofluorescence
These studies used 50,000-75,000 HFA per well, seeded in 6-well tissue-culture plates. NL-4-3, NLENY1, and VSV-pseudotyped HIV-1 viral stocks were prepared by transfection of 293T cells. NL4-3 virus at 0.1-1 μg/mL and VSV-NLENY1 at 10-100 ng/mL p24 concentrations were used for infection of HFA. About 800 μl of the working viral stock was added to 60%-70% confluent 6-well culture plates and kept for 2 h at 37°C with gentle mixing every 20 min. After 2 h of incubation, the inoculum was washed two times with DMEM and complete medium was added. Some cultures were treated with 10 μM chloroquine (CQ) for 12 h. The next day, cultures were washed two times and replenished with complete medium. The cultures were maintained for 10 days, then treated with PMA (1 nM).
Viral capsid protein 24 (p24) expression in HIV-1 infected astrocytes was detected by immunofluorescence [28], using HIV-1 p24 Gag monoclonal antibody (cat # 6458, NIH). On day 12 after HIV-1 infection, infected HFA were fixed in 2% paraformaldehyde in PBS (pH 7.2) for 15 min, then permeabilized with 0.2% Triton-X-100 (PBS) for 11 min. Blocking was then done with 2% bovine serum albumin in PBS for 1 h at 25°C. The treated cells were overlaid with primary antibodies at an optimum working dilution of GFAP 1:200 and p24 1:300 in a moist chamber at 4°C overnight. After washing four times with PBS (10 min each at 37°C), the secondary antibodies (Molecular probes) labeled with Alexa 488 and 568 (1:400 dilutions) were added to the specimens for 35 min at 25°C in the dark. Subsequently, the specimens were washed four times with phosphate buffer saline (PBS), stained with Hoechst dye (PBS) for 10 min during the last wash, rinsed with PBS, and kept in 2.0 mL PBS. The specimens were visualized using an ultraviolet microscope (Nikon). Images were captured with a digital camera (Nikon). The immunostained cells were counted for p24 positive cells in ten random fields under low power field in duplicate sets. Means were plotted and p values calculated using t test.
2.7. Virus release assay (p24)
Astrocytes seeded at 60%-70% confluency in culture dishes or on six-well culture plates were infected or transiently transfected using Lipofectamine 2000 (Invitrogen) as described [12]. At various time points, the culture fluids were monitored for viral capsid p24. Quantitative viral p24 titration was done by ELISA (ZeptoMetrix). Data were expressed as mean ± standard error of mean (SEM). Multiple infection and transfection experiments (n ≥ 5) were done.
2.8. Statistics
Results are represented as mean ± SEM for each bar set plotted using Sigma plot v12.0 with the associated p-values for each treatment group compared to its control. Statistical analysis was done using Origin 6.1 software. Significance between two groups was calculated using the two-tailed Student's t-test. p < 0.05 was considered significant.
3. Results and Discussion
In our earlier study we established that T- and M-tropic HIV-1 viruses were equally restricted at the viral entry level, leading to minimal infection in astrocytes [12]. To investigate latent HIV-1 infection in astrocytes, two T-tropic viruses, NL4-3 and NLENY1 were used (Fig. 1A). NLENY1 is a recombinant NL4-3 that expresses yellow fluorescent protein (YFP) reporter (Fig. 1A). NLENY1 virus faithfully expresses YFP in unison with viral proteins [36, 39]. Given that authentic HIV-1 infection in astrocytes was undetectable by ELISA in our earlier studies [12, 27], in this study we used a fluorescence approach, either immunostaining the viral p24 protein or direct YFP reporter fluorescence (NLENY1) in infected cells. The use of ELISA on HIV-1-infected culture supernatants from barely HIV-1(wild type)-infected astrocytes was not useful, given that primary human astrocytes adsorb the virus and release it for several days without its being actually replicated (pseudo-viral activity). ELISA cannot differentiate such slight virus infection. Further, flow cytometry was a limitation because wild type HIV-1 infection was minimal as well as occurrence of autofluorescence in astrocytes. Primary human fetal astrocytes (HFA) cultured in 6-well plates were infected with NL4-3 virus (0.1-1.0 μg/mL p24) for 2 h in the absence or presence of chloroquine (QC), PMA, or CQ plus PMA for 16-18 h. T-tropic virus was chosen because we found no viral tropism in astrocytes, given that T- and M-tropic viruses infect astrocytes equally [12, 27]. After virus infection in astrocytes, as in our earlier studies, wild-type HIV-1 or recombinant virus infection was minimum, as demonstrated by p24 immunostaining or YFP fluorescence in GFAP-positive astrocytes (Fig.1B).
Earlier, we reported that natural HIV-1 infection at the viral entry step is blocked equally to T- and M-tropic HIV-1. Irrespective of viral tropism, we also found that minimum HIV-1 infection occurred by pH- dependent endocytosis [12, 27]. In this study, however, as in our earlier ones, treatment with CQ profoundly increased HIV-1 infection in astrocytes compared to that in untreated infected astrocytes (Fig. 2). As reported, increase in HIV-1 infection in CQ-treated astrocytes was several folds higher, but did not exceed 0.5% [12]. Thus, it is possible that HIV-1 infects the majority of astrocytes unproductively, but that this infection could be in a latent state. To investigate others’ [19-21, 23] claims that the majority of HIV-1 infection in astrocytes is latent, we treated virus-infected astrocytes with potent latency reversing agent (LRA), PMA. PMA is a protein kinase C (PKC) agonist which reactivates the latent HIV infection [39, 40]. Because, in our earlier studies, several LRAs such as TNF-α, TSA, IL-1β, and other cytokines, failed to reactivate HIV-1 infection in astrocytes [12]. Thus, in the current study, PMA was used at 40-fold higher concentrations (1 nM) than our previously used concentrations on latently infected Jurkat and monocytic cells [39]. HFAs were infected with NL4-3 virus in six-well culture plates in duplicate sets, then treated with PMA or PMA plus CQ. CQ treatment was always done 6-8 h after infection for 12-14 h. PMA treatment was done on day 10 after viral infection because by this time residual viral activity has been washed out [12, 14]. Also, this was enough time for the HIV-1, if any occurred in astrocytes, to have undergone a latent state. At 48 h after PMA treatment (i.e., 12 days after infection), HFA were immunostained for p24 and GFAP; nuclei were stained with Hoechst dye. The stained cultures were examined under a UV microscope and counted. PMA at 40-fold less concentration on latently HIV-1-infected monocytic cells reactivated 100 percent of the cells as reported previously [39].
Figure 2.
Treatment of HIV-1-infected HFA with chloroquine (CQ) or PMA. (A) HFA seeded in 6-well culture plates were infected the next day with NL4-3; 6 h later, they were treated with CQ for 16 h. PMA treatment was done after 10 days of HIV-1 infection. After 12 days of infection, HFA were immunostained for p24 and GFAP; nuclei were stained with Hoechst dye. (B) p24 positive astrocytes were counted in 10 random fields in duplicate sets. Mean positive cells were plotted and analyzed by t-test. (C) PMA (0.025 nM) and DMSO were used as positive and negative controls on latently HIV-1-infected monocytic cells expressing GFP. Latently THP-p89 cells were cultured in RPMI 1640 medium for 24 h and next day treated with either PMA or DMSO for 16 h and images were captured after 24 h.
As shown above, HIV-1 infection alone was barely detectable, but was bolstered severalfold by CQ (Fig. 2). PMA treatment alone was not significantly different from untreated HIV-1-infected astrocytes. Co-treatment with PMA and CQ showed a few more virus-infected astrocytes than did CQ treatment alone (Fig. 2), but was not statistically significant (p ≤ 0.2) (Fig. 2B). Significant differences in viral infection were seen in CQ or CQ-plus-PMA-treated HIV-1-infected astrocytes from untreated HIV-1-infected or PMA-treated HIV-1-infected astrocytes (p ≤ 0.002) (Fig. 2B). Also, PMA at lower concentrations (0.025 nM) robustly reactivated control latently HIV-1-infected monocytic cells THP-p89 (Fig. 2C). It is clear from these observations that HIV-1 infection in astrocytes is not in a latent state, inducible to productive viral state (expression of viral p24 protein). In our earlier studies, we showed that productive HIV-1 infection in astrocytes occurs from integrated viral DNA and persists for several months [12, 27].
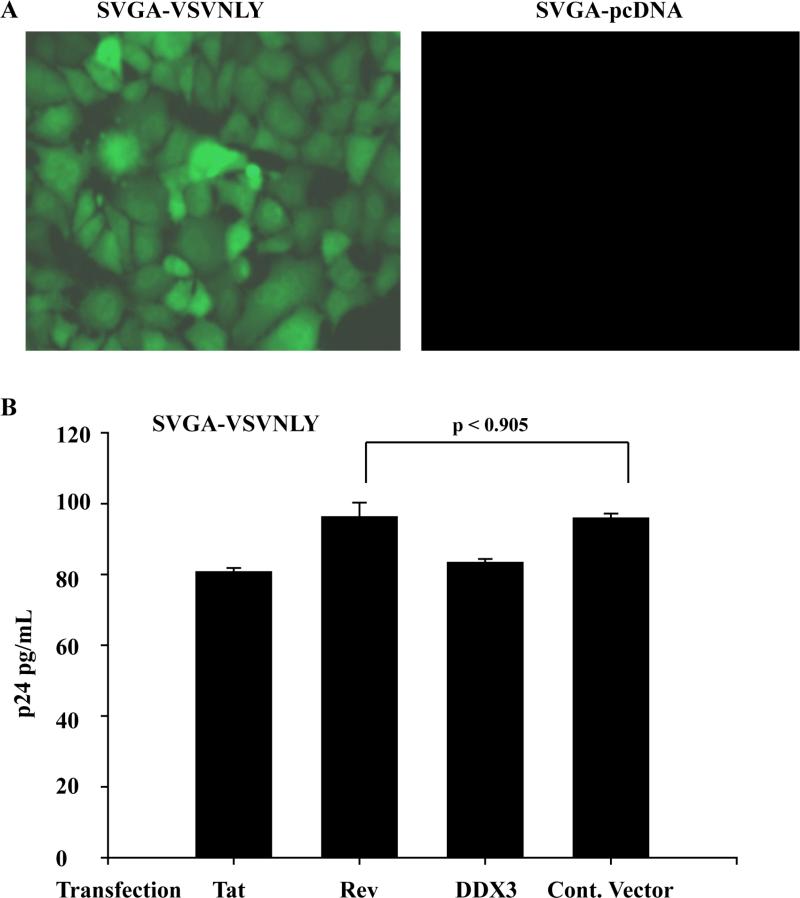
Two early HIV-1 regulatory proteins, Tat and Rev, which are produced upon multiple splicing from full-length viral transcripts, are indispensable for temporal regulation of the viral life cycle. Assuming that Tat and Rev proteins are naturally under expressed in HIV-1-infected astrocytes, the failure of PMA to reverse this latent viral infection could be one reason for latent infection in astrocytes. Rev, which has been the center of focus with respect to restricted HIV-1 infection in astrocytes [41-43], was investigated for its posttranscriptional activity. Further, [37] have elegantly demonstrated the critical role of a host RNA helicase DDX3 in Rev function and HIV-1 replication, but so far no evidence in astrocytes. Since, in our earlier study, DDX3 was barely detectable in astrocytes [28], the role of DDX3 in latent HIV-1 infection was also investigated. Also in our earlier study, treatment of persistently HIV-1-infected astrocytes with TSA, TNF-α, or IL-1β did not show any activity of long terminal repeat (viral promoter) and p24 above basal levels [12]. These persistently HIV-1 infected astrocytes (SVGA) were established after infection with VSV-pseudotyped NLENY1 and, after several weeks in culture, clonally selected for having the lowest green fluorescence (Fig. 3A) [12]. The HIV-1-infected SVGA cell model was chosen because it has the highest transfection efficiency compared to primary human astrocytes [35], and yields conclusive data. Thus, to explore whether under expression of Tat, Rev, or DDX3 has a role in latent HIV-1 infection in astrocytes, we ectopically expressed these proteins in persistently HIV-1-infected astrocytes (SVGA-VSVNLY1). Viral p24 protein levels in culture supernatants after 72 h of transfection of Tat, Rev, or DDX3 in SVGA-VSVNLY1 were quantified by ELISA. Ectopic expression of Tat, Rev, or DDX3 in SVGA-VSVNLY1 showed extracellular viral p24 levels similar to those of control vector or non-transfected cells (p<0.2) (Fig. 3B).
Figure 3.
Induction of persistently HIV-1 infected astrocytic cells. (A) SVGA were infected with VSV-NLENY1 and, after three weaks, were selected for GFP-postive cells by flow cytometry. (B) Persistently SVGA-NLENY1 cells were transfected with Tat, Rev, DDX3 or control vector. After 48 h of transfection, culture supernantants were collected and analyzed for p24 using quantitative ELISA. Mean p24 values were plotted and analyzed by t-test (p ≤ 0.9).
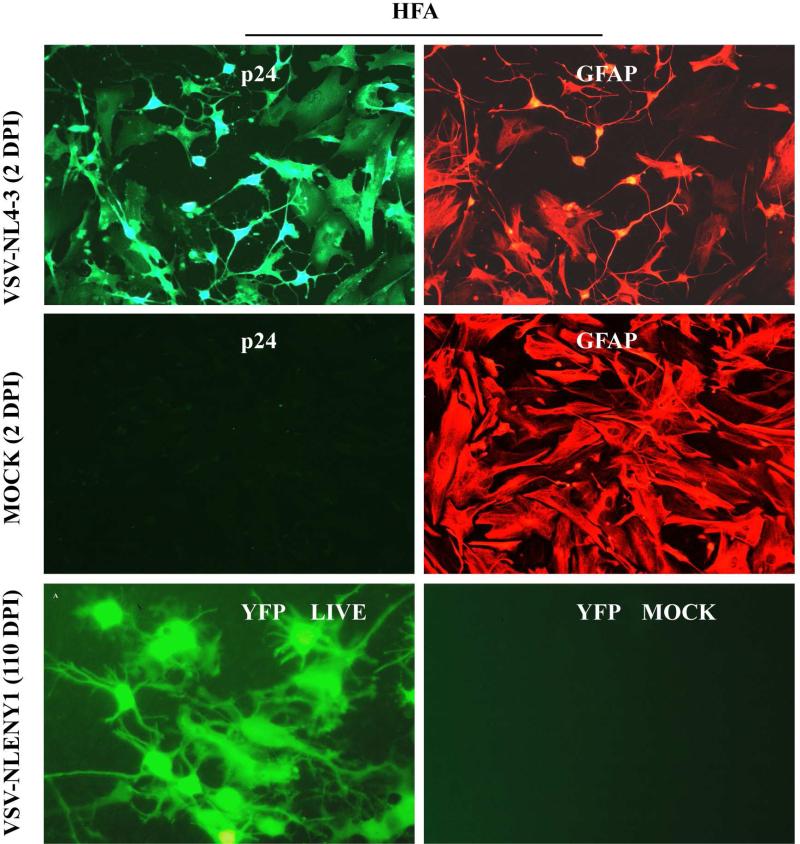
The presence of viral DNA ([20] but absence of viral RNA and p24 protein [24] in astrocytes in HIV-1-infected patients’ post-mortem brain tissues has been suggested as latent viral infection, but the significance of these findings is unknown. Thus, to investigate further the latent HIV-1 infection in primary astrocytes, a robust infection model of VSV-pseudotyped HIV-1 infected astrocytes was used (Fig. 4A). HFA cultures in T-25 flasks were infected with VSV-envelope-pseudotyped NLENY1 (VSV-NLEY1), then examined every 10 days by fluorescence microscopy (live). On follow-up of live NLENY1 infection, persistently HIV-1 infected astrocytes (green) were seen for several months (110 days) (Fig. 4B). In our previous studies, VSV-pseudotyped NLENY1 infected HFA cultures at 80 days of infection, after treatment with TNF-α showed non inducible but persistently productive virus infection [12], suggesting the occurrence of a non-latent state of the virus. Even ectopic expression of Tat in persistently HIV-1-infected astrocytes did not show p24 above basal levels in the culture supernatants [12]. It can be still argued that HIV infection in astrocytes is restricted due to untranslated products of unspliced or partially spliced HIV mRNA transcripts. However, in the current study robust VSV-pseudotyped HIV-1 infection of astrocytes has ruled out the above possibility corroborating the previous observations [12, 44]. Also, transfection of full length HIV infectious DNA clones lead to robust expression of viral products from unspliced, partially spliced, and multiply spliced mRNA transcripts, suggesting that viral infection is restricted at the viral entry [15, 50].
Figure 4.
Peristent infection in HFA. HFA seeded in 6-well culture plates or T-25 flasks were infected with VSV-NL4-3 or NLENY1. After 48 h of infection, HFA were immunostained for viral p24 protein and GFAP. Infected cultures in T-25 flasks were observed every 10 days for 110 days under UV fluorescent microscope and photographed by digital camera.
The current study demonstrated that there is no evidence of latent HIV-1 infection (reactivation to viral p24 protein expression), corroborating our earlier observations [12, 28]. However, keeping in mind the data obtained from HIV-infected brain tissues of patients, viral DNA was present in astrocytes without any evidence of viral replication. However, this situation may occur naturally as reported in a study on CD4 T-lymphocytes from HIV-1-infected patients undergoing suppressive antiviral treatment. In this study, integrated viral DNA was shown to be not reactivatable [40]. Thus, one caveat from the current study is that scantily HIV-1-infected astrocytes may serve as a stable reservoir that could hamper viral purging to cure the infection. Also, in contrast to earlier reports, the current study found no major latent state (reactivation to viral p24 protein expression) of HIV-1 infection in astrocytes. The use of CQ clearly showed that after endocytosis-mediated viral entry, virus is blocked in endosomes, while PMA treatment had no significant effect on HIV-1 infection in astrocytes, suggesting that the virus has a least chance of existing in the latent state (reactivation to viral p24 protein expression). In our earlier study, authentic viral infection was obtained by p24 immunostaining, but not free virus (p24) in culture supernatants, because infection was too small and occurrence of erroneous results with viral p24 ELISA. The inability of the viral detection technique (ELISA) to differentiate between true and residual infection in barely HIV-1-infected astrocytes came to light when p24 was measured serially in parallel with live fluorescent microscopy [12].
Even though long-term studies on HIV-1-infected astrocytes are lacking, virus infection has been shown to enter into a latent state that is amenable to reactivation [29-33]. In one study [30], HIV-1 latent infection in astrocytes was inducible, although active virus was recoverable only after co-culture with lymphocytes. Given the limited HIV-1 infection in astrocytes, the above scenario could be possible. A recent study has shown latent viral infection in the brain tissue of HIV-infected patients [21]. Immunocytochemical examination of these tissues showed elevated levels of B-cell lymphoma (Bcl) IIB, a factor for regulating HIV-1 silencing in microglia and astrocytes. In this study, latent infection was identified according to the criteria of the presence of viral DNA but the absence of viral RNA and p24 protein [21]. Although it was indicated that microglia and astrocytes had latent HIV-1 infection, the lack of precise evidence gave rise to a need for further investigation. In an in-vitro study, Bcl IIB protein was identified as a factor in silencing HIV-1 and was reported to regulate HIV-1 latency in U1 and microglia [45]. However, this study lacked data on true viral latency. Thus, even if Bcl IIB regulates HIV silencing in microglia [46], there is no evidence regarding its role, if any, in latent viral infection of astrocytes. In the current study, the PKC agonist PMA demonstrated a slight increase in HIV-1 infection that could have been caused by reactivation of latent HIV-1. However, PKC also has a role in endocytosis-mediated entry of non-enveloped [47] and enveloped viruses [48]. This small increase in HIV-1 infected cells by PMA could have been produced by increased endocytosis-mediated entry. In any case, the increase was not as impressive as that reported with CQ.
Given that it is the nature of productive HIV-1 to revert to a latent state in permissive cells (lymphocytes), it is possible that astrocytes may harbor similarly quiescent virus. The limited division of adult astrocytes in the brain is another reason to believe that HIV-1 infection may undergo a latent state in this cell type and points toward under expression of host factors required for viral replication. Several host factors, such as underexpression of DDX3 and TAR RNA binding protein (TRBP) and high basal levels of antiviral protein kinase R in astrocytes [28] could contribute to this latent state. In our previous study, low levels of DDX3 and TRBP did not affect HIV-1 replication in astrocytes except upon complete depletion of DDX3. Ectopic overexpression of DDX3 and TRBP and ablation of protein kinase R by RNAi did not provide any advantage to HIV-1 replication in astrocytes [12]. Intriguingly, in the current study, we found persistently productive HIV-1 infection in astrocytes (Fig. 4), as we did in our previous studies throughout the 110-160-days of follow-up with no response to LRA [12, 28]. Other studies, using wild type HIV-1 [49] or VSV-pseudotyped HIV-1 viruses [44, 50], have also found persistent productive infection in human astrocytes. Overall, given the current in-vitro evidence and the result of earlier investigations, there is no evidence of massive latent HIV-1 infection (reactivation to viral p24 protein expression) in astrocytes. However, to validate the invitro data, in-vivo investigations on simian immunodeficiency virus (SIV) nonhuman primate model using LRA intracisternally will provide clear picture of latent viral infection in astrocytes. Given that HIV-1 reservoirs other than resting memory CD4+ T-cells have been identified but not fully characterized, merely reversing HIV-1 latency in memory T-cells shall not cure HIV-1 infection. In particular, long-lived HIV-1-infected cells producing low amounts of virus will create the biggest impediment to curing HIV-1 infection. The scanty, elusive reservoir of HIV-1-infected astrocytes would hamper viral purging strategies. Thus, it is of the utmost importance that we identify and characterize HIV-1 reservoirs in particular CD4-negative cells as low-productive viral reservoirs in patients undergoing suppressive cART and implement a multi-pronged approach instead of targeting only latent T-cell reservoirs.
Acknowledgments
We thank Dr. David N. Levy (NYU School of Dentistry) for HIV-1 NLENY1 infectious molecular clone. We also acknowledge the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, for providing NL4-3 infectious molecular clone and monoclonal antibody to p24. Human fetal tissues at 10 to 12 weeks gestational age were obtained following written approval from adult female patients undergoing therapeutic abortion at the University of Washington, Seattle. This work was supported by NIH grant RO1 NS0064 (AC) and internal funding from the University of South Carolina School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Author declares no conflicts of interest
References
- 1.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–42. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 2.Chan P, Brew BJ. HIV Associated Neurocognitive Disorders in the Modern Antiviral Treatment Era: Prevalence, Characteristics, Biomarkers, and Effects of Treatment. Curr HIV/AIDS Rep. 2014;11:317–24. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- 3.Spudich SS. CROI 2014: neurologic complications of HIV infection. Top Antivir Med. 2014;22:594–601. [PMC free article] [PubMed] [Google Scholar]
- 4.Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, et al. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29:433–42. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:239. doi: 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siliciano RF. Opening Fronts in HIV Vaccine Development: Targeting reservoirs to clear and cure. Nat Med. 2014;20:480–1. doi: 10.1038/nm.3550. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–40. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 8.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 9.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345:169–74. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson M. CROI: basic science review. Top Antivir Med. 2014;22:574–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Speck RF, Esser U, Penn ML, Eckstein DA, Pulliam L, Chan SY, et al. A trans-receptor mechanism for infection of CD4-negative cells by human immunodeficiency virus type 1. Curr Biol. 1999;9:547–50. doi: 10.1016/s0960-9822(99)80241-3. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan A, Mehla R, Theophilus-Sunder VK, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the HIV-1 in astrocytes. Virology. 2014;456-457:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpless N, Gilbert D, Vandercam B, Zhou JM, Verdin E, Ronnett G, et al. The restricted nature of HIV-1 tropism for cultured neural cells. Virology. 1992;191:813–25. doi: 10.1016/0042-6822(92)90257-p. [DOI] [PubMed] [Google Scholar]
- 14.Clarke JN, Lake JA, Burrell CJ, Wesselingh SL, Gorry PR, Li P. Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology. 2006;348:141–155. doi: 10.1016/j.virol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987;61:3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An SF, Groves M, Giometto B, Beckett AA, Scaravilli F. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 1999a;98:481–7. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- 17.An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999b;58:1156–62. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, et al. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 20.Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 21.Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–23. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2013;13:95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20:28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 24.Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res. 2014;12:97–110. doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropathol Exp Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;12:8–31. doi: 10.1186/1471-2202-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijaykumar TS, Nath A, Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008;381:1–5. doi: 10.1016/j.virol.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauhan A. Unperturbed posttranscriptional regulatory Rev protein function and HIV-1 replication in astrocytes. PLoS One. 2014;9:e106910. doi: 10.1371/journal.pone.0106910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987;61:1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atwood WJ, Tornatore CS, Traub R, Conant K, Drew PD, Major EO. Stimulation of HIV type 1 gene expression and induction of NF-kappa B (p50/p65)-binding activity in tumor necrosis factor alpha-treated human fetal glial cells. AIDS Res Hum Retroviruses. 1994;10:1207–11. doi: 10.1089/aid.1994.10.1207. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–28. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol. 2014;88:3031–8. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F, Al-Harthi L. Role of ,β-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J Virol. 2012;86:1911–21. doi: 10.1128/JVI.06266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan A, Hahn S, Gartner S, Pardo CA, Netesan SK, McArthur J, et al. Molecular programming of endothelin-1 in HIV-infected brain: role of Tat in up-regulation of ET-1 and its inhibition by statins. FASEB J. 2007;21:777–89. doi: 10.1096/fj.06-7054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutsch O, Benveniste EN, Shaw GM, Levy DN. Direct and quantitative single cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol. 2002;76:8776–8786. doi: 10.1128/JVI.76.17.8776-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Mehla R, Chauhan A. Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus -1 preintegration complex (DNA). PLoS One. 2010;5:e15620. doi: 10.1371/journal.pone.0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PloS One. 2010;5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20:425–9. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorry PR, Howard JL, Churchill MJ, Anderson JL, Cunningham A, Adrian D, et al. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumann M, Afonina E, Ceccherini-Silberstein F, Schlicht S, Erfle V, Pavlakis GN, et al. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J Cell Sci. 2001;114:1717–29. doi: 10.1242/jcs.114.9.1717. [DOI] [PubMed] [Google Scholar]
- 43.Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005;336:299–307. doi: 10.1016/j.virol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26:412–23. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marban C, Redel L, Suzanne S, Van Lint C, Lecestre D, Chasserot-Golaz S, et al. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005;33:2318–31. doi: 10.1093/nar/gki529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Upla P, Marjomäki V, Kankaanpää P, Ivaska J, Hyypiä T, Van Der Goot FG, et al. Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol Biol Cell. 2004;15:625–36. doi: 10.1091/mbc.E03-08-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constantinescu SN, Cernescu CD, Popescu LM. Effects of protein kinase C inhibitors on viral entry and infectivity. FEBS Lett. 1992;292:31–3. doi: 10.1016/0014-5793(91)80826-o. [DOI] [PubMed] [Google Scholar]
- 49.Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21:2271–81. doi: 10.1097/QAD.0b013e3282f12f27. [DOI] [PubMed] [Google Scholar]
- 50.Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]