Abstract
Background:
Acellular dermal matrices are used in implant-based breast reconstruction. The introduction of contour fenestrated AlloDerm (Life-Cell, Branchburg, N.J.) offers sterile processing, a crescent shape, and prefabricated fenestrations. However, any evidence comparing reconstructive outcomes between this newer generation acellular dermal matrices and earlier versions is lacking.
Methods:
Patients undergoing implant-based breast reconstruction from 2010 to 2014 were identified. Reconstructive outcomes were stratified by 4 types of implant coverage: aseptic AlloDerm, sterile “ready-to-use” AlloDerm, contour fenestrated AlloDerm, or total submuscular coverage. Outcomes were compared with significance set at P < 0.05.
Results:
A total of 620 patients (1019 reconstructions) underwent immediate, implant-based breast reconstruction; patients with contour fenestrated AlloDerm were more likely to have nipple-sparing mastectomy (P = 0.0001, 0.0004, and 0.0001) and immediate permanent implant reconstructions (P = 0.0001). Those with contour fenestrated AlloDerm coverage had lower infection rates requiring oral (P = 0.0016) and intravenous antibiotics (P = 0.0012) compared with aseptic AlloDerm coverage. Compared with sterile “ready-to-use” AlloDerm coverage, those with contour fenestrated AlloDerm had similar infection outcomes but significantly more minor mastectomy flap necrosis (P = 0.0023). Compared with total submuscular coverage, those with contour fenestrated AlloDerm coverage had similar infection outcomes but significantly more explantations (P = 0.0001), major (P = 0.0130) and minor mastectomy flap necrosis (P = 0.0001). Significant independent risk factors for increased infection were also identified.
Conclusions:
Contour fenestrated AlloDerm reduces infections compared with aseptic AlloDerm, but infection rates are similar to those of sterile, ready-to-use AlloDerm and total submuscular coverage.
Implant-based breast reconstruction remains the most common reconstruction method.1 Acellular dermal matrices (ADMs) are used to overcome deficient muscular coverage, to improve contracture, and to enhance cosmetic results in a cost-effective manner.2–4 However, the complication profile of multiple ADMs continues to be investigated and defined in the literature.2,5–8 Although AlloDerm (LifeCell, Branchburg, N.J.) has been shown to offer improved outcomes in some studies,2,7 our institution identified increased complication risks from aseptic AlloDerm relative to total submuscular implant coverage.9
AlloDerm transitioned from aseptic to sterile “ready-to-use” ADM with component human cadaveric dermis being hydrated and terminally sterilized before distribution.8 Studies comparing aseptic and sterile “ready-to-use” AlloDerm have demonstrated mixed outcomes with one study finding decreased infection and need for explantation and others demonstrating either no difference in outcomes or increased cellulitis and seroma formation with sterile “ready-to-use” AlloDerm.8,10–12
In response to plastic surgeon feedback, contour fenestrated AlloDerm was developed specifically for use in breast reconstruction. Contour fenestrated AlloDerm uses the same processing methods as sterile “ready-to-use” AlloDerm, but the AlloDerm is crescent shaped with prefabricated fenestrations and various size dimensions.13 The “contour medium” size of 19.3 × 9.6 cm was used by the authors.13 The authors did not alter or create more fenestrations in the product once it was opened.
The authors have previously instituted strict indications for the use of ADM in breast reconstruction after an internal review revealed increasing complications with its use.9 Our institutional use of ADM has transitioned from aseptic to sterile “ready-to-use” and now contour fenestrated AlloDerm. As there have been no studies examining results with contour fenestrated AlloDerm to date, the authors aim to compare outcomes between aseptic, sterile “ready-to-use,” and contour fenestrated AlloDerm in breast reconstruction.
METHODS
All patients undergoing implant-based breast reconstruction at NYU Langone Medical Center from 2010 to 2014 were identified in this retrospective review of the authors’ practices. Patients undergoing reconstruction with tissue expander (TE) or permanent implant reconstruction were included for analysis. Patients undergoing delayed breast reconstruction or in whom SeriScaffold (Allergan, Irvine, Calif.) was used were excluded from analysis.
As discussed in our institution’s prior publication,1 strict institutional indications for the use of AlloDerm (LifeCell) were initiated in November 2010. Sterile “ready-to-use” AlloDerm has been in use since November 2011, whereas contour fenestrated AlloDerm was used from October 2014. Reconstructions were thus subdivided into 4 groups: submuscular, aseptic AlloDerm, sterile “ready-to-use” AlloDerm, and contour fenestrated AlloDerm implant coverage.
As in our previous study,8 all reconstructions were performed with either textured, shaped TEs or round, smooth implants. Implants were placed in a total submuscular position with elevation of the pectoralis and serratus anterior muscles when possible. AlloDerm was sewn in as an inferolateral sling when total submuscular placement was not possible secondary to inadequate pectoralis or serratus musculature, either due to congenital insufficiency or iatrogenic injury. This decision was made at the discretion of the plastic surgeon. In general, 2 drains were placed per breast in all patients. One drain was placed superiorly and one was placed inferiorly along the inframammary fold; all drains were placed above the level of the implant pocket in the subcutaneous plane. Drains were maintained until output was less than 30 mL in 24 hours. Patients remained on prophylactic antibiotics until all drains were removed as per the authors’ preferences.
Data regarding patient demographics, neoadjuvant therapy, type of reconstruction, nipple-sparing mastectomy (NSM) or skin-sparing mastectomy, type of implant coverage, intraoperative TE fill volume, and reconstructive complications, including infectious complications, seroma, hematoma, interventional radiology drainage, explantation, and mastectomy flap necrosis (MFN), were collected. Infections were stratified by minor infection requiring only oral (PO) antibiotics and major infection requiring hospital readmission and intravenous antibiotics. MFN was stratified by minor MFN requiring only local wound care and major MFN requiring debridement either in the office or in the operating room.
Measures of central tendency and descriptive statistics were used to describe absolute and mean outcomes. Chi-square analysis and Student’s t tests were used to compare categorical variables and to analyze means, respectively. Univariate logistic analysis was performed to assess independent risk factors for infection. P values less than 0.05 were deemed significant.
RESULTS
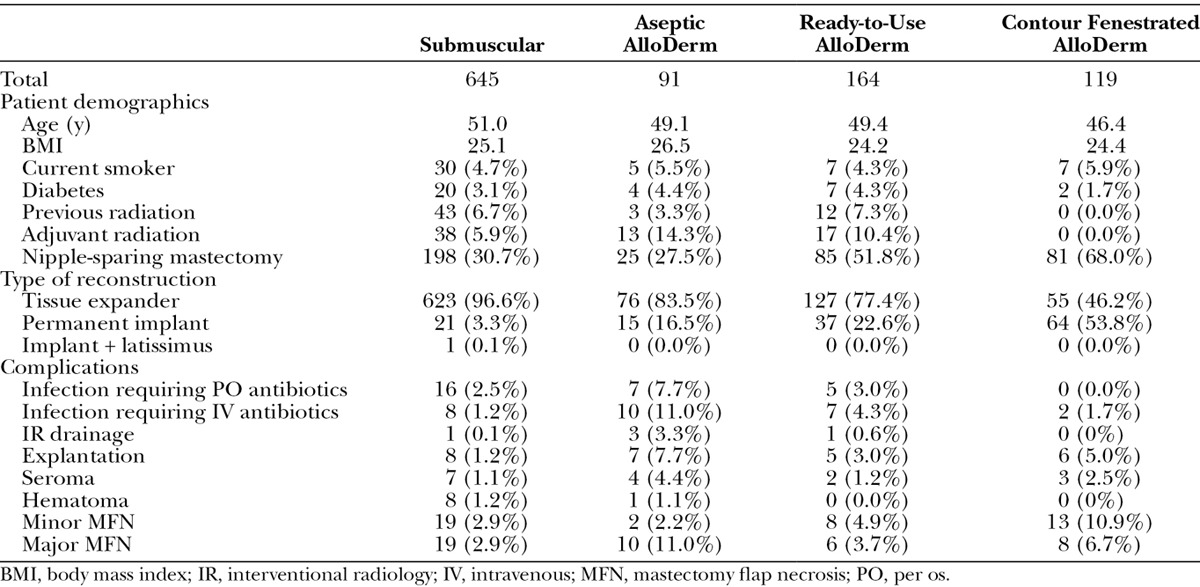
From November 2010 to October 2014, a total of 620 eligible patients (1019 reconstructions) underwent immediate, implant-based breast reconstruction. Overall patient demographics are presented in Table 1. About 86.5% of these reconstructions were with TEs while 13.4% were with permanent implants. The majority (63.3%) of reconstructions were performed with total submuscular implant coverage. Of the reconstructions requiring AlloDerm, 8.9%, 16.1%, and 11.7% were performed with aseptic, sterile “ready-to-use,” and contour fenestrated AlloDerm, respectively. Patient demographics and complications stratified by the type of implant coverage are presented in Table 2.
Table 1.
Overall Patient Demographics
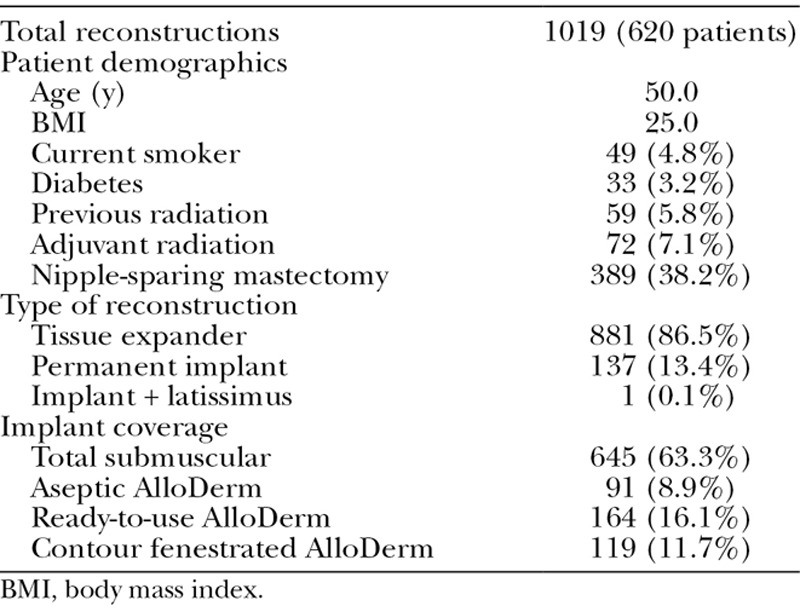
Table 2.
Demographics and Complications by Implant Coverage
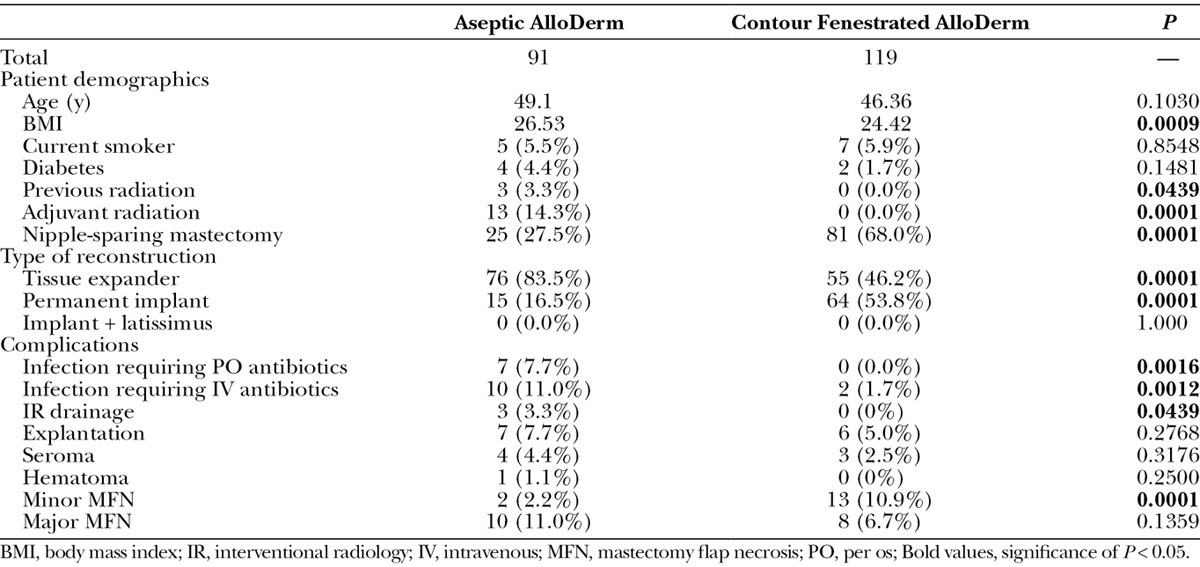
Patient characteristics and outcomes were compared between contour fenestrated and aseptic AlloDerm (Table 3). Patients who underwent contour fenestrated AlloDerm reconstruction were more likely to have lower body mass index (P = 0.0009), have less previous radiation (P=0.0439) and adjuvant radiation (P=0.0001), have an NSM (P=0.0001), and have permanent implant reconstructions (P=0.0001). Contour fenestrated AlloDerm was associated with lower rates of infection requiring oral (P = 0.0016) and intravenous antibiotics (P = 0.0012), interventional radiology drainage (P = 0.0439), and minor MFN (P = 0.0001) compared with those with aseptic AlloDerm coverage.
Table 3.
Comparison of Breasts with Aseptic AlloDerm and Contour Fenestrated AlloDerm Implant Coverage
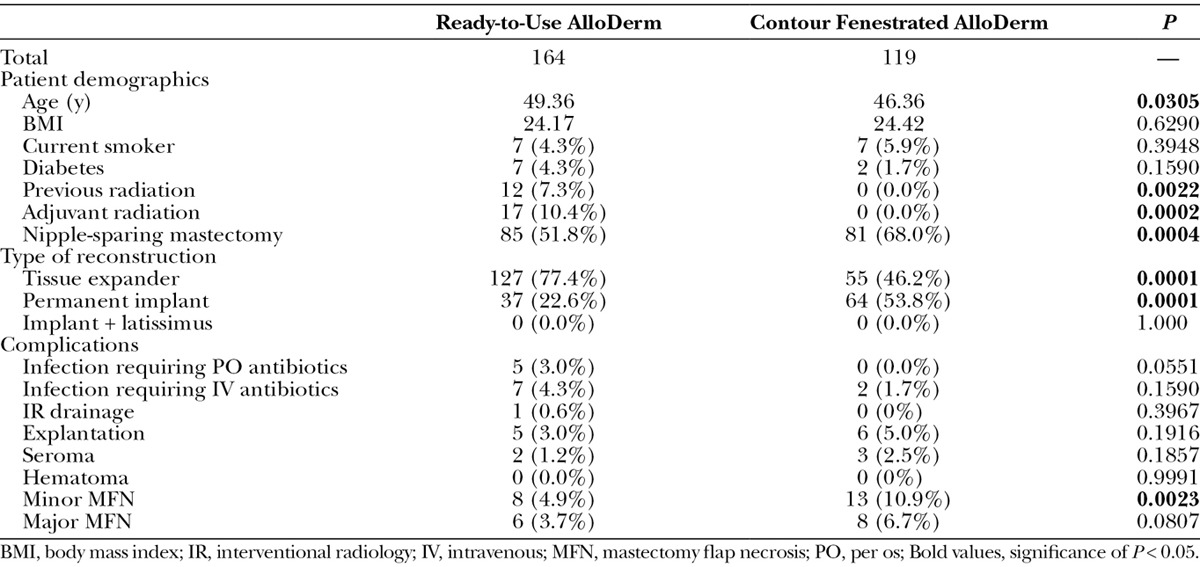
Patient characteristics and outcomes were compared between contour fenestrated and sterile “ready-to-use” AlloDerm (Table 4). Patients who underwent contour fenestrated AlloDerm reconstruction were more likely to be younger (P = 0.0305), have less previous radiation (P = 0.0022) and adjuvant radiation (P = 0.0002), have an NSM (P = 0.0004), and have permanent implant reconstructions (P = 0.0001). However, contour fenestrated AlloDerm was associated with greater minor MFN (P = 0.0023) compared with sterile “ready-to-use” AlloDerm.
Table 4.
Comparison of Breasts with Ready-to-Use AlloDerm and Contour Fenestrated AlloDerm Implant Coverage
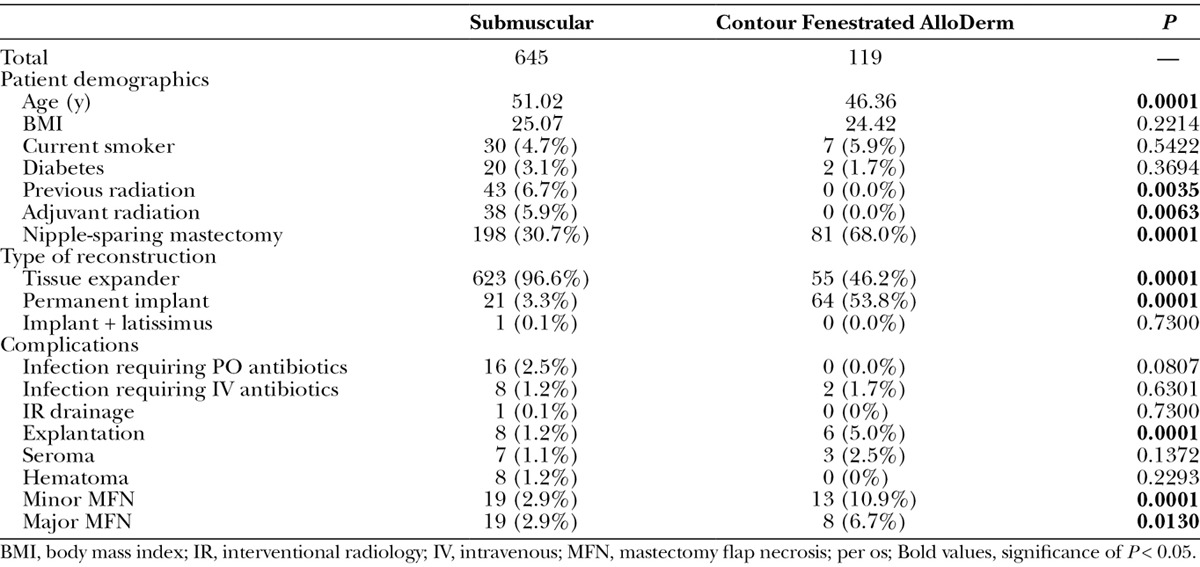
Finally, patient characteristics and outcomes were compared between contour fenestrated AlloDerm and total submuscular coverage (Table 5). Patients who underwent contour fenestrated AlloDerm reconstruction were more likely to be younger (P = 0.0001), have less previous radiation (P = 0.0035) and adjuvant radiation (P = 0.0063), have an NSM (P = 0.0001), and have permanent implant reconstructions (P = 0.0001). Contour fenestrated AlloDerm was associated with more explantations (P = 0.0001) as well as major (P = 0.0130) and minor MFN (P = 0.0001).
Table 5.
Comparison of Breasts with Total Submuscular and Contour Fenestrated AlloDerm Implant Coverage
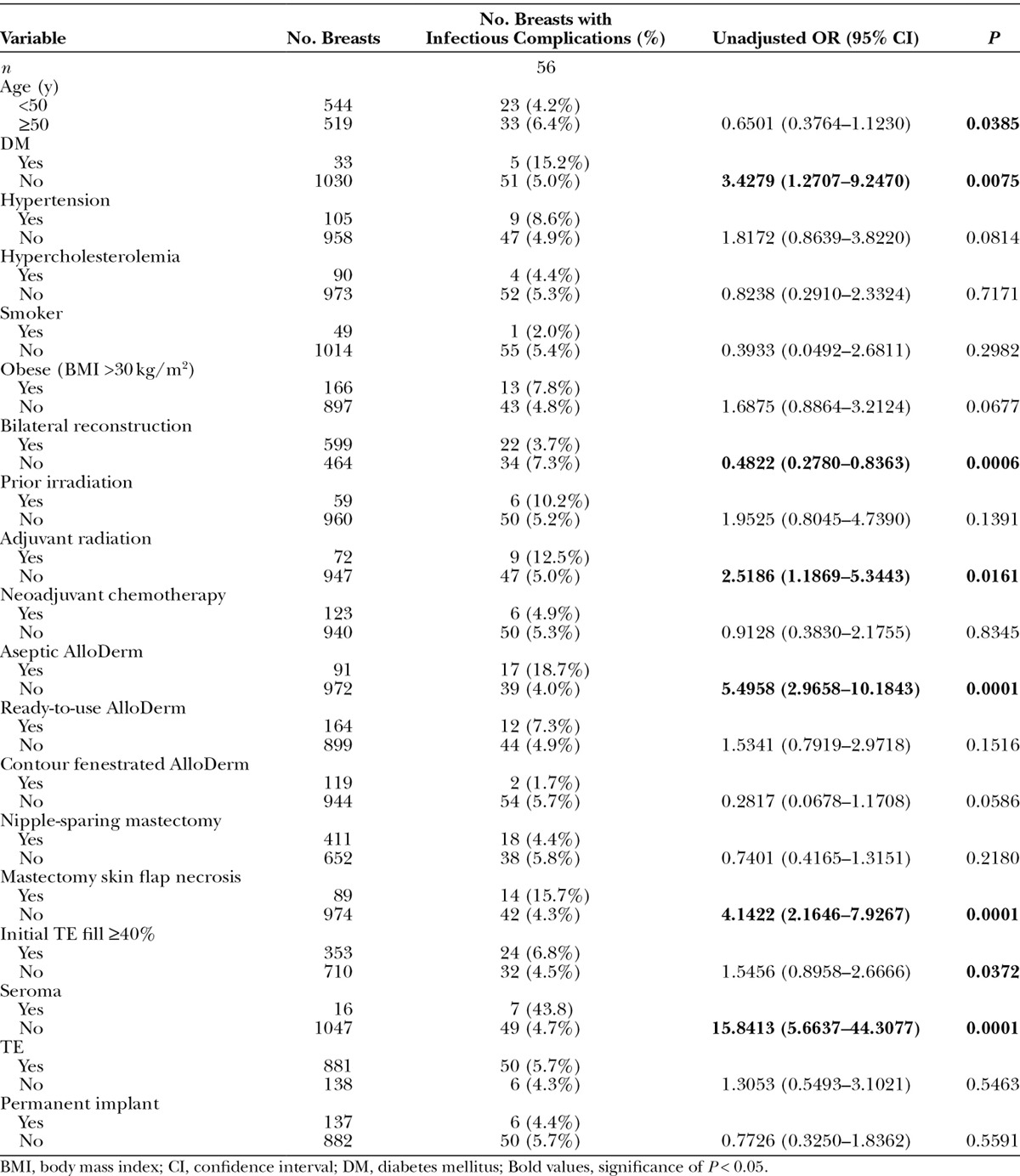
Independent risk factors for infection were identified using univariate analysis (Table 6). Age over 50 [odds ratio (OR) = not significant (NS); P = 0.0385], diabetes mellitus (OR = 3.4279; P = 0.0075), aseptic ADM (OR = 5.4958; P = 0.0001), mastectomy skin flap necrosis (OR = 4.1422; P = 0.0001), adjuvant radiation therapy (OR = 2.5186; P = 0.0161), initial TE fill ≥40% (OR = NS; P = 0.0372), and the presence of seroma (OR = 15.8413; P = 0.0001) were significant independent risk factors for increased infectious complications. Bilateral reconstruction was a significant independent predictor of decreased infectious complications (OR = 0.4822; P = 0.0006). Of note, the type of implant used, whether a TE or a permanent implant, was not a significant independent predictor of infectious complications (P = 0.5463 and P = 0.5591, respectively).
Table 6.
Independent Risk Factors for Infectious Complications
DISCUSSION
The use of ADM in breast reconstruction remains a contested subject. Increased complications with ADM have been published and purported by some, whereas others have demonstrated its benefits in reducing capsular contracture, improving cosmetic outcomes, and permitting stable implant coverage when pectoralis major and serratus anterior muscles are insufficient.2–8
AlloDerm is the predominant ADM used at our institution and many others.2–12 In response to increased complications from aseptic AlloDerm use,9 it has been refined into a sterile product8 and now includes the current contoured fenestrated form.13 Contour fenestrated AlloDerm is designed to allow the egress of fluid, to theoretically reduce periprosthetic fluid collection, and to provide greater ease of inset due to shape.13 To our knowledge, this is the first study to critically evaluate contour fenestrated AlloDerm against aseptic and sterile “ready-to-use” AlloDerm.
Although the majority of the implant-based reconstructions at our institution continue to be performed with total submuscular coverage, the proportion of patients who received contour fenestrated AlloDerm (11.7%) was comparable to the other AlloDerm subgroups. In general, patients who had reconstruction with contour fenestrated AlloDerm were more likely to have NSM and permanent implant reconstruction. This trend is due to the broadening indications for NSM and increased use of permanent implants that coincides with the introduction of contour fenestrated AlloDerm.
Consistent with our results comparing aseptic with sterile “ready-to-use” AlloDerm, contour fenestrated AlloDerm is associated with reduced infection.8 However, contour fenestrated AlloDerm, sterile “ready-to-use” AlloDerm, and total submuscular coverage have comparable infection and seroma rates. Given the power of our study, we did not identify fewer periprosthetic fluid collections from the prefabricated fenestrations. These results suggest that the sterilization process, rather than mechanical alterations in design, may be the most important aspect in decreasing infection with AlloDerm.
Contour fenestrated AlloDerm was associated with higher rates of minor MFN than sterile “ready-to-use” AlloDerm and higher rates of both major and minor MFN and implant explantation compared with total submuscular coverage. Once again, this may be related to the broader use of NSM, a higher risk procedure, and direct-to-implant reconstruction, which may stress the breast skin envelope.14–16 Minor MFN was treated with local wound care in all cases with relatively minor impact on final reconstructive result.15
Diabetes, seroma formation, MFN, and aseptic AlloDerm were again determined to be independent predictors of infection, confirming our previous findings.8 However, additional predictors emerged in this review includes age over 50, TE fill greater than 40%, and adjuvant radiation therapy. Bilateral reconstruction, meanwhile, emerged as a protector against infection. Although age and radiation are known predictors of poor wound healing17–19 and greater TE fill can increase tissue stress,20 the finding of unilateral reconstruction as a risk factor for infection requires further investigation. Possible explanations include an increased incidence of neoadjuvant radiation, chemotherapy, or permanent implant reconstruction in patients with unilateral reconstruction. Of note, contour fenestrated AlloDerm trended toward an independent predictor of decreased risk of infection (OR = NS; P = 0.0586) but failed to reach statistical significance. Although those undergoing reconstruction with contour fenestrated AlloDerm were significantly less likely to have previous radiation and adjuvant radiation, we do not expect that this alone accounts for this trend toward decreased risk of infection.
Limitations of this study include its retrospective nature, the inclusion of multiple surgeons in whom slight variations in technique may be present, the manner in which data were collected by chart review, and the significant trend of increased NSM and direct-to-implant reconstruction in the fenestrated AlloDerm group. Analyses of long-term outcomes using a prospectively maintained institutional database are forthcoming. Further, future directions of study will involve independent examinations of reconstructive outcomes and risk factors in NSM as well as immediate, permanent implant reconstruction.
CONCLUSIONS
We find that contour fenestrated AlloDerm, sterile “ready-to-use” AlloDerm, and total submuscular coverage have comparable infection profiles. Diabetes, seroma formation, MFN, and aseptic AlloDerm were again found to be independent predictors for infection, whereas age over 50, TE fill ≥40%, and unilateral reconstruction emerged as new predictors of infection.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Eltahir Y, Werners LL, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg. 2015;135:43–50. doi: 10.1097/PRS.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 2.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 3.Koltz PF, Frey JD, Langstein HN. The use of human acellular dermal matrix in the first stage of implant-based breast reconstruction simplifies the exchange procedure. Plast Reconstr Surg. 2013;132:691e–692e. doi: 10.1097/PRS.0b013e31829fe3d2. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan NM, Chatterjee A, Rosenkranz KM, et al. The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2014;67:468–476. doi: 10.1016/j.bjps.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim AM, Ayeni OA, Hughes KB, et al. Acellular dermal matrices in breast surgery: a comprehensive review. Ann Plast Surg. 2013;70:732–738. doi: 10.1097/SAP.0b013e31824b3d30. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg. 2012;130(5 Suppl 2):57S–66S. doi: 10.1097/PRS.0b013e31825f05b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendenhall SD, Anderson LA, Ying J, et al. The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast Reconstr Surg. 2015;135:29e–42e. doi: 10.1097/PRS.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 8.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 9.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 10.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg. 2013;70:497–499. doi: 10.1097/SAP.0b013e31827f52c8. [DOI] [PubMed] [Google Scholar]
- 11.Yuen JC, Yue CJ, Erickson SW, et al. Comparison between freeze-dried and ready-to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open. 2014;2:e119. doi: 10.1097/GOX.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth AK, Persing S, Connor CM, et al. A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg. 2013;70:632–635. doi: 10.1097/SAP.0b013e318250f0b4. [DOI] [PubMed] [Google Scholar]
- 13.LifeCell. Introducing AlloDerm RTM Ready To Use Contour. Branchburg, N.J.: LifeCell; 2015. [Google Scholar]
- 14.Spear SL, Shuck J, Hannan L, et al. Evaluating long-term outcomes following nipple-sparing mastectomy and reconstruction in the irradiated breast. Plast Reconstr Surg. 2014;133:605e–614e. doi: 10.1097/PRS.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 15.Dent BL, Small K, Swistel A, et al. Nipple-areolar complex ischemia after nipple-sparing mastectomy with immediate implant-based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J. 2014;34:560–570. doi: 10.1177/1090820X14528352. [DOI] [PubMed] [Google Scholar]
- 16.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506. doi: 10.1097/01.prs.0000438056.67375.75. [DOI] [PubMed] [Google Scholar]
- 17.Wiewiorski M, Barg A, Hoerterer H, et al. Risk factors for wound complications in patients after elective orthopedic foot and ankle surgery. Foot Ankle Int. 2015;36:479–487. doi: 10.1177/1071100714565792. [DOI] [PubMed] [Google Scholar]
- 18.Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23:1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:1–17. doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng YJ, Xu CQ, Yang J, et al. Biomechanical comparison between conventional and rapid expansion of skin. Br J Plast Surg. 2003;56:660–666. doi: 10.1016/s0007-1226(03)00217-0. [DOI] [PubMed] [Google Scholar]


