Abstract
Background:
Wound re-epithelialization has been traditionally described to occur from the dermal appendages of the wound edges. As such, the role of the dermal wound bed in re-epithelialization has been questioned. In a patient undergoing breast reconstruction with free tissue transfer, the buried portions of the free flap skin paddle could be either de-epithelialized or deskinned. In case of mastectomy skin flap loss, the role of de-epithelialized skin in wound healing has not been described before.
Methods:
We report a patient with bilateral mastectomies and bilateral deep inferior epigastric perforator flaps whose postoperative course was complicated by bilateral full-thickness mastectomy skin flap loss. Multiple debridements of nonviable skin resulted in exposure of previously buried de-epithelialized skin paddle of the deep inferior epigastric perforator flap.
Results:
Our study demonstrates self re-epithelialization of the dermal wound bed from the dermal appendages. We noticed multiple noncontiguous neoepidermal islands in the dermal wound bed, which did not communicate with the wound edges.
Conclusions:
In case of full-thickness mastectomy skin flap loss, deep vascular plexus present in the dermal bed of the underlying de-epithelialized skin paddle of the free flap converts an otherwise full-thickness wound to a partial-thickness wound. Our study demonstrates the self-epithelialization potential of the de-epithelialized dermal wound bed from the dermal appendages when exposed to air and in the presence of wound healing elements.
Re-epithelialization is an essential part of wound healing because it helps in reestablishing the barrier function of the skin.1,2 The traditional dogma regarding wound re- epithelialization involves skin appendages from the epidermis of wound edges.2,3 As such, the re-epithelialization ability of the dermis has been questioned. There have been few studies demonstrating successful re-epithelialization of wound with dermal grafts.4–6 However, the role of dermis in neoepithelialization could not be established in these studies.
We report a patient who underwent bilateral mastectomies and deep inferior epigastric perforator (DIEP) flap reconstruction. Her postoperative course was complicated by bilateral full-thickness mastectomy skin flap loss. Multiple debridements to viable skin resulted in exposure of previously buried de-epithelialized skin paddle of the DIEP flap. Our study demonstrates self re- epithelialization of the dermal wound bed from the dermal appendages.
INDEX PATIENT
Our patient, a 48-year-old woman, was initially diagnosed with left breast ductal carcinoma in-situ and underwent lumpectomy and radiation of her left breast. On follow-up imaging and biopsy, she was found to have invasive ductal carcinoma of the left breast for which she underwent neoadjuvant chemotherapy followed by left breast skin-sparing mastectomy and prophylactic right mastectomy. Immediate bilateral breast reconstruction was performed with DIEP flaps. All buried portions of the DIEP flap skin paddle were de-epithelialized and redundant mastectomy skin was excised. The patient had an uneventful recovery and was discharged on postoperative day 5. However, she returned to the clinic on postoperative day 8 with concerns of acute breast infection and skin in the left breast reconstruction area (Fig. 1). She reported purulent fluid drainage through a prior incision on the left chest wall and developed similar picture in the reconstructed right breast over the course of the next few days. She was hospitalized, started on intravenous vancomycin and clindamycin, and serial debridements of necrotic/infected mastectomy skin were begun. Although the skin paddles of the DIEP flaps were viable, the mastectomy skin flaps in the inferomedial and superolateral areas were compromised bilaterally. The mastectomy skin was debrided back until the healthy-appearing skin was evident at all the skin margins, requiring multiple operative procedures. This resulted in exposure of the de-epithelialized skin paddle of the DIEP flap (Fig. 2). Based on intraoperative culture data, the patient was switched to oral Bactrim and was discharged with a vacuum-assisted closure device. On 2-week follow-up, she was found to have multiple isolated islands of epithelialization on her exposed de-epithelialized dermal skin paddle, which were not confluent with the wound edges (Fig. 2). Wound care was switched to Aquacel Ag and her antibiotics were discontinued. During subsequent clinical visits, she continued to have more coalescing islands of epithelializations with about 50% of wound coverage in 5 weeks and almost complete epithelialization of the wound by 7 weeks (Fig. 3). Over the course of the next 2 months, the epithelialization of the exposed dermis was complete and the wound was almost indistinguishable from the surrounding area. Subsequently, she underwent a small revision of the right breast reconstruction scar and tattooing of her nipple–areolar complex. Ultimately, the neoepidermis matched the surrounding skin color well (Fig. 4).
Fig. 1.
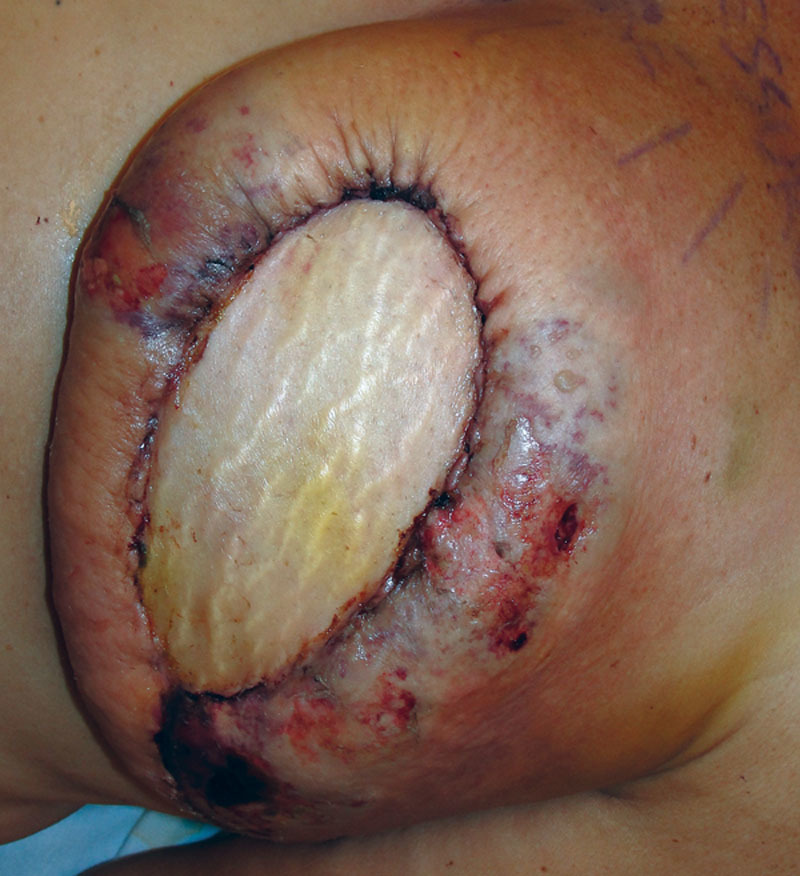
Concern for acute infection and mastectomy skin flap necrosis in the patient with bilateral mastectomies and immediate DIEP flap reconstruction.
Fig. 2.
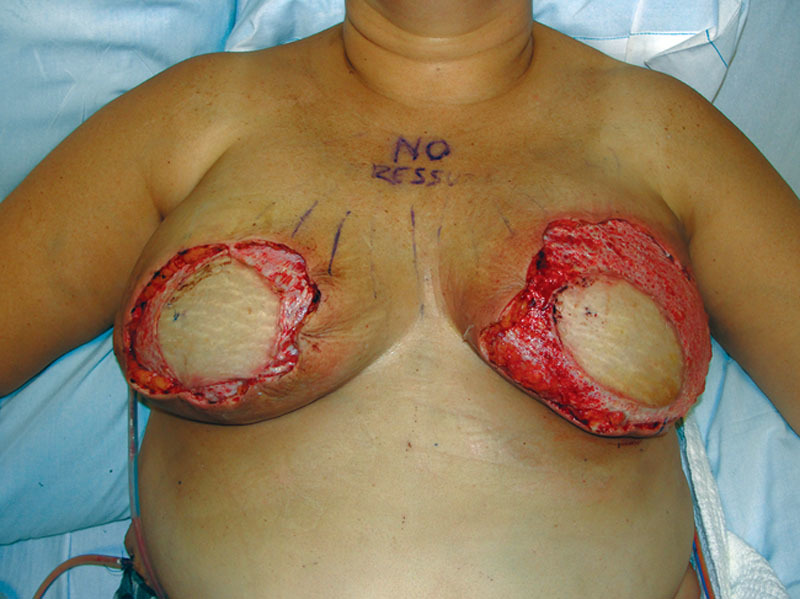
Debrided bilateral wounds with exposed dermal wound bed of the DIEP flaps.
Fig. 3.
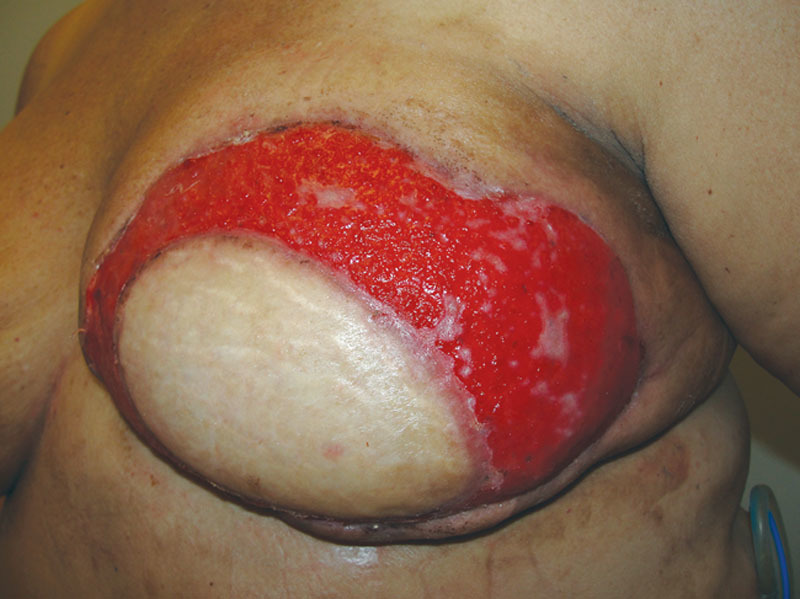
Multiple isolated skin islands that are noncommunicating with the wound edges.
Fig. 4.
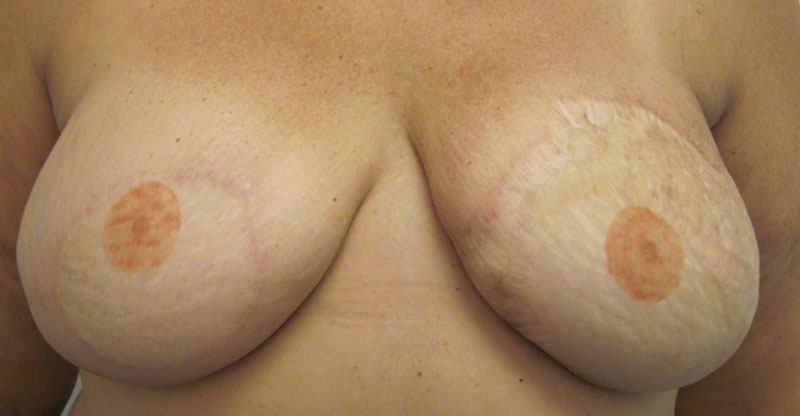
Re-epithelialized wound with matched skin color compared with the surrounding tissue.
DISCUSSION
Mastectomy skin flap necrosis is a common complication following mastectomy, occurring in almost 20% cases.7 In a patient undergoing breast reconstruction with free tissue transfer, the buried portions of the free flap skin paddle could be either de-epithelialized or deskinned.8,9 In the case of full-thickness mastectomy skin flap loss, the deep vascular plexus present in the dermal bed of the underlying de-epithelialized skin paddle of the free flap converts an otherwise full-thickness wound to a partial-thickness wound. This potentially promotes minimal wound contraction, which helps to maintain the desired breast. Also, the vascular dermal bed provides for a healthy platform for skin grafting, if needed. However, de-epithelializing a large skin paddle can be time consuming during the initial operation. In addition, the thicker de-epithelialized skin paddle may be palpable later compared with the softer, nonpalpable flap edges of a deskinned skin paddle.
In wounds healing with secondary intention, the epithelialization is believed to start from the skin appendages from the wound edges and gradually covers the entire wound as an advancing sheet.1–3 However, in our patient, there were distinct nonconfluent epidermal islands on the wound bed, which were discontinuous with the wound edges. Over next few weeks, these islands merged among themselves and with the wound edges to complete the neoepithelialization of the wound bed. This pattern of wound healing is in sharp contrast with the traditional concept of epithelialization from the wound edges. Histological analyses of the dermis have revealed skin appendages such as hair follicles, sweat glands, and sebaceous glands with their epithelial linings.10 This might be the source of epithelial cells that differentiate and migrate to the dermal wound bed for neoepithelialization.
In a canine model, Reed et al6 demonstrated that the epithelialization of dermal graft was from the cut surfaces of the skin appendages and not from the periphery of the wound. Tanabe et al4 also suggested this phenomenon in humans by demonstrating successful reconstruction of volar hand defect with a dermal graft from the plantar surface. However, it could not be definitively established in these studies whether the epithelialization was from the dermal graft or the wound edges. This patient uniquely demonstrates noncontiguous multiple skin islands on a dermal wound bed, which were discontinuous with the wound edges.
CONCLUSIONS
Buried de-epithelialized skin is commonplace in breast reduction surgery and autologous breast reconstruction. This buried skin does not re-epithelialize. This may be due to suppression of the dermal appendages when covered with an overlying tissue. It is possible that without contact inhibition these wound healing elements can be reactivated through an undiscovered mechanism to promote self re- epithelialization. Further study is ongoing to understand this clinical phenomenon.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Escámez MJ, García M, Larcher F, et al. An in vivo model of wound healing in genetically modified skin-humanized mice. J Invest Dermatol. 2004;123:1182–1191. doi: 10.1111/j.0022-202X.2004.23473.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe HY, Aoyagi A, Tai Y, et al. Reconstruction for palmar skin defects of the digits and hand using plantar dermal grafting. Plast Reconstr Surg. 1998;101:992–998. doi: 10.1097/00006534-199804040-00016. [DOI] [PubMed] [Google Scholar]
- 5.Yeow VK. Cleft lip scar camouflage using dermal micrografts. Plast Reconstr Surg. 1999;103:1250–1253. doi: 10.1097/00006534-199904040-00023. [DOI] [PubMed] [Google Scholar]
- 6.Reed GF, Zafra E, Ghyselen AL, et al. Self-epithelization of dermal grafts. Arch Otolaryngol. 1968;87:518–521. doi: 10.1001/archotol.1968.00760060520015. [DOI] [PubMed] [Google Scholar]
- 7.Kroll SS. The early management of flap necrosis in breast reconstruction. Plast Reconstr Surg. 1991;87:893–901. doi: 10.1097/00006534-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Iwuagwu OC, Drew PJ. Deskinning versus deepithelialization for inferior pedicle reduction mammoplasty: a prospective comparative analysis. Aesthetic Plast Surg. 2005;29:202–204. doi: 10.1007/s00266-004-0120-7. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo DA. Improving safety and aesthetic results in inverted T scar breast reduction. Plast Reconstr Surg. 1999;103:874–886; discussion 887. [PubMed] [Google Scholar]
- 10.Rubis BA, Danikas D, Neumeister M, et al. The use of split-thickness dermal grafts to resurface full thickness skin defects. Burns. 2002;28:752–759. doi: 10.1016/s0305-4179(02)00180-8. [DOI] [PubMed] [Google Scholar]


