Supplemental Digital Content is available in the text.
Keywords: estradiol, estrogen receptor alpha, mice, myocytes, smooth muscle
Rationale:
17β-Estradiol (E2) exerts numerous beneficial effects in vascular disease. It regulates gene transcription through nuclear estrogen receptor α (ERα) via 2 activation functions, AF1 and AF2, and can also activate membrane ERα. The role of E2 on the endothelium relies on membrane ERα activation, but the molecular mechanisms of its action on vascular smooth muscle cells (VSMCs) are not fully understood.
Objective:
The aim of this study was to determine which cellular target and which ERα subfunction are involved in the preventive action of E2 on neointimal hyperplasia.
Methods and Results:
To trigger neointimal hyperplasia of VSMC, we used a mouse model of femoral arterial injury. Cre-Lox models were used to distinguish between the endothelial- and the VSMC-specific actions of E2. The molecular mechanisms underlying the role of E2 were further characterized using both selective ERα agonists and transgenic mice in which the ERαAF1 function had been specifically invalidated. We found that (1) the selective inactivation of ERα in VSMC abrogates the neointimal hyperplasia protection induced by E2, whereas inactivation of endothelial and hematopoietic ERα has no effect; (2) the selective activation of membrane ERα does not prevent neointimal hyperplasia; and (3) ERαAF1 is necessary and sufficient to inhibit postinjury VSMC proliferation.
Conclusions:
Altogether, ERαAF1-mediated nuclear action is both necessary and sufficient to inhibit postinjury arterial VSMC proliferation, whereas membrane ERα largely regulates the endothelial functions of E2. This highlights the exquisite cell/tissue-specific actions of the ERα subfunctions and helps to delineate the spectrum of action of selective ER modulators.
Neointimal hyperplasia essentially arises when cells positive for smooth muscle markers cross the internal elastic lamina then migrate and proliferate.1,2 In human pathology, this process frequently occurs after the treatment of symptomatic atherosclerosis, which involves mechanical endovascular ballooning (angioplasty) followed by stenting. Neointimal hyperplasia leads to a narrowing of the arterial lumen and is thus termed restenosis.3
On the basis of both experimental and clinical data, we found that estrogens have been proposed to exert several protective arterial effects. In particular, 17β-estradiol (E2), the main endogenous estrogen, has a dual beneficial effect on the 2 facets of vascular healing after angioplasty because it both accelerates endothelial regrowth and inhibits the proliferation of vascular smooth muscle cells (VSMC), which otherwise leads to the narrowing of the arterial lumen (restenosis).4 Consistent with these functions, E2 has been shown to prevent neointimal hyperplasia in response to endovascular injury in various animal models and species, including rats, pigs, and sheep,5–7 but this action has not been reported to date in a mouse model and our understanding of the underlying mechanisms are limited.
Estrogens mediate most of their actions through the binding and activation of the intracellular estrogen receptors (ER) α and β. Their roles have been explored in vivo using transgenic mouse models. We8,9 and others10 have demonstrated that ERα, but not ERβ, is required for estrogen-dependent endothelial protection from vascular injury. Indeed, using a model of carotid artery electric injury, we demonstrated that ERα, and not ERβ, mediates the stimulatory effect of E2 on re-endothelialization through the action of both endothelial and hematopoietic ERα.8,11 Both ERα and ERβ act as transcription factors in the nucleus, where they modulate transcription by directly binding to estrogen response element sequences in the DNA. They can also modulate the activity of heterologous transcription factors through protein–protein interactions.12 Two activation functions, ERαAF1 and ERαAF2, have been shown to play crucial roles in the transcriptional effects of ERα9 through the recruitment of coactivators.13,14 Using mice selectively deficient for ERαAF1 or ERαAF2, we previously demonstrated that both of these functions are necessary for E2-mediated endometrial proliferation15 but are dispensable for the acceleration of reendothelialization by E2.16,17 In addition to the well-established role of the nuclear pool of ERα in its transcriptional (also named genomic) actions, a fraction of ERα is also present at or near the plasma membrane, where it has been found to elicit rapid nongenomic membrane-initiated steroid signaling (MISS) effects.18,19 Using a unique mouse model containing a disabled palmitoylation site within ERα that is essential for MISS, we recently demonstrated that MISS is essential for the endothelial effects of E2, including its acceleration of endothelial healing.15,20 In striking contrast, the responses of the uterus to E2, in particular epithelial proliferation and gene expression changes, depend on the action of nuclear ERα, whereas membrane ERα seems to play little if any role.15,20
The molecular and cellular mechanisms of the action of E2 on endothelial healing have been extensively described,8,11,20,21 in particular through the use of the electric injury of the carotid artery model. In contrast, much less information is available on the effects of E2 on VSMC. The effect of E2 on neointimal hyperplasia has been reported mostly in large- or medium-sized animal models. We recently developed a model of endovascular mechanical injury of the femoral artery which, in contrast to the carotid artery site model, induces strong neointimal hyperplasia in mice.2 The aim of this study was to evaluate (1) the effect of E2 in a mouse model of neointimal thickening, (2) the role of ERα and its subfunctions (nuclear versus membrane), and the cellular targets involved in the action of E2 on neointimal hyperplasia.
Methods
An expanded Methods section is available in the Online Data Supplement.
Mice
Wild-type female mice with a C57Bl/6J background were purchased from Charles River Laboratories (France). Tie2Cre−/− ERαlox/lox and Tie2Cre+/− ERαlox/lox mice were generated as described previously,22 and are further referred to as Tie2Cre− ERαlox/lox and Tie2Cre+ ERαlox/lox. ERαAF1+/+ and ERαAF10/0 mice were generated as described previously.16 To generate mice carrying a specific deletion of the ERα-encoding gene expressed under the control of the smooth muscle actin (SMA) promoter, ERαlox/lox mice were crossed with SMACreERT2 transgenic mice,23 further referred to αSMACreERT2+ ERαlox/lox (Figure 1B). αSMACreERT2- ERαlox/lox (control mice) and αSMACreERT2+ ERαlox/lox mice were injected daily with tamoxifen (1 mg/mouse per day; Sigma, France) during 5 days from 3 weeks of age to induce activation of the Cre recombinase (Figure 1A). Throughout all protocols, mice were housed at the animal facility of Rangueil (US06, Toulouse, France) and kept under SPF conditions. Mice were housed in a temperature-controlled room with a 12:12-hour light-dark cycle and maintained with access to food and water ad libidum. All animal procedures were conducted in accordance with institutional guidelines on animal experimentation and were under a French Ministry of Agriculture license.
Figure 1.
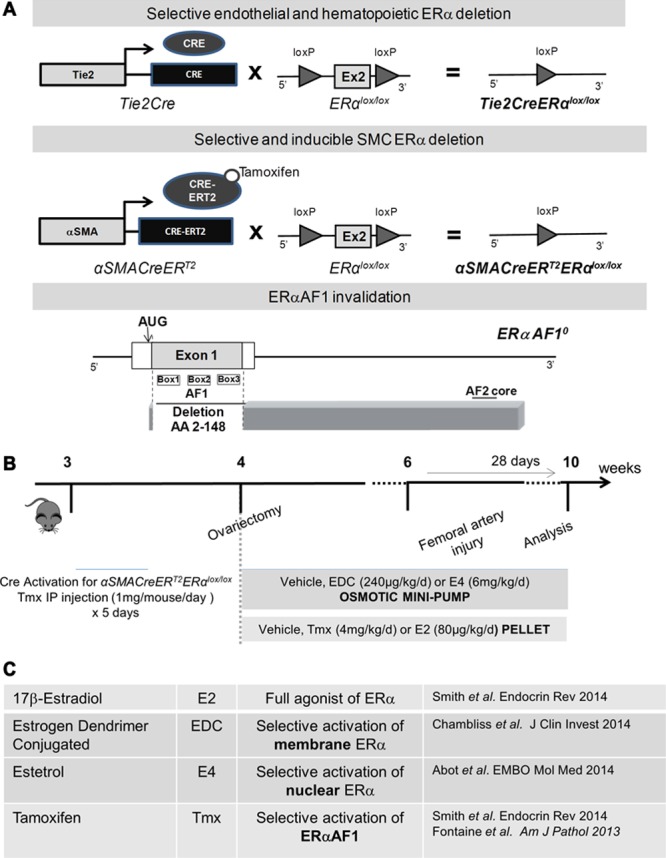
Estrogen ligands, mouse models, and intimal hyperplasia protocol used in the study. A, Schematic representation of the Tie2Cre+ ERαlox/lox, αSMACreERT2+ ERαlox/lox and ERαAF10/0 mouse model and (B) of the protocol used to evaluate neointimal hyperplasia. C, Mechanism of estrogen receptor α (ERα) activation by 17β-estradiol (E2), estetrol (E4), tamoxifen (Tmx) and estrogen dendrimer conjugate (EDC). CRE indicates Cre recombinase.
Ovariectomy and Treatments
Bilateral ovariectomy was performed at 4 weeks of age after anesthesia with a mixture of xylazine and ketamine, and mice concomitantly received estrogens or selective ER modulator treatments (Figure 1B and 1C; Online Figure I). Mice were submitted to a femoral artery wire injury 2 weeks after the start of the treatment (Figure 1C; Online Figure I).
Femoral Artery Wire Injury in Mice
The femoral artery wire injury was performed as previously described.2 For subsequent neointimal hyperplasia analysis, mice were euthanized 28 days later.
Femoral Artery Processing and Morphometry
To assess neointimal hyperplasia in the injured arteries, morphometry was performed on sections from paraffin-embedded arteries. Intimal hyperplasia was expressed as a ratio of ANI/Amed ANI:neointimal area; Amed:medial area).
Immunohistochemistry
SMC and T cells were, respectively, immunostained with anti-αSMA and anti-CD3 antibodies, followed by a standard ABC-peroxidase/DAB protocol. Endothelial cells were immunostained with an anti-CD31 antibody, followed by a standard immunofluorescence protocol.
Analysis of mRNA Levels by Real-Time Quantitative Polymerase Chain Reaction
After homogenization, total RNA was extracted from tissues with a classical phenol/chloroform extraction protocol. The derived cDNA was then submitted to real-time quantitative polymerase chain reaction analysis.
Statistical Analysis
To test the effect of treatments, groups were compared for statistical significance using Mann–Whitney U test. To test the respective roles of treatment and genotype (ERα deficiency), a 2-way ANOVA was performed. When an interaction was observed between the 2 factors, the effect or the treatment in each genotype was studied using a Bonferroni post-test. A value of P<0.05 was considered as statistically significant.
Results
Chronic E2 Treatment Decreases Neointimal Hyperplasia After Mechanical Injury of the Femoral Artery
To assess the effect of E2 on the development of arterial neointimal hyperplasia, ovariectomized wild-type female mice were chronically treated with either vehicle or E2 (Figure 1B and 1C). In response to mechanical injury of the femoral artery, neointimal hyperplasia, expressed as the neointima/media ratio, was reduced by 62% after E2 treatment (Figure 2A). This reduction was solely because of the prevention of neointimal proliferation because E2 treatment did not elicit any medial remodeling (Figure 2A). Histological analysis of the injured arteries (Figure 2B) showed a large neointima in control mice, whereas it was reduced to few cellular layers in E2-treated animals (Figure 2B, top). The neointima from both control and E2-treated mice was mostly composed of SMA-positive cells (Figure 2B, middle). The periadventitial CD3+-T-cell content remained unchanged between E2-treated mice and controls (Figure 2B, bottom).
Figure 2.
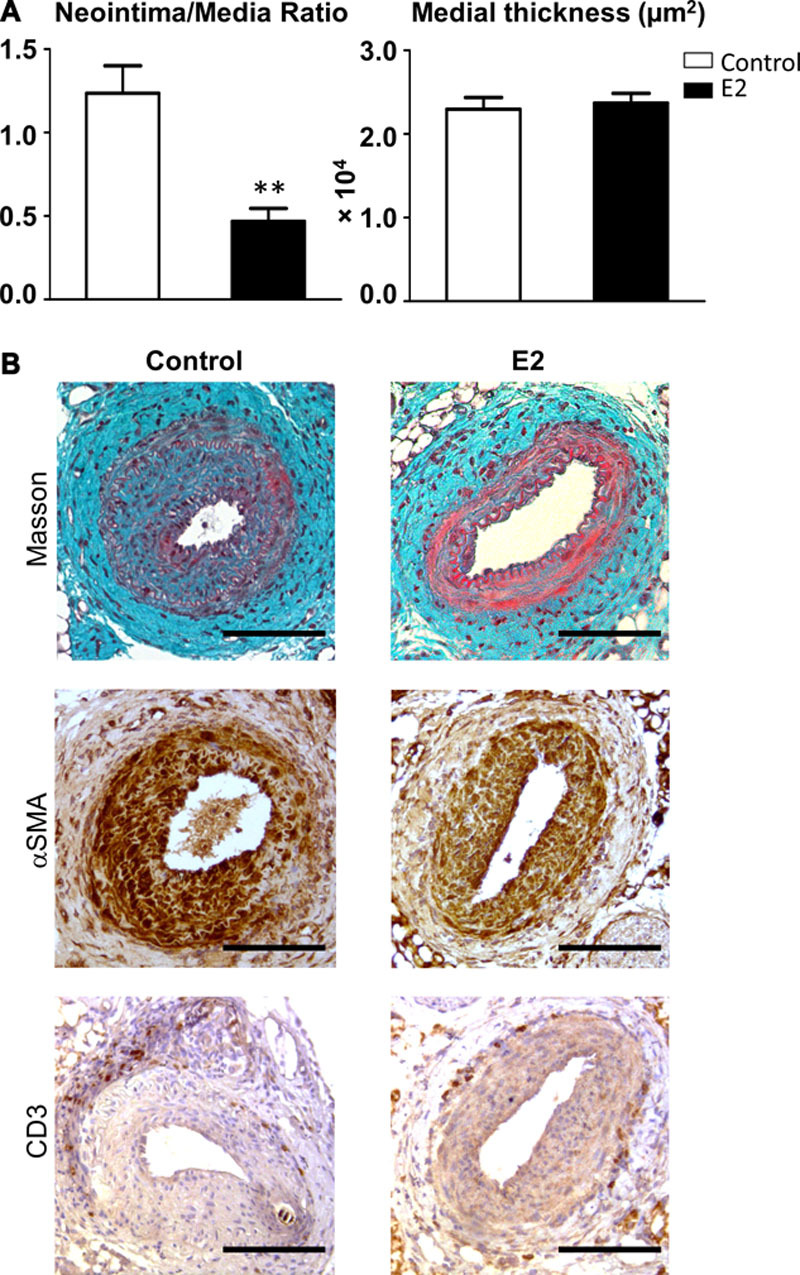
Chronic estradiol treatment decreases intimal hyperplasia after mechanical injury of the femoral artery without affecting medial thickness. Four-week-old wild-type female mice were ovariectomized and subcutaneously treated with placebo (control) or 17β-estradiol (E2) until the end of the protocol. At 6 weeks, mice were submitted to mechanical injury of the femoral artery. Twenty-eight days later, mice were euthanized, and arteries were harvested for morphometric and immunohistological analysis. A, Quantitative analysis of neointima/media ratio (left) and medial thickness (right) of control (white bars) and E2 treated (black bars) ovariectomized mice. Values are presented as mean±SEM (n=7–15), and statistically compared with Mann–Whitney U test. **P<0.01. B, Representative images of injured femoral arteries cross sections of control (left) and 17β-estradiol (E2, right) treated ovariectomized mice stained with Masson Trichrome (top), immunostained with anti–α smooth muscle actin (SMA) antibody to detect smooth muscle cells (middle) or with anti-CD3 antibody to detect T lymphocytes (bottom). Bars, 100 µm.
Simultaneous Endothelial and Hematopoietic ERα Deletion Does Not Affect the Action of E2 on Neointimal Hyperplasia but It Impairs E2-Induced Endothelial Healing
Given the importance of endothelial ERα in numerous vascular protective effects of E2, we investigated the potential effects of a deletion of ERα in this cell type on neointimal hyperplasia development. For this purpose, we used mice expressing the Cre recombinase under the control of the Tie2 promoter that carried a floxed ERα-encoding sequence (Figure 1A).22 In addition to the endothelial deletion of ERα, these mice also presented with an 80% decrease in ERα expression in the bone marrow.22 As expected, placebo-treated control Tie2Cre- ERαlox/lox mice displayed a large neointima, which was reduced by E2 treatment (Figure 3A). This beneficial effect of E2 was similar for Tie2Cre+ ERαlox/lox mice, as indicated by the 2-way ANOVA results, which show the absence of any interaction (P=0.86) and a highly significant effect of E2 treatment (P=0.0006).
Figure 3.
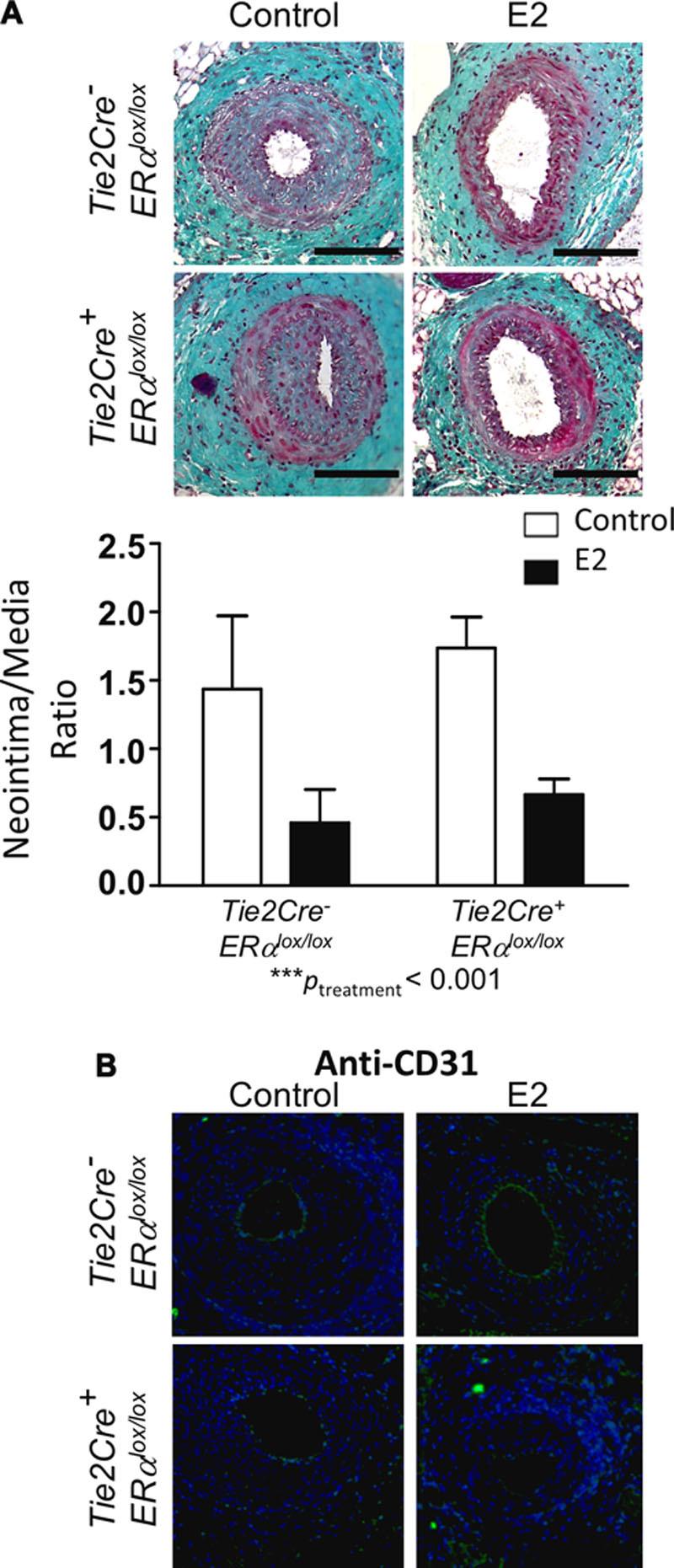
Loss of endothelial estrogen receptor α (ERα) expression does not affect the action of E2 action on intimal hyperplasia. Four-week-old Tie2Cre- ERαlox/lox and Tie2Cre+ ERαlox/lox female mice were ovariectomized and subcutaneously treated with placebo (control) or 17β-estradiol (E2) until the end of the protocol. At 6 weeks, mice were submitted to mechanical injury of the femoral artery. Twenty-eight days later, mice were euthanized, and arteries were harvested for morphometry (A) and immunostaining (B). A, Top, Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Bottom, Quantitative analysis of neointima/media ratio of indicated mice. Values are presented as mean±SEM (n=8 mice per group). A statistical 2-way ANOVA test revealed no significant interaction. The overall effect of the treatment was ***Ptreatment<0.001. B, Representative images of injured femoral arteries cross sections of indicated mice, stained with anti-CD31 antibody and counterstained with DAPI (blue). Bars, 100 µm.
Mechanical injury of the femoral artery leads to a loss in the endothelial integrity of the injured portion. This result was confirmed by anti-CD31 immunostaining of endothelial cells in the injured femoral arteries of placebo-treated Tie2Cre- ERαlox/lox and Tie2Cre+ ERαlox/lox mice (Figure 3B). E2 treatment accelerated endothelial coverage in Tie2Cre- ERαlox/lox, but not in Tie2Cre+ ERαlox/lox animals, confirming the importance of E2 in endothelial healing through a direct effect on the endothelium.
E2 Decreases Neointimal Hyperplasia by Directly Targeting Arterial SMC
To determine whether VSMC ERα is involved in the decrease in neointimal hyperplasia in response to E2, we established a new mouse model containing a selective deletion of ERα in SMC. For this purpose, we crossed previously described mice expressing the Cre-ERT2 fusion gene under the control of the αSMA promoter23 with ERαlox/lox mice (Figure 1A). We verified the specific deletion of ERα in the SMC compartment from αSMACreERT2+ ERαlox/lox mice in which the nuclear action of Cre recombinase had been induced by tamoxifen injection (Figure 4A). ERα mRNA expression was almost totally abrogated from the mediae dissected from the aortae of these mice, whereas its expression was preserved in the skeletal muscle tissue and in the cardiac muscle tissue (Figure 4A).
Figure 4.
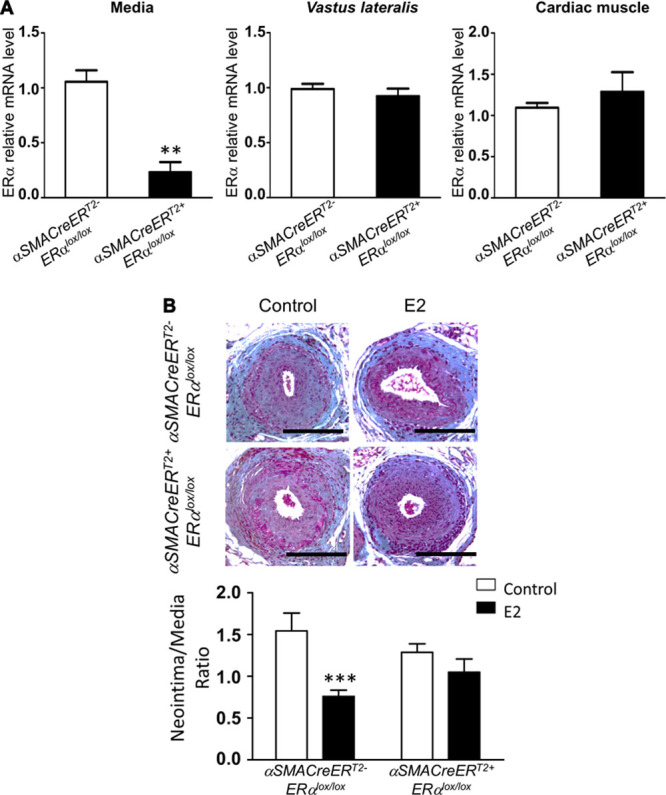
17β-Estradiol (E2) decreases intimal hyperplasia through its specific action on smooth muscle cells. A, mRNA levels from media, vastus lateralis muscle and heart from ovariectomized tamoxifen-injected αSMACreERT2+ ERαlox/lox vs αSMACreERT2- ERαlox/lox were measured by quantitative polymerase chain reaction and normalized to TPT1 expression. Results are expressed as mean±SEM. B, Four-week-old tamoxifen-injected αSMACreERT2+ ERαlox/lox and αSMACre-ERT2- ERαlox/lox female mice were ovariectomized and subcutaneously treated with placebo (control) or E2 until the end of the protocol. At 6 weeks, mice were submitted to mechanical injury of the femoral artery. Twenty-eight days later, mice were euthanized, and arteries were harvested for morphometric analysis. Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Quantitative analysis of neointima/media ratio of indicated mice. Values are mean±SEM (n=10–16 mice per group). As a statistical 2-way ANOVA revealed a significant interaction (P=0.04), it was followed by a Bonferroni post-test (***P<0.001).
We then addressed the effects of specific ERα deletion in SMC on injury-induced neointimal hyperplasia (Figure 1B). In both genotypes, placebo-treated mice displayed a large neointimal hyperplasia (Figure 4B). E2 treatment decreased neointimal hyperplasia in αSMACreERT2- ERαlox/lox control mice but failed to have such an effect in αSMACreERT2+ ERαlox/lox mice (Figure 4B).
Activation of Nuclear and Not Membrane ERα Is Sufficient for the Suppression of Neointimal Hyperplasia
Once we had identified SMC as the main target cell for the action of E2 in modulating neointimal hyperplasia, we sought to dissect the molecular mechanisms involved. Thus, we adopted a pharmacological approach using an estrogen dendrimer conjugate (EDC) that selectively activates MISS,21 and estetrol that selectively activates nuclear ERα (Figure 1C; Online Figure I).24 Whereas chronic EDC treatment failed to decrease neointimal hyperplasia (Figure 5A), estetrol prevented neointimal hyperplasia formation to the same extent as E2 (Figure 5B). These results strongly suggest that the nuclear effects of ERα, but not membrane ERα, are sufficient to decrease neointimal hyperplasia.
Figure 5.
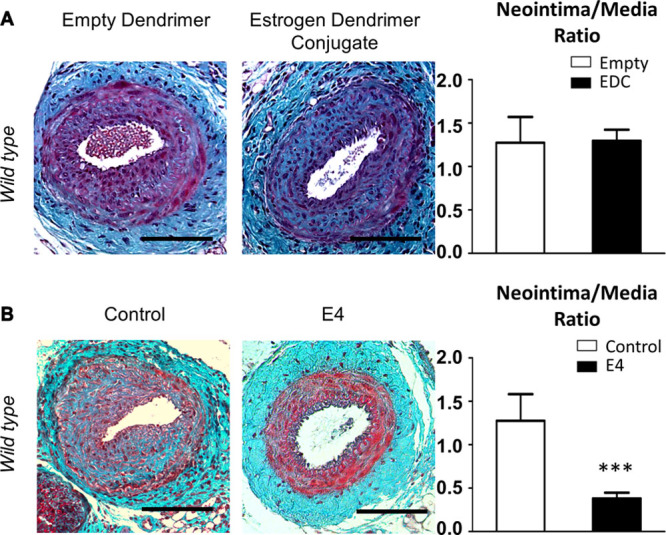
Genomic functions of estrogen receptor α (ERα) mediate protection from intimal hyperplasia. A, Four-week-old wild-type female mice were ovariectomized and subcutaneously implanted with estrogen dendrimer conjugate or empty dendrimer-eluting osmotic minipumps. Two weeks later, animals were submitted to mechanical injury of the femoral artery. Arteries were harvested 28 days after the injury for morphometric analysis. Left, Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Right, Quantitative analysis of neointima/media ratio of indicated mice. Values are presented as mean±SEM (n=10–12 mice per group), and statistically compared with Mann–Whitney U test. B, Four-week-old wild-type female mice were ovariectomized and subcutaneously implanted with control of estetrol (E4)-eluting osmotic minipumps. Two weeks later, animals were submitted to mechanical injury of the femoral artery. Arteries were harvested 28 days after the injury for morphometric analysis. Left, Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Right, Quantitative analysis of neointima/media ratio of indicated mice. Values are presented as mean±SEM (n=7–12 mice per group), and statistically compared with Mann–Whitney U test. ***P<0.001.
AF1 Is Both Necessary and Sufficient for the Reduction of Neointimal Hyperplasia
ERαAF1 has previously been found necessary for the proliferative effects of E2 on the endometrium, but it does not play a role in the accelerative effect of E2 on re-endothelialization.16 Therefore, we sought to evaluate the role of this key transcriptional function of ERα on the prevention of neointimal hyperplasia. Ovariectomized ERαAF1+/+ and ERαAF10/0 mice (Figure 1A), treated with E2 or vehicle control, were submitted to mechanical injury of the femoral artery. As expected, E2 decreased neointimal hyperplasia in control ERαAF1+/+ mice (Figure 6A). In striking contrast, we found that the antiproliferative effect of E2 on neointimal hyperplasia was not observed in mice genetically deficient in ERαAF1 function (Figure 6A), demonstrating that ERαAF1 is necessary for this effect. Second, we treated ovariectomized C57Bl/6J mice with tamoxifen (Figure 1C; Online Figure I), a selective ER modulator characterized as a selective agonist of AF1 function but an antagonist of AF2 function.13,25 The pronounced antiproliferative effect of tamoxifen highlighted that ERαAF1 activation is sufficient for preventing the development of neointimal hyperplasia (Figure 6B). At the same time, we verified that tamoxifen also elicited the growth of the uterus (Figure 6C), confirming the proliferative action of ERαAF1 activation that has previously been demonstrated in this tissue.15
Figure 6.
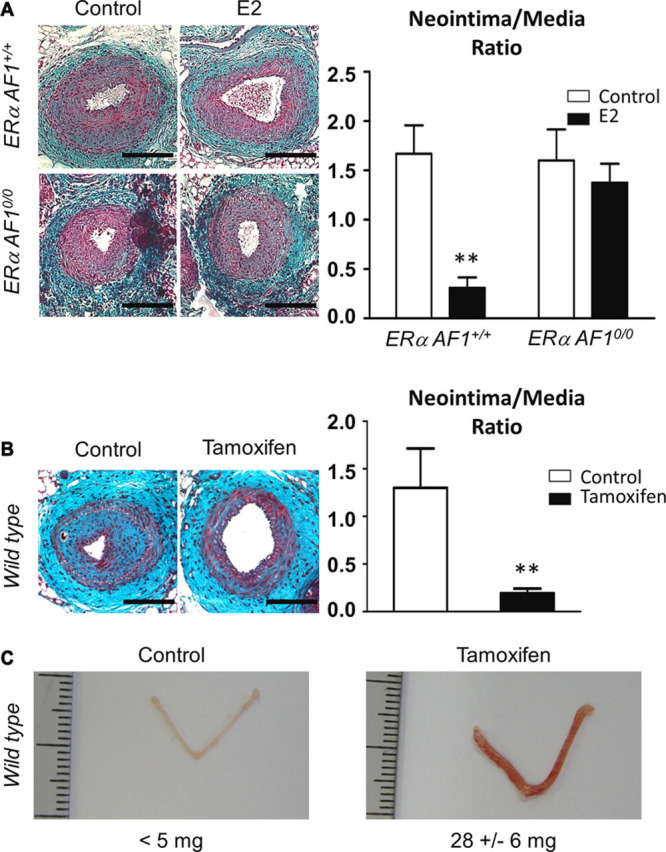
Activation function (AF) 1 of estrogen receptor α (ERα) is necessary for the prevention of intimal hyperplasia by 17β-estradiol (E2). A, Four-week-old ERαAF1+/+ and ERαAF10/0 female mice were ovariectomized and subcutaneously treated with placebo (control) or E2 until the end of the protocol. At 6 weeks, mice were submitted to mechanical injury of the femoral artery. Twenty-eight days later, mice were euthanized, arteries were harvested for morphometric analysis. Left, Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Right, Quantitative analysis of neointima/media tissue ratio of indicated mice. Values are presented as mean±SEM (n=8–15 mice per group). As a statistical 2-way ANOVA revealed a significant interaction (P=0.04), it was followed by a Bonferroni post-test (**P<0.01). B and C, Four-week-old wild-type female mice were ovariectomized and subcutaneously implanted placebo or tamoxifen (4 mg/kg per day) pellets. Two weeks later, animals were submitted to mechanical injury of the femoral artery. Arteries were harvested 28 days after the injury for morphometric analysis. B, Left, Representative images of cross sections of femoral arteries of indicated mice stained with Masson Trichrome. Bars, 100 µm. Right, Quantitative analysis of neointima/media ratio of indicated mice. Values are presented as mean±SEM (n=7–12 mice per group), and statistically compared with Mann–Whitney test. **P<0.01. C, Representative images of uterus from indicated mice.
Discussion
Our results show that E2 is able to widely prevent neointimal hyperplasia within the vascular wall using a mouse model of endovascular mechanical injury of the femoral artery. Because of the central role of the endothelium in the vascular wall, in particular, in the control of VSMC proliferation,4,26 it is commonly thought that triggering endothelial healing can have a beneficial action on neointimal hyperplasia. Using a Cre-Lox strategy, we have demonstrated here that ERα in the endothelium is not necessary for the suppression of SMC proliferation in response to E2, in contrast to the effects of E2 on the acceleration of endothelial healing and the prevention of atheroma.22 In addition, the preservation of the suppressive effects of E2 on neointimal hyperplasia in Tie2Cre+ ERαlox/lox mice suggests that ERα is also dispensable in bone marrow–derived cells, because in this model, medullar ERα expression is also largely abrogated.22 Consistent with this observation, immunostaining analysis of injured femoral arteries suggested the presence of similar numbers of CD3-positive T lymphocytes in E2- and placebo-treated mice. E2 has been described to also have direct inhibitory effect on SMC proliferation and migration in vitro.27 Accordingly, we have shown that ERα in SMC is essential for the prevention of femoral artery neointimal hyperplasia in vivo through the generation of a SMC-specific conditional knockout of ERα. Finally, using a combination of genetic and pharmacological approaches, we have demonstrated that nuclear activation involving ERαAF1 is both necessary and sufficient to prevent neointimal hyperplasia. This result contrasts with the dispensable role of ERαAF1 in mediating the effect of E2 on endothelial healing16 and the lack of tamoxifen activity on re-endothelialization.25
Taken together, our results confirm the crucial role of ERα in neointimal hyperplasia in response to E2 and show for the first time the direct action of E2 on SMC ERα. The role of ERβ is less clear, with several studies having demonstrated that the selective activation of ERβ is sufficient for inhibiting neointima formation.28–30 However, in ERβ−/− mice, the effects of E2 on vascular media area in injured carotids were found to be similar to those in control mice.31 This observation fits with the results obtained in the present study, which suggests that the expression of ERβ is not sufficient to mediate the effect of E2 in the absence of ERα. Overall, ERβ involvement in postinjury VSMC proliferation seems to be dependent on sex, age, extent of vascular injury and anatomic site (ie, carotid versus femoral arteries). In addition, the estrogen response could involve or even be mediated by the G-protein–coupled receptor GPR30, the activation of which has also been proposed to inhibit SMC proliferation.32 The aim of the present study was to focus on the cellular and molecular mechanisms of ERα, but it will be interesting in future studies to assess the possible role of ERβ and GPR30 in this model of neointimal hyperplasia. This was unfortunately beyond the scope of the present study because of the complexity of their inter-relationship and the controversy over the available animal models targeting both ERβ and GPR30.33,34
In an attempt to dissect the molecular mechanisms of E2 action on neointimal hyperplasia, we used EDC to selectively activate ERα MISS. EDC failed to prevent neointimal hyperplasia, but estetrol, a natural selective estrogen receptor modulator selectively activating nuclear ERα,24 was able to prevent neointimal hyperplasia to a similar extent as E2. Taken together, these results demonstrate that nuclear activation is necessary and sufficient to prevent neointimal hyperplasia after endovascular mechanical injury of the femoral artery in a normocholesterolemic context. It was previously shown that E2 failed to decrease injury-induced proliferation of medial SMC in the carotid artery in mice expressing a peptide that inhibits ERαMISS.35 In addition, Chambliss et al21 demonstrated that the formation of an atheromatous neointima could be prevented not only by E2 but also, at least in part, by EDC in a more complex model of carotid injury (as a consequence of hypercholesterolemia caused by ApoE deficiency). The discrepancies between our present findings and these studies may be attributed to differences in the models used because wire injury of the carotid artery results in a medial remodeling with little or no neointima formation.36,37 In contrast, femoral artery wire injury in the mouse leads to a large neointima formation, with massive proliferation of neointimal SMC.38,39 It is therefore likely that the observed differences in the molecular targets of E2 are attributable to the phenotype of the VSMC involved in the 2 models. The results of both studies demonstrate that the proliferation of neointimal VSMC is inhibited via nuclear ERαAF1 activation by E2, whereas ERαMISS controls medial SMC remodeling in the elastic carotid artery. Altogether, these findings seem complementary and enable us to discriminate between the molecular targets of E2 depending on the phenotype of VSMC (medial versus neointimal, ie, synthetic) and their anatomic origin (elastic versus muscular artery). It is also important to note that carotid injury in ApoE-deficient mice leads to the formation of a complex lesion with predominantly inflammatory cells and poor SMA-positive cells.40 Altogether, these studies emphasize that the roles of the ERα subfunctions (here MISS versus AF1) seem to vary according to the differentiation state of the SMC.
Thus, the results presented here show that whereas the effects of E2 on endothelial cells are essentially dependent on ERαMISS, the effects of E2 on SMC proliferation require the nuclear effect of ERα to prevent neointimal hyperplasia, particularly the ERαAF1 subfunction. We previously demonstrated that ERαAF1 is both necessary and sufficient for the proliferative effect of ERα on the epithelium of the uterus,15 and show here that this same function is able to mediate the antiproliferative effect of estrogens in another cell type and tissue. Importantly, in breast cancer, ERαAF1 is recognized as a convergence point for growth factor and hormonal activation.41 Thus, our in vivo data highlight ERαAF1 as a major functional element of ERα, one that appears to contribute to the integration of various signals that control cell proliferation in a strictly cell- and tissue-specific manner.
Altogether, these varied mouse models permit the dissection of the mechanisms of action of both estrogens and selective ER modulators. These findings also begin to highlight how ERα might best be modulated to optimize the expected benefit/risk ratio of its multitude of activities that could represent a novel facet of personalized medicine.
Acknowledgments
The staff of the animal facilities are acknowledged for their skillful technical assistance. Founding ERαlox/lox and ERαAF10/+ mice were kindly provided by Pr P. Chambon.
Sources of Funding
The work at the INSERM unit U1048 was supported by the INSERM, Université de Toulouse III and Faculté de Médecine Toulouse-Rangueil, Fondation de France, Conseil Régional Midi-Pyrénées, Fondation pour la Recherche Médicale, Fondation de l’Avenir and Agence Nationale de la Recherche. The Nuclear Magnetic Resonance facility is part of the genotoul-Ibisa Toulouse Drug Screening Platform platform and was funded by the National Scientific Research Center, région Midi-Pyrénées and European structural funds. This work was also supported by National Institutes of Health grants R01DK015556 (awarded to J.A. Katzenellenbogen) and P50AT006268 (awarded to B.S. Katzenellenbogen).
Disclosures
J.-M. Foidart was associated with Uteron, a division of Actavis, and now is associated with Mithra (Liège, Belgium). This work was supported, in part, by a grant from Uteron. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- activation function
- E2
- 17β-Estradiol
- EDC
- estrogen dendrimer conjugate
- ER
- estrogen receptor
- MISS
- membrane-initiated steroid signaling
- SMA
- smooth muscle actin
- SMC
- smooth muscle cell
- VSMC
- vascular smooth muscle cell
In July 2015, the average time from submission to first decision for all original research papers submitted to Circulation Research was 12.38 days.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.115.306416/-/DC1.
Novelty and Significance
What Is Known?
17β-estradiol (E2) prevents neointimal hyperplasia in response to endovascular injury in various animal models and species, including rats, pigs, and sheep.
Estrogen receptor alpha (ERα) mediates the action of E2 through regulation of gene transcription via 2 activation functions (AF), AF1 and AF2, and through activation of rapid signaling at the plasma membrane.
ERαAF1 and ERαAF2, but not membrane ERα, mediate uterine endometrial proliferation in response to E2.
Membrane ERα, but not ERαAF1 or ERαAF2, mediates the beneficial action of E2 on the endothelium.
What New Information Does This Article Contribute?
E2 prevents neointimal hyperplasia in mice.
Smooth muscle cell, but not endothelial, ERα is necessary to prevent neointimal hyperplasia in response to E2 in mice.
ERαAF1 is necessary and sufficient to prevent neointimal hyperplasia.
This study, using a mouse model of endovascular mechanical injury of the femoral artery, shows that E2 is able to prevent neointimal hyperplasia. Our results further establish the crucial role of ERα in the prevention of neointimal hyperplasia by E2 and show for the first time the key role of smooth muscle cell ERα. Although the effects of E2 on endothelial cells are essentially dependent on membrane ERα, the effects of E2 on smooth muscle cell proliferation require the nuclear effect of ERα to prevent neointimal hyperplasia, in particular the ERαAF1 subfunction. We previously demonstrated that ERαAF1 is both necessary and sufficient for the proliferative effect of ERα on the epithelium of the uterus and show here that this same function is able to mediate the antiproliferative effect of E2 on arterial smooth muscle cell. This approach highlights the exquisite cell/tissue-specific actions of the ERα subfunctions and will help to delineate the spectrum of action of selective ER modulators, as that of the selective ERαAF1 activator tamoxifen used in the present study.
References
- 1.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smirnova NF, Gayral S, Pedros C, Loirand G, Vaillant N, Malet N, Kassem S, Calise D, Goudounèche D, Wymann MP, Hirsch E, Gadeau AP, Martinez LO, Saoudi A, Laffargue M. Targeting PI3Kγ activity decreases vascular trauma-induced intimal hyperplasia through modulation of the Th1 response. J Exp Med. 2014;211:1779–1792. doi: 10.1084/jem.20131276. doi: 10.1084/jem.20131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekar B, Sirois MG, Geoffroy P, Lauzier D, Nattel S, Tanguay JF. Local delivery of 17beta-estradiol improves reendothelialization and decreases inflammation after coronary stenting in a porcine model. Thromb Haemost. 2005;94:1042–1047. doi: 10.1160/TH04-12-0823. doi: 10.1160/TH04-12-0823. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar B, Tanguay JF. Local delivery of 17-beta-estradiol decreases neointimal hyperplasia after coronary angioplasty in a porcine model. J Am Coll Cardiol. 2000;36:1972–1978. doi: 10.1016/s0735-1097(00)00940-2. [DOI] [PubMed] [Google Scholar]
- 6.Ishibahshi T, Obayashi S, Sakamoto S, Aso T, Ishizaka M, Azuma H. Estrogen replacement effectively improves the accelerated intimal hyperplasia following balloon injury of carotid artery in the ovariectomized rats. J Cardiovasc Pharmacol. 2006;47:37–45. doi: 10.1097/01.fjc.0000192149.83008.dc. [DOI] [PubMed] [Google Scholar]
- 7.Selzman CH, Gaynor JS, Turner AS, Johnson SM, Horwitz LD, Whitehill TA, Harken AH. Ovarian ablation alone promotes aortic intimal hyperplasia and accumulation of fibroblast growth factor. Circulation. 1998;98:2049–2054. doi: 10.1161/01.cir.98.19.2049. [DOI] [PubMed] [Google Scholar]
- 8.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. doi: 10.1161/01.CIR.103.3.423. [DOI] [PubMed] [Google Scholar]
- 9.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 10.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. doi: 10.1161/01.RES.0000021114.92282.FA. [DOI] [PubMed] [Google Scholar]
- 11.Toutain CE, Filipe C, Billon A, Fontaine C, Brouchet L, Guéry JC, Gourdy P, Arnal JF, Lenfant F. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol. 2009;29:1543–1550. doi: 10.1161/ATVBAHA.109.192849. doi: 10.1161/ATVBAHA.109.192849. [DOI] [PubMed] [Google Scholar]
- 12.Stender JD, Kim K, Charn TH, Komm B, Chang KC, Kraus WL, Benner C, Glass CK, Katzenellenbogen BS. Genome-wide analysis of estrogen receptor alpha DNA binding and tethering mechanisms identifies Runx1 as a novel tethering factor in receptor-mediated transcriptional activation. Mol Cell Biol. 2010;30:3943–3955. doi: 10.1128/MCB.00118-10. doi: 10.1128/MCB.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 14.Feng Q, O’Malley BW. Nuclear receptor modulation–role of coregulators in selective estrogen receptor modulator (SERM) actions. Steroids. 2014;90:39–43. doi: 10.1016/j.steroids.2014.06.008. doi: 10.1016/j.steroids.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Bergès H, Laurell H, Gourdy P, Lenfant F, Arnal JF. The AF-1 activation function of estrogen receptor α is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- 16.Billon-Galés A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci U S A. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billon-Galés A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–13316. doi: 10.1073/pnas.1105632108. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda K, Karas RH. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids. 2013;78:589–596. doi: 10.1016/j.steroids.2012.12.006. doi: 10.1016/j.steroids.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286:14737–14743. doi: 10.1074/jbc.R110.191791. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adlanmerini M, Solinhac R, Abot A, et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:E283–E290. doi: 10.1073/pnas.1322057111. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambliss KL, Wu Q, Oltmann S, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billon-Galés A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guéry JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 23.Wendling O, Bornert JM, Chambon P, Metzger D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14–18. doi: 10.1002/dvg.20448. doi: 10.1002/dvg.20448. [DOI] [PubMed] [Google Scholar]
- 24.Abot A, Fontaine C, Buscato M, et al. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor α modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;6:1328–1346. doi: 10.15252/emmm.201404112. doi: 10.15252/emmm.201404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontaine C, Abot A, Billon-Galés A, Flouriot G, Bergès H, Grunenwald E, Vinel A, Valera MC, Gourdy P, Arnal JF. Tamoxifen elicits atheroprotection through estrogen receptor α AF-1 but does not accelerate reendothelialization. Am J Pathol. 2013;183:304–312. doi: 10.1016/j.ajpath.2013.03.010. doi: 10.1016/j.ajpath.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, Mizuno K, Ino Y, Okada M, Kikkawa F, Mizutani S, Tomoda Y. Effects of 17 beta-estradiol and progesterone on growth-factor-induced proliferation and migration in human female aortic smooth muscle cells in vitro. Cardiovasc Res. 1996;32:516–523. [PubMed] [Google Scholar]
- 28.Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93:399–405. doi: 10.1161/01.RES.0000088640.18462.42. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- 29.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res. 2012;173:e1–10. doi: 10.1016/j.jss.2011.09.021. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krom YD, Pires NM, Jukema JW, de Vries MR, Frants RR, Havekes LM, van Dijk KW, Quax PH. Inhibition of neointima formation by local delivery of estrogen receptor alpha and beta specific agonists. Cardiovasc Res. 2007;73:217–226. doi: 10.1016/j.cardiores.2006.10.024. doi: 10.1016/j.cardiores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, Gustafsson JA, Korach KS, Smithies O, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proc Natl Acad Sci U S A. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas E, Bhattacharya I, Brailoiu E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Bernelot Moens SJ, Schnitzler GR, Nickerson M, Guo H, Ueda K, Lu Q, Aronovitz MJ, Nickerson H, Baur WE, Hansen U, Iyer LK, Karas RH. Rapid estrogen receptor signaling is essential for the protective effects of estrogen against vascular injury. Circulation. 2012;126:1993–2004. doi: 10.1161/CIRCULATIONAHA.112.124529. doi: 10.1161/CIRCULATIONAHA.112.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr, Lubahn DB, O’Donnell TF, Jr, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 37.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. doi: 10.1161/01.RES.73.5.792. [DOI] [PubMed] [Google Scholar]
- 38.Reis ED, Roqué M, Cordon-Cardo C, Drobnjak M, Fuster V, Badimon JJ. Apoptosis, proliferation, and p27 expression during vessel wall healing: time course study in a mouse model of transluminal femoral artery injury. J Vasc Surg. 2000;32:1022–1029. doi: 10.1067/mva.2000.109763. doi: 10.1067/mva.2000.109763. [DOI] [PubMed] [Google Scholar]
- 39.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 40.Manka D, Forlow SB, Sanders JM, Hurwitz D, Bennett DK, Green SA, Ley K, Sarembock IJ. Critical role of platelet P-selectin in the response to arterial injury in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1124–1129. doi: 10.1161/01.ATV.0000127619.04687.f4. doi: 10.1161/01.ATV.0000127619.04687.f4. [DOI] [PubMed] [Google Scholar]
- 41.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18:R1–14. doi: 10.1677/ERC-10-0070. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]


