Abstract
Over 383,900 individuals in the U.S. undergo maintenance hemodialysis that exposes them to water, primarily in the form of dialysate. The quality of water and associated dialysis solutions have been implicated in adverse patient outcomes and is therefore critical. The Association for the Advancement of Medical Instrumentation has published both standards and recommended practices that address both water and the dialyzing solutions. Some of these recommendations have been adopted into Federal Regulations by the Centers for Medicare and Medicaid Services as part of the Conditions for Coverage, which includes limits on specific contaminants within water used for dialysis, dialysate, and substitution fluids. Chemical, bacterial, and endotoxin contaminants are health threats to dialysis patients, as shown by the continued episodic nature of outbreaks since the 1960s causing at least 592 cases and 16 deaths in the U.S. The importance of the dialysis water distribution system, current standards and recommendations, acceptable monitoring methods, a review of chemical, bacterial, and endotoxin outbreaks, and infection control programs are discussed.
By the end of 2010, a total of 594,374 people had end-stage renal disease (ESRD) in the United States (1). Of the total number of people with ESRD, the prevalent dialysis (hemodialysis and peritoneal dialysis) and transplant population was 415,013 and 179,361, respectively (1). Of the patients treated by dialysis, over 383,900 receive maintenance hemodialysis. Patients undergoing hemodialysis ‘three times per week’ can be exposed to 300–600 l of water depending on their prescription (2,3). The volume of dialysis fluid increases for those on nocturnal treatments to 580–860 l per week (3). Ensuring the necessary quality of dialysate is a vital aspect of this type of treatment considering the repeated, large volumes each patient is subjected to. Specifically, chemical, bacterial, and associated endotoxin contamination can threaten a dialysis patient’s health. Dialysis patients often have additional comorbidities (e.g., diabetes, hypertension, cardiovascular disease, etc.) that can make them more vulnerable to adverse outcomes. Aging, obesity, and hypertension rates are also increasing in the U.S. population, which are associated with ESRD and chronic kidney disease (4). Thus, more individuals will probably need renal replacement therapy (maintenance hemodialysis, peritoneal dialysis, or transplantation). Asserting that water and dialysate quality is an important factor in protecting the health of hemodialysis patients is an understatement.
Drinking water is treated, purified, and transported through a distribution system within a dialysis center where it is used in the preparation of dialysate concentrates, as well as for proportioning concentrates at the dialysis machine to produce the final dialysate bath. All of these steps provide an opportunity for microbial growth or chemical exposure if the water is contaminated and not properly maintained. The main water sources for hemodialysis facilities, as well as for home dialysis treatments, are local drinking water suppliers. Municipalities and other drinking water suppliers are required to adhere to the U.S. Environmental Protection Agency (EPA) drinking water standards under the Safe Drinking Water Act (SDWA), which specifies chemical and microbiological contaminant levels. The dialysis staff must be cognizant of their incoming water quality and the provider’s treatment practices prior to beginning dialysis prep and dialysis treatment. Dialysis centers and their employees are also required to meet the Centers for Medicare and Medicaid Services (CMS) Conditions for Coverage, which includes various requirements intended to ensure the safe treatment of dialysis patients (5).
The current CMS rules were published in 2008 (5) and are based upon recommendations made in 2004 by the Association for the Advancement of Medical Instrumentation (AAMI) (6). While the 2008 CMS regulations are directed at maintenance of hemodialysis facilities and are the minimum standards for water for dialysis and dialysate quality, the 2009 and 2011 updated recommendations by AAMI are more stringent and are voluntary (7,8). All of these guidelines and recommendations focus on the quality management of dialysis treatment, of which water or dialysate are the main topic.
Guidelines and recommendations are necessary due to the potential health outcomes for dialysis patients if exposed to chemical or microbiological contaminants. Chemical contaminants can cause chemical toxicity and adverse effects if present at high enough concentrations. Chemical toxicity leads to a range of clinical outcomes, including but not limited to, speech and motor difficulties, seizures, nausea, hypotension, and diarrhea. Each chemical produces a specific reaction; for example, sulfate (>200 mg/l) is associated with nausea, vomiting, and metabolic acidosis (9), while lead (52–65 μg/l) has caused abdominal pain and muscle weakness (10). While there are defined ranges where toxicity is likely to occur, each person has a specific threshold before clinical symptoms will appear due to various physiological reasons and the individuals’ health status.
Some chemicals are not inherently toxic in nature, but, if present in high enough concentrations, they can cause adverse health effects. Calcium is one such example, where excessive amounts have been associated with renal disease (11). Meanwhile, microbial contaminated dialysis water and/or dialysate may produce bacteremia and chronic inflammation, which contributes to or complicates the leading cause of death for dialysis patients, cardiovascular disease (CVD). Endotoxin fragments or endotoxin in the dialysate bath may pass through the dialyzer membranes or cause transmembrane stimulation of circulating immune cells to produce symptoms of septicemia or a pyrogenic reaction. The presence of dialysate contaminants also triggers inflammatory markers, such as high-sensitivity C-reactive protein, interleukin (IL)-6, fibrinogen, and intercellular adhesion molecule (sICAM-1) (12). Chronic inflammation, in addition to contributing to CVD, has been linked to the following clinical outcomes: poor nutritional status, reduced response to erythropoietin therapy, decline in residual renal function, and carpal tunnel syndrome (13).
With two available guidance documents and continuing sporadic outbreaks, as well as a multitude of contaminants, hemodialysis options, and monitoring approaches, an updated review was necessary to consolidate the current information. This review describes the dialysis water distribution system and appropriate materials; dialysis quality with regard to current standards; methods for identifying chemical, bacterial, and endotoxin contaminants; outbreaks that were caused by chemical and microbiological agents; and the importance of infection control.
Dialysis Water Distribution System and Materials
Once water enters a hemodialysis center, the goal is to achieve high quality and safe hemodialysis water and dialysate. Water treatment, system design, and distribution material choices are contributing factors. Dialysis water treatment should remove chemical and microbial contaminants to below established allowable limits and is characterized by two phases: (i) pretreatment, where constituents are removed from the feed water to protect the downstream treatment components and (ii) water treatment, which is the process of physically removing and/or chemically inactivating remaining chemical and/or microbial contaminants. Details regarding water treatment options and typical designs have already been given (8,14,15), but are briefly described here. Pretreatment includes the following: a blend valve – i.e., temperature controller to aid in efficient treatment downstream; multimedia depth filtration – composed of sand and/or coal, where the goal is to remove solids; granular activated carbon (GAC) filter(s) – absorb(s) organic matter that influences taste, odor, color, toxicity, and mutagenicity; softener – reduces the presence of cations (Ca2+, Mg2+, Sr2+, Fe2+, and Mn2+), which is measured as the ‘hardness of water’ and is commonly expressed as the concentration (mg/l) of CaCO3 (16); and a prefilter – removes remaining particles (e.g., particulates and fine particles released from the GAC filters) prior to treatment. Water treatment includes reverse osmosis (RO) with/without deionization (DI) tanks, followed by these optional components: storage tank, ultraviolet (UV) irradiator, and ultrafilter/endotoxin-retentive filter (always used after storage tank, UV irradiator, or DI tank). RO is capable of excluding metal ions, aqueous salts, and molecules from the treated water. Ultrafiltration and endotoxin-retentive filters can be included after the deionizer, immediately after the storage tank, and/or before delivery to the dialyzer (depending on the design of the system) (13) to remove bacteria and endotoxin by using a positively charged filter surface and size exclusion.
There are two types of system designs, indirect and direct, for distribution of the treated water before the water is combined with the concentrates to make dialysate. Indirect systems constantly circulate water through the previously described pretreatment/treatment, even when the machines are not in use, and route the unused treated water back to the point before RO treatment or to a storage tank after the RO. Direct systems are one-way and when the machines are off, the water is stagnant. The direct system design is not recommended due to the opportunity for microbial growth and biofilm formation that can occur during periods of low or no flow. If a storage tank is incorporated into the loop, the tanks should have a conical/bowl shape base and tight lid with hydrophobic air filter (0.22–0.45 μm), and should be cleaned and disinfected regularly (e.g., weekly, bi-weekly, monthly, etc.) as determined by monthly (or established monitoring schedule) bacteriological results and visual assessment (8).
Although the exact role that biofilms in the water distribution system play is not defined for hemodialysis patients (17), outbreak data demonstrate that it is in the patients’ interest to have water and dialysis solutions that are as “microbiologically clean” as possible. Unfortunately, biofilms are highly resistant to disinfectants due to the mixed bacterial community’s structure and the formation of exopolysaccharides (EPS). Preventing the initial growth of a biofilm is recommended, and part of this tactic includes choosing appropriate materials for the dialysis water distribution system, in combination with compatible disinfection.
The AAMI 2011 recommendations include a material compatibility list in Table B.1 for commonly used materials in the distribution system and available disinfectants (8). The materials listed were polyvinylchloride (PVC), chlorinated polyvinylchloride (CPVC), polyvinylidene fluoride (PVDF), cross-linked polyethylene (PEX), stainless steel (SS), polypropylene (PP), polyethylene (PE), acrylonitrile butadiene styrene (ABS), and polytetrafluorethylene (PTFE). The disinfectants included were sodium hypochlorite (chlorine bleach), peracetic acid, formaldehyde, hot water, and ozone (dissolved in water). Peracetic acid was listed as compatible with all distribution materials. Incompatibilities between distribution materials and disinfectants were sodium hypochlorite with SS and ABS; formaldehyde with ABS; hot water with PVC, CPVC, PE, and ABS; and ozone with PP and ABS. The incompatibilities could cause leaching or corrosion of the materials, which may pose a risk to the patients or integrity of the system.
While the AAMI table offers a general list of materials, PVC (Type 1, Schedule 40 or 80) and SS (316L) are the two most used in hemodialysis systems. PVC is the more common substratum used due to availability and cost; however, an evaluation showed that purified water, chemical disinfection, and water flow ‘wore’ the material down over time (14 years) to create a surface that supported bacterial growth (18). In addition, the connections within a system must be welded or joined properly, so as to not create any rough edges where bacteria can proliferate; and proper angles are recommended to allow for an even flow (19).
Standards and Methods
When hemodialysis was introduced as a treatment for acute renal failure patients around 1945 (20), the importance of water and dialysate quality with regard to chemicals and microorganisms was not well recognized (21). This was in part because, until the 1960s, the procedure itself was not widespread and patients received a limited number of treatments. Even after hemodialysis became a mainstream therapy following the development of the Scribner shunt permanent vascular access in 1960, water quality was only factored in by controlling temperature and conductivity on the untreated source waters used for dialysis (21). This may have been a reflection of geography, as Ward pointed out, because in the beginning, the epicenter for hemodialysis was in the northwestern U.S. at the University of Washington in Seattle where the water quality is comparatively better than in other regions (21). Additionally, drinking water quality was not standardized until 1974 when the Safe Drinking Water Act (SDWA) was enacted.
The first chemicals of concern for dialysis water were those with a natural presence in the source waters or as drinking water additives for public health reasons (e.g., disinfection, preventing dental caries) (21). Thus, water treatment as part of hemodialysis became normal protocol and the first standards were published in 1981 by AAMI. While water treatment technology has advanced, assuring adequate quality of dialysate will continue to be complex. The continuous changes in municipal water treatment due to new EPA regulations or seasonal fluctuations, the use of new dialyzer designs (i.e., high-flux), and expansion of dialyzer reuse (21) are a few examples creating this complexity. As previously mentioned, the current Conditions for Coverage (42 CFR Parts 405, 410, 413, 414, 488, 494) published by CMS for water and dialysate quality became a Final Rule in 2008 (5) and were based in part upon the 2004 AAMI document, “Dialysate for Hemodialysis” (6).
AAMI has since updated the recommendations (8), specifically for the microbiological methods and standards for hemodialysis concentrates (11), water for hemodialysis (11), water treatment and related therapies (22), and dialysis fluids (7). Comparisons of these regulatory and recommended standards and methods are shown in Tables 1–3. The maximum allowable levels signify when a dialysis system should be taken offline (i.e., discontinuation of dialysis treatment in the facility), followed by appropriate treatment or measures applied to correct the contamination, quality assurance testing prior to reinstallation for patient treatment, and documentation of corrective actions in records. Action levels note the concentration at which steps should be taken to prevent the levels from increasing to the maximum allowable limits. To determine whether dialysis fluids meet the Conditions for Coverage or recommendations regarding chemicals and microbial contaminants, the water and dialysis fluids are to be tested for chemical and biological contaminants. The levels, if ‘in house’ testing capabilities are not adequate, can be measured by renal laboratories, such as DaVita Labs and Spectra laboratories, or hospital laboratories.
TABLE 1.
| Parameter | Municipal drinking water | Health effect | Dialysis water | Health effect |
|---|---|---|---|---|
| Toxic chemicals (mg/l) | ||||
| Aluminum1 | 0.05–0.2 | Anemia, osteomalacia | 0.01 | “Dialysis dementia” |
| Chloramine2 | 4.0 | Eyes, nose; GI discomfort, anemia | 0.1 3 | Acute hemolytic anemia |
| Chlorine2 | 4.0 | Eyes, nose; GI discomfort, anemia | 0.5 3 | |
| Total chlorine | – | 0.1 | ||
| Copper4 | 1.3 | GI distress, liver/kidney damage | 0.1 | |
| Fluoride | 4.0 | Bone disease | 0.2 | Toxicity, bone disease |
| Lead4 | 0.015 | Neurological damage, fatal hemolysis | 0.005 | GI pain, muscle weakness |
| Nitrate (as N) | 10 | Blue-baby syndrome, shortness breath | 2.0 | Methemoglobinemia |
| Sulfate | – | 100 | Nausea, metabolic acidosis | |
| Zinc | – | Nausea, vomiting, fever, anemia | 0.1 | |
| Trace elements (mg/l) | ||||
| Antimony | 0.006 | 0.006 | ||
| Arsenic | 0.010 | 0.005 | ||
| Barium | 2 | 0.1 | ||
| Beryllium | 0.004 | 0.0004 | ||
| Cadmium | 0.005 | 0.001 | ||
| Chromium | 0.10 | 0.014 | ||
| Mercury | 0.002 | 0.0002 | ||
| Selenium | 0.05 | 0.09 | ||
| Silver1 | 0.10 | 0.005 | ||
| Thallium | 0.002 | 0.002 |
This limit is part of the National Secondary Drinking Water Regulation, which is nonenforceable.
This is the highest allowable limit in drinking water, defined as the Maximum Residual Disinfectant Level.
Chloramine and Free Chlorine limits are only listed in the CMS standards, not in the ANSI/AAMI/ISO recommendations.
This limit is the action level if more than 10% of samples exceed this threshold.
TABLE 3.
Methods, media, incubation and time for optimal bacterial monitoring
| Dialysis Water and Fluids* | Methods | Media | Incubation parameters (°C, hours) |
|---|---|---|---|
| CMS Regulation1 | Membrane filtration Spread plate Dip samplers |
TSA2, TGYE3 | 35°C, 48 hours |
| ANS Recommended4 | Membrane filtration Spread plate Pour plate |
TGEA5, R2A6 | 17–23°C, 7 days |
Bicarbonate concentrates – methods, media, and incubation parameters are the same as listed above; however, for the recommended monitoring assay, the media should be supplemented with 4% sodium bicarbonate.
TSA = Tryptic soy agar.
TGYE = Standard methods and plate count agar.
The 2011 AAMI recommendations have been updated compared to the 2004 recommendations (8).
TGEA = Tryptone Glucose Extract Agar.
R2A = Reasoner’s Agar No. 2.
Chemical Standards and Methods
The maximum allowable limits for toxic chemicals (mg/l) and trace elements (mg/l) in municipal drinking water (23) and for dialysate water (11) are shown in Table 1. The chemical contaminants that can be found in water or the dialyzing fluid and have produced toxicity in patients are aluminum, chloramine, copper, fluoride, lead, nickel, nitrate, sulfate, and zinc (9,10,24–32). AAMI has recommended lower maximum allowable limits of these potential contaminants for dialysis water and associated fluids to below the levels associated with dialysis toxicity, and has also included limits for chemical contaminants (trace elements) based on regulations for drinking water. The trace elements limits in Table 1 were adopted from the U.S. EPA drinking water standards (33) with one-tenth of the maximum allowable limits for all but selenium and chromium. Selenium and chromium have higher allowable limits because “a restriction is not needed below the level at which there is no passage from the dialysis fluid to the blood” (11).
The initial AAMI standard in 2003 (34) included limits for barium, selenium, chromium, silver, cadmium, mercury, and arsenic. The SDWA included new restrictions on chemical contaminants (antimony, beryllium, and thallium) and decreased the allowable concentration of cadmium, thus these were incorporated into the dialysis chemical limitations (11). Electrolytes normally included in dialysis fluids are also monitored for maximum allowable levels and are as follows: calcium ≤2 mg/l, magnesium ≤4 mg/l, potassium ≤8 mg/l, and sodium ≤70 mg/l (11). Approved methods for monitoring toxic chemicals and trace elements are described in the guidance documents of the American Public Health Association (35) and U.S. EPA (33). If a facility is unable to process samples using these approved methods, there are equivalent analytical methods available (11). Compliance can be met by comparing the source waters to the regulations set by the World Health Organization or local regulations, where the total heavy metals measure below 0.1 mg/l, or even with a reverse osmosis system complying with >90% rejection based on conductivity, resistivity, or total dissolved solids (11).
Microbial Standards and Methods
Due to scientific evaluation, outbreak data dissemination, and industrial influence, the U.S. microbial standards have evolved over time since the first recommendations in 1981. For example, a small study of pyrogenic reactions (i.e., reactions characterized by the onset of chills/shaking within approximately one hour of treatment followed by a fever one to two hours after treatment, as well as hypotension, headache, and muscle ache) in a single dialysis center by Favero et al. (1974) demonstrated that when dialysate had bacterial counts lower than 102 CFU/ml, there was a 4% attack rate; however, when concentrations exceeded 104 CFU/ml, the attack rate for pyrogenic reactions increased to 24% (36). Additional studies and outbreak investigations demonstrated that the incoming water and final dialysis fluids should not exceed a maximum contaminant level (MCL) of 100–1000 CFU because of possible pyrogenic or septicemic complications (37,38). The limits were established by consensus as 2000 CFU/ml for the dialysate bath and one log10 lower for water used to prepare the dialysate as a result of these findings. However, the MCL for dialysate was lowered again to 200 CFU/ml in 2004 to match the water limit (39).
Standards for ultrapure dialysate were also determined, but the renal provider industry claimed that the cost would be too great to achieve this level of purity and pressed for the ultrapure qualifications to be voluntary. CMS was also responsive to their stakeholders in the provider community regarding the issues around potential costs associated with ultrapure fluids. In response to these concerns, levels were created with varying bacterial limits: conventional, ultrapure, and substitution fluid. CMS adopted the conventional dialysate as the minimum requirement. Yet, the U.S. standards were not harmonized with the international standards. One basis for the difference between the U.S. and international standards is that all limits outside the U.S. are set by pharmacopeial conventions because dialysate is a drug, while the U.S. categorizes dialysate as a device. For example, the European Renal Association recommends 100 CFU/ml total viable counts (TVC) and 0.25 EU/ml for endotoxin for regular water, while the limits for ultrapure water are <1 CFU/10 ml TVC and <0.03 IU/ml endotoxin (40). The Japanese Society for Dialysis Therapy (JSDT) also recommends <100 CFU/ml TVC for water, but only 0.05 EU/ml endotoxin for regular water and standard dialysis fluid (41). The JSDT limits for ultrapure dialysate are <1 CFU/10 ml TVC and <0.001EU/ml (41).
The current AAMI recommendations have been harmonized with the international dialysate community, although the CMS Conditions for Coverage do not recognize these stringent levels despite numerous studies supporting ultrapure hemodialysis having reduced chronic inflammation (13,42–56). The CMS Conditions for Coverage requires conventional dialysis to have <200 colony-forming unit (CFU)/ml (action level: 50 CFU/ml) for TVC for dialysis water and dialysate/dialysis fluid (Table 2) (5,6). However, the 2011 AAMI recommendations lowered the acceptable TVC to <100 CFU/ml (action level: 50 CFU/ml) for dialysis water and dialysate (8) in an effort to move toward international standards. If a facility chooses ultrapure dialysis, this should be clearly stated in their policies and procedures. They must also meet the recommended standards set by AAMI (6) according to the Final Rule (5) regardless of whether they meet the less stringent limits of the conventional dialysis. Ultrapure dialysis requires the TVC for the dialysate to be <0.1 CFU/ml (no action level; Table 2) (2,8). Ultrapure dialysate is an ideal level of quality for patients, as studies have proved a reduction in inflammation and oxidative stress, improvement of iron utilization and erythropoietin response, and other positive benefits (51,53,57–66).
TABLE 2.
Microbial standards for municipal drinking water, dialysis water, and dialysate (standard and ultrapure) (2,5–8,11,23). The heterotrophic bacteria (HPC) and Total Viable Count are comparable when using Reasoners 2A (R2A) for 7 days at 17–23°C
| Parameter | Municipal drinking water | Conventional dialysis water |
Conventional dialysate/ Dialysis fluid |
Ultrapure dialysate |
|---|---|---|---|---|
| Heterotrophic bacteria (HPC) | ≤500 CFU/ml | – | – | – |
| Total Viable Count | ||||
| CMS max allowable limit1 | – | <200 CFU/ml | <200 CFU/ml | <0.1 CFU/ml |
| CMS action level1,2 | 50 CFU/ml | 50 CFU/ml | – | |
| ANS max allowable limit3 | – | <100 CFU/ml | <100 CFU/ml | <0.1 CFU/ml |
| ANS action level2,3 | 50 CFU/ml | 50 CFU/ml | – | |
| Endotoxin | ||||
| CMS max allowable limit1 | – | <2 EU/ml | <2 EU/ml | <0.03 EU/ml |
| CMS action level1,2 | 1 EU/ml | 1 EU/ml | – | |
| ANS max allowable limit3 | – | <0.25 EU/ml | <0.5 EU/ml | <0.03 EU/ml |
| ANS action level2,3 | 0.125 EU/ml | 0.25 EU/ml | – |
The Centers for Medicare and Medicaid Services (CMS), Department of Health and Human Services set the regulations for maximum allowable limits and action levels for dialysis facilities to be certified under the Medicare program (5); these are currently based upon the 2004 recommendations from the Association for the Advancement of Medical Instrumentation (AAMI) (6).
The action level is the concentration at which corrective measures are to be immediately conducted to reduce the bacteria and/or endotoxin levels, which are typically 50% of the maximum allowable level.
The recommended methods (e.g., pour plate, spread plate, membrane filtration), media, and incubation ranges allow each dialysis center to accommodate their facility with a monitoring program of their choice (Table 3). The methods and associated commercially available assays have been shown to be comparable (67), while different media types and incubation periods can result in varying colony concentrations (68–71). Reasoner’s 2A agar (R2A) results in higher colony counts than plate count agar (PCA) and tryptic soy agar (TSA) in water samples and dialysis fluids (69–71). Tryptone glucose extract agar (TGEA), an additional low nutrient media, also shows higher colony counts than TSA (68). These findings led to the updated recommendations for using TGEA or R2A for microbiological monitoring of dialysis water and fluids. However, for bicarbonate related samples, media containing salt (i.e., TSA, TSA-NaCl, standard methods agar (SMA+), SMA-NaCl, and R2A-NaCl) demonstrated higher colony counts due to the organisms thriving in saline conditions similar to the bicarbonate solutions (72). Specifically, the recommendation states to add 4% sodium bicarbonate to either TGEA or R2A (8).
Endotoxin Standard and Methods
Endotoxin is also included in the updated recommendations by AAMI. Conventional dialysis requires the endotoxin concentration in the dialysis water and dialysate to be <2 EU/ml with an action level of 1 EU/ml (Table 2) (5,6). However, the 2011 AAMI recommendations lowered the acceptable endotoxin concentration to <0.25 EU/ml in dialysis water and <0.5 EU/ml in the dialysate (8). Ultrapure dialysis requires the endotoxin concentration in the dialysate to be <0.03 EU/ml (no action level; Table 2) (2,8). The standard method for measuring endotoxin concentrations is the Limulus amoebocyte lysate (LAL) test. While two LAL approaches (kinetic and gel-clot assay) are approved in the Final Rule (5,6), the 2011 recommendations mention six different testing techniques (8).
Monitoring and Reporting
The parameters described above are to be monitored on a “regular” basis after validation has been completed and the systems are functioning properly. Validation of the water treatment and dialysis fluid production systems is a documenting process that occurs once a new system is installed and operated according to the manufacturer’s recommendations to determine whether it consistently produces fluids of the required quality. Validation of a dialysis system is vital for establishing that the system can both provide the necessary water quality and whether the disinfection processes are sufficient at keeping the microbial contaminants below the maximum allowable limits.
The recommendations for routine monitoring mentioned within this section are from the updated 2011 recommendations by AAMI (8). The chemical contaminants within the water system are to be tested at least annually in combination with the evaluation of the source water (incoming feed water). Total chlorine should be monitored prior to each patient shift after the primary carbon tank to confirm that the concentration is below 0.01 mg/l. The dialysis storage water tanks and water distribution piping system should be monitored once a month, or as determined from the validation process, for microorganisms and endotoxin. The standard dialysis fluid from each dialysis machine within a facility should be tested at least once a year for bacteria and endotoxin, where regular testing is conducted on a different machine each month (machines are tested on a rotation). Ultrapure dialysis fluid is also tested monthly, but only for endotoxin. However, if the bacteria- and endotoxin-retentive filter is validated, operated, and monitored according to the manufacturer’s instructions, this testing may not be necessary. Specifically for endotoxin-retentive filters, daily monitoring of the pressure across the filter is adequate in assuring endotoxin levels are within the limit.
Additionally, for anyone who develops signs and symptoms during the dialysis session, blood cultures and dialysate (from the patient’s machine) for cultures and endotoxin should be obtained as a routine part of the patient workup. Clinical symptoms may include fever (≥38.3°C/101°C), septic shock, chills (visible rigors), malaise, abdominal pain, nausea, vomiting, diarrhea, anxiety, confusion, and shortness of breath. Unlike in pyrogenic reactions, symptoms of septicemia typically do not resolve on cessation of dialysis treatment.
Monitoring is only a small part of assuring quality dialysis, but required for reimbursement by Medicare (5). Dialysis facilities need to have up-to-date logs that allow the technicians or supervisor to trend the chemical, bacterial, and endotoxin data. Facilities should also be proactive in disinfecting or conducting corrective measures when action levels have been reached, and certainly before the maximum contamination levels have been exceeded. Regular maintenance of the machines, knowledge of factors that impact dialysis quality, and pathways for corrective measures to be successful are additional keys for effective and safe dialysis. The monitoring data stay within the facility, but if there is an issue and State or CMS surveyors request reports, these data are required to be available for review.
Outbreaks
Overall, chemical and microbial contaminants have caused 13 and 20 outbreaks in the U.S., respectively (based on outbreaks reported to CDC). There were 197 patients who experienced a total of 217 episodes of chemical intoxication and 14 deaths as a result of the chemical outbreaks that were investigated by CDC between 1960 and 2007 (Table 4). Microbial-associated outbreaks have resulted in a total of 375 cases (patients and episodes combined) and 2 deaths (Table 5). Bacteria caused 10 outbreaks (including mycobacteria) with 145 cases and 2 deaths between 1969 and 2008; endotoxin was responsible for 6 outbreaks with 177 cases and no deaths between 1973 and 1987. Four outbreaks were due to contamination with both bacteria and endotoxins with 53 cases and zero deaths. While outbreaks have decreased over time due to efforts to improve patient outcomes through professional practice guidelines and development of standards and regulatory oversight (see Fig. 1), chemicals, microorganisms, and endotoxins remain potential health threats. The peak years for outbreak investigations were between 1980 and 1989 and outbreaks investigated during this time were primarily associated with the introduction of high-flux dialysis, dialyzer reprocessing/reuse becoming a common practice among dialysis facilities, and errors in dialyzer reprocessing.
TABLE 4.
Outbreaks and adverse events caused by chemical intoxication associated with water in the dialysis setting within the United States (modified from Arduino et al. (15)). The 13 events listed below occurred between 1960 and 2007 for a total of 217 cases and 14 deaths
| Contamination | Description; cause | References |
|---|---|---|
| Aluminum | Intoxication and seizures in 7 patients; exhausted deionization tanks unable to remove aluminum in incoming tap water |
(73) |
| Intoxication neurologic symptoms, dementia and elevated serum levels in 64 patients, 3 deaths; aluminum pump was used to transfer acid concentrate to the treatment area |
(76) | |
| Elevated serum levels detected in 10 patients during routine screening; replacement pump used to pump acid concentrate contained aluminum components |
(77) | |
| Chloramine | Hemolytic anemia in 41 patients; residual disinfectant was not removed completely by the carbon tank when the facility increased the capacity of the water treatment system |
(74) |
| Copper | Hemolytic syndrome in 12 patients, 32 episodes with 4 fatalities; six hemodialysis centers had partially exhausted deionization system resulting in low pH water causing the formation of copper ions |
(28) |
| Fluoride | Intoxication in 8 patients, 1 death; accidental spill in hydrofluosilic acid at drinking water plant lead to excessive fluoride levels entering dialysis unit, insufficient treatment prior to dialysis |
(25) |
| Intoxication in 9 patients, 3 deaths; exhausted deionization tanks discharged a bolus of fluoride |
(24) | |
| Formaldehyde | Intoxication in 5 patients, 1 death; disinfectant not properly rinsed from the distribution system |
(105) |
| Intoxication in 12 patients; new filtration system was installed and not properly rinsed |
(80) | |
| Hydrogen peroxide | Decreased hemoglobin in 3 pediatric dialysis patients; H2O2 used to disinfect the system was not adequately rinsed from the system due to a flat bottom storage tank that could not be rinsed |
(78) |
| Nitrate | Patient developed methemoglobinemia; home dialysis using well water that contained nitrate nitrogen (94 mg/l) |
(27) |
| Sodium azide | Severe hypotension in 9 patients; dialysate contaminated with sodium azide used as a preservative from new ultrafilters, which were labeled “not for medical use” |
(79) |
| Sulfate(s) | Nausea, vomiting, chills, some with fever in 16 patients, 2 deaths; source water used to prepare dialysate contained volatile organic compounds (CS2, CH3, etc.) and additional failures |
(75) |
TABLE 5.
Outbreaks and adverse events caused by bacterial and/or endotoxin-associated contamination in water in the dialysis setting within the United States (modified from Arduino et al. 2010 (15)). The 20 events listed below span between 1969 and 2008 (events where reprocessing of dialyzers was a main contributor to the outbreak are noted by an asterisk)
| Contamination | Description; cause | References |
|---|---|---|
| Bacterial | Gram-negative bacteria bloodstream infections in 8 patients (Burkholderia cepecia
complex, Ralstonia sp., Pseudomonas aeruginosa, or Stenotrophomonas maltophilia); Burkholderia cepacia complex found in reverse osmosis water, gram-negative organisms detected in a patient dialyzer and solution distribution system |
(106) |
| Bacteremia episodes (~30) with the main gram-negative organisms being P. aeruginosa, Proteus, and Flavobacterium; bacteria was found in tap water and dialyzer resins, while no chlorine residual was detected after deionizer columns |
(83) | |
|
Pseudomonas cepacia recovered from 10 patients (13 cases of peritonitis); insufficient disinfection of contaminated tap water that was used for cleaning dialysis machines |
(84) | |
| Nontuberculous mycobacterial (NTM) infection (Mycobacterium chelonae subspecies abcessus), 27 cases; detected in water samples |
(85) | |
| Pyrogenic reactions in 14 patients, 2 with bacteremia and 1 death; reverse osmosis water storage tank contaminated with bacteria |
(36) | |
| *Intradialytic sepsis in 9 patients; gram-negative organisms detected in predialysis saline rinse, the source was either the dialysis fluid or water used for rinsing the dialyzers between uses |
(98) | |
| *Bacteremia in 6 patients; likely source(s) of the gram-negative bacteria were the dialysis fluid or water used for rinsing dialyzers prior to reuse, as well as the improper preparation of the new disinfectant |
(86) | |
| *Bloodstream infections of Klebsiella pneumonia in 6 patients; inadequate disinfection of reprocessed dialyzers, as technicians’ gloves were cross contaminating from infected patient |
(87) | |
| Endotoxin | Pyrogenic reaction in 49 patients; untreated tap water used to prepare the dialysate contained high levels of endotoxin |
(107) |
| Pyrogenic reaction in 45 patients; inadequate disinfection of the fluid distribution system |
(89) | |
| *Pyrogenic reactions in 13 patients; bacteria was detected in tap water and water used to prepare the bicarbonate dialysate, endotoxin was detected in the faucet of the reprocessing room and the water-spraying device used for rinsing |
(99) | |
| Pyrogenic reactions in 23 patients (49 episodes); increased endotoxin levels found in the tap water used to prepare the dialysate |
(100) | |
| *Pyrogenic reactions in 3 patients; change in reprocessing methods potentially altered the permeability characteristics allowing endotoxins to pass through membrane |
(96) | |
| *Pyrogenic reactions in 16 patients (18 episodes); endotoxin is the believed cause during reuse of dialyzers, water used to rinse dialyzers and dilute the disinfect was contaminated with high concentrations of endotoxins (>6 ng/ml) and bacteria (>104 CFU/ml) |
(88) | |
|
Combined:
Bacterial & Endotoxin |
Pyrogenic reactions and bacteremia in 5 patients (2 with Klebsiella pneumonia, 1 with K. pneumonia and P. aeruginosa); distribution systems and machines were inadequately disinfected with sodium hypochlorite when a pump failed 2 weeks prior to the outbreak |
(90) |
| Pyrogenic reactions (9 episodes) and gram-negative bacteremia (5 episodes) in 11 patients; water distribution system was not routinely disinfected, machine was not disinfected according to manufacturer’s instructions, poor bacterial assay resolution |
(91) | |
| *Pyrogenic reactions (~20) due to bacteria and/or endotoxins; reverse osmosis water was believed to be the source of contamination |
(92) | |
| *Pyrogenic reactions in 9 and gram-negative bacteremias in 5 patients; inadequate mixing of Renalin disinfectant |
(93) | |
| Nontuberculous mycobacteria | *A total of 27 cases with various infections: bacteremia in 14, soft-tissue infections in 3, and 1 with an access-graft infection, while 9 others had widely disseminated disease. Mycobacterium chelonae ssp. abscessus was identified in 26 isolates and the remaining isolate was a M. chelonae-like organism; the water treatment system showed widespread contamination and the processed dialyzers were contaminated with viable mycobacterium |
(95) |
| *Systemic M. chelonae abscessus infections in 5 patients, 1 patient died during antimicrobial therapy; a hose with a spray device was contaminated with M. abscessus and the Renalin disinfectant concentration was not high enough |
(94) |
Fig. 1.
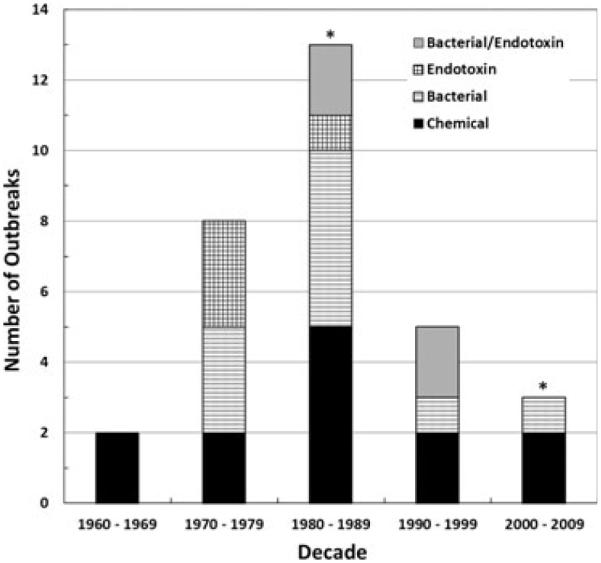
Reported outbreaks during dialysis treatment caused by water-associated contamination, where the specific causes of chemical, bacterial, endotoxin, and a combination of bacterial/endotoxin agent(s) are noted in the legend. The asterisk over the column for the decades 1980–1989 and 2000–2009 highlights where the first regulatory standard was mandated (2001) and the most recent Association for the Advancement of Medical Instruments (AAMI) recommendations were published (2008).
Chemical
Outbreaks of chemical toxicity or reported adverse events in dialysis patients are listed in Table 4. The reasons for patients being exposed to such toxic chemicals were water treatment failures at the drinking water treatment plant or dialysis center, incompatible dialysis solutions and distribution equipment/materials, and inadequate rinsing of dialysis systems after disinfection or newly installed dialysis systems. Aluminum (73), fluoride (24,25), chloramines (74), sulfur (75), and nitrates (27) have caused toxicity in patients due to water treatment failures. Water treatment failures at the drinking water provider level allowed aluminum and fluoride to be released at levels beyond the maximum allowable limits; and subsequent dialysis water treatment was inadequate in removing these substances. As a result, aluminum exposure resulted in seven cases of dialysis encephalopathy (characterized by speech and motor difficulties, seizures) and eight cases of fluoride exposure causing a variety of adverse effects (e.g., nausea, hypotension, substernal pain/pressure, diarrhea) (25,73).
Water treatment failure at the dialysis facility has been responsible for fluoride, chloramine, and sulfur chemical intoxication due to improper maintenance of internal treatment equipment and failure to perform monitoring (e.g., carbon filter, exhausted resins in deionized systems) prior to dialysis (24,74,75). The chemical intoxication caused by nitrates was due to lack of water treatment prior to home dialysis, as nitrates leached into the well water supply (27). Dialysis equipment containing aluminum parts has been responsible for aluminum toxicosis due to the incompatibility of the equipment with the acid concentrate component of the bicarbonate-based dialysis fluids (76,77). Hydrogen peroxide (78), sodium azide (79), and formaldehyde (25,80), however, were linked to chemical intoxication due to inadequate rinsing of the dialysis machine after disinfection procedures or of newly installed water treatment system components.
Microbiological
Bacteria are often detected in the water of dialysis systems and health risks are present when the concentrations are high enough. Microbes that have been detected and pose a threat are as follows (81): Burkholderia cepacia, Enterobacter cloacae, Flavobacterium spp., Klebsiella pneumonia, Pseudomonas spp. including P. aeruginosa, Ralstonia picketti, Sphingomonas paucimobilis, Stenotrophomonas maltophilia, and nontuberculous mycobacteria (NTM) species. Fungi, specifically Candida albicans, and Phialemonium curvatum, have also been found in dialysis systems, but are uncommonly present or associated with health impacts. Candida parapsilosis, however, has been associated with bloodstream infections (82).
A majority of microbial-caused dialysis outbreaks (13 of 20) were associated with inadequate disinfection, which were directly linked to bacteria (83–87), endotoxin (88,89), bacteria/endotoxin mix (90–93), and NTM species (94,95) (Table 5). Inadequate disinfection allowed concentrations of bacteria to propagate and endotoxin to increase in the following scenarios: inconsistent cleaning/disinfection of the facility’s tap water and commercial deionizer resins (every 1–3 weeks) (84), improper disinfection of the water distribution system when the flow meter valves were left open (89), and poorly mixing the dialyzer disinfectant with water to create a disinfectant solution with a >230% gradient differential between the top and bottom of the working solution container (93). There were also issues in microbial-associated outbreaks with what was believed to be an alteration of the dialyzers’ permeability characteristics when multiple disinfectants (e.g., 4% formaldehyde followed with peracetic acid) were being used to disinfect the dialyzers (86,96), or the introduction of a new chemical disinfectant (i.e., RenNew-D, Alcide Corporation, Norwalk, CT) that caused holes in dialyzer membranes, thereby allowing organisms to pass from dialysate into the blood stream (97), in addition to other complicating factors.
A significant finding is that the reuse of dialyzers has been associated with 50% of the microbial-associated outbreaks (79,86–88,93–96,98,99). Reprocessing or reuse of dialyzers renders the dialyzers vulnerable to contamination from water used for rinsing, inadequate disinfection, and potential alterations to the permeability of the membrane. Additionally, the combination of using reprocessed dialyzers and subsequent inadequate disinfection had led to NTM outbreaks (94,95). Poor infection control practices (77) and a gram-negative contaminated RO water storage tank (37) were also implicated in outbreaks. Seasonality has also been observed with an endotoxin-associated outbreak in a dialysis center lacking an RO water system (100). Drought conditions had caused an algal bloom in the water source, thus endotoxin-rich blue-green algae was present at high concentrations in the water used to prepare the dialysate (100).
Infection Control
Waterborne outbreaks in dialysis are connected to infection control practices due to the potential for cross-contamination. For example, if the technician had contaminated water or dialysate on their gloves, it is feasible for the technician to transfer microorganisms to the patient during treatment. Another example would include dialysis machinery being contaminated from droplets or inoculated hands, which then could potentially infect the patient. The CMS Conditions for Coverage (5) follows the Centers for Disease Control and Prevention publication, “Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis Patients” (101), also including the “Recommendations” narrative section for clarification.
Basic infection control practices include, but are not limited to, the following: staff wearing gloves while working with a dialysis patient, washing hands between patients, assuring items are cleaned before being introduced to a patient’s station, cleaning stations between patients, staff wearing personal protection equipment when appropriate (e.g., gowns), and keeping waste contained (101–103). Common breaches in infection control practice during hemodialysis treatment relevant to waterborne outbreaks include, but are not limited to, errors in dialyzer processing, backflow into blood lines from WHO ports, cross-contamination with dialysis fluids (e.g., wet hands and vascular access), and occasional undetected membrane leaks. A successful infection control program requires properly trained staff (104). Unfortunately, a majority of outpatient dialysis centers (>80%) may still lack well-developed infection control programs due to not being associated with a hospital (104). Additionally, the training for dialysis technicians and surveillance are limited (104). Dialysis centers, however, are required to follow certain recommendations for infection control, training requirements, and surveillance to be covered by CMS (5).
Summary
As technology and the clinical science on renal replacement therapy improves with an increasing patient population, hemodialysis therapies will continue to be an evolving, yet increasing, health treatment in the U.S. The changing water treatment at municipalities due to the nation’s variable water quality, rapid developments in membrane technology and water disinfection, and strains on our health system are important discussion points for the future. Dialysis centers should emphasize the importance of education and training to their employees, as well as supporting adequately resourced infection control programs. Requiring centers to report their water quality surveillance data, with regard to chemicals and microorganisms, would also instill accountability among the dialysis technicians and allow for trends to be determined in the U.S. However, the patient should be their own best advocate by being knowledgeable about the potential hazards that poor water quality can cause in hemodialysis. For improved patient outcomes, the ultimate goal is to eventually transition to the use of ultrapure fluids as the technology improves and to move toward a common evidence-based standard that is accepted internationally.
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.USRDS . U.S. Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [Google Scholar]
- 2.AAMI . TIR43: ultrapure dialysate for hemodialysis and related therapies. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2011. [Google Scholar]
- 3.Ward RA. Avoiding toxicity from water-borne contaminants in hemodialysis: new challenges in an era of increased demand for water. Adv Chronic Kidney Dis. 2011;18:207–213. doi: 10.1053/j.ackd.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.HHS CMS . Conditions for Coverage for End-Stage Renal Disease Facilities. 42 CFR Parts 405, 410, 413, 414, 488, and 494. Department of Human and Health Services, Centers for Medicare and Medicaid Services; Baltimore, MD: 2008. [Google Scholar]
- 6.ANSI/AAMI/ISO . Dialysate for hemodialysis RD-52:2004. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2004. [Google Scholar]
- 7.ANSI/AAMI/ISO . Quality of dialysis fluid for hemodialysis fluid and related therapies 11663:2009. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2009. [Google Scholar]
- 8.ANSI/AAMI/ISO . Guidance for the preparation and quality management of fluids for hemodialysis and related therapies 23500:2011. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2011. [Google Scholar]
- 9.Comty C, Luehmann D, Wathen R, Shapiro FL. Prescription water for chronic hemodialysis. Trans Am Soc Artif Intern Organs. 1974;10:189–196. [PubMed] [Google Scholar]
- 10.Kathuria P, Nair B, Schram D, Medlock R. Outbreak of lead poisoning in a hemodialysis unit. J Am Soc Nephrol. 2004;15:617A. [Google Scholar]
- 11.ANSI/AAMI/ISO . Water for hemodialysis and related therapies 13959:2009. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2009. [Google Scholar]
- 12.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 13.Ward RA. Ultrapure dialysate. Semin Dial. 2004;17:489–497. doi: 10.1111/j.0894-0959.2004.17617.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S. Essentials of water treatment in hemodialysis. Hemodial Intern. 2005;9:127–134. doi: 10.1111/j.1492-7535.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 15.Arduino MJ, Patel PR, Thompson ND, Favero MS. Hemodialysis-Associated Infections. Elsevier; Philadelphia, PA: 2010. [Google Scholar]
- 16.Association AWW . Water Quality and Treatment: A Handbook of Community Water Supplies. McGraw-Hill, Inc.; New York, NY: 1999. [Google Scholar]
- 17.Cappelli G, Ravera F, Ricardi M, Ballestri M, Perrone S, Albertazzi A. Water treatment for hemodialysis: a 2005 update. Cardiovasc Disorders Hemodial. 2005;149:42–50. doi: 10.1159/000085422. [DOI] [PubMed] [Google Scholar]
- 18.Cappelli G, Ballestri M, Facchini F, Carletti P, Lusvarghi E. Leaching and corrosion of polyvinyl-chloride (PVC) tubes in a dialysis water distribution system. Int J Artif Organs. 1995;18:261–263. [PubMed] [Google Scholar]
- 19.Andrysiak P. Design requirements for a water distribution system in a hemodialysis center. Dial Transpl. 2002;31:683–690. [Google Scholar]
- 20.Fagette P. Hemodialysis 1912–1945: no medical technology before its time – part I. Am Soc Artif Intern Organs J. 1999;45:238–249. [PubMed] [Google Scholar]
- 21.Ward RA. Water processing for hemodialysis. Part I: a historical perspective. Semin Dial. 1997;10:26–31. doi: 10.1111/j.1525-139X.1997.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 22.ANSI/AAMI/ISO . Water treatment equipment for hemodialysis appliations and related therapies 26722:2009. Association for the Advancement of Medical Instrumentation; Arlington, VA: 2009. [Google Scholar]
- 23.USEPA . National Primary Drinking Water Regulations Fact Sheet. EPA 816-F-09-004. Office of Water, United States Environmental Protection Agency; Washington, DC: May, 2009. [Google Scholar]
- 24.Arnow PM, Bland LA, Garcia-Houchins S, Fridkin S, Fellner SK. An outbreak of fata fluoride intoxication in a long-term hemodialysis unit. Ann Intern Med. 1994;121:339–344. doi: 10.7326/0003-4819-121-5-199409010-00005. [DOI] [PubMed] [Google Scholar]
- 25.CDC Fluoride intoxication in a dialysis unit – Maryland. Morb Mortal Wkly Rep. 1980;29:134–136. [Google Scholar]
- 26.Eaton JW, Kolpin CF, Swofford HS, Kjellstrand C-M, Jacob HS. Chlorinated urban water: a cause of dialysis-induced hemolytic anemia. Science. 1973;181:463–464. doi: 10.1126/science.181.4098.463. [DOI] [PubMed] [Google Scholar]
- 27.Carlson DJ, Shapiro FL. Methemoglobin from well water nitrates. A complication of hemodialysis. Ann Intern Med. 1970;73:757–759. doi: 10.7326/0003-4819-73-5-757. [DOI] [PubMed] [Google Scholar]
- 28.Ivanovich PA, Manzler A, Drake R. Acute hemolysis following hemodialysis. Trans Am Soc Artif Intern Organs. 1969;15:316–320. [PubMed] [Google Scholar]
- 29.Kovalchik MT, Kaehny WD, Higg AP. Aluminum kinetics during hemodialysis. J Lab Clin Med. 1978;92:712–720. [PubMed] [Google Scholar]
- 30.Masumaya J, Tachibana Y. Effects of water purification on renal osteodystrophy in the patients with regular hemodialysis therapy. J Jpn Soc Kidney Dis. 1984;26:407–416. [PubMed] [Google Scholar]
- 31.Petrie JJB, Row PG. Dialysis anemia caused by subacute zinc toxicity. Lancet. 1977;1:1178–1180. doi: 10.1016/s0140-6736(77)92718-0. [DOI] [PubMed] [Google Scholar]
- 32.Rao RKS, Friedman EA. Fluoride and bone disease in uremia. Kidney Int. 1975;7:125–129. doi: 10.1038/ki.1975.19. [DOI] [PubMed] [Google Scholar]
- 33.USEPA Methods for the determination of metals in environmental samples. 1994. Supplement 1 EPA-600/R-94/111.
- 34.ANSI/AAMI Hemodialysis systems. 2003. RD5:2003 (Revision of RD5:1992)
- 35.APHA/AWWA/WEF . Standard Methods For the Examination of Water and Wastewater. American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WEF); Washington, D.C.: 2012. [Google Scholar]
- 36.Favero MS, Petersen NJ, Boyer KM, Carson LA, Bond WW. Microbial contamination of renal dialysis systems and associated health risks. Trans Am Soc Artif Intern Organs. 1974;20A:175–183. [PubMed] [Google Scholar]
- 37.Favero MS, Carson LA, Bond WW, Petersen NJ. Factors that influence microbial contamination of fluids associated with hemodialysis machines. Appl Microbiol. 1974;28:822–830. doi: 10.1128/am.28.5.822-830.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favero MS, Petersen NJ, Carson LA, Bond WW, Hindman SH. Gram-negative water bacteria in hemodialysis systems. Health Lab Sci. 1975;12(4):321–324. [PubMed] [Google Scholar]
- 39.Amato RL. Water treatment for hemodialysis–updated to include the latest AAMI standards for dialysate (RD52: 2004) continuing. Nephrol Nurs J. 2005;32:151. [PubMed] [Google Scholar]
- 40.European Renal Association – European Dialysis and Transplant Association European best practice guidelines for heamodialysis (Part 1), Section IV: Dialysis fluid purity. Nephrol Dial Transplant. 2002;17:45–62. [PubMed] [Google Scholar]
- 41.Kawanishi H, Masakane I, Tadashi T. The new standard of fluids for hemodialysis in Japan. Blood Purif. 2009;27:5–10. doi: 10.1159/000213490. [DOI] [PubMed] [Google Scholar]
- 42.Canaud BJM. Changing paradigms of renal replacement therapy in chronic kidney disease patients: ultrapure dialysis fluid and high-efficiency hemodiafiltration for all? Kidney Int. 2009;76:591–593. doi: 10.1038/ki.2009.249. [DOI] [PubMed] [Google Scholar]
- 43.Masakane I. Review: clinical usefulness of ultrapure dialysate – recent evidence and perspectives. Ther Apher Dial. 2006;10:348–354. doi: 10.1111/j.1744-9987.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 44.Schiffl H. High-flux dialyzers, backfiltration, and dialysis fluid quality. Semin Dial. 2011;24:1–4. doi: 10.1111/j.1525-139X.2010.00786.x. [DOI] [PubMed] [Google Scholar]
- 45.Canaud B, Granger-Vallee A. Should ultrapure dialysate be part of standard therapy in hemodialysis? Semin Dial. 2011;24:426–427. doi: 10.1111/j.1525-139X.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- 46.Ledebo I. Ultrapure dialysis fluid” improving conventional and daily dialysis. Hemodial Intern. 2004;8:159–166. doi: 10.1111/j.1492-7535.2004.01090.x. [DOI] [PubMed] [Google Scholar]
- 47.Ledebo I. Ultrapure dialysis fluid – how pure is it and do we need it? Nephrol Dial Transplant. 2007;22:20–23. doi: 10.1093/ndt/gfl574. [DOI] [PubMed] [Google Scholar]
- 48.Tao J, Sun Y, Li X, Li H, Liu S, Wen Y, Duan L, Li Y, Li X. Conventional versus ultrapure dialysate for lowering serum lipoprotein(a) levels in patients on long-term hemodialysis: a randomized trial. Int J Artif Organs. 2010;33:290–296. [PubMed] [Google Scholar]
- 49.Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant. 2002;17:1814–1818. doi: 10.1093/ndt/17.10.1814. [DOI] [PubMed] [Google Scholar]
- 50.Schiffl H, Lang SM. Effects of dialysis purity on uremic dyslipidemia. Ther Apher Dial. 2009;14:5–11. doi: 10.1111/j.1744-9987.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 51.Arizono K, Nomura K, Motoyama T, Matsushita Y, Matsuoka K, Miyazu R, Takeshita H, Fukui H. Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood Purif. 2004;22(suppl 2):26–29. doi: 10.1159/000081870. [DOI] [PubMed] [Google Scholar]
- 52.Lederer SR, Schiffl H. Ultrapure dialysis fluid lowers the cardiovascular morbidity in patients on maintenance hemodialysis by reducing continuous microinflammation. Nephrology. 2002;91:452–455. doi: 10.1159/000064286. [DOI] [PubMed] [Google Scholar]
- 53.Schiffl H, Lang SM, Stratakis D, Fischer R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol Dial Transplant. 2001;16:1863–1869. doi: 10.1093/ndt/16.9.1863. [DOI] [PubMed] [Google Scholar]
- 54.Ward RA. Ultrapure dialysate: a desirable and achievable goal for routine hemodialysis. Semin Dial. 2000;13:378–380. doi: 10.1046/j.1525-139x.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- 55.Lacson E, Jr, Levin NW. C-reactive protein and end-stage renal disease. Semin Dial. 2004;17:438–448. doi: 10.1111/j.0894-0959.2004.17604.x. [DOI] [PubMed] [Google Scholar]
- 56.Glorieux G, Neirynck N, Veys N, Vanholder R. Dialysis water and fluid purity: more than endotoxin. Nephrol Dial Transplant. 2012;27:4010–4021. doi: 10.1093/ndt/gfs306. [DOI] [PubMed] [Google Scholar]
- 57.Sitter T, Bergner A, Schiffl H. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 2013;15:1207–1211. doi: 10.1093/ndt/15.8.1207. [DOI] [PubMed] [Google Scholar]
- 58.Sato T, Kurosawa A, Kurihaha T. Preparation of ultrapure dialysate in Japan – clinical usefulness and short-term future. Blood Purif. 2004;22:55–59. doi: 10.1159/000081876. [DOI] [PubMed] [Google Scholar]
- 59.Furuya R, Kumagai H, Takahashi M, Sano K, Hishida A. Ultrapure dialysate reduces plasma levels of beta(2)-microglobulin and pentosidine in hemodialysis patients. Blood Purif. 2005;23:311–316. doi: 10.1159/000086554. [DOI] [PubMed] [Google Scholar]
- 60.Honda H, Suzuki H, Hosaka N, Hirai Y, Sanada D, Nakamura M, Nagai H, Ashikaga E, Matsumoto K, Mukai M, Watanabe M, Akizawa T. Ultrapure dialysate influences serum myeloperoxidase levels and lipid metabolism. Blood Purif. 2009;28:29–39. doi: 10.1159/000210035. [DOI] [PubMed] [Google Scholar]
- 61.Schiffl H, Lang SM. Effects of dialysis purity on uremic dyslipidemia. Ther Apher Dial. 2010;14:5–11. doi: 10.1111/j.1744-9987.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 62.Izuhara Y, Miyata T, Saito K, Ishikawa N, Kakuta T, Nangaku M, Yoshida H, Saito A, Kurokawa K, de Strihou CV. Ultrapure dialysate decreases plasma pentosidine, a marker of “carbonyl stress”. Am J Kidney Dis. 2004;43:1024–1029. doi: 10.1053/j.ajkd.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Tielemans C, Husson C, Schurmans T, Gastaldello K, Madhoun P, Delville JP, Marchant A, Goldman M, Vanherweghem JL. Effects of ultrapure and non-sterile dialysate on the inflammatory response during in vitro hemodialysis. Kidney Int. 1996;49:236–243. doi: 10.1038/ki.1996.33. [DOI] [PubMed] [Google Scholar]
- 64.Lamas JM, Alonso M, Sastre F, Garcia-Trio G, Saavedra J, Palomares L. Ultrapure dialysate and inflammatory response in haemodialysis evaluated by darbepoetin requirements – a randomized study. Nephrol Dial Transplant. 2006;21:2851–2858. doi: 10.1093/ndt/gfl322. [DOI] [PubMed] [Google Scholar]
- 65.Susantitaphong P, Riella C, Jaber BL. Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: a meta-analysis. Nephrol Dial Transplant. 2013;28:438–446. doi: 10.1093/ndt/gfs514. [DOI] [PubMed] [Google Scholar]
- 66.Hsu PY, Lin CL, Yu CC, Chien CC, Hsiau TG, Sun TH, Huang LM, Yang CW. Ultrapure dialysate improves iron utilization and erythropoietin response in chronic hemodialysis patients – a prospective cross-over study. J Nephrol. 2004;17:693–700. [PubMed] [Google Scholar]
- 67.Arduino MJ, Bland LA, Aguero SM, Carson L, Ridgeway M, Favero MS. Comparison of microbiologic assay-methods for hemodialysis fluids. J Clin Microbiol. 1991;29(3):592–594. doi: 10.1128/jcm.29.3.592-594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ledebo I, Nystrand R. Defining the microbiological quality of dialysis fluid. Artif Organs. 1999;23:37–43. doi: 10.1046/j.1525-1594.1999.06275.x. [DOI] [PubMed] [Google Scholar]
- 69.Pass T, Wright R, Sharp B, Harding GB. Culture of dialysis fluids on nutrient-rich media for short periods at elevated temperatures underestimate microbial contamination. Blood Purif. 1996;14:136–145. doi: 10.1159/000170255. [DOI] [PubMed] [Google Scholar]
- 70.van der Linde K, Lim BT, Rondeel JMM, Antonissen L, de Jong GMT. Improved bacteriological surveillance of haemodialysis fluids: a comparison between Tryptic soy agar and Reasoner’s 2A media. Nephrol Dial Transplant. 1999;14:2433–2437. doi: 10.1093/ndt/14.10.2433. [DOI] [PubMed] [Google Scholar]
- 71.Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arduino MJ, Bland LA, Aguero SM, Favero MS. Effects of incubation-time and temperature on microbiologic sampling procedures for hemodialysis fluids. J Clin Microbiol. 1991;29(7):1462–1465. doi: 10.1128/jcm.29.7.1462-1465.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.CDC A Cluster of Seizures in a Hemodialysis Unit – Louisiana (EPI 81-39) 1982.
- 74.Tipple MA, Shusterman N, Bland LA, McCarthy MA, Favero MS, Arduino MJ, Reid MH, Jarvis WR. Illness in hemodialysis patients after exposure to chloramine contaminated dialysate. Trans Am Soc Artif Intern Organs. 1991;37(4):588–591. [PubMed] [Google Scholar]
- 75.Selenic D, Alvarado-Ramy F, Arduino M, Holt S, Cardinali F, Blount B, Jarrett J, Smith F, Altman N, Stahl C, Panlilio A, Pearson M, Tokars J. Epidemic parenteral exposure to volatile sulfur-containing compounds at a hemodialysis center. Infect Control Hosp Epidemiol. 2004;25:256–261. doi: 10.1086/502387. [DOI] [PubMed] [Google Scholar]
- 76.Burwen DR, Olsen SM, Bland LA, Arduino MJ, Reid MH, Jarvis WR. Epidemic aluminum intoxication in hemodialysis patients traced to use of an aluminum pump. Kidney Int. 1995;48:469–474. doi: 10.1038/ki.1995.315. [DOI] [PubMed] [Google Scholar]
- 77.CDC Elevated serum aluminum levels in hemodialysis patients associated with use of electric pumps – Wyoming, 2007. Morb Mortal Wkly Rep. 2008;57:689–691. [PubMed] [Google Scholar]
- 78.Gordon SM, Bland LA, Alexander SR, Newman HF, Arduino MJ, Jarvis WR. Hemolysis associated with hydrogen peroxide at a pediatric dialysis center. Am J Nephrol. 1990;10:123–127. doi: 10.1159/000168066. [DOI] [PubMed] [Google Scholar]
- 79.Gordon SM, Drachman J, Bland LA, Reid MH, Favero M, Jarvis WR. Epidemic hypotension in a dialysis center caused by sodium azide. Kidney Int. 1990;37:110–115. doi: 10.1038/ki.1990.15. [DOI] [PubMed] [Google Scholar]
- 80.Orringer EP, Mattern WD. Formaldehyde-induced hemolysis during chronic hemodialysis. N Engl J Med. 1976;294:1416–1420. doi: 10.1056/NEJM197606242942602. [DOI] [PubMed] [Google Scholar]
- 81.Arduino MJ. Dialysis-Associated Complications and Their Control. In: Jarvis WR, editor. Bennett and Brachman’s Hospital Infections. 5th Lippincott Williams and Wilkins; Philadelphia, PA: 2007. pp. 341–371. [Google Scholar]
- 82.Rosenberg J. Primary bloodstream infections associated with dialyzer reuse in California dialysis centers; 43rd Meeting of the Infectious Diseases Society of America.2005. [Google Scholar]
- 83.CDC Gram-negative infections in a hemodialysis unit – Miami, FL (EPI-71-42-2) Epidemic Investigations Report. 1971.
- 84.Berkelman RL, Godley J, Weber JA, Anderson RL, Lerner AM, Petersen NJ, Allen JR. Pseudomonas cepacia peritonitis associated with contamination of automatic peritoneal dialysis machines. Ann Intern Med. 1982;96:456–458. doi: 10.7326/0003-4819-96-4-456. [DOI] [PubMed] [Google Scholar]
- 85.CDC Epidemiologic notes and reports nontuberculous mycobacterial infections in hemodialysis patients – Louisianna, 1982. Morb Mortal Wkly Rep. 1983;32:244–246. [PubMed] [Google Scholar]
- 86.CDC Clusters of bacteremia and pyrogenic reactions in hemodialysis patients – Georgia (EPI-86-65-2) Epidemic Investigations Reports. 1987.
- 87.Welbel SF, Schoendorf K, Bland LA, Arduino MJ, Groves C, Schable B, O’Hara CM, Tenover FC, Jarvis WR. An outbreak of Gram-negative bloodstream infections in chronic hemodialysis patients. Am J Nephrol. 1995;15:1–4. doi: 10.1159/000168793. [DOI] [PubMed] [Google Scholar]
- 88.Gordon SM, Tipple M, Bland LA, Jarvis WR. Pyrogenic reactions associated with the reuse of disposable hollow-fiber hemodialyzers. J Am Med Assoc. 1988;260:2077–2081. [PubMed] [Google Scholar]
- 89.Petersen NJ, Boyer KM, Carson LA, Favero MS. Pyrogenic reactions from inadequate disinfection of a dialysis unit fluid distribution system. Dial Transplant. 1978;7:52–57. [Google Scholar]
- 90.CDC Epidemiolgic notes and reports: an outbreak of bacteremia and progenic reactions in a dialysis unit – Pennsylvania. Morb Mortal Wkly Rep. 1978;27:307–309. [Google Scholar]
- 91.Jackson BM, Beck-Sague CM, Bland LA, Arduino MJ, Meyer L, Jarvis WR. Outbreak of pyrogenic reactions and gram-negative bacteremia in a hemodialysis center. Am J Nephrol. 1994;14:85–89. doi: 10.1159/000168694. [DOI] [PubMed] [Google Scholar]
- 92.CDC Pyrogenic reactions in hemodialysis patients, California (EPI-92-34) Epidemic Investigations Report. 1992.
- 93.Beck-Sague CM, Jarvis WR, Bland LA, Arduino MJ, Aguero SM, Verosic G. Outbreak of gram-negative bacteremia and pyrogenic reactions in a hemodialysis center. Am J Nephrol. 1990;10:397–403. doi: 10.1159/000168155. [DOI] [PubMed] [Google Scholar]
- 94.Lowry PW, Beck-Sague CM, Bland LA, Aguero SM, Arduino MJ, Minuth AN, Murray RA, Swenson JM, Jarvis WR. Mycobacterium chelonae infection among patients receiving high-flux dialysis in a hemodialysis clinic in California. J Infect Dis. 1990;161:85–90. doi: 10.1093/infdis/161.1.85. [DOI] [PubMed] [Google Scholar]
- 95.Bolan G, Reingold AL, Carson LA, Silcox VA, Woodley CL, Hayes PS, Hightower AW, McFarland L, Brown JW, III, Petersen NJ, Favero MS, Good RC, Broome CV. Infections with Mycobacterium chelonae in Patients Receiving Dialysis and Using Processed Hemodialyzers. J Infect Dis. 1985;152:1013–1019. doi: 10.1093/infdis/152.5.1013. [DOI] [PubMed] [Google Scholar]
- 96.CDC Pyrogenic reactions in patients undergoing high-flux hemodialysis – California (EPI-86-80-2) Epidemic Investigations Report. 1987.
- 97.Centers for Disease Control and Prevention Epidemiologic notes and reports bacteremia associated with reuse of disposable hollow-fiber hemodialyzers. Morb Mortal Wkly Rep. 1986;32:417–418. [PubMed] [Google Scholar]
- 98.CDC Bacteremia associated with reuse of disposable hollow-fiber dialyzers (EPI-86-44-2) Epidemic Investigations Report. 1986.
- 99.CDC Pyrogenic reactions in hemodialysis patients on high-flux hemodialysis – California (EPI-87-12-2) Epidemic Investigations Report. 1987.
- 100.CDC Pyrogenic reactions in patients undergoing hemodialysis – Camp Springs, Maryland (EPI-75-24-2) Epidemic Investigations Report. 1976.
- 101.Centers for Disease Control and Prevention DoHaHS Recommendations for preventing transmission of infections among chronic hemodialysis patients. Morb Mortal Wkly Rep. 2001;50(RR-5) [PubMed] [Google Scholar]
- 102.Tokars JI, Arduino MJ, Alter MJ. Infection control in hemodialysis units. Infect Patients Chronic Ren Fail. 2001;15:797–812. doi: 10.1016/s0891-5520(05)70173-2. [DOI] [PubMed] [Google Scholar]
- 103.Arduino MJ, Tokars JI, Lyeria R, Alter MJ. Prevention of healthcare-associated transmission of bloodborne viruses in hemodialysis facilities. Semin Infect Control. 2001;1:49–60. [Google Scholar]
- 104.Kallen AJ, Arduino MJ, Patel PR. Preventing infections in patients undergoing hemodialysis. Expert Rev Anti Infect Ther. 2010;8:643–655. doi: 10.1586/eri.10.47. [DOI] [PubMed] [Google Scholar]
- 105.CDC Formaldehyde intoxication associated with hemodialysis – California (EPI-81-73-2) Epidemic Investigations Report. 1984.
- 106.CDC Outbreak of bloodstream infections at an outpatient dialysis center – Ohio, 2008 (EPI-2008-072) Epidemic Investigations Report. 2008.
- 107.Hindman SH, Favero MS, Carson LA, Petersen NJ, Schonberger LB, Solano JT. Pyrogenic reactions during haemodialysis caused by extramural endotoxin. Lancet. 1975;2(7938):732–734. doi: 10.1016/s0140-6736(75)90721-7. [DOI] [PubMed] [Google Scholar]


