Abstract
New derivatives of verapamil (1) modified with nitroxides and their precursors were synthesized and screened for reactive oxygen species (ROS) scavenging activities. The basic structure was modified by changing the nitrile group to an amide or the methyl substituent on tertiary nitrogen with nitroxides and their reduced forms (hydroxylamine and secondary amines). Among the new verapamil derivatives compound 16B (Mohan, I. K. et al. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 140.),1 modified with hydroxylamine salt of 2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridine-1-yloxyl proved to be the best ROS scavenger in vitro and protected HSMC and CHO cells against H2O2 induced damage.
Keywords: antioxidant activity, cardioprotective activity, EPR, free radicals, Langendorff-heart, nitroxide, verapamil
Introduction
The five- and six-membered nitrogen heterocyclic nitroxides and their diamagnetic derivatives (sterically hindered amine and hydroxylamine) are known as low-molecular-weight nonenzymatic multifunctional antioxidants as they can participate in one-electron redox processes. Nitroxides have attenuated oxidative damage in various experimental models, such as cultured cells,2 brain injury,3 lipid peroxidation in liver microsomes,4 postischemic reperfusion injury in isolated organs,5 and ionizing irradiation of rats and mice.6,7 This antioxidant activity is attributed to scavenging of oxygen-, nitrogen-, and carbon-centered radicals,8,9 and oxidation of reduced transition metal ions (such as Fe2+) pre-empting their participation in Fenton-reaction.10 The sterically hindered secondary amines oxidized to non-toxic nitroxides by ROS11 and hydroxylamines can act as a proton-donor antioxidants.9
Recently we have synthesized and investigated several biologically active molecules modified with nitroxides and their amine precursors, which showed antioxidant activity owing to the possible scavenging of various free radicals which cause deleterious biological processes such as oxidative stress.12 The paramagnetic analogues of ebselen,13 amiodarone,14,15 trimetazidine16,17 and mexiletine5 retained their essential activity and they exhibited increased antioxidant activity.14,17,18 These studies suggested, that nitroxides and their precursors’ antioxidant activity can be exploited when the nitroxide is attached to a drug molecule, e.g. this allows the binding of a nitroxide to a certain receptor or its accumulation in a membrane to scavenge the generated free radicals in statu nascendi. The superoxide-scavenging is a representative example of how doxorubicin-induced cardiomyopathy was prevented by a pyrroline ring containing experimental drug (H-2545) in a rat heart model.19
Verapamil, a known calcium-channel blocker, is used for the treatment of hypertension, angina pectoris, arrhythmia and an effective multi-drug resistance (MDR) reversing agent.20 Several new verapamil analogues were synthesized and tested21-27 and these studies concluded that both aromatic ring, nitrile group attached to a quaternary carbon and tertiary amine are important, while isopropyl group and substituents of the aminoethyl aromatic ring are less important. The substituents on the aromatic ring with benzyl cyanide group on carbon influences the calcium antagonistic activity. Therefore, verapamil seemed to be a well established model for modification with nitroxides and their precursors not only as calcium-channel antagonist acting on the L-type channels28 but as an MDR reversing agent. It was also elucidated that S-verapamil is 10-fold more potent calcium antagonist than R-verapamil, while this enantioselectivity was not observed in case of MDR reversing activity.29,30 It is interesting to note that Omote and Al-Shawi used a spin-labeled verapamil to study the transport mechanism of P-glycoprotein, which mediates multi drug resistance through its ability to transport various hydrophobic compounds across the plasma membrane.31 We now report modification of the racemic calcium antagonist verapamil (1) with nitroxides and their precursors on tertiary amine and on primary amine moiety; the latter was achieved by reduction of nitrile group. The new molecules might be important both for structure-cardiovascular activity relationship studies and MDR-reversing investigations, although in this paper only the former topic is outlined.
Chemistry
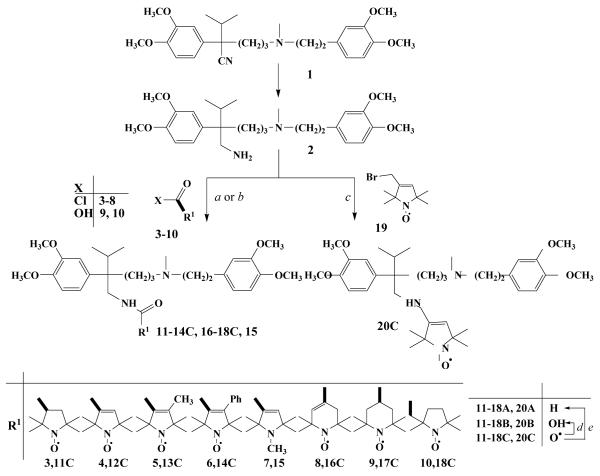
To achieve new verapamil (1) derivatives the nitrile group of the parent compound was reduced to primary amine with Red-Al in THF to yield compound 232 which was then acylated with 3-8 paramagnetic acid chlorides33 in CH2Cl2 in the presence of Et3N to afford 11C-16C nitroxide amides. Instead of unstable paramagnetic acid chlorides paramagnetic acids (9, 10) activated with DCC were used in synthesis of amides 17C-18C. The more water soluble hydroxylamines 11B-18B were prepared by refluxing 11C-18C with EtOH saturated previously with HCl gas9 and reduction of 11C-18C with iron powder in glacial acetic acid34 yielded 11A-18A. For further structure-activity relationship studies, the basicity of side chain was treasured. Therefore the primary amino group of compound 2 was alkylated with 19 paramagnetic allylic bromide35 in CHCl3 in the presence of K2CO3 to yield compound 20C which was converted to the more water soluble 20A and 20B derivatives as described above. (Scheme 1).
Scheme 1. Reagents and conditions.
(a) 3-8 (1.1 equiv.), Et3N (1.1 equiv.), CH2Cl2 0°C→ rt., 1 h; (b) 9, 10 DCC (1.0 equiv.), 4-dimethylaminopyridin (0.05 equiv.), rt., 24h (c) 19 (1.1 equiv.), K2CO3 (1.1 eq), 18-crown-6 (cat.), CHCl3, reflux, 8h; (d) EtOH/HCl, reflux, 20 min.; (e) Fe (10 equiv.), AcOH, 70 °C, 1 h, then K2CO3 to pH=9.
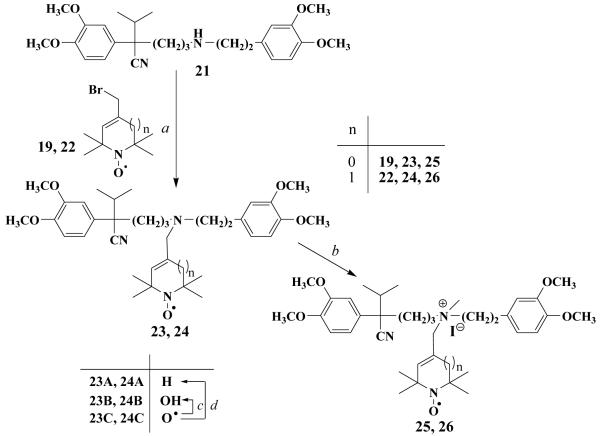
An evident procedure for getting further new verapamil derivatives was the alkylation of nor-verapamil (21) on the secondary nitrogen atom with allylic bromide 19 and with its six-membered analogue (22)36 in acetonitrile in the presence of K2CO3 to afford 23C and 24C. These derivatives were further reduced to 23B and 24B hydroxylamines by refluxing nitroxides in ethanolic HCl solution and to 23A and 24A amines by treatment of nitroxides with iron powder and glacial acetic acid. Methylating the tertiary nitrogen atom of compound 23C and 24C with methyl iodide, using methyl iodide as a solvent, in a sealed tube under pressure afforded the quaternary salts 25 and 26 as water-soluble compounds with an intact (non-reduced) nitroxide function (Scheme 2). Carboxylic acids (29, 30) were prepared by oxidation of aldehydes (27, 28) with H2O2/NaClO2 system in a buffer solution (Scheme 3).
Scheme 2. Reagents and conditions.
(a) 19 or 22 (1.1 equiv.), K2CO3 (1.1 eq), 18-crown-6 (cat.), acetonitrile, reflux, 3-8 h; (b) 23 or 24, MeI (5.0 equiv.), 50 °C, 48h; (c) EtOH/HCl, reflux, 20 min.; (d) Fe (10 equiv.), AcOH, 70 °C, 1 h, then K2CO3 to pH=9.
Scheme 3. Reagents and conditions.
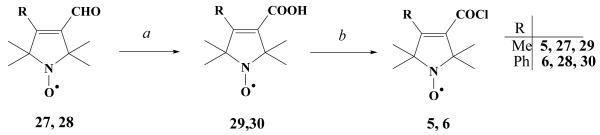
(a) NaClO2 (1.1 equiv.), H2O2 (0.9 equiv.), H2O-MeCN, KH2PO4, 0°C, 1 h, r. t., then Na2S2O5, H+; (b) SOCl2 (1.23 equiv.), pyridine (1.25 equiv.), benzene, 0°C, 10 min., then r. t., 40 min.
Results and discussion
The compounds synthesized were screened for superoxide and peroxyl radical-scavenging activity and protection of human aortic smooth muscle HSMC and Chinese hamster ovary CHO cells against H2O2-mediated cytotoxicity.
The protective effect of verapamil against oxygen free radical damage is well documented. Verapamil preserved myocite viability during exposure to hypoxantine and xantine oxidase.37
Recently Kovács et al. reported that in a Langendorff-perfused heart model experiment during ischemia and reperfusion verapamil decreased oxidative damage attributed to activation of Akt and ERK1/2 pathways, hence significantly contribute to cardioprotective effects.38 Villari et al. reported the benefical effect of Ca2+ antagonists to decreased myocardial demand during ischemia.39
Verapamil does not exhibit any superoxide-scavenging capability while all the other derivatives exhibit such ability. The primary amine acylated with pyrroline nitroxide 12A and 12B exhibits better protection than compound 1, however the N-hydroxylamine (12B) proved to be a better superoxide scavenger and was more effective than sterically hindered amine (12A); therefore, mainly the hydroxylamines were prepared and tested (Table 1). Among the acylated derivatives containing a saturated five-membered ring 11B, the six-membered rings (16B, 17B), 2-substituted saturated ring (18B) and unsaturated ring with 4-methyl or 4-phenyl groups (13B, 14B) have proven better superoxide scavenger than 12B. When N-hydroxylamine function is blocked as in case of compound 15 the superoxide-scavenging activity is decreased. When primary amine group is alkylated with five-membered ring (20A and 20B) the superoxide scavenging activity is better than 12A or 12B amide analogues. The derivatives where the tertiary amine of verapamil is modified with five- or six-membered nitroxide reduced form (N-hydroxylamine, 23B and 24B) are better superoxide scavengers than the amine derivatives (23A and 24A), while the quaternary salts such as compound 25 and 26 with nitroxide function exhibit more limited superoxide-scavenging capability than compounds 23B or 24B (Table 2 and Table 3).
Table 1.
Biological activity of new verapamil derivatives
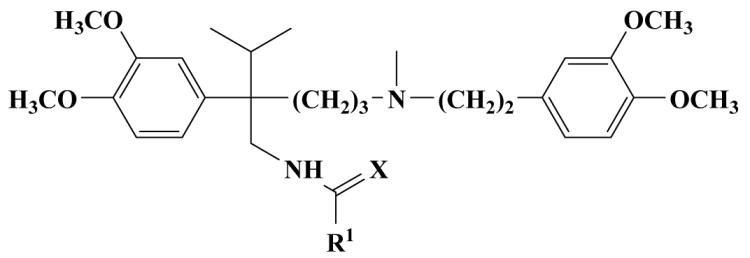
|
||||||
|---|---|---|---|---|---|---|
| Compound | R1 | X | Superoxide Scavenging (%) |
Peroxyl Scavenging (%) |
Cytotoxicity CHO (%) |
Cytotoxicity HSMC (%) |
|
1 verapamil |
- | - | 2.1 ± 4.2a | 60.0 ± 3.5a | 61.03 ± 6.2 | 55.95 ± 7.87 |
|
11B HO-4149 |
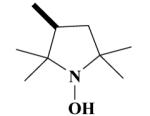
|
O | 100a 84.2±4.4b |
100a 98.0±0.5b |
62.1 ± 9.1 | 40.2 ± 3.8 |
|
12A H-3009 |
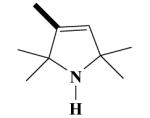
|
O | 34.9 ± 2.5 a | 84.1 ± 3.8a | 53.5 ± 11.0 | 53.7 ± 4.5 |
|
12B H-3010 |
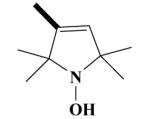
|
O | 55.2 ± 3.2 a | 14.9 ± 17.8 a | 48.9 ± 4.8 | 59.1 ± 4.5 |
|
13B HO-4148 |
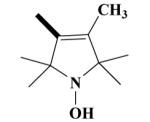
|
O | 100 a 78.2±4.9b |
100 a 100b |
60.0 ± 3.6 | 39.7 ± 4.2 |
|
14B HO-4099 |
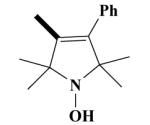
|
O | 100 a 79.3±1.2b |
100 a 86.1±0.1b |
68.3 ± 7.4 | 47.5 ± 3.0 |
|
15 HO-4087 |
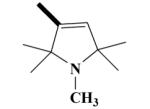
|
O | 60.7 ± 0.2 a | 89.8 ± 1.8 a | 55.5 ± 8.0 | 48.7 ± 5.2 |
|
16B HO-4038 |
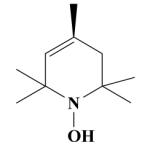
|
O | 100 a 66.5 ± 1.2 b |
100 a 91.4 ± 0.3 b |
46.1 ± 12.4 | 33.1 ± 6.9 |
|
17B HO-4218 |
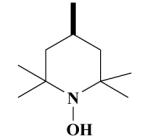
|
O | 100 a 76.5±4.3 b |
90.1 ± 3.4 a | 50.3 ± 10.4 | 40.2 ± 3.5 |
|
18B HO-4152 |
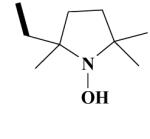
|
O | 100a 70.7±4.3b |
100a 89.2±0.1b |
54.0 ± 6.4 | 44.0 ± 6.2 |
|
20B HO-3886 |
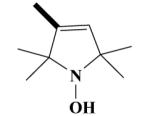
|
H2 | 100 a 88.5±4.2b |
100 a 92.9±1.0b |
63.6 ± 6.5 | 43.3 ± 7.3 |
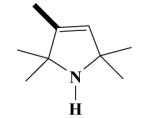
|
20A HO-3887 |
|
H2 | 100 a 73.8±1.0b |
100 a 89.8±3.9b |
71.2 ± 4.1 | 39.1 ± 4.6 |
Measured using 1-mM compound in presence of 1-mM DEPMPO
Measured using 100-μM compound in presence of 1-mM DEPMPO
Table 2.
The biological activity of N-alkyl derivatives achieved from nor-verapamil
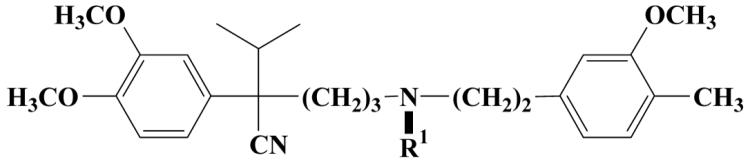
|
|||||
|---|---|---|---|---|---|
| Compound | R1 | Superoxide Scavenging (%) |
Peroxyl Scavenging (%) |
Cytotoxicity CHO (%) |
Cytotoxicity HSMC (%) |
|
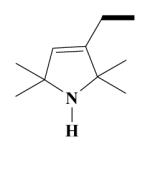
23A HO-3940 |
|
70.6 ± 3.3a | 92.2 ± 0.5a | 74.0 ± 3.4 | 64.8 ± 7.1 |
|
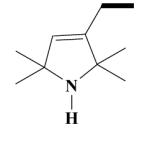
23B HO-3939 |
|
100a 78.1±2.6b |
100a 66.5±9.2b |
66.7 ± 3.0 | 58.6 ± 4.8 |
|
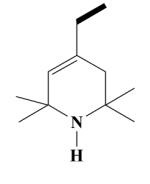
24A HO-3945 |
|
68.2 ± 7.6a | 90.6 ± 1.5a | 95.3 ± 0.5 | 91.1 ± 0.5 |
|
24B HO-3944 |
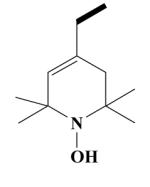
|
100a 82.5±2.7b |
100a 75.2±1.3b |
64.8 ± 5.6 | 57.2 ± 5.4 |
Measured using 1-mM compound in presence of 1-mM DEPMPO
Measured using 100-μM compound in presence of 1-mM DEPMPO
Table 3.
The biological activity of quaternary salts of verapamil
|
|||||
|---|---|---|---|---|---|
| Compound | R1 | Superoxide Scavenging (%) |
Peroxyl Scavenging (%) |
Cytotoxicity CHO (%) |
Cytotoxicity HSMC (%) |
|
25 HO-3983 |
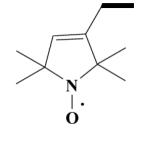
|
73.8 ± 3.0a | 47.9 ± 5.3a | 48.3 ± 11.0 | 41.9 ± 5.1 |
|
26 HO-3975 |
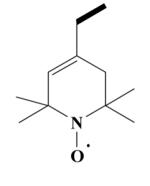
|
48.1 ± 2.7a | 40.4 ± 1.8a | 61.9 ± 5.7 | 33.4 ± 6.6 |
Measured using 1-mM compound in presence of 1-mM DEPMPO.
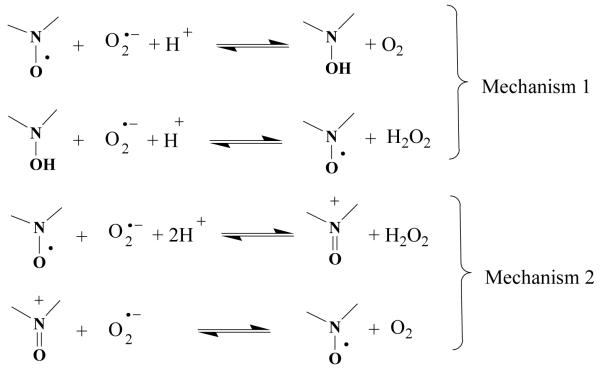
A similar tendency was observed for peroxyl radical-scavenging except that verapamil itself can scavenge peroxyl radicals up to 60% while the quaternary salts 25 and 26 and unexpectedly 12B are worse scavengers than verapamil itself. The superoxide-scavenging feature of hydroxylamines can be interpreted easily as hydroxylamines are spontaneously deprotonated in a buffer solution and oxidized back to nitroxide. Then nitroxide can dismutate superoxide via oxidation to oxoammonium cation (Figure 1). This ability is highly influenced by the redox potential depending on the substituent of the nitroxide rings. The in vitro results of superoxide-scavenging capability are in good accordance that saturated five-membered rings (such 11B, 18B), six-membered rings (such 16B, 17B) has lower redox potential40 and an electron-donating group on nitroxide ring also decreases the redox potential41,42 (13B, 14B, 20A, 20B, 23A, 23B, 24A, 24B), however when an electron-withdrawing group is attached - such as quaternary salt with permanent positive charge in case of compounds 25 and 26 - results in higher redox potential and hence worse capability of superoxide scavenging. The superoxide-scavenging abilities of new compounds were lowered by 20-30% by decreasing the concentration of verapamil derivatives from 1 mM to 100 μM, and 10-20% decrease were resulted in peroxyl-scavenging abilities. The competitive reaction with DEPMPO shows that the verapamil and derivatives are in general comparable or even better scavengers of peroxyl radicals than superoxide. This may indicate that the verapamils have a relatively higher reactivity toward peroxyl radicals when compared to superoxide in competing with DEPEMPO. However, a more rigorous evaluation of the reaction kinetics is required for further understanding of the contributing factors to the observed differences in the scavenging abilities.
Figure 1.
OD mimetic mechanisms of cyclic nitroxides
At first glance this would inspire to accomplish the synthesis only leading to six-membered rings with amine function only, however in case of amines the in vivo protonation and toxicity also should be considered. When comparing the cell-viability data in CHO cells and in vitro superoxide-scavenging capability, an obvious relationship can not be drawn. Compounds 20A, 23A, 24A behave as sensitizers and worse than the protection of verapamil, while N-hydroxy compounds (16B, 17B, 12B) and quaternary salt 25 exhibit good protection. HSMC cells are protected by quaternary salts 25, 26 N-hydroxylamines 13B, 14B, 20B, 16B, 17B and 20A sterically hindered amine. In the protection HSMC cells against H2O2 mediated toxicity, only compounds 23A and 24A behave as sensitizers, e. g. worse than verapamil.
Based on these results, compound 16B was chosen as the lead compound which exhibited fairly good superoxide and peroxyl-radical scavenging and cell-protection ability. The compound 16B was recently demonstrated to protect hearts against ischemia-reperfusion (I/R) injury in a Langendorf model. Hearts were subjected to 30 min of global ischemia followed by 45 min of reperfusion. Coronary flow (CF), (left ventricular developed pressure (LVDP), and rate pressure product (RPP) were continuously monitored before the induction of global ischemia and during the reperfusion. verapamil or 16B was administered to the heart via a side arm infusion for 1 min before the onset of ischemia. While hearts pre-treated with the verapamil showed a significant improvement in the recovery of contractile functions compared with the I/R hearts, hearts treated with 16B showed a significantly better recovery in cardiac function compared to verapamil alone.1 Similar studies performed using an in vivo model of I/R injury showed that 16B markedly attenuated superoxide production, increased nitric oxide generation, and enhanced Akt and Bcl-2 levels in the reperfused myocardium.
Conclusions
New verapamil derivatives were synthesized by modification on nitrile group and on tertiary nitrogen. The new compounds were tested on superoxide radical and peroxyl radical-scavenging and cell protection assays. Among the synthesized compounds, 16B compound modified on nitrile group with tetrahydropyridine ring was chosen as lead compound. Overall, the results demonstrated that 16B significantly protected hearts against I/R-induced cardiac dysfunction and damage through the combined beneficial actions of calcium-channel blocking, antioxidant, and prosurvival signaling activities.
Experimental
Melting points were determined with a Boethius micro melting point apparatus and are uncorrected. Elemental analyses (C, H, N, S) were performed on Fisons EA 1110 CHNS elemental analyzer. Mass spectra were recorded on a Thermoquest Automass Multi and VG TRIO-2 instruments in the EI mode and ESI-TOF MS measurements were performed with a BioTOF II instrument (Bruker Daltonics, Billerica, MA). 1H NMR spectra were recorded with Varian UNITYINOVA 400 WB spectrometer. Chemical shifts are referenced to Me4Si. Measurements were run at 298K probe temperature in CDCl3 solution. ESR spectra were taken on Miniscope MS 200 in 10−4 M CHCl3 solution and all monoradicals gave triplet line, aN = 14.7-16.4 G. Flash column chromatography was performed on Merck Kieselgel 60 (0.040-0.063 mm). Qualitative TLC was carried out on commercially prepared plates (20 × 20 × 0.02 cm) coated with Merck Kieselgel GF254. Compounds 2,32 4,33 9,43 10,44 19,35 2236 were prepared according to published procedures. Acid chlorides 3, 5, 6, 7, 8 were prepared from the corresponding carboxylic acids33,36 analogously for the preparation of compound 4 and used immediately in the acylation step without isolation. Compound 1, 21 and all other reagents were purchased from Aldrich and Sigma or received as a kind donation of Sanofi-Aventis (Budapest, Hungary).
Acylation of compound 2 with acid chlorides, General procedure (11-16C)
To a solution of compound 2 (917 mg, 2.0 mmol) and Et3N (222 mg, 2.2 mmol) in CH2Cl2 (30 mL) 3-8 acid chlorides (2.22 mmol) dissolved in CH2Cl2 (5 mL) were added dropwise at 0°C. After stirring at r.t. for 1 h, the solvent was washed with brine (10 mL), the organic phase was separated, dried (MgSO4), filtered and evaporated. The residue was purified by flash column chromatography (hexane/EtOAc) to give the title compounds in 50-69 %.
1-Oxyl-2,2,5,5-tetramethyl-pyrrolidine-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (11C)
Yield 664 mg 53%; brown oil. MS (EI) m/z (%): 626 (M+, 9), 475(16), 594(2), 151(100). Anal. Calcd for C36H56N3O6: C, 68.98; H, 9.00; N, 6.70. Found: C, 69.18; H, 8.90; N, 6.66.
1-Oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (12C)
Yield 850 mg 68%; pale-brown oil. MS (EI) m/z (%): 624 (M+, 3), 594(10), 458(59), 151(100). Anal. Calcd for C36H54N3O6: C, 69.20; H, 8.71; N, 6.73. Found: C, 69.16; H, 8.80; N, 6.68.
1-Oxyl-2,2,4,5,5-pentamethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4- dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (13C)
Yield 690 mg 54%; brownish-yellow oil. MS (EI) m/z (%): 638 (M+, 1), 487(9), 303(54), 151(66), 43(100). Anal. Calcd for C37H56N3O6: C, 69.56; H, 8.84; N, 6.58. Found: C, 69.55; H, 8.61; N, 6.51
1-Oxyl-2,2,5,5-tetramethyl-4-phenyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (14C)
Yield 700 mg 50%; brown solid; mp. 75-77oC. MS (ESI): 701 [M+1]+. Anal. Calcd for C42H58N3O6: C, 71.97; H, 8.34; N, 5.99. Found: C,71.89; H, 8.11; N, 5.71.
1,2,2,5,5-Pentamethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimethoxy-phenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide (15)
Yield 711 mg 57%; orange oil. MS (EI) m/z (%): 623 (M+, <1), 608(1), 472(20), 151(45), 43(100). Anal. Calcd for C37H57N3O5: C, 71.23; H, 9.21; N, 6.74. Found: C, 71.25; H, 8.90; N, 6.63. 1H NMR ( D2O) : 6.91-6.87 (d, ArH, 2H); 6.85-6.79 (d, ArH, 2H); 6.70 (d, ArH, 2H); 6.16 (s, CH, 1H); 3.75, 3.74, 3.73, 3.71 (4s, 4OCH3, 12H); 3.65-3.55 (t, NCH2, 2H); 3.14-3.0 (m, 2NCH2, 4H); 2.85-2.80 (t, CH2, 2H); 2.79 (s, NCH3, 3H); 2.5 (s, NCH3, 3H); 2.0-1.85 (m, CH2, 2H); 1.82-1.70 (t, CH2, 2H); 1.50, 1.43, 1.38, 1.31 (4s, 4CH3, 12H); 1.25-1.19 (m, CH, 1H); 0.73 (d, J = 6,6 Hz, CH3, 3H); 0.70 (d, J = 6,2 Hz, CH3, 3H).
1-Oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydro-pyridine-4-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (16C)
Yield 638 mg 50%; brownish solid; mp 106-108°C. MS (EI) m/z (%): 638 (M+, <1), 487(7) 472(2), 156(62), 43(100). Anal. Calcd for C37H56N3O6: C, 69.56; H, 8.84; N, 6.58. Found: C, 69.48; H, 8.90; N, 6.46.
Acylation of compound 2 with acids, General procedure (17C, 18C)
The solution of the acids (2.0 mmol), 2 amine (917mg, 2.0 mmol) and 4-dimethylamino-pyridine (12 mg, 0.1 mmol) in dry ethyl-acetate (20 mL) was stirred for 10 min. at room temperature, then DCC (412 mg, 2.0 mmol) dissolved in EtOAc (10 mL) was added, and the mixture was stirred at r.t. for 24h. The mixture was filtered, the filtrate was evaporated, the residue was dissolved in CHCl3 (30 mL), washed with brine (20 mL) and separated. The organic phase was dried (MgSO4), filtered and evaporated. The residue was purified by flash column chromatography (CHCl3/Et2O) to give the compounds as yellow or red oils.
1-Oxyl-2,2,6,6-tetramethyl-piperidine-4-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide Radical (17C)
Yield 600 mg 46%; orange solid, mp. 52-64°C. (Turbo Spray) m/z (%): 641 ([M+H]+, 100). Anal. Calcd for C37H58N3O6: C, 69.34; H, 9.12; N, 6.56. Found: C, 69.54; H, 9.56; N, 6.54.
N-(2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentyl-2-(1-oxyl-2,5,5-trimethyl-pyrrolidin-2-yl)-acetamide Radical (18C)
Yield 538 mg 42%; yellow oil. (EI) m/z (%): 626 (M+, 1), 475(5) 151(100). Anal. Calcd for C36H56N3O6: C, 68.98; H, 9.00; N, 6.70. Found: C, 68.74; H, 8.96; N, 6.53.
3-[(2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-methylamino]-2-isopropyl-pentylamino)methyl]-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl Radical (20C)
A solution of compound 2 (917 mg, 2.0 mmol), 19 allylic bromide (513 mg, 2.2 mmol), K2CO3 (303 mg, 2.2 mmol), 18–crown-6 (50 mg) in CHCl3 (30 mL) was stirred and refluxed for 8h, the inorganic salt was filtered off, the filtrate was washed with water (10 mL), the organic phase was separated, dried (MgSO4), filtered and evaporated. The residue was purified by flash column chromatography (CHCl3/Et2O) to give the title compound 450 mg (36 %). Pale yellow solid, mp 65-67°C. (EI) m/z (%): 610 (M+, 6), 459(2) 278(33), 181(38), 151(100). Anal. Calcd for C36H56N3O5: C, 70.78; H, 9.24; N, 6.88. Found: C, 70.75; H, 9.20; N, 6.71.
Alkylation of nor-verapamil (21), general procedure (23C, 24C)
A mixture of nor-verapamil 21 (880 mg, 2.0 mmol), finely powdered K2CO3 (203 mg, 2.2 mmol) and 18-crown-6 (50 mg) in dry acetonitrile (40 mL) was stirred for 10 min., then paramagnetic alkyl-halogenide 19 or 22 (2.2 mmol) dissolved in acetonitrile (15 mL) was added in one portion, the mixture was stirred and refluxed for 8 h. After cooling the inorganic salts were filtered off, the acetonitrile was evaporated, the residue was dissolved in CHCl3 (40 mL), the organic phase was washed with brine (20 mL), separated, dried (MgSO4), filtered and evaporated. The remaining oil was purified by flash column chromatography (hexane/EtOAc) to produce the title compounds in 48–61% yield.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-ylmethyl)-amino]-2-isopropyl-pentanenitrile Radical (23C)
Yield 723 mg 61%; orange oil. (EI) m/z (%): 592 (M+, 1), 441(22), 426(19), 151(67), 43(100). Anal. Calcd. for C35H50N3O5: C, 70.91; H, 8.50; N, 7.09. Found: C, 70.87; H, 8.43; N, 7.22.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(1-oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-4-ylmethyl)amino]-2-isopropyl-pentanenitrile Radical (24C)
Yield 580 mg 48%; brown oil. (EI) m/z (%): 606 (M+, 1), 455(33) 289(85), 151(100). Anal. Calcd. for C36H52N3O5. C, 71.25; H, 8.64; N, 6.92. Found: C, 71.29; H, 8.54; N, 7.09.
Synthesis of quaternary salts, general procedure (25, 26)
The solution of compounds 23C or 24C (2.0 mmol) dissolved in iodomethane (5 mL) in sealed tube was heated at 50°C for 48 h. After cooling, iodomethane was removed by evaporation, then the residue was triturated with Et2O, the salt was filtered off, to give yellow crystals in 60-70% yield.
[4-Cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl]-[2-(3,4-dimethoxyphenyl)ethyl]-(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-ylmethyl)methylammonium Iodide Salt Radical (25)
Yield 1.028 g 70%; pale-yellow solid; mp 110-112°C. Anal. Calcd. for C36H53IN3O5: C, 58.85; H, 7.27; N, 5.72. Found: C, 58.56; H, 7.17; N, 5.67.
[4-Cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl]-[2-(3,4-dimethoxyphenyl)ethyl]-(1-oxyl-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-4-ylmethyl)methylammonium Iodide Salt Radical (26)
Yield 0.928 g 62%; yellow solid; mp 167-170°C. Anal. Calcd. for C37H55IN3O5: C, 59.35; H, 7.40; N, 5.61. Found: C, 59.15; H, 7.25; N, 5.69.
General procedure for N-hydroxylamine salt formation (11B, 12B, 13B, 14B, 16B, 17B, 18B, 20B, 23B, 24B)
A solution of radicals 11C or 12C or 13C or 14C or 16C or 17C or 18C or 20C or 23C or 24C (1.0 mmol) was heated under reflux for 20 min in EtOH (10mL) saturated with HCl previously. The solvent was evaporated off; the residue was crystallized with acetone or Et2O to give white or pale yellow solids in 60-78% yield.
1-Hydroxy-2,2,5,5-tetramethyl-pyrrolidine-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide HCl Salt (11B)
Yield 470 mg 67%; white solid, mp. 122-124°C. 1H NMR (D2O): 6.90 (d, J = 8 Hz, ArH, 2H); 6.87 (s, ArH, 2H); 6.73 (d, J = 8.7 Hz, ArH, 2H); 4.79-4.71 (t, CH, 1H); 3.78, 3.76, 3.75, 3.72 (4s, 4OCH3, 12H); 3.52-3.46 (t, NCH2, 2H); 3.31-3.19 (t, NCH2, 2H); 3.12 (s, NCH2, 2H); 2.86-2.81 (t, CH2, 2H); 2.79 (s, NCH3, 3H); 2.09 (t, CH2, 2H); 2.14 (d, J = 7.5 Hz, CH2, 2H); 1.99-1.92 (m, CH2, 2H); 1.78-1.69 (m, CH2, 2H); 1.37 (s, CH3, 3H); 1.335 (d, J = 6 Hz, CH3, 3H); 1.27 (s, CH3, 3H); 1.15 (d, J = 6.5 Hz, CH3, 3H); 1.10-1.02 (m, CH, 1H); 0.77 (d, J = 6 Hz, CH3, 3H); 0.70 (d, J = 6.6 Hz, CH3, 3H). Anal. Calcd for C36H58Cl2N3O6: C, 61.79; H, 8.35; N, 6.00. Found: C, 61.58; H, 8.23; N, 5.87.
1-Hydroxy-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimeth-oxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide HCl Salt (12B)
Yield 530 mg 76%; white solid, mp. 120-121°C. 1H NMR (DMSO-d6) : 6.93 (d, J = 11 Hz, ArH, 2H); 6.85 (d, J = 8.3 Hz, ArH, 2H); 6.68, 6.70 (2s, ArH, 2H); 6.15 (d, J = 5.5 Hz, CH, 1H); 3.77, 3.73, 3.71, 6.70 (4s, 4OCH3, 12H); 3.28 (s, NCH2, 2H); 3.20-3.01 (m, NCH2, 4H); 2.88-2.84 (t, CH2, 2H); 2.63 (s, NCH3, 3H); 2.20-2.12 (m, CH2, 4H); 2.06 (s, CH3, 6H); 1.60 (s, CH3, 3H); 1.51 (s, CH3, 3H); 1.32-1.20 (m, CH, 1H); 1.09 (d, J= 6.5 Hz, CH3, 3H); 0.68 (d, J = 6.8 Hz, CH3, 3H). Anal. Calcd for C36H56Cl2N3O6: C, 61.97; H, 8.09; N, 6.02. Found: C, 61.86; H, 8.01; N, 5.81.
1-Hydroxy-2,2,4,5,5-pentamethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-di-methoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide HCl Salt (13B)
Yield 505 mg 71%; white solid, mp. 123-125°C. 1H NMR (D2O) : 6.92 (s, ArH, 2H); 6.86 (d, J = 8.9 Hz, ArH, 2H); 6.76 (d, J = 9.1 Hz, ArH, 2H); 3.77 (s, 2OCH3, 6H); 3.75 (s, 2OCH3, 6H); 3.65-3.55 (t, NCH2, 2H); 3.30-3.25 (m, NCH2, 2H); 3.09-3.06 (t, NCH2, 2H); 2.92-2.88 (m, CH2, 2H); 2.83 (s, NCH3, 3H); 2.78 (s, CH3, 3H); 2.0-1.92 (m, CH2, 2H); 1.71-1.64 (t, CH2, 2H); 1.55, 1.42, 1.37, 1.34 (4s, 4CH3, 12H); 0.96-0.92 (m, CH, 1H); 0.76 (d, J = 6.6 Hz, CH3, 3H); 0.72 (d, J = 5.5 Hz, CH3, 3H). Anal. Calcd for C37H58Cl2N3O6: C, 62.44; H, 8.21; N, 5.90. Found: C, 62.25;H, 8.08; N, 5.74.
1-Hydroxy-2,2,5,5-tetramethyl-4-phenyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide HCl Salt (14B)
Yield 503 mg 65%; white solid, mp. 121-123°C. 1H NMR (D2O) : 7.40 (d, J = 7 Hz, ArH, 2H); 7.33-7.30 (m, ArH, 2H); 7.10 (d, J = 6 Hz, ArH, 2H); 6.94-6.86 (m, ArH, 4H); 6.63-6.56 (t, ArH, 1H); 3.78 (s, OCH3, 6H); 3.77, 3.75 (2s, 2OCH3, 6H); 3.63-3.56 (t, NCH2, 2H); 3.49-3.42 (t, NCH2, 2H); 3.25 (s, NCH2, 2H); 2.96-2.89 (m, CH2, 2H); 2.80 (s, NCH3, 3H); 1.67-1.60 (m, CH2, 4H); 1.58, 1.48, 1.46, 1.40 (4s, 4CH3, 12H); 0.86-0.75 (m, CH, 1H); 0.59 (d, J = 7.2 Hz, CH3, 6H). Anal. Calcd for C42H60Cl2N3O6: C, 65.19; H, 7.81; N, 5.43. Found: C, 64.99; H, 7.66; N, 5.29.
1-Hydroxy-2,2,6,6-tetramethyl-1,2,3,6-tetrahydro-pyridine-4-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide HCl Salt (16B)
Yield 448 mg 63%; brownish white solid, mp. 125-127°C. 1H NMR (D2O) : 6.94 (d, J = 9 Hz, ArH, 2H); 6.87 (d, J = 8.6 Hz, ArH, 2H); 6.81, 6.73 (2s, ArH, 6H); 6.14 (s, CH, 1H); 3.76, 3.74 (2s, 4OCH3, 12H); 3.67 (s, NCH2, 2H); 3.28-3.16 (t, NCH2, 2H); 3.11-2.98 (t, NCH2, 2H); 2.93-2.85 (m, CH2, 2H); 2.79 (s, NCH3, 3H); 1.96 (s, CH2, 2H); 1.76-1.65 (t, CH2, 2H); 1.60-1.54 (m, CH2, 2H); 1.50-1.40 (m, CH3, 12H); 1.20-1.11 (m, CH, 1H); 0.75 (d, J = 6.6 Hz, CH3, 6H). Anal. Calcd for C37H58Cl2N3O6: C, 62.44; H, 8.21; N, 5.90. Found: C, 62.31; H, 8.15; N, 5.75.
1-Hydroxy-2,2,6,6-tetramethyl-piperidine-4-carboxylicacid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxy-phenyl)ethyl]-methyl-amino]-2-isopropyl-pentyl-amide HCl Salt (17B)
Yield 428 mg 60%; pale yellow solid, mp. 143-145°C. 1H NMR (D2O) : 6.90 (d, J = 6.6 Hz, ArH, 2H); 6.84 (d, J = 6.9 Hz, ArH, 2H); 6.74 (s, ArH, 2H); 4.75-4.60 (m, CH, 1H); 3.76-3.73 (2s, 4OCH3, 12H); 3.65-3.55 (t, NCH2, 3H); 3.29-3.21 (t, NCH2, 2H); 3.10 (s, NCH2, 2H); 2.90-2.83 (m, CH2, 2H); 2.81 (s, NCH3, 3H); 2.19-2.13 (t, CH2, 2H); 1.99-1.91 (d, CH2, 2H); 1.76-1.69 (m, CH2, 4H); 1.50, 1.39, 1.30, 1.28 (4s, 4CH3, 12H); 1.20-1.11 (m, CH, 1H); 0.99 (d, J = 6.8 Hz, CH3, 3H); 0.72 (d, J = 6.6 Hz, CH3, 3H). Anal. Calcd for C37H60Cl2N3O6: C, 62.26; H, 8.47; N, 5.89. Found: C, 62.13; H, 8.38; N, 5.71.
N-(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentyl-2-(1-hydroxy-2,5,5-trimethyl-pyrrolidin-2-yl)-acetamide HCl Salt (18B)
Yield 440 mg 63%; white solid, mp. 108-110°C. 1H NMR (D2O) : 6.98 (d, J = 8.3 Hz, ArH, 2H); 6.88 (d, J =11.0 Hz, ArH, 2H); 6.82, 6.73, (2s, ArH, 2H); 3.80, (d, J = 2.3 Hz, OCH3, 3H); 3.75, (d, J = 3.1 Hz, OCH3, 3H); 3.72, 3.67 (2s, 2OCH3, 6H); 3.50-3.47 (m, CH2CO, 2H); 3.45 (s, NCH2, 2H); 3.33-3.19 (m, NCH2, 2H); 3.18-3.04 (m, NCH2, 2H); 2.83-2.86 (t, CH2, 2H); 2.69 (s, NCH3, 3H); 2.05-1.98 (m, CH2, 2H); 1.85-1.72 (m, CH2, 2H); 1.42, 1.39, 1.30 (3CH3, 9H); 1.26-1.21 (m, CH, 1H); 1.20 (t, J = 7 Hz, CH2, 2H); 1.10 (t, J = 7,0 Hz, CH2, 2H); 0.78 (d, J = 6.8 Hz, CH3, 3H); 0.74 (d, J = 5.6 Hz, CH3, 3H). Anal. Calcd for C36H58 l2N3O6: C, 61.79; H, 8.35; N, 6.00. Found: C, 61.68; H, 8.20; N, 5.82.
1-Hydroxy-3-[(2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxy-phenyl)ethyl]-methylamino]-2-isopropyl-pentylamino)methyl]-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol, HCl Salt (20B)
Yield 530 mg 73%; white solid, mp. 122-124°C. 1H NMR (D2O) : 6.93 (d, J = 9.7 Hz, ArH, 2H); 6.82 (d, J = 6.1 Hz, ArH, 2H); 6.76 (s, ArH, 2H); 5.20 (s, CH, 1H); 3.80, 3.78, 3.74, 3.73 (4s, 4OCH3,12H); 3.69 (s, NCH2, 2H); 3.40 (s, NCH2, 2H); 3.38-3.30 (m, NCH2, 2H); 3.20-3.10 (m, NCH2, 2H); 2.84 (NCH3, 3H); 2.78 (t, CH2, 2H); 2.28-2.18 (q, CH2, 2H); 1.87-1.80 (t, CH2, 2H); 1.55, 1.42, 1.48, 1.42 (4s, 4CH3, 12H); 1.28-1.20 (m, CH, 1H); 0.90 (d, J = 6.0 Hz, CH3, 3H); 0.79 (d, J = 5.9 Hz, CH3, 3H). Anal. Calcd for C36H59Cl3N3O5: C, 60.03; H, 8.26; N, 5.83. Found: C, 59.94; H, 8.19; N, 5.73.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(1-hydroxy-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-ylmethyl)-amino]-2-isopropyl-pentanenitrile HCl Salt (23B)
Yield 525 mg 79%; white solid, mp. 122-124°C. 1H NMR (D2O) : 6.95 (d, J = 7.2 Hz, ArH, 2H); 6.85 (d, J = 9.1 Hz, ArH, 2H); 6.72 (s, ArH, 2H); 5.85 (s, CH, 1H); 3.77 (s, 2OCH3, 6H); 3.75, 3.74 (2s, 2OCH3, 6H); 3.38 (s, NCH2, 2H); 3.29-3.20 (t, NCH2, 2H); 3.17-3.09 (t, NCH2, 2H); 2.90-2.80 (t, CH2, 2H); 2.22-2.10 (q, CH2, 2H); 1.98-1.90 (m, CH2, 2H); 1.43, 1.40, 1.37, 1.32 (4s, 4CH3, 12H); 1.30-1.20 (m, CH, 1H); 1.06 (d, J = 6.5 Hz, CH3, 3H); 0.63 (d, J = 5.9 Hz, CH3, 3H). Anal. Calcd. for C35H52Cl2N3O5: C, 63.15; H, 7.87; N, 6.31. Found: C, 63.07; H, 7.74; N, 6.15.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(1-hydroxy-2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-4-ylmethyl)amino]-2-isopropyl-pentanenitrile HCl Salt(24B)
Yield 468 mg 69%; white solid, mp. 164-166°C. 1H NMR (D2O) : 6.93 (d, J= 9.5 Hz, ArH, 2H); 6.85 (d, J = 8.9 Hz, ArH, 2H); 6.72 (s, ArH, 2H); 5.79 (s, CH, 1H); 3.77 (s, 2 OCH3, 6H); 3.75, 3.74 (2s, 2 OCH3, 6H); 3.40 (s, NCH2, 2H); 3.27-3.20 (t, NCH2, 2H); 3.19-3.14 (t, NCH2, 2H); 2.89-2.80 (t, CH2, 2H); 2.23 (s, CH2, 2H), 2.20-2.10 (q, CH2, 2H); 1.96-1.90 (m, CH2, 2H); 1.45, 1.40, 1.39, 1.35 (4s, 4CH3, 12H); 1.24-1.16 (m, CH, 1H); 1.06 (d, J = 6.2 Hz, CH3, 3H); 0.66 (s, J = 6.0 Hz, CH3, 3H). Anal. Calcd. for C36H54Cl2N3O5. C, 63.61; H, 8.01; N, 6.18. Found: C, 63.44; H, 7.82; N, 6.02.
Reduction of nitroxides to amines; general procedure (12A, 20A, 23A, 24A)
To a solution of nitroxide 12C or 20C or 23C or 24C (2.0 mmol) in AcOH (10 mL) Fe powder (1.12 g, 20.0 mmol) was added and the mixture was warmed up to 70°C until the reaction started. The mixture was stirred at room temperature for 1 h, diluted with water (30 mL), decanted and the decanted aq. solution made alkaline with solid K2CO3 (intensive foaming!). The mixture was extracted with CHCl3/MeOH (9:1) (3 × 15 mL), dried (MgSO4), filtered, evaporated and chromatographic purification (CHCl3/MeOH) offered the title amines in 38–51% yield.
2,2,5,5-Tetramethyl-2,5-dihydro-1H-pyrrol-3-carboxylic acid-[(2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamide (12A)
Yield 620 mg 51%; white solid, mp 198-190°C (2 HCl salt). (EI) m/z (%): 609 (M+, 4), 594(10), 458(59), 151(100). 1H NMR (CDCl3): 6.81 (d, J = 8.7 Hz, ArH, 2H); 6.78 (d, J = 7.9 Hz, ArH, 2H); 6.69 (s, ArH, 2H); 6.38 (s, CH, 1H); 3.86, 3.85, 3.84, 6.83 (4s, 4 OCH3,12H); 3.56-3.52 (s, NCH2, 2H); 3.15-3.10 (t, NCH2, 2H); 3.04-2.98 (t, NCH2, 2H); 2.97-2.90 (t, CH2, 2H); 2.65 (s, NCH3, 3H); 2.25-2.16 (m, CH2, 2H); 1.81-1.78 (m, CH2, 2H); 1.48 (d, J = 6.0 Hz, CH3, 6H); 1.62 (s, CH3, 3H); 1.57 (s, CH3, 3H); 1.21-1.17 (m, CH, 1H); 0.80 (d, J = 6.2 Hz, CH3, 3H); 0.77 (d, J = 6.0 Hz, CH3, 3H). Anal. Calcd for C36H55N3O5: C, 70.90; H, 9.09; N, 6.89. Found: C, 70.84; H, 9.12; N, 6.93.
3-[(2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]-2-isopropyl-pentylamino)methyl]-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol (20A)
Yield 548 mg 46%; yellowish oil. (EI) m/z (%): 590 (M+, < 1), 580 (2), 444 (40), 278 (94), 151 (100). 1H NMR (D2O) for HCl salt: 6.95 (d, J = 8.7 Hz, ArH, 2H); 6.82 (d, J = 7.1 Hz, ArH, 2H); 6.79 (s, ArH, 2H); 5.32 (s, CH, 1H); 3.80, 3.78, 3.74, 3.73 (4s, 4OCH3,12H); 3.65 (s, NCH2, 2H); 3.42 (s, NCH2, 2H); 3.40-3.30 (m, NCH2, 2H); 3.25-3.14 (m, NCH2, 2H); 2.86 (NCH3, 3H); 2.82 (t, CH2, 2H); 2.28-2.19 (m, CH2, 2H); 1.88-1.82 (t, CH2, 2H); 1.50, 1.42, 1.48, 1.42 (4s, 4CH3, 12H); 1.26-1.14 (m, CH, 1H); 0.90 (d, J = 6.2 Hz, CH3, 3H); 0.79 (d, J = 5.9 Hz, CH3, 3H). Anal. Calcd for C36H57N3O4: C, 72.57; H, 9.64; N, 7.05. Found: C, 72.45; H, 9.53; N, 7.13.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-ylmethyl)-amino]-2-isopropyl-pentanenitrile (23A)
Yield 462 mg 40%; brown oil. (EI) m/z (%): 577 (M+, <1), 562(1), 426(58), 151(63), 138(100). 1H NMR (D2O) : 6.86 (d, J = 7.0 Hz, ArH, 2H); 6.77 (d, J = 6.6 Hz, ArH, 2H); 6.63 (s, ArH, 2H); 5.30 (s, CH, 1H); 3.88, 3.87 (2s, OCH3, 6H); 3.84 (s, OCH3, 6H); 2.92 (s, NCH2, 2H); 2.59-2.57 (t, 2NCH2, 4H); 2.46-2.42 (m, CH2, 2H); 2.07- 2.03 (k, CH2, 2H); 2.05-1.99 (t, CH2, 2H); 1.50-1.49 (2s, CH3, 12H); 1.24-1.20, (m, CH, 1H); 1.18, (d, J = 7 Hz, CH3, 3H); 0.79 (d, J = 6.8 Hz, CH3, 3H). Anal. Calcd for C35H51N3O4: C, 72.75; H, 8.90; N, 7.27. Found: C, 72.77; H, 8.83; N, 7.22.
2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl]-(2,2,6,6-tetramethyl-1,2,3,6-tetrahydropyridin-4-ylmethyl)amino]-2-isopropyl-pentanenitrile (24A)
Yield 450 mg 38%; pale-brown oil. (EI) m/z (%): 591 (M+, <1), 576(1), 440(30), 151(100). 1H NMR (D2O) : 6.84 (d, J = 8.7 Hz, ArH, 2H); 6.76 (d, J = 6.9 Hz, ArH, 2H); 6.64, 6.61 (2s, ArH, 2H) 5.35 (s, CH, 1H); 3.87, 3.86 (2s, OCH3,6H); 3.83 (s, OCH3, 6H); 2.90 (s, NCH2, 2H); 2.59-2.54 (t, NCH2, 2H); 2.53-2.49 (t, NCH2, 2H); 2.48-2.38 (m, CH2, 2H); 2.21 (s, CH2, 2H); 2.07-2.03 (k, CH2, 2H); 2.02-1.96 (t, CH2, 2H); 1.52 (s, CH3, 6H); 1.45, 1.42 (2s, CH3, 6H); 1.26-1.22 (m, CH, 1H); 1.16 (d, J = 6.5 Hz, CH3, 3H). 0.78 (d, J = 7 Hz, CH3, 3H). Anal. Calcd for C36H53N3O4: C, 73.06; H, 9.03; N, 7.10. Found: C, 72.96; H, 9.04; N, 7.08.
Synthesis of carboxylic acids (29, 30); general procedure
To the stirred solution of aldehydes 27, 28 (2.0 mmol), KH2PO4 (140 mg, 1.03 mmol) in MeCN-H2O (5:3, 16 mL) and aq. H2O2 (30%, 0.2 mL, 1.76 mmol), NaClO2 (400 mg, 4.4 mmol) dissolved in H2O (10 mL) at 0° 2 C was added dropwise over 30 min. After 1 h stirring at r.t. Na2S2O5 (200 mg, 1.05 mmol) was added and the solution was acidified with aq. HCl (1.0 M). The solution was extracted with EtOAc (2 × 30 mL), the organic phase was separated, dried (MgSO4) and evaporated, the residue was purified by flash column chromatography (hexane-EtOAc) to give the title acids (45-55%).
3-Carboxy-4-methyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl Radical (29)
Yield 217 mg 55%; yellow solid; mp 205°C (subl.) MS (EI) m/z (%): 198 (M+, 88), 183 (100), 168 (46), 153 (21). Anal. Calcd for C10H16NO3: C, 60.59; H, 8.14; N, 7.07. Found: C, 60.7; H 7.98; N 7.27.
3-Carboxy-4-phenyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxyl Radical (30)
Yield 234 mg 45%; pale-yellow solid; mp 190-192°C. MS (EI) m/z (%): 260 (M+, 100), 245(70), 230 (17), 91(42), 77(36). Anal. Calcd for C15H18NO3: C, 69.21; H, 6.97; N, 5.38. Found: C, 69.4; H, 6.8; N, 5.18.
Biology
Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and TritonX were obtained from Sigma. Cell-culture medium (RPMI 1640), smooth-muscle-cell basal medium, fetal bovine serum, antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline (PBS) were purchased from Invitrogen. All other reagents and compounds were analytical grades.
Cell lines and cultures
Chinese hamster ovary (CHO) cells and human aortic vascular smooth muscle cells (HSMCs) were obtained at passage 3 (AoSMC; Lonza Walkersville, Inc., Walkersville, MD) were used. Cells were grown in RPMI 1640 and SMC basal medium supplemented with 10% fetal bovine serum, 2% sodium pyruvate, 1% penicillin, and streptomycin. Cells were grown in a 100-mm dish or T-75 Flask to 80% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated cell counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Cell viability assay
Cell viability was determined using the conversion of MTT to formazan via mitochondrial oxidation. CHO and HSMC cells were grown in T-75 flasks to 80% confluence. They were then trypsinized, counted, and seeded in 96-well plates with an average of 7,000 cells/well. Cells were incubated for 24 to 48 h and then treated with 50 μM verapamil derivatives for 2 h and then treated with 1mM H2O2 for 1 h. All experiments were repeated at least 6 times.
Measurement of superoxide and alkylperoxyl radicals by EPR spectroscopy
The superoxide and alkylperoxyl radical-scavenging ability of verapamil derivatives were determined by using EPR spectroscopy. A mixture of xanthine (0.2 mM) and xanthine oxidase (0.02 U/ml) in PBS (pH 7.4) was used to generate superoxide radicals. Alkylperoxyl radicals were generated through the thermolytic fission of 2,2-azobis-2-amidonopropane dihydrochloride (25 mM) in aerobic PBS solution at 37°C. All EPR measurements were performed in PBS (pH 7.4) containing DEPMPO (1 mM) and diethylenediaminepentaacetate (0.1 mM) in the presence or absence of verapamil derivatives (1 mM or 100μM). The superoxide and peroxyl radicals were detected as DEPMPO-OOH and DEPMPO-OOR adducts, respectively. The attenuation of DEPMPO adduct generation was quantified by double-integration and expressed as percentage of untreated (without verapamil derivatives) levels.
Compound characterization checklist
| Compound | Microanalysis | MS | 1H-NMR | Compound | Microanalysis | MS | 1H-NMR |
|---|---|---|---|---|---|---|---|
| 11B | X | - | X | 20A | X | X | X |
| 11C | X | X | - | 20B | X | - | X |
| 12A | X | X | X | 20C | X | X | - |
| 12B | X | - | X | 23A | X | X | X |
| 12C | X | X | - | 23B | X | - | X |
| 13B | X | - | X | 24A | X | X | X |
| 13C | X | X | - | 24B | X | - | X |
| 14B | X | - | X | 23C | X | X | - |
| 14C | X | X | - | 24C | X | X | - |
| 15 | X | X | X | 25 | X | - | - |
| 16B | X | - | X | 26 | X | - | - |
| 16C | X | X | - | 29 | X | X | - |
| 17B | X | - | X | 30 | X | X | - |
| 17C | X | X | - | ||||
| 18B | X | - | X | ||||
| 18C | X | X | - |
“X”: investigated, “-”not applicable or non-investigated
Acknowledgment
This work was supported by a grant from Hungarian National Research Fund (OTKA K81123, OTKA-NKTH K67597) and National Institutes of Health (EB006153). The authors thank, Dr. Zoltán Berente (Dept of. Biochemistry and Medicinal Chemistry, Univ. of Pécs) for NMR measurement and Ms. Krisztina Kish for elemental analysis. We thank Prof. I. Hermecz for (Chinoin Ltd., Hungary) for donation of verapamil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohan IK, Kahn M, Wisel S, Selvendiran K, Sridhar A, Carnes CA, Bognár B, Kálai T, Hideg K, Kuppusamy P. Am. J. Physiol. Heart Circ. Physiol. 2009;296:140. doi: 10.1152/ajpheart.00687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A. Biochemistry. 1990;29:2802. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- 3.Cuzzocrea S, McDonald MC, Mazzon E, Siriwadena D, Costantino G, Fulia F, Cucinotta G, Giotto E, Cordaro S, Barberi I. Brain Res. 2000;875:96. doi: 10.1016/s0006-8993(00)02582-8. [DOI] [PubMed] [Google Scholar]
- 4.Miura Y, Utsumi H, Hamada A. Arch. Biochem. Biophys. 1993;300:148. doi: 10.1006/abbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Xu KY, Zhou L, Kálai T, Zweier JL, Hideg K, Kuppusamy P. J. Pharmacol Exp. Ther. 2000;295:563. [PubMed] [Google Scholar]
- 6.Mitchell JB, DeGraff WG, Kaufman D, Krishna MC, Samuni A, Finkelstein E, Ahn MS, Hahn SM, Gamson J, Russo A. Arch. Biochem. Biophys. 1991;289:62. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- 7.Hahn SM, Wilson L, Krishna MC, Liebman J, DeGraff WG, Gamson J, Samuni A, Venzon D, Mitchell JB. Radiat. Res. 1992;132:87. [PubMed] [Google Scholar]
- 8.Goldstein S, Samuni A, Hideg K, Merenyi G. J. Phys. Chem. A. 2006;110:3679. doi: 10.1021/jp056869r. [DOI] [PubMed] [Google Scholar]
- 9.Krishna MC, Degraff W, Hankovszky HO, Sár PC, Kálai T, Jekő J, Russo A, Mitchell JB, Hideg K. J. Med. Chem. 1998;41:3477. doi: 10.1021/jm9802160. [DOI] [PubMed] [Google Scholar]
- 10.Glebska J, Pulaski L, Gwozdinsky K, Skolimowski J. BioMetals. 2001;14:159. doi: 10.1023/a:1016689607579. [DOI] [PubMed] [Google Scholar]
- 11.Twomey P, Taira J, DeGraff W, Mitchell JB, Russo A, Krishna MC, Hankovszky HO, Frank L, Hideg K. Free Rad. Biol. Med. 1997;22:909. doi: 10.1016/s0891-5849(96)00477-7. [DOI] [PubMed] [Google Scholar]
- 12.Singh KK, editor. Oxidative Stress, Disease and Cancer. Imperial College Press; London: 2005. [Google Scholar]
- 13.Kálai T, Mugesh G, Roy G, Sies H, Berente Z, Hideg K. Org. Biomol. Chem. 2005;3:3564–3569. doi: 10.1039/b509865c. [DOI] [PubMed] [Google Scholar]
- 14.Kálai T, Várbíró G, Bognár Z, Pálfi A, Hantó K, Bognár B, Ősz E, Sümegi B, Hideg K. Bioorg. Med. Chem. 2005;13:2629–2636. doi: 10.1016/j.bmc.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Bognár Z, Kálai T, Pálfi A, Hantó K, Bognár B, Márk L, Szabó Z, Tapodi A, Radnai B, Sárszegi Zs., Szántó Á, Gallyas F, Jr., Hideg K, Sümegi B, Várbíró G. Free Rad. Biol. Med. 2006;41:835. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Kutala VK, Khan M, Mandal R, Ganesan LP, Tridandapani S, Kálai T, Hideg K, Kuppusamy P. J. Pharm. Exp. Ther. 2006;317:921. doi: 10.1124/jpet.105.100834. [DOI] [PubMed] [Google Scholar]
- 17.Kálai T, Khan M, Balog M, Kutala KV, Kuppusamy P, Hideg K. Bioorg. Med. Chem. 2006;14:5510. doi: 10.1016/j.bmc.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Marton Zs., Halmosi R, Horváth B, Alexy T, Késmárky G, Vékási J, Battyány I, Hideg K, Tóth K. J. Cardiovasc. Pharmacol. 2001;38:745. doi: 10.1097/00005344-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Deres P, Halmosi R, Tóth A, Kovács K, Pálfi A, Habon T, Czopf L, Kálai T, Hideg K, Sümegi B, Tóth K. J. Cardiovasc. Pharmacol. 2005;45:36. doi: 10.1097/00005344-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Mannhold R, Steiner R, Haas W, Kaufmann R. Naunyn Scmiedeberg’s Arch. Pharmacol. 1978;302:217. doi: 10.1007/BF00517988. [DOI] [PubMed] [Google Scholar]
- 21.Mannhold R, Zierden P, Bayer R, Rodenkirchen R, Steiner R. Arzneim. Forsch./Drug Res. 1983;5:773. [PubMed] [Google Scholar]
- 22.Mannhold R, Bayer R, Ransdorf M, Martens L. Arzneim. Forsch. 1987;37:419. [PubMed] [Google Scholar]
- 23.Mitani K, Yoshida T, Sakurai S, Morikawa K, Iwanaga Y, Koshinaka E, Kato H, Ito Y. Chem. Pharm. Bull. 1988;36:373. doi: 10.1248/cpb.36.373. [DOI] [PubMed] [Google Scholar]
- 24.Dei S, Romanelli MN, Scapecchi S, Teodori S, Chiarini A, Gualtieri F. J. Med. Chem. 1991;34:2219. doi: 10.1021/jm00111a043. [DOI] [PubMed] [Google Scholar]
- 25.Butora G, Bláha L, Rajsner M, Helfert I. Collect. Czeh. Chem. Commun. 1992;57:1967. [Google Scholar]
- 26.Teodori E, Ettori D, Garnier-Suriellot A, Gualtieri F, Manetti D, Romanelli MN, Scapecchi S. Bioorg. Med. Chem. 1999;7:1873. doi: 10.1016/s0968-0896(99)00104-2. [DOI] [PubMed] [Google Scholar]
- 27.Triggle JD. Biochem. Pharmacol. 2007;74:1. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Ford JM, Hait WN. Pharmacol. Rev. 1990;42:155. [PubMed] [Google Scholar]
- 29.Höllt V, Kouba M, Dietel M, Vogt G. Biochem. Pharmacol. 1992;43:2601. doi: 10.1016/0006-2952(92)90149-d. [DOI] [PubMed] [Google Scholar]
- 30.Toffoli G, Simone F, Corona G, Raschack M, Capelletto B, Gigante M, Boiocchi M. Biochem. Pharmacol. 1995;50:1245. doi: 10.1016/0006-2952(95)02003-u. [DOI] [PubMed] [Google Scholar]
- 31.Al-Shawi MK, Omote H. J. Biol. Chem. 2002;277:45688. doi: 10.1074/jbc.M206479200. [DOI] [PubMed] [Google Scholar]
- 32.Theodore LJ, Nelson WL. J. Org. Chem. 1987;52:1309. [Google Scholar]
- 33.Rozantsev EG. Free Nitroxyl Radicals. PlenumPress; New York: 1970. [Google Scholar]
- 34.Sár PC, Kálai T, Bárácz MN, Jerkovich Gy., Hideg K. Synth. Commun. 1995;25:2929. [Google Scholar]
- 35.Hankovszky HO, Hideg K, Lex L. Synthesis. 1980:914. [Google Scholar]
- 36.Hideg K, Csekő J, Hankovszky HO, Sohár P. Can J. Chem. 1986;64:1482. [Google Scholar]
- 37.Unterberg C, Buchwald AB, Mindel L, Kreuzer H. Basic Res. Cardiol. 1992;87:148–160. doi: 10.1007/BF00801962. [DOI] [PubMed] [Google Scholar]
- 38.Kovács K, Hantó K, Bognár Z, Tapodi A, Bognár E, Kiss NG, szabó A, Rappai G, Kiss T, Sümegi B, Gallyas F., Jr. Mol. Cell Biochem. 2009;321:155. doi: 10.1007/s11010-008-9929-8. [DOI] [PubMed] [Google Scholar]
- 39.Villari B, Ambrosio G, Golino P, Ragni M, Foccacio A, Tritto I, Salvatore M, Chiariello M. Am. Heart J. 1993;125:11. doi: 10.1016/0002-8703(93)90051-a. [DOI] [PubMed] [Google Scholar]
- 40.Blinco JP, Hodgson JL, Morrow BJ, Walker JR, Will GD, Coote ML, Bottle SE. J. Org. Chem. 2008;73:6763. doi: 10.1021/jo801099w. [DOI] [PubMed] [Google Scholar]
- 41.Manda S, Nakanishi I, Ohkubo K, Yakumaru H, Matsumoto K, Ozawa T, Ikota N, Fukuzumi S, Anzai K. Org. Biomol. Chem. 2007;5:3951. doi: 10.1039/b714765a. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein S, Samuni A, Hideg K, Merényi G. J. Phys. Chem. A. 2006;110:3679. doi: 10.1021/jp056869r. [DOI] [PubMed] [Google Scholar]
- 43.Rauckman EJ, Rosen GM, Abou-Donia MB. J. Org. Chem. 1976;41:564. [Google Scholar]
- 44.Hideg K, Lex L. J. Chem. Soc., Perkin Trans. 1. 1987:1117. [Google Scholar]