Abstract
PURPOSE
To compare measurements of corneal epithelial thickness using optical coherence tomography (OCT) and very high-frequency digital ultrasound (VHFDU).
METHODS
Retrospective analysis of 189 virgin corneas and 175 post-laser refractive surgery (LRS) corneas that had corneal epithelial thickness measurement with RTVue Fourier-domain OCT (Optovue, Inc., Fremont, CA) (tear film included) and Artemis VHFDU (ArcScan Inc., Morrison, CO) (tear film excluded). Averages were calculated for the central 2-mm diameter zone and for two further concentric annuli of 1.5- and 0.5-mm width, each divided into eight sectors. Agreement was analyzed by mean difference (OCT – VHFDU), 95% limits of agreement (LoA) (1.96 standard deviation of the difference), and Bland–Altman analysis.
RESULTS
In virgin epithelium, mean central thickness was 53.4 ± 3.20 μm (range: 46 to 62 μm) with OCT and 54.1 ± 2.96 μm (range: 48 to 61 μm) with VHFDU; OCT measured thinnest in 70% with a mean difference of −0.71 μm (95% LoA of ±3.94 μm, P < .001). In post-LRS epithelium, mean central thickness was 57.9 ± 6.08 μm (range: 42 to 77 μm) with OCT and 60.5 ± 6.47 μm (range: 42 to 79 μm) with VHFDU; OCT measured thinnest in 88%, with a mean difference of −2.48 μm (95% LoA of ±5.33 μm, P < .001). A larger difference between methods was more common with thicker epithelium.
CONCLUSIONS
Corneal epithelial thickness measurements using OCT were found to be slightly thinner than for VHFDU. In contrast to VHFDU, OCT measurement includes the tear film, so the true difference is probably approximately 4 μm more than reported. The difference was greatest inferiorly and higher for post-LRS eyes and in thicker epithelium.
Measurement of the corneal epithelial thickness is becoming recognized as an important part of diagnosis in refractive surgery, with many important clinical applications. As described by Alfred Vogt in 1921,1 the corneal epithelium is known to have the ability to alter its thickness profile to try to reestablish a smooth, symmetrical optical surface to compensate for changes to the stromal surface.2-4 For example, central epithelial thickening after myopic5-7 and paracentral epithelial thickening after hyperopic8 excimer laser ablation are well-established observations. Understanding and being able to better predict these changes may improve the predictability of corneal refractive procedures, whereas knowledge of the preoperative epithelial thickness profile9 may also influence the refractive outcome or be useful in planning transepithelial photorefractive keratectomy.
Measurement of the epithelial thickness profile can be critical for obtaining the true diagnosis in cases of irregular astigmatism.2-4 Any irregularity on the stromal surface will be partially or totally masked by compensatory epithelial thickness changes from front corneal surface topography. Epithelial thickness data can therefore be used to plan and predict the outcome of a transepithelial phototherapeutic keratectomy smoothing procedure.3 It is also possible to derive the stromal surface topography by subtracting the epithelial thickness data from front corneal surface elevation, which can be used to generate a stromal surface topography-guided custom ablation.4
Another major application for epithelial thickness mapping is keratoconus screening10,11 because the epithelium compensates by thinning over the cone and thickening around the cone.12-16 In early keratoconus, it is possible for the epithelium to fully compensate for the sub-surface cone,10 whereby the front corneal surface topography appears normal; the epithelial thickness profile will show a region of focal epithelial thinning, thus revealing the presence of the otherwise sub-clinical cone.10 Epithelial thickness changes have also been reported after corneal collagen cross-linking for keratoconus and ectasia, demonstrating how the stromal surface may continue to change and regularize despite there being no detectable change in surface topography.17,18
Reinstein et al.5 first published in vivo epithelial thickness mapping over the central 3-mm diameter area in 1994 using rectilinear three-dimensional very high-frequency (VHF) digital ultrasound. The area of epithelial mapping was increased to 10 mm in 1998 by development of a multi-meridional arc-scanning prototype,19 and the U.S. Food and Drug Administration– approved Artemis VHF digital ultrasound scanner (ArcScan Inc., Morrison, CO) was developed later with a central epithelial thickness measurement repeatability of 0.58 μm.20 In 2012, the RTVue optical coherence tomographer (OCT) (Optovue, Inc., Fremont, CA) became the first commercially available OCT-based epithelial mapping device producing 6-mm diameter mapping14 with a central repeatability of 0.70 μm for OCT.21
The aim of the current study was to compare corneal epithelial thickness measurements between Fourier-domain OCT and VHF digital ultrasound across the central 6-mm diameter area in normal corneas and corneas after laser refractive surgery. We were interested to see whether there was bias in epithelial measurements between the two platforms, whether there was spatial variation in the bias between different zones of the cornea, and how each technology differs when assessing the cornea after laser refractive surgery.
PATIENTS AND METHODS
This study was a retrospective case series of consecutive eligible patients before or after undergoing laser refractive surgery at the London Vision Clinic between July 2012 and November 2013. LASIK procedures were performed using the MEL80 (Carl Zeiss Meditec, Jena, Germany) excimer laser with either the VisuMax (Carl Zeiss Meditec) femtosecond laser or Hansatome zero compression microkeratome (Bausch & Lomb, Salt Lake City, UT). Small incision lenticule extraction (SMILE) procedures were performed using the VisuMax femtosecond laser. All patients were evaluated according to the standard protocol for corneal refractive surgery assessment of the London Vision Clinic, which includes a full ophthalmologic examination performed by one of our in-house optometrists.6 Patients were included in the study if they had epithelial thickness data available in a 6-mm diameter zone using both the RTVue OCT and Artemis VHF digital ultrasound arc-scanner, less than 40 days apart. Eyes after laser refractive surgery were scanned at least 3 months after surgery, by which time no further changes in epithelial thickness profile would be expected.6 Exclusion criteria included patients with keratoconus, corneal scarring, corneal dystrophies, or other significant previous ocular surgery. Informed consent and permission to use their data for retrospective analysis and publication was obtained from each patient prior to surgery as part of our routine clinical protocol for all patients seen at our clinic.
OCT Epithelial Thickness Measurements
OCT epithelial thickness data were obtained using the RTVue Fourier-domain OCT system with a corneal adaptor module working at 830-nm wavelength, as described previously.14,21 In the Pachymetry+CPwr scan pattern (software version 6.11.0.12), eight radial scans repeated five times for within-meridian averaging are obtained, each with 1,024 A-scan lines over a 6-mm diameter. The scans were centered on the coaxially fixating corneal light reflex identified by the central bright reflection on the OCT scan such that during scanning the center of rotation of the system is coaxial with the corneal vertex. The epithelial thickness map was generated by an automatic algorithm and divided into a total of 17 zones: a central 2-mm diameter zone, eight zones equally distributed within an annulus between the 2- and 5-mm diameter rings, and eight zones equally distributed within an annulus between the 5- and 6-mm diameter rings.
VHF Digital Ultrasound Epithelial Thickness Measurements
Artemis VHF digital ultrasound scanning was performed using sterile normal saline (Baxter, Newbury, United Kingdom) (0.90% w/v NaCl) at 33°C, an ultrasonic standoff medium with the patient in a sitting position. Immersion scanning means that the tear film is not included in the measurement of the epithelium, which has been shown to include the epithelial layer alone, excluding Bowman’s layer.22 Details of the measurement have been described in detail previously.9,19,20 The scan set consisted of four single meridional scans (ie, with no repeated averaging) at 45° intervals each with 128 A-scan lines. Scans were centered on the corneal vertex by the patient fixating on a narrowly focused aiming beam, which is coaxial with the infrared camera, the corneal vertex, and the center of rotation of the scanning system. During scanning, the center of rotation of the system is coaxial with the coaxially fixating corneal vertex. Epithelial thickness data were produced by an automated algorithm in a 10 × 10-mm Cartesian matrix in 0.1-mm steps. These data were limited to the 6-mm diameter and converted into zonal averages to match exactly the values produced by the RTVue device.
Data Analysis
Epithelial thickness data for each device were transformed using vertical mirrored symmetry superimposition; epithelial thickness values for left eyes were reflected in the vertical axis and superimposed onto the right eye values so that nasal/temporal characteristics could be combined. For each of the 17 zones, descriptive statistics including mean, minimum, maximum, range, and standard deviation were calculated. Both myopic and hyperopic treated eyes were included in the post-laser refractive surgery group to include a wide range of epithelial thicknesses both centrally and paracentrally, given the opposite epithelial remodeling after myopic6 and hyperopic8 ablations. Therefore, the zonal map of mean epithelial thicknesses should not be interpreted as an average epithelial response due to the mixture of myopic and hyperopic cases.
Difference (bias) in thickness readings for each zone was calculated as OCT minus VHF digital ultrasound (ie, a negative difference indicated a thinner reading on OCT compared to VHF digital ultrasound). The mean and 95% limits of agreement (1.96 × standard deviation) of the bias were calculated for each zone. Bland–Altman charts were created for the central, minimum, and maximum epithelial thicknesses for both the virgin cornea group and post-laser refractive surgery group. The mean bias and limits of agreement were displayed on the charts.
Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) was used for data entry and statistical analysis. A P value of less than .05 was considered to be statistically significant. Demographic data are displayed in Table 1.
TABLE 1.
Patient Demographics
| Characteristic | Virgin Corneas | Post-LRS Corneas |
|---|---|---|
| Patients | 103 | 96 |
| Eyes | 189 | 175 |
| Gender (male:female) | 43:57 | 31:69 |
| Mean age (range) | 39 (19 to 60) | 40 (20 to 68) |
| Median days after surgery (range) |
– | 396 (27 to 3,296) |
| Spherical equivalent refraction myopic/ hyperopic (range) |
– | 93%/6% (−14.95 to +4.75 D) |
| LASIK/SMILE | – | 41%/59% |
| Mean central thickness ± SD (μm) |
||
| OCT | 53.4 ± 3.20 | 57.9 ± 6.08 |
| VHFDU | 54.1 ± 2.96 | 60.5 ± 6.47 |
| Range (μm) | ||
| OCT | 46 to 62 | 42 to 77 |
| VHFDU | 48 to 61 | 42 to 79 |
| Mean minimum thickness ± SD (μm) |
||
| OCT | 50.3 ± 3.00 | 52.0 ± 5.09 |
| VHFDU | 50.9 ± 2.65 | 51.2 ± 5.02 |
| Range (μm) | ||
| OCT | 43 to 58 | 26 to 63 |
| VHFDU | 44 to 59 | 33 to 64 |
| Mean maximum thickness ± SD (μm) |
||
| OCT | 56.9 ± 3.69 | 63.5 ± 6.08 |
| VHFDU | 59.6 ± 3.56 | 67.9 ± 6.25 |
| Range (μm) | ||
| OCT | 49 to 69 | 50 to 85 |
| VHFDU | 52 to 67 | 55 to 87 |
| Mean days apart | 6 | 3 |
| Median days apart | 1 | 0 |
| Modal days apart | 0 | 0 |
| Range of days apart | 0 to 37 | 0 to 40 |
LRS = laser refractive surgery; D = diopters; SMILE = small incision lenticule extraction; SD = standard deviation; OCT = optical coherence tomography; VHFDU = very high-frequency digital ultrasound; days apart = number of days between OCT and VHFDU scans
RESULTS
Virgin Corneas
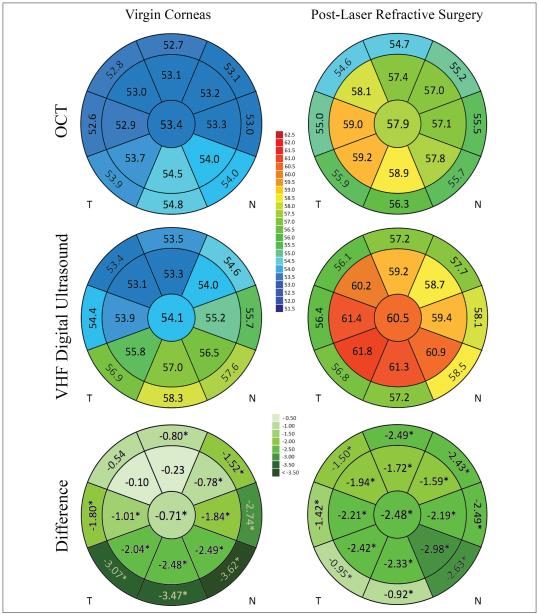
Figure 1 shows the mean epithelial thickness within each of the 17 analyzed zones for both VHF digital ultrasound and OCT, as well as a similar map for the bias between the two instruments. Limits of agreement are displayed in Table 2. OCT measured the central epithelium thinner than VHF digital ultrasound in 70% of cases. The mean OCT measurement was statistically significantly thinner in 14 of the 17 zones.
Figure 1.
Schematic corneas displaying the central 2-mm diameter zone, inner annulus 5-mm diameter, and outer annulus 6-mm diameter. Epithelial maps for left eyes were reflected about the vertical axis. The figures in each zone represent mean epithelial thickness for the optical coherence tomography (OCT) and very high-frequency (VHF) digital ultrasound rows and the bias (mean difference) between OCT and VHF digital ultrasound in the difference row (OCT minus VHF digital ultrasound). An asterisk denotes significant difference in mean thickness reading for this epithelial zone between OCT and VHF digital ultrasound (P < .05). T = temporal; N = nasal.
TABLE 2.
OCT vs VHF Digital Ultrasounda
| 95% Limits of Agreement (μm) |
|||||
|---|---|---|---|---|---|
| Parameter | Bias (μm) | SD (μm) | Upper | Lower | Width |
| Virgin corneas | |||||
| Central zone | −0.71 | 2.01 | 3.23 | −4.65 | 7.88 |
| Zone with greatest bias (inferonasal outer annulus) | −3.62 | 4.93 | 6.04 | −13.3 | 19.3 |
| Zone with smallest bias (superotemporal inner annulus) | −0.10 | 4.27 | 8.27 | −8.47 | 16.7 |
| Postoperative laser refractive surgery corneas | |||||
| Central zone | −2.48 | 2.72 | 2.85 | −7.81 | 10.7 |
| Zone with greatest bias (inferonasal inner annulus) | −2.98 | 2.79 | 2.49 | −8.45 | 10.9 |
| Zone with smallest bias (inferior outer annulus) | −0.91 | 4.14 | 7.20 | −9.02 | 16.2 |
OCT = optical coherence tomography; VHF = very high-frequency; SD = standard deviation
Difference (bias) in epithelial thickness measurements was calculated as OCT minus VHF digital ultrasound (ie, a negative difference indicated a thinner reading on OCT compared to VHF digital ultrasound).
Both methods measured epithelium thinner superiorly and thicker inferiorly in a trend that was more pronounced with VHF digital ultrasound. Therefore, this was also reflected in the bias that was greater inferiorly.
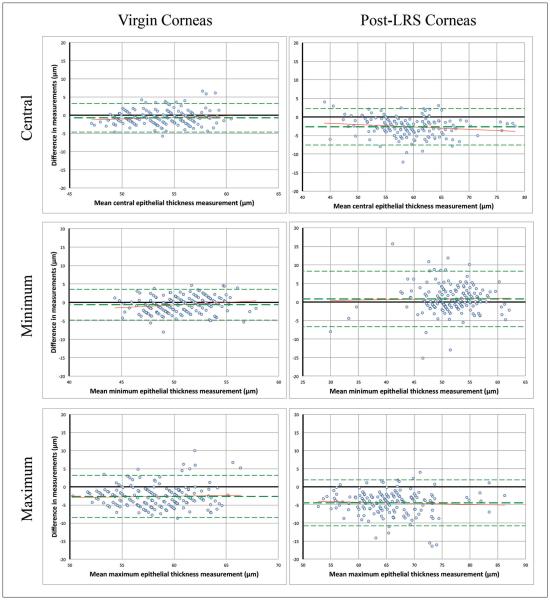
Bland–Altman plots (Figure A, available in the online version of this article) revealed no relationship between epithelial thickness and central bias, bias in minimum thickness readings, or bias in maximum thickness readings. Pearson correlation between the two methods for central epithelial thickness was 0.79 (P < .001).
Figure A.
Bland–Altman charts showing difference in central, minimum, and maximum corneal epithelial thickness measurements in relation to mean measurement between the two methods (optical coherence tomography [OCT] minus very high-frequency digital ultrasound). A negative difference shows OCT measuring thinner. Bias ± 95% limits of agreement are displayed (see Table 2). No relationship between difference in central, minimum, or maximum thickness measurements with epithelial thickness was observed. LRS = laser refractive surgery
Post-laser Refractive Surgery Corneas
Figure 1 shows the mean epithelial thickness within each of the 17 analyzed zones for both VHF digital ultrasound and OCT, as well as a similar map for the bias between the two instruments for the post-laser refractive surgery group. Limits of agreement are displayed in Table 2. OCT measured central epithelium thinner than VHF digital ultrasound in 85% of cases. In the post-laser refractive surgery group, all 17 zones were measured statistically significantly thinner by OCT.
Both methods measured the epithelium in the central and inner zones to be thicker than the outer zones. Both methods also found an asymmetry diagonally with the inner inferotemporal zones thicker than the inner superonasal zones. This agreement between methods was reflected in the map of the bias, which was a similar value for all zones except for the outer temporal zones.
Similarly to virgin corneas, Bland–Altman plots (Figure A) showed no relationship between epithelial thickness and central bias, bias in minimum thickness readings, or bias in maximum thickness readings. Pearson correlation between the two methods for central epithelial thickness was 0.94 (P < .001).
DISCUSSION
This study found OCT and VHF digital ultrasound to be in fairly close agreement for epithelial thickness measurement. However, because one of the main applications for epithelial thickness is keratoconus screening, where changes need to be measured on the scale of a few microns,10,11 differences between systems should be considered on this scale. There was a small bias in corneal epithelial thickness measurement with OCT tending to obtain thinner values than VHF digital ultrasound. The magnitude of this bias between methods was greater on average in post-laser refractive surgery corneas; centrally the mean bias was −0.71 μm for virgin corneas (53.4 μm for OCT and 54.1 for VHF digital ultrasound) compared to −2.48 μm for post-laser refractive surgery corneas (58.0 μm for OCT and 60.5 for VHF digital ultrasound). However, spatial variation in bias was more prominent in virgin corneas with larger differences between methods in inferior zones. Overall, the mean thickness was not significantly different between methods (P > .05) in only three zones (inner superior and the two superotemporal zones) in virgin corneas, although the large population meant that even small differences would be statistically significant.
OCT measurements include the tear film, reported to be between 2 and 7 μm,23,24 whereas the tear film is not included by VHF digital ultrasound due to the scans being performed under normal saline immersion. Therefore, it would be expected that OCT epithelial thickness measurements would be thicker than by VHF digital ultrasound; however, the opposite was found and OCT tended to measure the epithelium to be thinner. Therefore, the actual difference between methods is likely to be approximately 3 to 4 μm more, which translates to OCT measuring the central epithelium approximately 4 μm thinner in virgin corneas and approximately 6 μm thinner in post-laser refractive surgery corneas. The tear film has also been shown to act as a low-pass filter to compensate to a small degree for irregularities on the front corneal surface25 (ie, the surface of the epithelium), similar to the way that the epithelium compensates for stromal surface irregularities.2-4 Therefore, it may be that a small amount of detail in the epithelial thickness profile is lost by including the tear film in the measurement.
There are several factors that might potentially affect the accuracy of both methods. One potential source of error for VHF digital ultrasound is epithelial swelling during immersion; however, this can be safely ignored because it has been demonstrated that serial VHF digital ultrasound measurements over a period of 5 minutes of standard immersion resulted in no change in epithelial thickness.26
Another potential source of error is identification of the epithelial/stromal interface. Although the 5-μm axial resolution of the RTVue is sufficient to resolve the surfaces of the 10-μm thick Bowman’s layer, precise identification of the surfaces is problematic because pixels are at approximately 5-μm intervals. Ultrasonically, we have shown that the anterior surface of Bowman’s membrane presents a much larger acoustic impedance discontinuity relative to the epithelium than does anterior stroma,22 and thus produces a much larger echo than does the posterior surface of Bowman’s layer. Up to now, we have assumed that the posterior boundary of the epithelial thickness measurement by VHF digital ultrasound is the anterior surface of Bowman’s layer. However, given the approximately 30-μm ultrasound wavelength, phase interference between reflections from the anterior and posterior surfaces of Bowman’s layer may result in the point of peak reflection amplitude occurring slightly below, rather than at, the surface, resulting in a small systematic overestimation of epithelial thickness. We are currently investigating the precise location of the epithelial/stromal interface by VHF digital ultrasound by mathematical modeling of interference patterns.
Both instruments also include a source of error due to the different optical and ultrasonic speed constants of the epithelium and the stroma.
The RTVue uses the nominal refractive index of 1.376 for all corneal layers without differentiating between epithelium and stroma. However, the epithelial refractive index has been shown in bovine corneas to be significantly different (1.401).27 Using this value of 1.401, instead of the nominal 1.376 value, we estimate would result in the epithelial thickness being 0.9 μm (1.8%) thinner (in a 50-μm thick epithelium).
The Artemis uses the nominal speed of sound of 1640 m/s for all corneal layers; however, the speed of sound has not, to our knowledge, been measured specifically for the corneal epithelium. Given the higher water content in the epithelium compared to the stroma, one would expect the speed of sound to be slower in the epithelium. In a previous error analysis, we assumed a lower limit of 1,610 m/s based on the speed of sound of 1,616 m/s that has been reported in ex vivo bovine corneas.28 This predicts a maximum potential error of 1.8% for the accuracy of VHF digital ultrasound epithelial thickness measurements (a maximum error of 0.9 μm for a 50-μm epithelium). However, it is possible that the speed of sound constant for epithelium is less than 1,610 m/s, which would increase this potential error. We are currently investigating an in vitro method of measuring the speed of sound independently in epithelium, Bowman’s layer, and stroma using scanning acoustic microscopy.29
If we combine sources of error for OCT, of approximately 3.5 μm for including the tear film and 0.9 μm for adjusting the refractive index of the epithelium, the adjusted epithelial thickness value in the current study for virgin eyes would be 49.9 μm. Similarly, if we assume that the epithelial/stromal interface using VHF digital ultrasound is actually 2 μm below the anterior surface of Bowman’s layer, the adjusted epithelial thickness value would be 52.1 μm. This leaves a 2.5-μm difference remaining between OCT and VHF digital ultrasound. If the OCT measurement is accurate, it is possible that this difference is due to the speed of sound constant for epithelium that was used for the VHF digital ultrasound measurements. Under this assumption, the speed of sound constant for epithelium would be predicted to be 1,563 m/s (4.7% less than 1,640 m/s, because 2.5 μm is a 4.7% decrease in epithelial thickness), to result in a 2.4-μm decrease in the epithelial thickness measurement and align the two measurement methods.
Variability in bias of epithelial thickness measurement was found to be greatest in virgin corneas, where less agreement between methods was found in the inferior zones. The range in virgin corneas was 3.52 μm between the outer inferonasal zone (−3.62 μm) and inner superotemporal zones (−0.10 μm) in comparison to 1.80 μm in post-laser refractive surgery corneas. The mean epithelial thickness maps in Figure 1 show that this variability in virgin corneas was caused by greater vertical asymmetry measured by VHF digital ultrasound, with inferior epithelium measured to be 4.8 μm thinner than superiorly. This agreed with our previous report, where this difference was 5.7 μm (measured at a point on the 3-mm diameter rather than averaged over a zone).9 Although this asymmetry was also observed with OCT, and has been previously reported with OCT,13,14 it was less pronounced, suggesting a difference in sensitivity to small changes in epithelial thickness across the diameter of the measured zone. This may be partly due to tear film smoothing as described earlier.
The average bias was different between the two groups, with bias generally greater in the post-laser refractive surgery group; the all-zones mean bias was −2.19 μm for the post-laser refractive surgery group compared to −1.72 μm in virgin corneas. The greater bias in the post-laser refractive surgery group is similar to the differences previously reported for corneal thickness after laser refractive surgery between optical and ultrasound measurement methods. Studies have reported this for Orbscan (Bausch & Lomb),30,31 as well as (but less so) Pentacam (Oculus Optikgeräte, Wetzlar, Germany),30,31 implying that post-laser refractive surgery corneas have an altered refractive index that may not be accounted for. Interestingly, the mean corneal thickness measurement between OCT and ultrasound measurements reported in post-laser refractive surgery eyes is similar, but there appears to be greater scatter in the post-laser refractive surgery group.32,33
The repeatability of VHF digital ultrasound for epithelial thickness measurement across the 6-mm diameter of the cornea has been well established (1.1 μm coefficient or repeatability centrally and similar out to the 6-mm diameter).20 Given this demonstrated repeatability of VHF digital ultrasound, the degradation of the limits of agreement between OCT and VHF digital ultrasound moving away from the central cornea suggests that OCT measurements suffer a loss of repeatability themselves. One possible explanation is that OCT maps the image space in a planar fashion. Due to the specular nature of corneal surfaces, the reflected OCT beam stays focused (unlike when imaging diffuse tissue such as the retina). As the planar scan moves away from the central zone, the incoming beam becomes increasingly non-orthogonal to the corneal surface. As a still focused beam, the reflected signal moves away from the OCT detector axis, thus significantly reducing its signal strength and hence reducing repeatability. This effect can be seen directly in OCT corneal images as Bowman’s interface becomes more difficult to discern paracentrally. Oversampling is used to account for this effect, but only partially. VHF digital ultrasound avoids this by moving the transducer in an arcuate fashion, keeping the detection beam orthogonal to the cornea across the entire surface of the cornea.
One weakness of this study is the time lapse between OCT and VHF digital ultrasound scans on the same eye; 54% of virgin corneas and 14% of post-laser refractive surgery corneas were not scanned on the same day with both methods (median days apart: virgin corneas 1 day, post-laser refractive surgery corneas 0 day). Theoretically the effect of environmental factors on the tear film may have altered pachymetric characteristics for OCT, especially in the post-laser refractive surgery group; however, it has been shown that the epithelial thickness changes mostly in the first month after laser refractive surgery and only minimally 3 months thereafter.6
The current study has demonstrated only a small discrepancy between OCT and VHF digital ultrasound in measuring corneal epithelial thickness, although this was found to vary across the central 6-mm zone and was different for virgin and post-laser refractive surgery corneas. This finding could have important implications for the way in which epithelial thickness measurements are used diagnostically in screening for keratoconus preoperatively, as well as assessing and planning therapeutic refractive surgery postoperatively. Further study is required to characterize the accuracy of epithelial thickness measurements by OCT independent of the tear film and by use of accurate refractive indices. Similarly, further study is currently being undertaken to pinpoint the position of boundaries and verify the speed of sound constant of the corneal epithelium for ultrasonic measurements.
Acknowledgments
Supported in part by NIH grant EY019055.
Footnotes
Drs. Reinstein and Silverman have a proprietary interest in the Artemis technology (ArcScan Inc., Morrison, Colorado) through patents administered by the Center for Technology Licensing at Cornell University, Ithaca, New York. Dr. Reinstein is also a consultant for Carl Zeiss Meditec AG, Jena, Germany. The remaining authors have no proprietary or financial interest in the materials presented herein.
Prepared in part fulfillment of the requirements for the doctoral thesis of Dr. Reinstein for University of Cambridge.
AUTHOR CONTRIBUTIONS
Study concept and design (TJA, MG, DZR, RHS, TEY); data collection (TJA, DZR, TEY); analysis and interpretation of data (TJA, MG, DZR, RHS, TEY); writing the manuscript (TJA, DZR, RHS, TEY); critical revision of the manuscript (MG); statistical expertise (TJA, DZR)
Contributor Information
Dan Z. Reinstein, London Vision Clinic, London, United Kingdom; Columbia University Medical Center, New York, New York; Centre Hospitalier National d’Ophtalmologie, Paris, France.
Timothy E. Yap, London Vision Clinic, London, United Kingdom.
Timothy J. Archer, London Vision Clinic, London, United Kingdom.
Marine Gobbe, London Vision Clinic, London, United Kingdom.
Ronald H. Silverman, Columbia University Medical Center, New York, New York; F.L. Lizzi Center for Biomedical Engineering, Riverside Research, New York, New York.
REFERENCES
- 1.Vogt A. Textbook and Atlas of Slit Lamp Microscopy of the Living Eye. Wayenborgh Editions; Bonn: 1981. [Google Scholar]
- 2.Reinstein DZ. Therapeutic refractive surgery. J Refract Surg. 2015;31:6–8. doi: 10.3928/1081597X-20141223-01. [DOI] [PubMed] [Google Scholar]
- 3.Reinstein DZ, Archer TJ, Dickeson ZI, Gobbe M. Transepithelial phototherapeutic keratectomy protocol for treating irregular astigmatism based population on epithelial thickness measurements by Artemis very high-frequency digital ultrasound. J Refract Surg. 2014;30:380–387. doi: 10.3928/1081597X-20140508-01. [DOI] [PubMed] [Google Scholar]
- 4.Reinstein DZ, Gobbe M, Archer TJ, Youssefi G, Sutton HF. Stromal surface topography-guided custom ablation as a repair tool for corneal irregular astigmatism. J Refract Surg. 2015;31:54–59. doi: 10.3928/1081597X-20141218-06. [DOI] [PubMed] [Google Scholar]
- 5.Reinstein DZ, Silverman RH, Trokel SL, Coleman DJ. Corneal pachymetric topography. Ophthalmology. 1994;101:432–438. doi: 10.1016/s0161-6420(94)31314-5. [DOI] [PubMed] [Google Scholar]
- 6.Reinstein DZ, Archer TJ, Gobbe M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2012;28:195–201. doi: 10.3928/1081597X-20120127-02. [DOI] [PubMed] [Google Scholar]
- 7.Kanellopoulos AJ, Asimellis G. Longitudinal postoperative LASIK epithelial thickness profile changes in correlation with degree of myopia correction. J Refract Surg. 2014;30:166–171. doi: 10.3928/1081597X-20140219-01. [DOI] [PubMed] [Google Scholar]
- 8.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness after hyperopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26:555–564. doi: 10.3928/1081597X-20091105-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–581. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604–610. doi: 10.3928/1081597X-20090610-06. [DOI] [PubMed] [Google Scholar]
- 11.Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Invest Ophthalmol Vis Sci. 2014;55:1580–1587. doi: 10.1167/iovs.13-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial, stromal and corneal thickness in the keratoconic cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26:259–271. doi: 10.3928/1081597X-20100218-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013;29:173–179. doi: 10.3928/1081597X-20130129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119:2425–2433. doi: 10.1016/j.ophtha.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandali O, El Sanharawi M, Temstet C, et al. Fourier-domain optical coherence tomography imaging in keratoconus: a corneal structural classification. Ophthalmology. 2013;120:2403–2412. doi: 10.1016/j.ophtha.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Kanellopoulos AJ, Aslanides IM, Asimellis G. Correlation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL: is overall epithelial thickness a very early ectasia prognostic factor? Clin Ophthalmol. 2012;6:789–800. doi: 10.2147/OPTH.S31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinstein DZ, Gobbe M, Archer TJ, Couch D. Epithelial thickness profile as a method to evaluate the effectiveness of collagen cross-linking treatment after corneal ectasia. J Refract Surg. 2011;27:356–363. doi: 10.3928/1081597X-20100930-01. [DOI] [PubMed] [Google Scholar]
- 18.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. Epithelial and stromal remodeling after corneal collagen cross-linking evaluated by spectral-domain OCT. J Refract Surg. 2014;30:122–127. doi: 10.3928/1081597X-20140120-08. [DOI] [PubMed] [Google Scholar]
- 19.Reinstein DZ, Silverman RH, Raevsky T, et al. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16:414–430. doi: 10.3928/1081-597X-20000701-04. [DOI] [PubMed] [Google Scholar]
- 20.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Repeatability of layered corneal pachymetry with the Artemis very high-frequency digital ultrasound arc-scanner. J Refract Surg. 2010;26:646–659. doi: 10.3928/1081597X-20091105-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma XJ, Wang L, Koch DD. Repeatability of corneal epithelial thickness measurements using Fourier-domain optical coherence tomography in normal and post-LASIK eyes. Cornea. 2013;32:1544–1548. doi: 10.1097/ICO.0b013e3182a7f39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinstein DZ, Silverman RH, Coleman DJ. High-frequency ultrasound measurement of the thickness of the corneal epithelium. Refract Corneal Surg. 1993;9:385–387. [PubMed] [Google Scholar]
- 23.Azartash K, Kwan J, Paugh JR, Nguyen AL, Jester JV, Gratton E. Pre-corneal tear film thickness in humans measured with a novel technique. Mol Vis. 2011;17:756–767. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Wang J, Tao A, Shen M, Jiao S, Lu F. Ultrahigh-resolution measurement by optical coherence tomography of dynamic tear film changes on contact lenses. Invest Ophthalmol Vis Sci. 2010;51:1988–1993. doi: 10.1167/iovs.09-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albarran C, Pons AM, Lorente A, Montes R, Artigas JM. Influence of the tear film on optical quality of the eye. Cont Lens Anterior Eye. 1997;20:129–135. doi: 10.1016/s1367-0484(97)80011-2. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Archer TJ, Gobbe M. Stability of epithelial thickness during 5 minutes immersion in 33 degrees C 0.9% saline using very high-frequency digital ultrasound. J Refract Surg. 2012;28:606–607. doi: 10.3928/1081597X-20120815-03. [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Marshall J, Fitzke FW. Refractive index of the human corneal epithelium and stroma. J Refract Surg. 1995;11:100–105. doi: 10.3928/1081-597X-19950301-09. [DOI] [PubMed] [Google Scholar]
- 28.Silverman RH, Patel MS, Gal O, et al. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound Med Biol. 2009;35:839–846. doi: 10.1016/j.ultrasmedbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohrbach D, Lloyd HO, Silverman RH, Mamou J. Fine-resolution maps of acoustic properties at 250 MHz of unstained fixed murine retinal layers. J Acoust Soc Am. 2015;137:EL381. doi: 10.1121/1.4916790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SW, Byun YJ, Kim EK, Kim TI. Central corneal thickness measurements in unoperated eyes and eyes after PRK for myopia using Pentacam, Orbscan II, and ultrasonic pachymetry. J Refract Surg. 2007;23:888–894. doi: 10.3928/1081-597X-20071101-04. [DOI] [PubMed] [Google Scholar]
- 31.Hashemi H, Mehravaran S. Central corneal thickness measurement with Pentacam, Orbscan II, and ultrasound devices before and after laser refractive surgery for myopia. J Cataract Refract Surg. 2007;33:1701–1707. doi: 10.1016/j.jcrs.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Prospero Ponce CM, Rocha KM, Smith SD, Krueger RR. Central and peripheral corneal thickness measured with optical coherence tomography, Scheimpflug imaging, and ultrasound pachymetry in normal, keratoconus-suspect, and post-laser in situ keratomileusis eyes. J Cataract Refract Surg. 2009;35:1055–1062. doi: 10.1016/j.jcrs.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Grewal DS, Brar GS, Grewal SP. Assessment of central corneal thickness in normal, keratoconus, and post-laser in situ keratomileusis eyes using Scheimpflug imaging, spectral domain optical coherence tomography, and ultrasound pachymetry. J Cataract Refract Surg. 2010;36:954–964. doi: 10.1016/j.jcrs.2009.12.033. [DOI] [PubMed] [Google Scholar]