Abstract
Background
Following the introduction of meningococcal serogroup C conjugate vaccine in Italy in 2005, changes in the epidemiology of Invasive Meningococcal Disease (IMD) were expected. The study aims were to describe the epidemiological trend and to characterize the isolates collected during the period 2008/09-2012/13 by multilocus sequence typing (MLST). Data on laboratory confirmed meningococcal diseases from National Surveillance System of IMD were reported.
Methods
Poisson regression models were used to estimate the incidence rate over time. Serogrouping and MLST were performed following published methods.
Results
The incidence rate of laboratory confirmed meningococcal disease decreased from 0.33 per 100,000 population in 2008/09 to 0.25 per 100,000 population in 2012/13. The serogroup B incidence rate was significantly higher (p<0.01) than that of other serogroups, among all age groups. The significant decrease of the IMD incidence rate (p = 0.01) reflects the decrease of serogroup B and C, in particular among individuals aged 15–24 years old (p<0.01). On the other hand, serogroup Y incidence increased during the period (from 0.01/100,000 in 2008/09 to 0.02/100,000 in 2012/13, p = 0.05). Molecular characterizations revealed that ST–41/44 cc and ST–11 cc were the main clonal complexes identified among serogroup B and C isolates, respectively. In particular, ST–41/44 cc was predominant in all age groups, whereas ST–11 cc was not identified in infants less than 1 year of age.
Conclusions
IMD incidence declined in Italy and serogroup B caused most of the IMD cases, with infants having the highest risk of disease. Continued surveillance is needed to provide information concerning further changes in circulating meningococci with special regard to serogroup distribution. Moreover, knowledge of meningococcal genotypes is essential to detect hyper-invasive strains.
Introduction
Invasive Meningococcal Disease (IMD) remains an important public health concern worldwide, for its global epidemiology and the burden of IMD in different countries [1, 2]. IMD may be severe, often disabling, and sometimes fatal, especially among children, [3]. Although IMD occurs sporadically and outbreaks are rare, in some regions of the “African meningitis belt” devastating epidemic waves have been reported, [4].
The majority of Neisseria meningitidis strains which cause the invasive disease in Europe belongs mainly to serogroups B and C [5]. Of note, the groups at risk for IMD are patients less than 5 years of age, adolescents and young adults, [6].
Following the implementation of the meningococcal serogroup C conjugate (MCC) vaccination in many European countries, a decline in meningococcal serogroup C disease incidence has been observed, even if this serogroup is still circulating and is even responsible of small outbreaks, [7, 8, 9,10].
The MCC vaccine in Italy, introduced in 2005, is currently recommended in the National Immunization Plan (NIP 2012–2014), following regional policies, to all children from the 13th to the 15th month of age, and to 11–18 years old individuals, if not previously vaccinated, [11]. Data relating to MCC vaccine coverage was already described and estimated around 36.9% at the 12th - 24th months for the cohort 2006 and around 72% at 24th months for the cohort 2009, [12, 13].
In Italy, in a previous analysis IMD due to serogroup C meningococci affected mostly children less than 4 years of age and adolescents, [14], but now the serogroup B is highly predominant, [15].
With regard to the licensure of the multicomponent vaccine against serogroup B vaccine (Bexsero®) in Europe [16], it is recommended following regional immunization strategies throughout the country.
Since several reports evidenced the antigenic and genetic diversity among N. meningitidis isolates, it is important to identify the molecular characteristics of the circulating meningococci [17].
In Italy, the National Surveillance System of IMD, as part of the National Surveillance System for Invasive Bacterial Diseases, and its European networking (European Centre for Disease Prevention and Control, ECDC), is a long-standing surveillance through the country.
In this study, we used the information derived from the National Surveillance System with the following aims: a) to describe IMD cases occurring in Italy from 2008/09 to 2012/13; b) to describe the clonal complexes of the circulating disease-associated meningococci.
Methods
Data sources
In Italy, all cases of IMD is based on mandatory notification. Information regarding age, sex, clinical picture, vaccination status, outcome of the disease, municipality of residence, nationality, municipality where the case occurred is routinely collected and managed using a dedicated database. Clinical isolates or samples are collected and stored at -80°C at the National Reference Laboratory (NRL) of the Istituto Superiore di Sanità (ISS).
Bacterial strains and serogrouping
Meningococci are cultured on Thayer Martin agar plate with IsoVitalex 2% (Oxoid, Italy) and incubated in 5% CO2 atmosphere at 37°C for 18h. Serogroup identification, performed at local level, is confirmed by the NRL; moreover, the NRL performs serogroup identification when needed. Serogroup identification is carried out by slide agglutination with commercial antisera (Remel Europe Ltd, UK) or by PCR testing [18].
DNA Extraction and MLST
Genomic DNA was extracted by QIAamp DNA minikit (Qiagen, Germany), according to the manufacturer’s protocol for Gram-negative bacteria.
Detection of DNA of N. meningitidis from sterile body site (cerebrospinal fluid or blood) was performed by real-time TaqMan PCR assay commercial kit (BIOSENSE, Italy).
MLST was performed following the guidelines included in the Neisseria pubMLST website to identify sequence type and clonal complex (cc) (http://neisseria.org/neisseria/) [17].
Ethical Statement
Ethical approval for the study was not required, because all the examined cases were managed as part of routine diagnostics (standard care). Patient’s data were recorded anonymously in separated files under the umbrella of the national surveillance system.
Statistical analysis
Incidence rates were calculated using as denominator the Italian population size provided in the web-site (www.demo.istat.it) of the National Bureau of Census. These numbers are available per calendar year, sex, age, region, and municipality. Incidence rates were calculated stratifying cases by epidemiological year, age class (i.e., <1, 1–4, 5–14, 15–24, ≥25 years old), serogroup and regions with low (<20%) (n = 8) and high (≥20%) (n = 12) percentage of unknown (UNK) serogroup [19].
Poisson regression models were used to evaluate the temporal trend of the incidence rates (both overall, and stratified by regions with low and high percentage of UNK serogroup, and by age group).
Statistical analysis was performed using STATA 12.0 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Invasive Meningococcal Disease
Out of 794 laboratory confirmed cases of meningococcal disease in Italy, from 2008/09 to 2012/13 epidemiological years, 410 meningococcal isolates and 31 positive clinical samples were sent to the NRL.
The clinical presentation of IMD was reported for 100% of cases. As expected, meningitis and septicaemia represented the main clinical syndromes: in particular, meningitis was reported in 55% (436/794) of the cases, sepsis in 29% (230/794) and meningitis and sepsis in 15.3% (122/794). Sepsis was more frequently associated with serogroup C (32.5%; 57/162) compared to serogroup B (29.8%; 107/359) and Y (25.8%; 16/62); however, there was no statistically significant association between clinical manifestation and serogroup (p = 0.07), (S1 Table).
Laboratory diagnosis was known for 97.8% (777/794) of cases and was confirmed in 66.5% (517/777) by culture, in 14% (107/777) by PCR, in 7.5% (58/777) by both culture and PCR, and in 12% (95/777) by microscopy or antigen test.
The overall incidence of laboratory confirmed meningococcal disease during the period was 0.26 per 100,000 inhabitants (Table 1).
Table 1. Number of cases and incidence of meningococcal disease in Italy by epidemiological year.
| 2008/09 | 2009/10 | 2010/11 | 2011/12 | 2012/13 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Italy | N | % | N | % | N | % | N | % | N | % | N | % | |
| Known serogroup | 170 | 85.4 | 109 | 75.2 | 121 | 78.6 | 107 | 72.8 | 102 | 68.5 | 609 | 76.7 | |
| Unknown serogroup | 29 | 14.6 | 36 | 24.8 | 33 | 21.4 | 40 | 27.2 | 47 | 31.5 | 185 | 23.3 | |
| Total cases | 199 | 145 | 154 | 147 | 149 | 794 | |||||||
| Population | 59832179 | 60192698 | 60483385 | 60010325 | 60010325 | 300528911 | |||||||
| Incidence | 0.33 | 0.24 | 0.25 | 0.24 | 0.25 | 0.26 | |||||||
| Regions with <20% of cases with unknown serogroup | N | % | N | % | N | % | N | % | N | % | N | % | |
| Known serogroup | 123 | 93.9 | 79 | 82.3 | 69 | 87.3 | 76 | 85.4 | 80 | 83.3 | 427 | 87.0 | |
| Unknown serogroup | 8 | 6.1 | 17 | 17.7 | 10 | 12.7 | 13 | 14.6 | 16 | 16.7 | 64 | 13.0 | |
| Total cases | 131 | 96 | 79 | 89 | 96 | 491 | |||||||
| Population | 26901733 | 27131868 | 27317071 | 27118025 | 27118025 | 135586720 | |||||||
| Incidence | 0.49 | 0.35 | 0.29 | 0.33 | 0.35 | 0.36 | |||||||
| Regions with ≥20% of cases with unknown serogroup | N | % | N | % | N | % | N | % | N | % | N | % | |
| Known serogroup | 47 | 69.1 | 30 | 61.2 | 52 | 69.3 | 31 | 53.4 | 22 | 41.5 | 182 | 60.1 | |
| Unknown serogroup | 21 | 30.9 | 19 | 38.8 | 23 | 30.7 | 27 | 46.6 | 31 | 58.5 | 121 | 39.9 | |
| Total cases | 68 | 49 | 75 | 58 | 53 | 303 | |||||||
| Population | 32930447 | 33060830 | 33166315 | 32892300 | 32892300 | 164942191 | |||||||
| Incidence | 0.21 | 0.15 | 0.23 | 0.18 | 0.16 | 0.18 | |||||||
The serogroup was unknown in 185 cases (185/794; 23%) out of the total (Table 1). For this reason, the analysis regarding the serogroups was performed on the overall country and on the Italian Regions divided in two groups: regions with <20% and regions with ≥20% of annual cases with UNK serogroup. A total of 491 IMD cases occurred in regions <20% of annual cases with UNK serogroup, and 303 cases in regions with a rate of annual cases with UNK serogroup ≥20% (Table 1).
The total incidence of laboratory confirmed meningococcal disease significantly declined from 0.33 per 100,000 inhabitants in 2008/09 to 0.25 per 100,000 inhabitants in 2012/13 (p = 0.01), (S1 Fig).
A similar trend was observed in regions with <20% of annual cases with UNK serogroup (p = 0.02) (S1 Fig). No significant changes in the incidence in the regions with ≥20% of annual cases with UNK serogroup was observed (p = 0.40), (data not shown).
Over the five years, serogroup B cases occurred most frequently (0.12 per 100,000), followed by serogroup C (0.05 per 100,000), serogroup Y (0.02 per 100,000), and other serogroups (including A, cnl, E, W, X) (0.01 per 100,000), (Table 2).
Table 2. Number and incidence of IMD cases by serogroup and age groups.
| B | C | Y | Other a | UNK | Total | ||||||||
| Italy | Age group | N | rate | N | rate | N | rate | N | rate | N | rate | N | rate |
| <1 | 58 | 2.09 | 12 | 0.43 | 2 | 0.07 | 2 | 0.07 | 21 | 0.76 | 95 | 3.42 | |
| 1–4 | 81 | 0.71 | 28 | 0.25 | 4 | 0.04 | 3 | 0.03 | 24 | 0.21 | 140 | 1.24 | |
| 5–14 | 63 | 0.22 | 17 | 0.06 | 21 | 0.07 | 3 | 0.01 | 32 | 0.11 | 136 | 0.48 | |
| 15–24 | 64 | 0.21 | 34 | 0.11 | 9 | 0.03 | 3 | 0.01 | 32 | 0.11 | 142 | 0.47 | |
| >25 | 93 | 0.04 | 71 | 0.03 | 26 | 0.01 | 15 | 0.01 | 76 | 0.03 | 281 | 0.12 | |
| Total | 359 | 0.12 | 162 | 0.05 | 62 | 0.02 | 26 | 0.01 | 185 | 0.06 | 794 | 0.26 | |
| B | C | Y | Other a | UNK | Total | ||||||||
| Regions with <20% of cases with unknown serogroup | Age group | N | rate | N | rate | N | rate | N | rate | N | rate | N | rate |
| <1 | 36 | 2.87 | 5 | 0.40 | 0 | 0.00 | 1 | 0.08 | 8 | 0.64 | 50 | 3.98 | |
| 1–4 | 59 | 1.16 | 17 | 0.34 | 1 | 0.02 | 2 | 0.04 | 5 | 0.10 | 84 | 1.66 | |
| 5–14 | 50 | 0.42 | 10 | 0.08 | 15 | 0.13 | 0 | 0.00 | 10 | 0.08 | 85 | 0.71 | |
| 15–24 | 48 | 0.40 | 25 | 0.21 | 3 | 0.02 | 2 | 0.02 | 16 | 0.13 | 94 | 0.78 | |
| >25 | 74 | 0.07 | 51 | 0.05 | 15 | 0.01 | 13 | 0.01 | 25 | 0.02 | 178 | 0.17 | |
| Total | 267 | 0.20 | 108 | 0.08 | 34 | 0.03 | 18 | 0.01 | 64 | 0.05 | 491 | 0.36 | |
Othera serogroup: A, cnl, E, W and X
UNK, cases with unknown serogroup
A decrease of incidence rates for serogroup B (from 0.16 in 2008/09 to 0.09 in 2012/13 per 100,000; p<0.01) and for serogroup C (from 0.10 in 2008/09 to 0.05 in in 2012/13 per 100,000; p<0.01) was also observed. However, when excluding the epidemiological year 2008/09, serogroup C showed a stable trend (from 0.03 in 2009/10 to 0.05 in 2012/13 per 100,000, p = 0.17).
The incidence rate of serogroup Y showed a significant increase during the study period (from 0.01 in 2008/09 to 0.02 in 2012/13 per 100,000; p = 0.05).
Infants under 1 year of age (3.42/100,000) and children aged 1 to 4 years, (1.24/100,000) were the most affected. The lowest of the total IMD incidence rate was observed in adults ≥ 25 years (0.12/100,000), (Table 2).
Serogroup B incidence rate was significantly more common than all other serogroups, in all age groups (p<0.01) although a low difference in terms of incidence rate in the population aged ≥ 25 years was found. Serogroup C incidence rate decreased with the increase of the age. Serogroup Y incidence rate was higher among infants>1 year and children aged 5–9 years (0.07/100,000), Table 2.
A similar age distribution of IMD cases was observed in regions with <20% of annual cases with UNK serogroup, (Table 2).
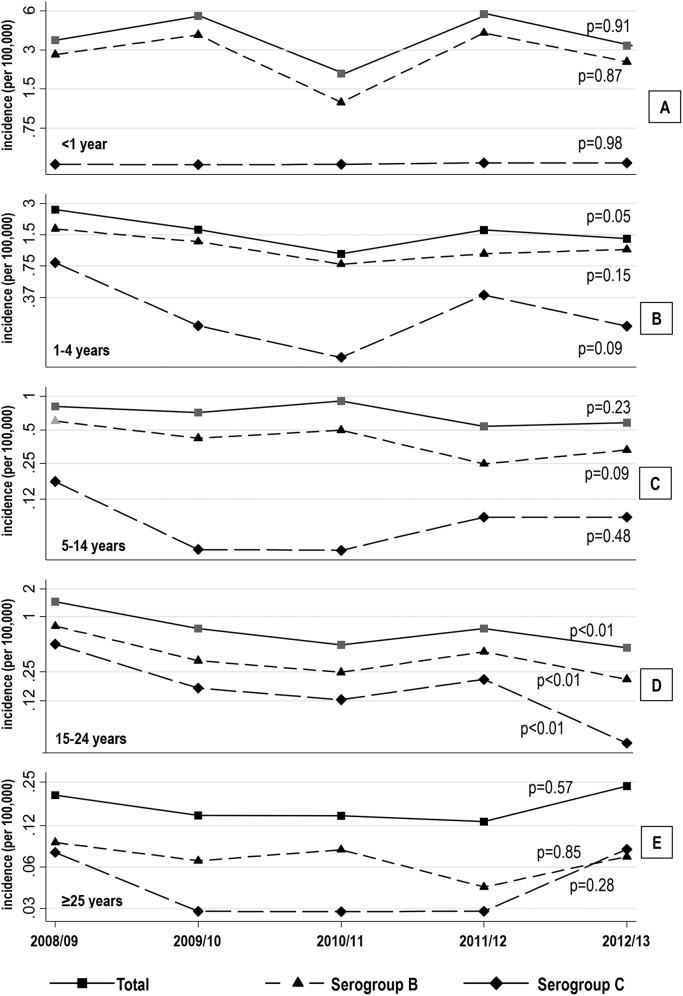
Fig 1 shows the temporal trend of the incidence rate for all serogroups combined and for the serogroup B and C, separately. For the age group <1 year, no significant changes in the incidence rates of all serogroups combined as well as for the serogroup B and C, respectively were observed (Fig 1A). For the age group 1–4, there was a significant decline for all serogroups combined; a similar decrease was observed both for serogroup B and C, respectively, but this decline was not statistically significant (Fig 1B). Regarding the other three age groups (Fig 1C, 1D and 1E), only for age group 15–24 years (Fig 1D) a statistically significant decline for the overall incidence rate, as well as, for the serogroup B and C, respectively, was observed.
Fig 1. Annual incidence, on logarithmic scale, of IMD in Italian Regions with <20% of annual cases with UNK serogroup by age group: A) <1, B) 1-to–4, C) 5-to–14, D) 15-to–24, E) ≥25 years.
p-values from Poisson univariate regression models with epidemiological year as covariate are in the right side.
Molecular investigation
A total of 21 known clonal complexes (ccs) were identified. The most commonly detected were ST–41/44 cc (76/344, 22%), ST–11 cc (66/344, 19%), and ST–23 cc (44/344, 13%).
Among serogroup B, 13 ccs were identified and the most common were ST–41/44 cc (73/182, 40%), ST–32 cc (22/182, 12%), ST–162 cc (22/182, 12%) and ST–269 cc (14/182, 8%).
Among serogroup C, 8 ccs were identified and the most common were: ST–11 cc (62/101, 61%), and ST–334 cc (19/101, 19%).
The majority of cases due to serogroup Y belonged to ST–23 cc (43/45, 96%).
Four ccs exclusively found among serogroup B were: ST- 162 cc, ST–865 cc, ST–213 cc and ST–461 cc. The ST–334 and ST–8 cc were restricted to serogroup C.
A total of 23 isolates (23/344; 6.7%), belonging to sequence types not currently assigned to any clonal complex, were classified as unknown (UNK).
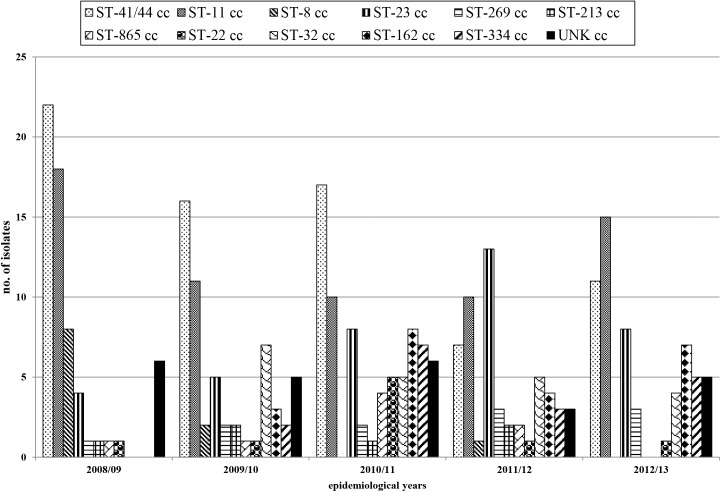
The ST–41/44 cc and the ST–11 cc tended to decrease from 2008/09 to 2012/13. The ST–8 cc decreased and disappeared from 2008/09 to 2012/13, with 1 ST–8 cc strain isolated in 2011/12. The ST–23 cc increased from 2008/09 to 2012/13, with a peak in 2011/12. Since 2009/10, ST–32 cc, ST–162 cc, and ST–334 cc, were identified (Fig 2).
Fig 2. Distribution of the main clonal complexes (ccs) identified among meningococci collected in the study period.
Among infants <1 year and among children 1–4 years, ST–41/44 cc was the prevalent; ST–23 cc and ST–41/44 cc were also the prevalent in subjects from 5 to 14 years; in subjects from 15 to 24 years, ST–41/44 cc and ST–11 cc were both the main ccs. Moreover, the majority of ST–32 cc isolates were found among individuals aged from 15 to 24years. In adults ≥25 years, ST–11 cc and ST–23 cc were the most frequently identified (Table 3).
Table 3. Age distribution of the most common clonal complexes identified in Italy, from 2008/09 to 2012/13.
| Age group (number per serogroup) | ||||||
|---|---|---|---|---|---|---|
| Clonal complex | <1 | 1–4 | 5–14 | 15–24 | >25 | Total |
| ST–41/44 | 13(12B, 1C) | 14 (14B) | 15 (14B, 1C) | 17 (17B) | 17 (16B, 1C) | 76 |
| ST–11 | 0 | 9 (9C) | 6 (6C) | 15 (14C, 1B) | 36 (33C, 2B, 1W) | 66 |
| ST–8 | 3 (3C) | 3 (3C) | 2 (2C) | 3 (3C) | 0 | 11 |
| ST–334 | 4 (4C) | 3 (3C) | 2 (2C) | 2 (2C) | 8 (8C) | 19 |
| ST–23 | 0 | 2 (2Y) | 18 (18Y) | 3 (3Y) | 21 (20Y, 1C) | 44 |
| ST–32 | 3 (3B) | 5 (5B) | 3 (3B) | 9 (8B, 1C) | 2 (2B) | 22 |
| ST–162 | 4 (4B) | 4 (4B) | 4 (4B) | 1 (1B) | 9 (9B) | 22 |
| ST–269 | 3 (3B) | 4 (4B) | 3 (3B) | 1 (1C) | 4 (4B) | 15 |
Discussion
Invasive meningococcal disease (IMD) is a serious illness that despite an early intervention and modern intensive care can become fatal in few hours. Vaccination is the only strategy to prevent the disease and to improve the herd protection in the population, thus reducing morbidity and mortality. According to IMD surveillance data reported by ECDC, the incidence of confirmed cases in 28 European countries continues to decrease: from 0.95 per 100,000 in 2008 to 0.68 per 100,000 in 2012 [5].The main reason for this decline could be the introduction of MCC vaccination in several countries [20]. Moreover, the effect of vaccination on carriage status results in a long-term reduction of the disease [21].
In Italy, IMD incidence rate showed cyclical fluctuations, in particular regarding to the total number of cases due to serogroup B and C, respectively [22, 14]. Moreover, other factors such as climate and geographical variation have been associated to a gradient in the IMD incidence in the country [23].
The risk of meningococcal disease clearly varies with age and serogroup. Meningococcal carriage is most common in teenagers whereas the invasive disease is reported mainly in infants and in a secondary peak observed in teenagers [24].
The serogroup distribution observed in this study was similar to that reported in most European countries, with serogroup B and C responsible for the majority of disease, followed by serogroup Y [5]. Serogroup B was responsible for the majority of IMD cases in all age groups, with a statistically significant higher incidence compared to any other serogroup. As already reported in Italy, serogroup B is the predominant among infants [15]. The Bexsero has been recently introduced in Italy even if with differences among the Regions. In a previous study [25], the potential Bexsero strain coverage has been estimated at 87% (95% CI 70–93).
In comparison to other serogroups, the number of IMD cases due to serogroups A and W is rare, in Italy. Whereas, an increase of IMD due to serogroup W have been reported in England and Wales since 2009 due to the emergence and clonal expansion of a single clone [26]. Serogroup Y increased as already reported in other European countries and worldwide [27, 28, 29]. These results may contribute in the revision of catch-up or booster vaccination, hopefully with the quadrivalent conjugate vaccine ACWY. The vaccine is available and recommended in Italy for at risk groups and for people who live or travel to countries where meningococcal disease is hyperendemic or epidemic. Moreover, it can be administered to children from 12 months of aged who have not received MCC vaccine or to adolescents aged between 12 and 16 years, as booster of MCC vaccine and for a complete coverage.
In the present analysis, serogroup B meningococci resulted more heterogeneous than serogroup C. In particular, the majority of clonal complexes among serogroup B were: ST–41/44 cc, ST–32 cc, ST–162 cc and ST–269 cc. These clonal complexes were distributed in all age groups.
The clonal complexes among serogroup C meningococci isolated from 2008/09 to 2012/13 were: ST–11 cc, ST–334 cc and ST–8 cc. Serogroup C ST–8 cc, mainly in persons aged less than 25 years, decreased and the serogroup C ST–334 cc, identified in adults, still increased. The majority of serogroup C ST–11 cc isolates were identified in all age groups except for infants less than 1 year of age, as already described [30].
Previously, serogroup C IMD cases in Italy were mainly associated with ST–8 cc (2003–2005) [31] and ST-11cc (2007–2008) [32].
As with any national surveillance study, the estimation is likely to represent a possible underreporting of disease incidence. Moreover, the relatively high proportion of cases with UNK serogroup (23.3%) could affect the data. Nevertheless, results from Regions with a low rate of UNK serogroup in our dataset are consistent with those collected in the rest of the country.
In conclusion, data from the national surveillance system provides information on IMD in the country up to 2013, before the introduction of meningococcal B vaccination in the country. Molecular typing analysis permits to monitor the changes in the distribution of those hyper-virulent clonal complexes recognized as responsible of outbreaks or rapid endemic expansion, demanding a continuous surveillance for the possible genetic pressure due to the immunization policies.
Supporting Information
(DOCX)
(TIF)
Acknowledgments
We are grateful to the following Collaborators of National Surveillance System of Invasive Meningococcal Disease: Anna Maria Barbui, Microbiology and Virology Laboratory, Molinette Hospital, Torino, Italy; Daniela Lombardi, SeREMI, ASL AL, Regional reference Centre for Infectious Diseases surveillance, Alessandria, Italy; Maria Laura Garlaschi, Microbiology Laboratory, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy; Laura Daprai, Microbiology Laboratory, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy; Lucia Rossi, Microbiology and Virology Unit, University Hospital, Padova, Italy; Giorgio Mucignat, Department of Clinical Pathology Cytogenetics and Molecular Biology Lab, General Hospital S.M.A., Pordenone, Italy; Paolo Lanzafame, Microbiology and Virology Unit, Provincial Health Services, S. Chiara Hospital, P.A. Trento, Italy; Richard Aschbacher, Microbiology and Virology Laboratory, Central Hospital, P.A. Bolzano, Italy; Christine Spitaler, Merano Hospital, P.A. Bolzano, Italy; Giovanna Renna, Laboratory of Microbiology, Ospedale San Giuseppe, Empoli, Italy; Maria Paola Landini, Unit of Clinical Microbiology, St. Orsola Malpighi University Hospital, Bologna, Italy; Caterina Vocale, Unit of Clinical Microbiology, St. Orsola Malpighi University Hospital, Bologna, Italy; Iolanda Santino, Department of Clinical and Molecular Medicine, Faculty of Medicine and Psychology, University “Sapienza”, Rome, Italy; Carlo Tascini, U.O. Infectious Diseases, University Hospital, Pisa, Italy; Patrizia Isola, Clinical Pathology Department, Azienda USL 6, Livorno, Italy; Antonella Mencacci, University of Perugia, Italy; Esther Manso, Department of Microbiology, University of Ancona/Azienda Ospedaliera Umberto I, Ancona, Italy; Paolo Fazii, Clinical Microbiology and Virology Unit, Santo Spirito Hospital, Pescara Italy; Anna Di Taranto, Clinical Pathology Department, UOC II Azienda Mista Ospedaliero-Universitaria OO.RR Foggia, Italy; Maria Chironna, Biomendical Sciences and Human Oncology Departement, University Hospital, Bari, Italy; Paolo Castiglia, Hygiene and Preventive Medicine, Department of Biomedical Sciences, AOU-University of Sassari, Italy.
This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/ neisseria/) developed by Keith Jolley and sited at the University of Oxford (Jolley & Maiden 2010, BMC Bioinformatics, 11:595). The development of this site has been funded by the Wellcome Trust and European Union.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Ministry of Health-CCM Project 4M01, Sorveglianza delle malattie invasive da Neisseria meningitidis, Streptococcus pneumoniae ed Haemophilus influenzae, 2013. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rosenstein NE, Perkins BA, Stephens DS, Popovic T. Meningococcal disease. N Engl J Med. 2001. May 3;344(18):1378–88. [DOI] [PubMed] [Google Scholar]
- 2. Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009. June 24;27 Suppl 2:B51–63. 10.1016/j.vaccine.2009.04.063 Epub 2009 May 27. [DOI] [PubMed] [Google Scholar]
- 3. Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010. May;10 (5):317–28. 10.1016/S1473-3099(10)70048-7 [DOI] [PubMed] [Google Scholar]
- 4. Memish ZA, Goubeaud A, Bröker M, Malerczyk C, Shibl AM. Invasive meningococcal disease and travel. J Infect Public Health. 2010. Dec; 3 (4):143–51. 10.1016/j.jiph.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control, E., Annual epidemiological report. Reporting on 2011 surveillance data and 2012 epidemic intelligence data, in Surveillance report, E. European Centre for Disease Prevention and Control, Editor. 2013, European Centre for Disease Prevention and Control, (ECDC): Stockholm. [PubMed]
- 6.Invasive meningococcal disease—All cases—Notification rate Data by Country and Year. Current time period: 2012. http://www.ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx.
- 7. Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001. October 15; 20 Suppl 1:S58–67. [DOI] [PubMed] [Google Scholar]
- 8. Cano R, Larrauri A, Mateo S, Alcalá B, Salcedo C, Vázquez JA. Impact of the meningococcal C conjugate vaccine in Spain: an epidemiological and microbiological decision. Euro Surveill. 2004. July; 9(7): 11–5. [PubMed] [Google Scholar]
- 9. de Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J. 2006. January; 25(1): 79–80. [DOI] [PubMed] [Google Scholar]
- 10. Deghmane AE, Parent du Chatelet I, Szatanik M, Hong E, Ruckly C et al. Emergence of new virulent Neisseria meningitidis serogroup C sequence type 11 isolates in France. J Infect Dis. 2010. July 15; 202 (2): 247–50. 10.1086/653583 [DOI] [PubMed] [Google Scholar]
- 11.www.salute.gov.it/imgs/c_17_pubblicazioni_1721_allegato.pdf
- 12.Istituto Superiore di Sanità, Gruppo di lavoro ICONA. ICONA 2008: Indagine di Copertura vaccinale Nazionale nei bambini e negli adolescenti 2009; 8:118. Rapporti ISTISAN 09/29.
- 13.D’Ancona F, Cerquetti M, Pantosti A, Caporali M, Fazio C, Camilli R et al. Surveillance of invasive bacterial diseases (N meningitidis, H influenza, S pneumoniae) and evaluation of vaccine coverage in 7 Italian regions, 2008–2012. Poster abstract 24th ECCMID, Barcelona, Spain, 10–13 May 2014.
- 14. Stefanelli P, Fazio C, Sofia T, Neri A, Mastrantonio P. Serogroup C meningococci in Italy in the era of conjugate MenC vaccination. BMC Infection Disease. 2009. August 22;9:135 10.1186/1471-2334-9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stefanelli P, Fazio C, Neri A, Boros S, Renna G, Pompa MG et al. Changing epidemiology of Infant Meningococcal Disease after the introduction of meningococcal serogroup C vaccine in Italy,2006–2014 Vaccine. 2015. Jul 17;33(31):3678–81. 10.1016/j.vaccine.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 16.http://www.ema.europa.eu/docs/it_IT/document_library/EPAR_-_Product_Information/human/002333/WC500137881.pdf
- 17. Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Zhang Q. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998. March 95: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Theodore MJ, Mair R, Trujillo-Lopez E, du Plessis M, Wolter N. Clinical validation of multiplex real-time PCR assays for detection of bacterial meningitis pathogens. J Clin Microbiol. 2012. March; 50 (3):702–8. 10.1128/JCM.06087-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinlin LM, Spain CV, Ng V, Johnson CC, Fisman DN. Environmental exposures and invasive meningococcal disease: an evaluation of effects on varying time scales. Am J Epidemiol. 2009. March 1;169 (5):588–95. 10.1093/aje/kwn383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007. January;31(1):101–7. [DOI] [PubMed] [Google Scholar]
- 21. Maiden MC. The impact of protein-conjugate polysaccharide vaccines: an endgame for meningitis? Philos Trans R Soc Lond B Biol Sci. 2013. June 24;368(1623):20120147 10.1098/rstb.2012.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastrantonio P, Stefanelli P, Fazio C, Sofia T, Neri A, La Rosa G et al. Serotype distribution, antibiotic susceptibility, and genetic relatedness of Neisseria meningitidis strains recently isolated in Italy. Clin Infect Dis. 2003. February;36(4):422–8. Epub 2003 Jan 30. [DOI] [PubMed] [Google Scholar]
- 23. Vescio F, Busani L, Mughini Gras L, Fazio C, Neri A, Avellis L. Climate, demographic factors and geographical variations in the incidence of invasive meningococcal disease in Italy. Epidemiol Infect. 2014. October 13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trotter CL, Gay NJ, Edmunds WJ. The natural history of meningococcal carriage and disease. Epidemiol Infect. 2006. June;134(3):556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013. May;13(5):416–25. 10.1016/S1473-3099(13)70006-9 [DOI] [PubMed] [Google Scholar]
- 26. Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015. February 15;60(4):578–85. 10.1093/cid/ciu881 [DOI] [PubMed] [Google Scholar]
- 27. Lahra MM, Enriquez RP. Annual report of the Australian Meningococcal Surveillance Programme, 2011. Commun Dis Intell Q Rep. 2012. September 30;36(3): E251–62. [DOI] [PubMed] [Google Scholar]
- 28.Bröker M, Emonet S, Fazio C, Jacobsson S, Koliou M, Kuusi M et al. Meningococcal serogroup Y disease in Europe continuation of high importance in some European regions in 2013. Hum Vaccin Immunother 2015 Jun 2:0. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 29. Ines-Agudelo C, Sanabria OM, Ovalle MV. Serogroup Y meningococcal disease, Colombia. Emerg Infect Dis 2008;14:990–1. 10.3201/eid1406.071357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skoczynska A, Waoeko I, Kuch A, Kadłubowski M, Gołębiewska A, Foryś M. A decade of invasive meningococcal disease surveillance in Poland. PLoS One 2013. August 20;8(8): e71943 10.1371/journal.pone.0071943 eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mastrantonio P, Sofia T, Neri A, Fazio C, Stefanelli P. Characterization of invasive meningococcal isolates from Italian children and adolescents. Clin Microbiol Infect 2007; 13: 100–103. [DOI] [PubMed] [Google Scholar]
- 32. Fazio C, Neri A, Sofia T, Carannante A, Caporali MG, Salmaso S et al. Characterization of Neisseria meningitidis C strains causing two clusters in the north of Italy in 2007 and 2008. Eurosurveillance 2009. 14; 23 April;14(16). pii: 19179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.