Abstract
Clostridium tetani, the etiologic agent of tetanus, produces a toxin that causes spastic paralysis in humans and other vertebrates. This study was aimed for isolation, identification, and determination of antimicrobial susceptibility of C. tetani from clinically diagnosed tetanus patients. Isolation was done from deep-punctured tissues of the foot and arm injuries of 80 clinically diagnosed tetanus patients from the Pakistan Institute of Medical Sciences hospital. We successfully screened out five C. tetani isolates out of 80 samples based on the strain-specific characteristics confirmed through biochemical testing and toxin production. A disc diffusion method was used for antimicrobial susceptibilities and C. tetani isolates showed susceptibility to cefoperazone, chloramphenicol, metronidazole, penicillin G, and tetracycline, but were found to be resistant to erythromycin and ofloxacin. During animal testing, all the infected mice developed symptoms of tetanus. The results showed that identification of C. tetani is possible using biochemical and molecular tools and that the strains of C. tetani isolated had not developed resistance against the antibiotics most often used for the treatment of tetanus.
Introduction
Despite the existence of a routine vaccination program, a World Health Organization (WHO) survey has shown that tetanus in developing countries caused about 200,000 fatalities per year.1 Because of a highly effective vaccination program, tetanus has been eradicated from the developed countries, however, the hundreds of thousand incidences of Clostridium tetani-mediated infection are still being reported in some developing countries of Africa and Asia including Pakistan. According to estimates presented by WHO, tetanus accounts for more than 400,000 of the neonatal fatalities reported in developing countries.1,2 Furthermore, surveys performed by the Centers for Disease Control and Prevention showed that neonatal tetanus was a significant problem worldwide with an estimated global death rate of 6.5 per 1,000 live births.3
Neonatal tetanus is one of the major health concerns in remote areas of the countries such as Baluchistan and Khayber PakhtunKhwa due to unhygienic condition and poor vaccination practices.4 About 22,000 neonatal deaths occur every year due to maternal tetanus in Pakistan.5 Maternal vaccination with diphtheria, tetanus, and pertussis is recommended and practiced worldwide and has resulted in a decline in the incidence of maternal and neonatal tetanus.1
C. tetani, an obligate anaerobe and spore-forming Gram-negative bacteria, is the causative agent of the disease called tetanus.6 The spores of C. tetani can withstand anaerobic and extreme temperature conditions in both indoor and outdoor environments, and can be isolated from both human or animal fecal samples, and from soil.7
The transmission route associated with C. tetani is via exposure of a deep tissue wound to the spores present in soil or fecal matter from animals or humans. The anaerobic conditions in the tissue wound provide an ideal environment for replication and growth of C. tetani. Following extensive bacterial replication at the wound site and expression of the genes encoding the tetanus toxins, neurotoxin, and tetanolysin; the tetanus patients exhibit typical symptoms of disease8 such as rigidity and severe muscular spasm in the head and neck regions.8,9 Under optimized conditions, C. tetani releases two toxins tetanolysin and tetanospasmin at the wound site. The tetanolysin is a hemolysin, which is not known to contribute toward tetanus symptoms, however, the tatanospasmin, a tetanus neurotoxin affects the central nervous system causing the appearance of clinical tetanus symptoms.6,10 To understand the mechanism and transmission of disease extensively, the reviews published by Farrar and others11 and Cook and others12 can be useful for readers.
Because of the easy symptomatic diagnosis of tetanus, fewer microbiological research studies have been conducted on the isolation of the organism from clinically diagnosed patients during past two decades. Furthermore, there has been an inadequate assessment of antimicrobial susceptibility patterns of C. tetani.13 Several organizations in Pakistan—the Pakistan Ministry of Health, the Global Alliance for Vaccination and Immunization and Health, and the Education and Literacy Program—are actively involved in the eradication of tetanus from Pakistan. These efforts can only bear fruit if the microbiological and antimicrobial susceptibility patterns of C. tetani are determined.14 So far as, the existing literature does not contain any single report about the isolation of C. tetani from clinically diagnosed patients in Pakistan.
Most of the previous tetanus studies have focused on toxins and development of vaccines,15 however, Campbell and others16 found that clinical isolates of C. tetani were susceptible to penicillin and metronidazole, but they were resistant to co-trimoxazole. Previously, penicillin was considered as the standard therapy for tetanus worldwide,16 however, metronidazole is now considered to be the first line of safe treatment and is an alternative to penicillin therapy.17,18 Tetracycline, macrolides, clindamycin, cephalosporin, and chloramphenicol are all effective in treating tetanus.18,19
In this study, we aimed to isolate C. tetani from clinically diagnosed tetanus patients, to determine both the antimicrobial sensitivity pattern and the toxicological profile.
Methodology
Isolation and identification of the culture.
Wound swabs taken from the arm and foot injuries of 80 patients diagnosed with tetanus were collected in sterilized Carry–Blair transport medium20 and were transported to the tetanus toxoid laboratory at the National Institutes of Health, Islamabad.
Samples were inoculated directly into thioglycolate broth medium and incubated at 37°C for 48 hours. The isolates were purified in cooked meat medium and then maintained at 4°C till further analysis. However, the C. tetani isolates were stored in 15% glycerol stock at −80°C for long-term storage. Bacterial isolates obtained on the selective media were then identified by Gram staining, colony morphology, swarming phenotype, and various biochemical tests such as gelatin hydrolysis, nitrate reduction, starch hydrolysis, hydrogen sulfide (H2S) production, DNase production, lipase, and lecithinase activities.21
Toxin production.
For the preparation of an induced inoculum, bacterial cells were inoculated aseptically into Mueller–Miller medium and incubated at 37°C for 48 hours. Subsequently, this seed culture was inoculated into the production medium (modified Mueller Miller media) in conical flasks and incubated at 37°C under shaking conditions for 6 days. The toxins were isolated from the debris of the cells (autolysis due to nutrient depletion) using a buckler filter and a vacuum pump.
Tetanus toxin detection in mouse.
Serology is often used as a screening tool and has good negative predictive value, however, due to the complex nature of bacterial strain this assay may yield false-positive results. To confirm toxin production by C. tetani, animal toxicity testing was performed following the recommendations and guidelines laid down by Public Health Laboratory Network Case Definitions released in 2000. Six mice having weights in the range of 18–20 g were selected and inoculated intramuscularly with 300 μL of tetanospasmin/tetanus toxin. Control mice were injected with 500 μL (1500 U) of tetanus anti-toxin an hour before toxin inoculation to neutralize the effect of any tetanospasmin injected into the mouse. Post-injection observations were documented for 72 hours. All experiments involving mice were conducted at the Animal Health Research Laboratories in the National Agricultural Research Center in compliance with ethical guidelines recommended by the board of governance of the respective institutions.
Antimicrobial susceptibility.
Because of a lack of clear guidelines for studies on C. tetani antimicrobial susceptibility testing, the Clinical Laboratories Standards Institute (CLSI) methodology was followed with certain modification.22 Fresh isolates were used to avoid any experimental error borne by the long-term storage of samples. Blood agar plates (3% agar and 7% sheep blood) were prepared; the agar concentration was kept higher than normal to ensure that the C. tetani would not swarm on the media. Antibiotic discs (Oxoid, United Kingdom) were impregnated with the following antibiotics: erythromycin (15 μg), metronidazole (5 μg), ciprofloxacin (75 μg), tetracycline (30 μg), penicillin G (10 μg), ofloxacin (5 μg), and chloramphenicol (30 μg). The antibiotic-impregnated discs were placed on top of the solidified agar before incubation at 37°C, for 24 hours. The diameters of the zones were measured to determine the minimum inhibitory concentrations (MICs) of given antibiotics.
Colony polymerase chain reaction for confirmation of TetX gene in C. tetani clinical isolates.
The presence of the TetX gene was determined to confirm the presumptive clinical isolates from tetanus diagnosed patients. DNA was extracted from C. tetani isolates by plating them on blood agar, picking the colonies by toothpick, and then resuspending the colonies thoroughly in 50 μL distilled water. The resultant cell suspension was boiled for 10 minutes, followed by centrifugation to pellet the cell debris and obtain the supernatant containing the C. tetani DNA. Polymerase chain reaction (PCR) was performed using primers Tet-F and Tet-R. Sample DNAs were added to a mixture containing PCR buffer, dNTPs, reverse and forward primers (20 μmol/L each), and Taq polymerase using the standard concentrations for a 50 μL reaction (Sigma, United Kingdom). The resulting PCR amplicons were examined on a 1% agarose gel with ethidium bromide.
Results
Wound swabs from 80 patients with clinically diagnosed tetanus were cultured anaerobically for screening C. tetani isolates using the biochemical tests. The biochemical examination of the culture in Mueller–Miller medium revealed the presence of anaerobic bacteria with characteristic gas production in five of the 80 culture tubes. Anaerobic plates were examined for the presence of swarming colonies and those observed were subcultured and examined using Gram staining; this showed the C. tetani bacteria as Gram-positive bacilli containing spores at their termini. In addition, the bacterial isolates were observed to produce a thin transparent film of swarming growth on the blood agar exhibiting hemolysis. The spore-forming rods showed the characteristic round, terminal, distending drumstick-like spores typical of C. tetani. Out of 80 samples only five samples (16%), were found to be positive for C. tetani; of these, three were from arm wounds and two from foot wounds. During biochemical characterization, the isolates were found to be positive for H2S and DNase in culture medium and liquefied the gelatin; however, there was no expression of nitrate reductase, amylase, lipase, and lecithinase activities.
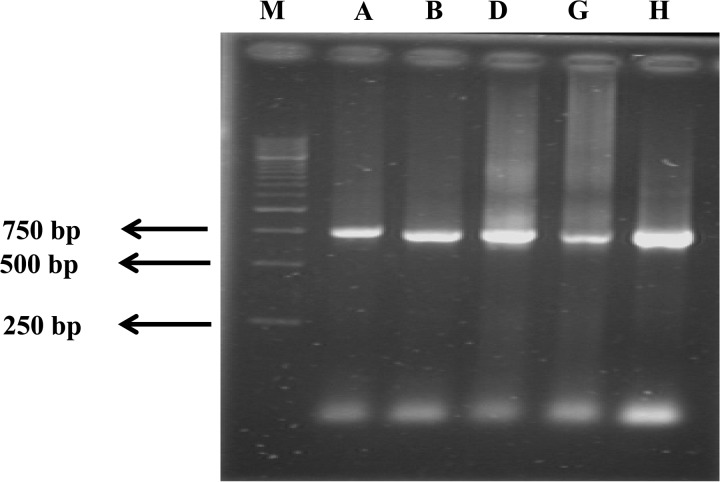
Colony PCR was conducted on all five isolates to confirm that they contained the TetX gene, which encodes tetanus neurotoxin. Previously, Campbell and others16 used TetX gene to isolate the infecting C. tetani strains by performing molecular diagnosis. Similarly, in Japan, the presence of the TetX gene was used to diagnose clinical tetanus in tetanus patients.23 We sought to ensure that all isolated strains were tetanus causing agents as suggested by clinical diagnosis, because not all of the strains of C. tetani harbored the pE88 plasmid (74 kbps) encoding TetX.24 In the selected clinical isolates, we successfully amplified a 750 bp fragment using PCR primers, Tet-F and Tet-R, which target the 5′ prime end of the 3.9 kb TetX gene (Figure 1 ). This confirmed that these clinical isolates were correctly identified as C. tetani; and that they could produce tetanus-specific neurotoxin.
Figure 1.
Detection of tetanospasmin gene (TetX) by PCR. Lanes A–H represent five clinical isolates of Clostridium tetani: C. tetani strains A, B, D, G, H, respectively. The size for tetanospasmin gene is 750 bp for lanes 2–6, lane M shows the molecular weight size marker (1 Kb marker, Fermentas).
Animal toxicity testing.
Mice that were injected with a dose of C. tetani or purified crude tetanus toxin showed a minor effect of rigidity in the tail muscles after 12 hours of injection. After 24 hours, the mice showed clear symptoms of tetanus with severe stiffness in the hind portion of the body especially in the limbs (Figure 2B ). After 48 hours, all of the body muscles showed escalating tautness that resulted in the death of all mice by 72 hours (Figure 2C).
Figure 2.
Mice showing the effects of injected cultured toxin (A) after 12 hours, (B) after 24 hours, with stiffness in tails and limbs, (C) at 72 hours, with death of mice.
Antimicrobial susceptibility.
Because of the lack of standard protocols for antimicrobial susceptibility testing on clinical isolates of C. tetani, we faced a significant challenge in our interpretation of the results as there are many factors that may compound the interpretation. For example, the CO2 gas production during the anaerobic growth of C. tetani may result in a drop in the pH and thereby subsequently disrupt the mechanism of antibiotic action and reduce the potency of antimicrobial agents. Moreover, as antimicrobial testing of anaerobic organisms is not practiced routinely, with the exception of specialist laboratories, this resulted in a lack of standards for setting the breakpoints for MICs for C. tetani. Therefore, the recommendations and guidelines stipulated by CLSI for setting breakpoints for anaerobic bacteria and non-Enterobacteriaceae were followed, and we correlated our results with them.22 Various standard parameters were used to categorize the C. tetani strains being susceptible to or resistant to antimicrobial agents, such as concentrations of the antimicrobial agents, conditions of growth, interpretation of zone diameters for susceptibility, and resistance based on the tentative breakpoints as per recommended for the anaerobes by CLSI. The selected antimicrobial agents, present on impregnated paper discs placed on the surface of a homogenized blood agar plate, diffused into the medium and inhibited the propagation of C. tetani around the discs, forming a zone of inhibition, hence showing the susceptibility of the isolates to antimicrobial agents commonly used in the treatment of tetanus. The experiment was repeated twice, each in triplicate and average values of zone diameters for all antimicrobial agents were calculated. Of particular note was the sensitivity of C. tetani to penicillin G, as this is the most recommended antibiotic for tetanus remedy. With the exception of erythromycin and ofloxacin, all five clinical isolates of C. tetani exhibited similar patterns of sensitivity to the selected antimicrobial agents (Table 1).
Table 1.
Antibiotic sensitivity pattern of Clostridium tetani
| Isolates† | Zones of inhibition* | ||||||
|---|---|---|---|---|---|---|---|
| E | MZ | CFP | T | P | OFX | C | |
| C. tetani A | ≤ 0.1 (R) | 1.2 (S) | 1.0 (S) | 0.9 (S) | 1.0 (S) | ≤ 0.1 (R) | 0.7 (S) |
| C. tetani B | ≤ 0.1 (R) | 1.1 (S) | 1.0 (S) | 1.0 (S) | 1.3 (S) | ≤ 0.1 (R) | 0.7 (S) |
| C. tetani D | ≤ 0.1 (R) | 1.5 (S) | 1.5 (S) | 1.0 (S) | 1.2 (S) | ≤ 0.1 (R) | 1.5 (S) |
| C. tetani G | ≤ 0.1 (R) | 1.2 (S) | 1.5 (S) | 1.3 (S) | 0.9 (S) | ≤ 0.1 (R) | 1.4 (S) |
| C. tetani H | ≤ 0.1 (R) | 1.0 (S) | 0.7 (S) | 1.2 (S) | 1.0 (S) | ≤ 0.1 (R) | 0.8 (S) |
CFP = cefoperazone; C = chloramphenicol; E = erythromycin; MZ = metranidazole; OFX = ofloxacin; P = penicillin G; R = resistance; S = sensitivity; T = tetracycline.
Cutoff values for E and C are: ≥ 0.7 = susceptible and ≤ 0.3 = resistance, while the cutoff values for metronidazole, CFP, T, P, OFX include ≥ 1 = susceptible and < 0.5 = resistance, ≥ 0.5 = susceptible and ≤ 0.1 = resistance, ≥ 0.8 = susceptible and ≤ 0.2 = resistance, ≥ 0.8 = susceptible and ≤ 0.3 = resistance, and ≥ 0.7 = susceptible and ≤ 0.2 = resistance, respectively.
Zone of inhibition (measured in cm) to E, MZ, CFP, T, P, OFX, and C.
Five C. tetani strains isolated from the clinical samples of tetanus diagnosed patients.
Discussion
The isolation and screening of C. tetani, from the clinical sample, is a most difficult and challenging practice for microbiologists. Previously, the isolation of the C. tetani from the different soil samples was carried out by Bukar and his colleagues using an anaerobic culturing technique.25 In this study, the isolation of the clinical bacterial isolates of C. tetani was performed from clinically diagnosed tetanus patients.26 As a site of infection was obvious in our patients, this increased the isolation rate of C. tetani by inoculating the primary media with C. tetani isolates.16
The screening and identification of the clinical isolates were confirmed through a number of different morphological, biochemical tests, and animal model. C. tetani is a Gram-positive bacillus, an obligate anaerobe, and readily produces endospores.27 Our isolates were all Gram-positive anaerobic bacilli, in agreement with earlier findings.10,15 Furthermore, PCR results obtained from amplification of the TetX gene clearly established the identity of the five clinical isolates as C. tetani. The presence of the TetX gene also showed they had the capability to produce the neurotoxin. Furthermore, upon challenging mice with pure neurotoxin obtained from these isolates, this caused stiffness in the hind portion of their bodies (limbs and tails), which is a clinical symptom of tetanus. This also indicated that the clinical isolates were C. tetani, which were capable of producing neurotoxin, which agrees with similar findings as reported by previous studies.28
Our isolated strains were found to be positive for H2S gas production and DNase activity; however, they showed negative results for starch, nitrate, lecithinase, and lipase activities; the results obtained from these biochemical tests corroborated those of the Public Health Laboratory Network case definitions (2002).
One of the main aims of the study was to examine the susceptibility of our C. tetani isolates to the routinely used antibiotics and to report any antimicrobial resistance developed by C. tetani, which might lead to poor treatment of the disease. There are very few studies reported on the antimicrobial susceptibility of clinically isolated C. tetani.12,16,26 As far as authors' knowledge is concerned; this is the first study performed concerning isolation and antimicrobial susceptibility of clinical isolates of C. tetani in Pakistan.
Despite its low accuracy, the disc diffusion method was ideal for use in this study. This method is reported to be inexpensive, easy to use, and can be applied for rapid screening of antibiotic susceptibility among both aerobic and anaerobic bacteria in developing countries. Also, this method is recommended by CLSI for anaerobes growing within 24 hours; in addition enough data about where to set breakpoints for different antibiotics is available for Clostridium species.22 Therefore, we have used the disc diffusion method for this study.
A variable pattern of antimicrobial susceptibility was observed with the different antibiotics tested in our study, that is, cefoperazone, tetracycline, penicillin G, metronidazole, and chloramphenicol all exhibited zones of inhibition against C. tetani, whereas erythromycin and ofloxacin did not. Cook and others12 previously reported the sensitivity of C. tetani to erythromycin, gentamycin, metronidazole, tetracycline, and chloramphenicol, which supports the findings obtained in our study. In another study, C. tetani sensitivity was observed with penicillin and metronidazole. However, it showed resistance to cotrimoxazole.16 In contrast to other species of Clostridia, such as C. difficile, which is an inhabitant of the intestinal tract, there is a distinct lack of antibiotic resistance mechanisms in C. tetani.29 The data produced from our study revealed the effectiveness of cefoperazone, metronidazole, tetracycline, penicillin G, and chloramphenicol against C. tetani-mediated infection in Pakistan. Simultaneously, the findings also suggested less sensitivity of C. tetani to ofloxacin and erythromycin.
The ineffectiveness of ofloxacin and erythromycin, for instance, can be related to the concentration of the effective dose over a crucial time frame of exposure. Public health agency of Canada30 found the viability of C. tetani in the wounds over a period of 2 weeks treatment with high doses of penicillin contrary to 24 hours exposure in our case. Furthermore, the ineffectiveness of the relevant antimicrobial agents can be explained by taking into account the fact that concentrations of antimicrobial agents reaching the target site might be less than required to be effective, possibly due to poor vascularization of the site of infection, which therefore provides a favorable conditions for the survival of C. tetani. This study suggests that the application of erythromycin and ofloxacin should be recommended cautiously to avoid any treatment failure in tetanus diagnosed patients in Pakistan. The findings of our study suggested the choice of penicillin, metronidazole, cefoperazone, and tetracycline on their own or in combination for treating clinically diagnosed tetanus patients in Pakistan.
Further study is required to explore the molecular basis of the antibiotic resistance observed in the C. tetani strains in Pakistan. Moreover, this study also emphasizes the application of mice as a model system to determine the effect of toxicity of the neurotoxin produced by C. tetani.
Despite much work that has been performed on the development of tetanus vaccine, very little data are available on local C. tetani isolates and their antimicrobial susceptibility. This study is the only study in Pakistan, which addresses isolation, identification, and antimicrobial susceptibility testing of C. tetani clinical isolates. This study has a great significance in terms of offering a base of preliminary evidence regarding the antimicrobial susceptibility patterns for C. tetani; and hopefully it will lead to further research on the molecular determinants that give rise to such patterns in Pakistan.
ACKNOWLEDGMENTS
We would like to thank the administration and all of the team at the Tetanus Toxoid Laboratory, National Institute of Health, Pakistan for providing the technical support and facilities during the research work. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: This research was supported financially by the Department of Microbiology, Quaid-i-Azam University, Islamabad, Pakistan.
Authors' addresses: Hajra Hanif, Naeem Ali, Asif Jamal, Muhammad Imran, and Muhammad Ishtiaq Ali, Department of Microbiology, Quaid-i-Azam University, Islamabad, Pakistan, E-mails: hajrahanif@hotmail.com, naeemali95@gmail.com, asifjamall@yahoo.com, m_imran766@hotmail.com, and ishi_ali@hotmail.com. Awais Anjum, Department of Food Sciences, University of Nottingham, Nottingham, United Kingdom, E-mail: awais.anjum@nottingham.ac.uk. Bashir Ahmad, Department of Bioinformatics and Biotechnology, International Islamic University Islamabad, Islamabad, Pakistan, E-mail: bashir.ahmad@iiu.edu.pk.
References
- 1.Dietz V, Milstein JB, Loon FV, Bennett J. Performance and potency of tetanus toxoid implications for eliminating neonatal tetanus. WHO. 1996;74:619–628. [PMC free article] [PubMed] [Google Scholar]
- 2.Thwaites CL, Yen LM, Nga NT, Parry J, Binh NT, Loan HT, Thuy TT, Bethell D, Parry CM, White NJ, Day NP, Farrar JJ. Impact of improved vaccination programme and intensive care facilities on incidence and outcome of tetanus in southern Vietnam, 1993–2002. Trans R Soc Trop Med Hyg. 2004;98:671–677. doi: 10.1016/j.trstmh.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Whitman C, Belgharbi L, Gasse F, Torel C, Mattei V, Zoffmann H. Progress towards the global elimination of neonatal tetanus. World Health Stat Q. 1992;45:248–256. [PubMed] [Google Scholar]
- 4.Quddus AS, Luby M, Rahbar Y, Pervaiz Y. Neonatal tetanus: mortality and risk factor in Loralai district Pakistan. Int J Epidemiol. 2002;31:648–653. doi: 10.1093/ije/31.3.648. [DOI] [PubMed] [Google Scholar]
- 5.Owusu-Darko S, Diouf K, Nour N. Elimination of maternal and neonatal tetanus. A 21st-century challenge. Rev Obstet Gynecol. 2012;5:151–157. [PMC free article] [PubMed] [Google Scholar]
- 6.Udwadia FE. Tetanus. Oxford, United Kingdom: Oxford University Press; 1994. [Google Scholar]
- 7.Bruggemann H, Baumer S, Fricke WF, Wiezer A, Liesegang H, Decker I, Herzberg C, Martinez-Arias R, Merkl R, Henne A, Gottschalk G. The genome sequence of Clostridium tetani the causative agent of tetanus disease. Proc Natl Acad Sci USA. 2003;100:1316–1321. doi: 10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellanby J, Green J. How does tetanus toxin act? Neuroscience. 1981;6:281–300. doi: 10.1016/0306-4522(81)90123-8. [DOI] [PubMed] [Google Scholar]
- 9.Hatheway CL. Toxigenic clostridia. J Micro Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard F, Edlich MD, Lisa G, Chandra A, Daniel G, Larry S. Management and prevention of tetanus. J Long Term Eff Med Implants. 2003;13:139–154. [PubMed] [Google Scholar]
- 11.Farrar JJ, Yen LM, Cook T, Fairweather N, Binh N, Parry JCM. Neurological aspects of tropical disease: tetanus. J Neurol Neurosurg Psychiatry. 2000;69:292–301. doi: 10.1136/jnnp.69.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87:477–487. doi: 10.1093/bja/87.3.477. [DOI] [PubMed] [Google Scholar]
- 13.Bruggemann H, Baumer S, Fricke WF, Wiezer A. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci USA. 2003;100:1316–1321. doi: 10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambo JA, Memon MI, Khahro ZH. Epidemiology of neonatal tetanus in rural Pakistan. J Pak Med Assoc. 2011;61:1099–1103. [PubMed] [Google Scholar]
- 15.Fairweather NF, Lyness VA. The complete nucleotide sequence of tetanus toxin. Nucleic Acids Res. 1986;14:7809–7812. doi: 10.1093/nar/14.19.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell JI, Lam TM, Huynh TL, To SD, Tran TT, Nguyen VM, Le TS, Nguyen vV, Parry C, Farrar JJ, Tran TH, Baker S. Microbiologic characterization and antimicrobial susceptibility of Clostridium tetani isolated from wounds of patients with clinically diagnosed tetanus. Am J Trop Med Hyg. 2009;80:827–831. [PubMed] [Google Scholar]
- 17.Sanford JP. Tetanus-forgotten but not gone. N Engl J Med. 1995;332:812–813. doi: 10.1056/NEJM199503233321209. [DOI] [PubMed] [Google Scholar]
- 18.WHO Technical Note . Current Recommendations for Treatment of Tetanus During Humanitarian Emergencies. Geneva, Switzerland: WHO Communicable Diseases Working Group on Emergencies, Communicable Diseases Surveillance and Response, WHO Regional Office for the Americas; 2010. [Google Scholar]
- 19.Abrutyn E. Tetanus. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. Harrison's Principles of Internal Medicine. New York, NY: McGraw-Hill; 1995. [Google Scholar]
- 20.Cary SG, Blair EB. New transport medium for shipment of clinical specimens. J Bacteriol. 1964;88:96–98. doi: 10.1128/jb.88.1.96-98.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. Manual of Clinical Microbiology. 6th edition. Washington, DC: ASM Press; 1995. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; 17th Informational Supplement. MS100-S17. Wayne, PA: CLSI; 2007. [Google Scholar]
- 23.Nagao K, Mori T, Sawada C, Sasakawa C, Kanezaki Y. Detection of the tetanus toxin gene by polymerase chain reaction: a case study. Jpn J Infect Dis. 2007;60:149–150. [PubMed] [Google Scholar]
- 24.Bruggemann H. Genomics of clostridial pathogens: implication of extrachromosomal elements in pathogenicity. Curr Opin Microbiol. 2005;8:601–605. doi: 10.1016/j.mib.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Bukar A, Mukhtar MD, Adam SA. Current trend in antimicrobial susceptibility pattern of Clostridium tetani isolated from soil samples in Kano Bay. J Pure and App Sci. 2008;1:112–115. [Google Scholar]
- 26.Brazier JS, Duerden BI, Hall V, Salmon JE. Isolation and identification of Clostridium spp. from infections associated with the injection of drugs: experiences of a microbiological investigation team. J Med Microbiol. 2002;51:985–989. doi: 10.1099/0022-1317-51-11-985. [DOI] [PubMed] [Google Scholar]
- 27.Izurieta HS, Sutter RW, Strebel PM. Tetanus surveillance—United States 1991–1994. In: CDC Surveillance Summaries. MMWR Morb Mortal Wkly Rep. 1997;46:15–25. [PubMed] [Google Scholar]
- 28.Mukhtar MD, Adam SA, Almustapha H. Study on the toxigenicity of C. tetani isolated from soil around Kano. Nig J Microbiol. 2008;20:1466–1471. [Google Scholar]
- 29.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 30.Public health agency of Canada Clostridium tetani. 2012. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/clostridium-tetani Available at. Accessed April 28, 2015.