Abstract
Brucellosis remains a serious public health issue in developing countries, including China. On August 8, 2013, four cases of brucellosis from one extended family were reported at Shuyang County, Jiangsu Province, China. Active case finding was performed to identify the source and the risk factors of the infection and to prevent additional cases. Multiple-locus variable number tandem repeat analysis (MLVA) was used for molecular subtyping analysis. Six people from two extended families met the case definition for brucellosis infection; four were blood culture positive for Brucella melitensis biotype 3. Four additional family members were found seropositive by using a serological test. Isolates from the four patients were indistinguishable by MLVA profiling, displaying a unique type for Jiangsu Province. Field epidemiological data combined with MLVA genotyping supported a common source of the isolates from the different patients. We recommend stronger reinforcement measures for animal quarantine practices, enhanced cooperation with veterinary service organizations, and implementation of measures that strengthen public education on brucellosis to prevent further human outbreaks in Jiangsu Province.
Brucellosis is recognized globally as one of the most common zoonotic diseases and accounts for more than 500,000 human cases every year.1 Humans are infected mainly through contact with infected animals or by consumption of contaminated dairy products.2 Brucellosis remains a serious public health issue in developing countries, including China. At present, brucellosis is endemic in 30 provinces or autonomous regions in China; the incidence increased substantially from 1.41 per 100,000 population in 2005 to 2.7 per 100,000 population in 2009.3 The major epidemic species in China is Brucella melitensis.4 With an incidence of 0.01 per 100,000 population, Jiangsu Province is one of the lowest incidence areas of brucellosis in eastern China.3
On August 8, 2013, clinicians at the First Affiliated Hospital of Nanjing Medical University in Nanjing, Jiangsu Province, reported to Jiangsu Provincial Center for Disease Control and Prevention that four patients from the same family had contracted brucellosis. An investigation was initiated to determine the extent of spread, source of infection, and possible mechanisms of pathogen introduction into this area.
The four family members are residents of Shuyang County, located in the north of Jiangsu (Figure 1 ), where no cases of brucellosis have previously been reported. Active case finding was conducted by searching local hospital records and outpatient clinical files, and by interviewing brucellosis cases, family members, and neighbors dwelling in the same villages. Information about demography, clinical symptoms, possession of or contact with animals, participation in common meals, consumption of dairy products, or traveling to brucellosis-endemic areas was collected at the same time. The study was approved by the Ethics Committee of the Jiangsu Center for Disease Control and Prevention (CDC). Informed consent was voluntarily obtained from all participants.
Figure 1.
Map of Jiangsu Province including the location of Nanjing, Suzhou, Xuzhou, Changzhou, Zhenjiang, and Shuyang.
We defined confirmed cases as any resident displaying positive by serum agglutination test (SAT) (Brucella agglutination titer greater than or equal to 1:160) or blood culture and at least one of the following symptoms: fever, night sweats, arthralgia, malaise, and headache. In total, six brucellosis cases from two families were identified in Shuyang County from January 1 to August 15, 2013. Five cases came from one extended family referred to as “family A” (Table 1). The remaining cases came from a family referred to as “family B.” Details of the six patients at presentation were described below.
Table 1.
Epidemiological and clinical features and laboratory results of 10 cases with brucellosis
| Case no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Epidemiological | ||||||||||
| Family | A | A | A | A | A | A | A | B | B | B |
| Sex | M | M | M | F | F | M | F | M | M | F |
| Age | 31 | 52 | 25 | 52 | 29 | 30 | 31 | 40 | 64 | 62 |
| Filiation | Index case | Father | Cousin | Mother-in-law | Wife | Brother | Sister-in-law | Index case | Father | Mother |
| Clinical findings | ||||||||||
| Days hospitalized | 5 | 10 | 7 | 10 | 8 | / | / | 10 | / | / |
| Fever | Yes | Yes | Yes | Yes | Yes | / | / | Yes | / | / |
| Chills | No | Yes | Yes | Yes | Yes | / | / | No | / | / |
| Sweating | Yes | Yes | No | No | No | / | / | Yes | / | / |
| Pain | Arthralgia | shoulder and back pain | No | Body ache | No | / | / | No | / | / |
| Headache | No | No | No | Yes | Yes | / | / | No | / | / |
| Clinical outcome | Cured | Cured | Cured | Cured | Cured | / | / | Cured | / | / |
| Laboratory results | ||||||||||
| WBC (3,500–9,500/μL) | 5,080 | 6,320 | 4,400 | 5,600 | 3,000 | / | / | 3,500 | / | / |
| PLT (125–350 × 103/μL) | 184 | 176 | 180 | 248 | 135 | / | / | 130 | / | / |
| ESR (0–15 mm/H) | 8 | 70 | 31 | / | 25 | / | / | 6 | / | / |
| ALT (0–50 U/L) | 16.8 | 29.8 | 68 | 14.7 | 24.8 | / | / | 126.4 | / | / |
| AST (0–50 U/L) | 19.6 | 20.2 | 64 | 17.1 | 41.2 | / | / | 65.0 | / | / |
| TBIL (5.1–19 μmol/L) | 19.5 | 15.6 | 12.0 | 3.4 | 9 | / | / | 11.4 | / | / |
| SAT | / | / | / | 1:200 | / | 1:400 | 1:400 | 1:800 | 1:400 | 1:400 |
| Isolation | Brucella melitensis | B. melitensis | B. melitensis | Negative | B. melitensis | / | / | B. melitensis | / | / |
| Strain's no. | JS1312 | JS1313 | JS1315 | / | JS1314 | / | / | Destroyed | / | / |
F = female; M = male; n.d. = not done; SAT = serum agglutination test; / = NA; WBC = white blood cell; PLT = platelets; ESR = erythrocyte sedimentation rate; ALT = alanine aminotransferase; AST = aspartate aminotransferase; TBIL = total bilirubin.
Family A
Case 1 (Table 1), a 31-year-old male considered the index case became ill on March 5, 2013 suffering from symptoms including fever, severe arthralgia, and sweating. Case 2, the index case's 52-year-old father became ill on April 16, was admitted with fever, shoulder and back pain, nausea, and chills. Case 3, a 25-year-old male who lived in an urban area in Shuyang County and was a relative of Case 1. Symptoms including fever and weakness were reported beginning on June 20. On July 20, another relative of Case 1, his mother-in-law (Case 4) presented to the hospital complaining of fever and body ache. Case 5, Case 1's 25-year-old wife, developed a fever on August 12.
Family B
A 40-year-old male (Case 8) was a civil servant. He became ill on June 13 with fever, night sweats, and weakness.
Brucella isolates were recovered from blood samples collected from four cases (Cases 1, 2, 3, and 5). The four isolates were identified as B. melitensis biotype 3 using a biochemical assay (bioMérieux VITEK 2, bioMérieux, Inc. Durham, NC), serum test (SAT), and molecular diagnostics (polymerase chain reaction [PCR] amplication for bcsp-31 and IS711).5,6 The Brucella spp. isolate recovered from Case 8 was isolated from blood and identified using a biochemical assay. Unfortunately, the isolate was lost during processing.
Serum samples from 69 persons including 16 family members and 53 neighbors were collected for Brucella serological screening by SAT.7 Blood specimens of 20 local villagers in Shuyang County who reared goats were also collected. Two members of family A, a brother and a sister-in-law of Case 1, had positive SAT titers (Cases 6 and 7) (Table 1). The father and mother of Case 8 also had positive SAT titers (Cases 9 and 10). Among the 53 neighbors and 20 local villagers, no one was seropositive.
A retrospective cohort study was conducted to identify risk factors for Brucella infection among a group of brucellosis patients from two extended families and their family members. In family A, Case 1 raised a herd of goats purchased from family B. Among these goats, pregnant females experienced abortions very soon after purchase. The goats were raised in captivity near Case 1's parents and his brother's homes before March of 2013. During this period, Case 1's parents and brother helped care for the goats and his father (Case 2) also had direct contact with the aborted fetuses. His cousin (Case 3), who resides primarily in an urban area in Shuyang, visited his parents once and was only occasionally exposed to the goats but without direct contact. However, the goats were moved to a shed nearby Case 3's house; subsequently, the goats were mainly raised by himself and his wife (Case 5). His mother-in-law (Case 4) who lived in another village had helped him take care of the goats for about 1 week when he was ill and in hospital. With respect of Family B, Case 8's parents were goat traders. Case 8 only had occasional exposures to his parents' goats without direct contact. The goats his parents sold to Case 1 originated from Shandong province with a higher incidence of brucellosis on the north of Jiangsu province (Figure 1), and had been raised mainly by his parents and brother. Before the onset of illness, all the family members from Family A and Family B denied having consumed lamb or goat's milk, or having traveled to brucellosis-endemic areas. The attack rate of Family A (77.8%) was much higher than that of Family B (23.1%) (relative risk [RR] = 3.4; 95% confidence interval [CI] = 1.2–9.7). Of the persons who had direct contact with the goats, 80% were infected with Brucella (RR = 4.8; 95% CI = 1.3–17.7), and the RR for direct contact with aborted fetuses was 6.0 (95% CI = 1.7–21.3).
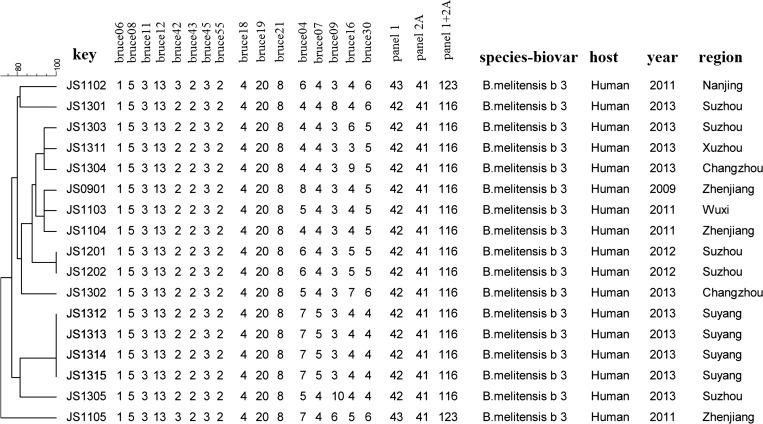
Multiple-locus variable number tandem repeat analysis (MLVA) was performed to study the genetic relationship of isolates recovered from cases according to a proposed international scheme.8,9 Sixteen microsatellite loci divided into three groups, eight loci in panel 1, three loci in panel 2A, and five loci in panel 2B, are used for the analysis. All four isolates recovered in this outbreak were identified as the same genotype: type 42 (1-5-3-13-2-2-3-2) in panel 1; this type has been included in the previously recognized “East Mediterranean” group. Genotype 42 in panel 1 and type 41 (4-20-8) in panel 2A are recognized as the major genotypes in Jiangsu, and are also widely distributed throughout China.10 However, type (7-5-3-4-4) in panel 2B is a unique genotype never before reported in China, but is the same type as that of BRU-S125 B. melitensis recovered from a person in Turkey.11 By combining panel1, 2A, and 2B markers, the four isolates were indistinguishable to one another but different from the genotypes of other Jiangsu isolates (Figure 2 ).
Figure 2.
Dendrogram based on multiple-locus variable number tandem repeat analysis (MLVA) results at 16 loci; 8 loci in panel 1, 3 loci in panel 2A, and 5 loci in panel 2B, showing relationships of the Brucella melitensis biovar 3 isolates from Jiangsu. Comparison of calculated type was based on unweighted pair group method with arithmetic mean (UPGMA) by BioNumerics software. Population modeling coefficient was categorical.
Collectively, B. melitensis biotype 3 was the causative pathogen of this outbreak, and its transmission was attributed to contact with imported infected goats. MLVA genotyping results provided evidence that four human cases may be infected from a common source. Together with the epidemiological investigation, our evidence indicated the link between the four human cases and the herd of imported goats. In conclusion, our results provide evidence supporting the usefulness of genotyping for epidemiological investigation.
Based on our findings, we recommend that promoting several public health measures to prevent brucellosis in the community. These include educating residents about Brucella infections, routes of transmission, and the main clinical manifestations of brucellosis as well as basic preventive measures for brucellosis. Screening animals sold commercially for brucellosis and vaccination of animals should be strengthened in this county. Reinforcing supervision of animal quarantine regulations, cooperating with veterinary authorities, and strengthening measures increasing knowledge for the prevention of brucellosis among people in non-epidemic areas were also suggested.
In this outbreak, support by local veterinarians was critical for epidemiological investigation. It is important for the local and provincial CDC staff to work collaboratively with veterinary counterparts in a “One Health” framework to control and prevent human brucellosis in epidemic areas, as well as other zoonotic disease challenges.12 Effective collaboration among different agencies will help to rapidly identify and remove potential sources of outbreaks.
One major limitation of the investigation was despite the epidemiological evidence that strongly supported the goats as the source of the outbreak, no specimen from the goats was available for Brucella testing, and the Brucella isolate recovered from Case 8 in Family B was inadvertently destroyed. These setbacks hampered the microbiological confirmation of the apparent epidemiological association between two families.
ACKNOWLEDGMENTS
We thank Ian J. Miller of the University of Wisconsin, Madison for his help with editing, and John Klena from the US Centers for Disease Control and Prevention for modifying our manuscript.
Footnotes
Financial support: This work was supported by the Jiangsu Province Health Development Project with Science and Education (ZX201109 and RC2011085), Chinese Field Epidemiology Training Program, and Jiangsu Province Science and Technology project of Clinical medicine (BL2014081).
Authors' addresses: Zhongming Tan, Weizhong Zhou, Fenyang Tang, and Yefei Zhu, Jiangsu Provincial Center for Disease Control and Prevention, Acute Infectious Disease Control and Prevention, Nanjing, China, E-mails: jstzm@jscdc.cn, jszwz@jscdc.cn, tfyepi@163.com, and jszyf@jscdc.cn. Yong Huang, Tongling Center for Disease Control and Prevention, Acute Infectious Disease Control and Prevention, Tongling, China, E-mail: topgun_hy@aliyun.com. Genyan Liu, The First Affiliated Hospital of Nanjing Medical University, Clinical laboratory, Nanjing, China, E-mail: lgyps@163.com. Xilou Xu and Zibing Zhang, Shuyang Center for Disease Control and Prevention, Acute Infectious Disease Control and Prevention, Suqian, China, E-mails: shuyangsongcdc@163.com and shycdczzb@163.com. Qing Shen, Karolinska Institutet, Institute of Environmental Medicine, Stockholm, Sweden, E-mail: qing.shen@ki.se.
References
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2013;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 3.Chen JD, Ke CW, Deng X, Jiang S, Liang W, Ke BX, Li B, Tan H, Liu M. Brucellosis in Guangdong Province, People's Republic of China, 2005–2010. Emerg Infect Dis. 2013;19:817–818. doi: 10.3201/eid1905.120146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man T, Wang D, Cui B, Wang Y, Ding F, Li TF. Analysis on surveillance data of brucellosis in China, 2009 [in Chinese] Dis Surveill. 2009;25:944–946. [Google Scholar]
- 5.Mukherjee F, Jain J, Patel V, Nair M. Multiple genus-specific markers in PCR assays improve the specificity and sensitivity of diagnosis of brucellosis in field animals. J Med Microbiol. 2007;56:1309–1316. doi: 10.1099/jmm.0.47160-0. [DOI] [PubMed] [Google Scholar]
- 6.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–2666. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob. 2010;36:S12–S17. doi: 10.1016/j.ijantimicag.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Le Flèche P, Jacques I, Grayon M, AI Dahouk S, Bouchon P, Denoeud F, Nöckler K, Neubauer H, Guilloteau LA, Vergnaud G. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006;6:9. doi: 10.1186/1471-2180-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whatmore AM, Shankster SJ, Perrett LL, Murphy TJ, Brew SD, Thirlwall RE, Cutler SJ, MacMillan AP. Identification and characterization of variable-number tandem-repeat markers for typing of Brucella spp. J Clin Microbiol. 2006;44:1982–1993. doi: 10.1128/JCM.02039-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Fan M, Chen J, Mi J, Yu R, Zhao H, Piao D, Ke C, Deng X, Tian G, Cui B. MLVA genotyping of Chinese human Brucella melitensis biovar 1, 2 and 3 isolates. BMC Microbiol. 2011;11:256. doi: 10.1186/1471-2180-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K𝚤l𝚤ç S, Ivanov IN, Durmaz R, Bayraktar MR, Ayaslioglu E, Uyanik MH, Aliskan H, Yasar E, Bayramoglu G, Arslantürk A, Vergnaud G, Kantardjiev TV. Multiple-locus variable-number tandem-repeat analysis genotyping of human Brucella isolates from Turkey. J Clin Microbiol. 2011;49:3276–3283. doi: 10.1128/JCM.02538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plumb G, Olsen S, Buttke D. Brucellosis: ‘One Health' challenges and opportunities. Rev Sci Tech Oie. 2013;32:271–278. doi: 10.20506/rst.32.1.2195. [DOI] [PubMed] [Google Scholar]