Abstract
While diffuse involvement of liver and spleen is frequently seen in brucellosis, suppurative abscesses caused by Brucella are less common but well described. With the increased availability of cross-sectional imaging techniques, reports have become more frequent. Four patients with hepatosplenic abscesses caused by Brucella spp. are described and included in a review of 115 previously published cases. Clinical characteristics and patterns on ultrasound (US) and computed tomography imaging were analyzed. Furthermore, the proportion of patients with brucellosis affected by suppurative hepatosplenic lesions was estimated. Hepatosplenic abscesses were seen in 1.2% of patients with brucellosis and were mostly caused by Brucella melitensis. Imaging analysis revealed two main distinct patterns. Solitary abscesses involving liver more frequently than spleen, and showing characteristic central calcifications, characterize the first pattern. Multiple smaller abscesses, frequent spleen involvement, and absence of calcifications characterize the second pattern. Blood and aspirate cultures were frequently negative, however, the positivity rate increased over the past years. Indirect Coombs test was positive in 96%. Half of the patients were cured by antibiotic treatment; case fatality in this series was 1.9%. Hepatosplenic abscesses due to Brucella infections have characteristic imaging findings. Clinicians should be aware of these and the proactive use of cross-sectional imaging, particularly US, should be encouraged in endemic regions.
Introduction
Brucellosis is the most common zoonosis worldwide with more than 500,000 cases annually and a wide geographic distribution.1 Brucella is endemic in the Mediterranean area and the Middle East as well as in Central Asian countries and to a lesser extent in South America. Brucellosis commonly presents as a systemic infection with fever and symptoms such as anorexia and night sweats. Focal lesions may occur in almost any organ, by far the most common localization being osteoarticular sites.2
Diffuse involvement of liver and spleen is frequently seen in human cases3; this may be attributed to intracellular survival and replication of bacteria in the mononuclear-phagocytic system of these organs. Hepatosplenomegaly and mild elevation of liver enzyme levels are observed as signs of this diffuse inflammation.
Suppurative abscess formation in liver and spleen is far less common but nevertheless well described in literature. Following the first reported case, diagnosed postmortem in 1904,4 only few further cases have been described in subsequent decades. This was partly due to the difficulty in making the diagnosis without imaging techniques such as those available today. Clinical signs and symptoms are nonspecific; plain radiographs of the abdomen may show calcifications in the hepatic area, but these are difficult to interpret and also nonspecific.5 With the advent of cross-sectional imaging techniques such as ultrasound (US) and computed tomography (CT), case reports have become more frequent during the past three decades.
In this article, we describe four cases of Brucella liver abscesses diagnosed by US. We also review published cases of hepatic and splenic abscesses in which imaging with US and/or CT were used in the diagnostic workup.
Methods
For all patients with hepatosplenic abscesses due to Brucella spp., diagnosed in the Department of Infectious and Tropical diseases, Istituto Di Ricovero e Cura a Carattere Scientifico San Matteo, Pavia, Italy, in the past 15 years, information on US and CT imaging as well as clinical data was collected.
A literature review using the key words “Brucella,” “liver,” “spleen,” and “abscess” as well as their extensions, was performed in Medline. Publications containing information on diagnostic findings on CT or US were selected and references of the articles were reviewed. Publications in English, Spanish, French, Italian, Portuguese, and German were included. Data were analyzed using χ2, Fisher exact and Mann–Whitney U tests, as appropriate.
Results
Four cases were identified in our department.
Case 1.
A 34-year old, previously healthy male butcher from Tuscany, Italy, was transferred to our hospital with a history of fever up to 39°C, joint, and abdominal pain. Laboratory tests revealed hemoglobin of 11.5 g/dL, white blood cells (WBC) of 11,500/μL with 75.9% neutrophils, and an erythrocyte sedimentation rate of 113 mm/hour. Liver function tests showed an increased alkaline phosphatase (ALP) (335 U/L) and gamma-glutamyl transferase (GGT) (151 U/L). Blood cultures were negative. Abdominal US revealed a lesion in liver segment VII of 6 cm in diameter and without significant doppler flow in or around the lesion. A calcification was seen in the central part of the lesion (Figure 1 ). Abdominal CT confirmed the presence of a hypo-dense lesion and the central calcification. An US-guided aspiration was performed, which yielded 25 mL of purulent material. The material was sent for microbiological analysis; culture results were negative after 20 days of incubation. Serological Wright test was negative, Coombs serology for incomplete Brucella antibodies was positive with a titer of 1:400. Therapy was started with doxycycline, rifampicin, and penfloxacin. The anti-Brucella titers rose to 1:640 in the Wright test and to 1:3,200 in Coombs' serology after initiation of therapy. After clinical improvement, the patient was discharged from hospital and antibiotic treatment was continued for a period of 3 months.
Figure 1.
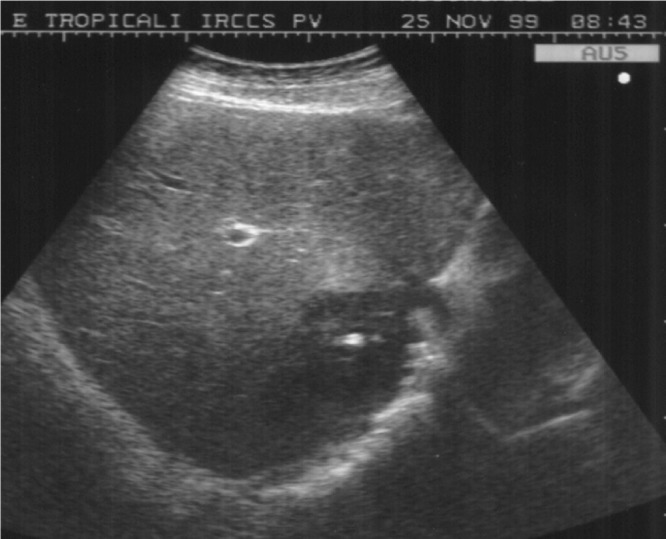
Ultrasonography of the liver (patient 1) showing a solitary brucellar abscess with typical echogenic calcification in the central area.
Case 2.
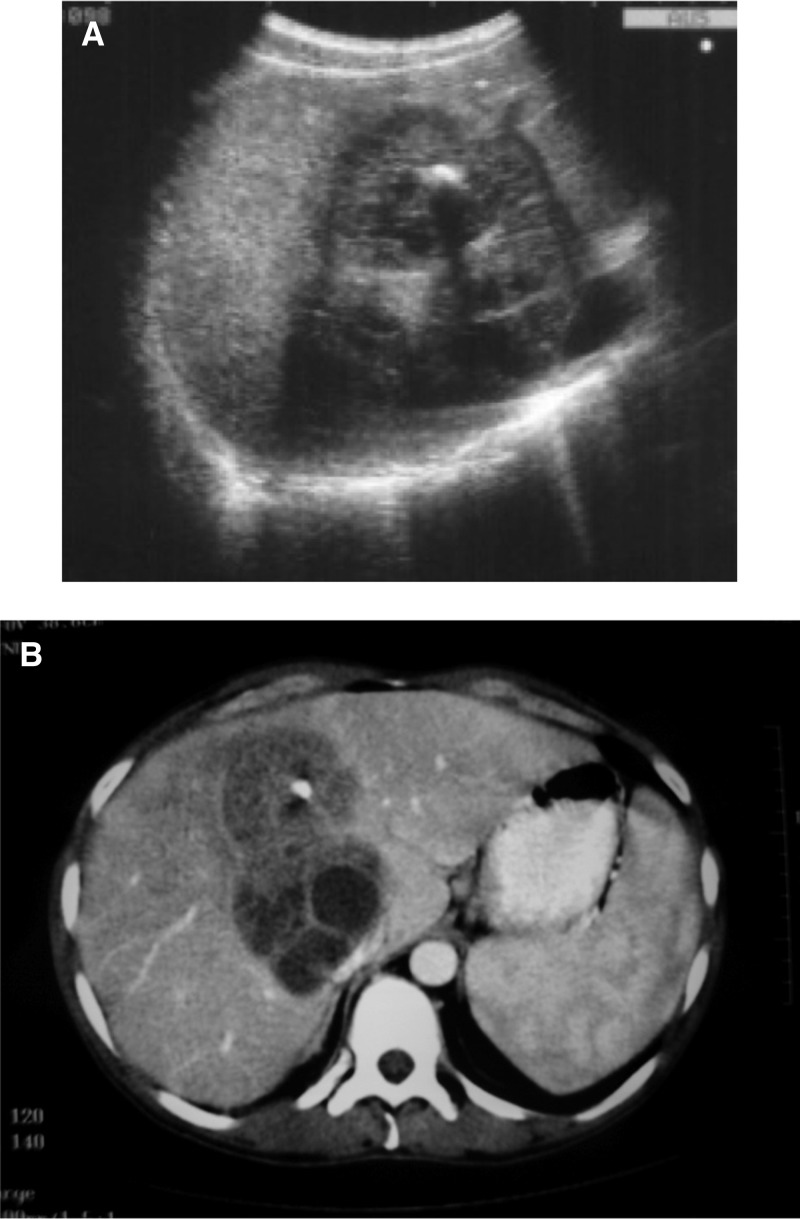
A 35-year-old male patient from Campania, Italy, was seen for suspected cystic echinococcosis (CE). Laboratory tests showed elevated WBC and GGT, and ALP. An abdominal US confirmed a mass in liver segments VII–VIII, with complex contours, a clear-cut border and a pattern of mixed echogenecity with hypo- and hyper-echoic areas and a central calcification of 1 cm diameter (Figure 2A ). A CT scan of the abdomen confirmed the lesion (Figure 2B). Aspiration biopsy produced granulomatous material; microbiological cultures remained sterile. Wright serology was positive with a titer of 1:640, Coombs-incomplete Brucella antibodies were positive with a titer of 1:1,600. The patient improved clinically during treatment (doxycycline/rifampicin). On an US performed 3 years after the initial diagnosis, the liver still showed a lesion, which was smaller than at diagnosis and homogenous; the central calcification had resolved.
Figure 2.
(A) Ultrasonography of the liver (patient 2) showing hepatic mass with complex contours, a well demarcated border and a pattern of mixed echogenecity with hypo- and hyper-echoic areas. A central calcification of approximately 1 cm diameter is visible as often seen in brucellar abscesses (B) Computer tomography scan of the same abscess showing a large lobulated hypodense lesion in liver segments VII–VIII with a central calcification.
Case 3.
A 33-year-old female patient from Lombardy, Italy, presented with an external US report compatible with a liver abscess in the left hepatic lobe with a central calcification, as well as a fluid collection in the abdominal wall with a diameter of approximately 6 cm. The CT scan confirmed an abscess in the left abdominal wall with an intraperitoneal component connected with the hepatic lesions in segment III and a cutaneous incision was performed in the surgical department, yielding large amounts of pus-like material. The patient was then referred to our clinic for suspected CE, but the central calcification immediately raised the suspicion of Brucella abscess and serological tests were performed (Wright negative; Coombs-incomplete antibodies 1:1,600). After treatment (doxycycline/rifampicin), the patient improved; the hepatic lesion had disappeared on a follow-up US after 1 year.
Case 4.
A 44-year-old female Italian patient with known sickle-cell anemia was seen for fever of unknown origin. Abdominal sonography showed a round lesion in liver segment VI (2.5 × 3 cm) with a large central calcification (1.5 cm). Following the experience of the previous cases, Brucella abscess was immediately suspected. Wright serology was negative, incomplete Brucella antibodies were highly positive (1:800) strengthening the diagnosis. Treatment with doxycycline/rifampicin was successful. On sonographic follow-up after 1 year, the hepatic abscess had disappeared, only the calcification remained visible.
Results from literature review of previously reported cases.
Literature review identified 80 reports (Supplemental Table 1 and references 1–80) published since 1979 describing diagnostic findings on US and/or CT imaging in patients with focal hepatic or splenic brucellosis. Including our own four cases, data on 119 cases of hepatosplenic Brucella abscesses were analyzed. Demographic information and the findings of diagnostic investigations are summarized in Table 1.
Table 1.
Demographic and clinical information on 119 patients with hepatosplenic abscesses due to Brucella spp. (proportion in % and 95% confidence interval)
| Demographic | Age, median years (range) | 43 (3–78) |
| Sex, male/female (ratio) | 65/49 (1,3:1) | |
| Clinical presentation | Abdominal pain and fever | 57% (48–67%) |
| Fever only | 37% (28–46%) | |
| Abdominal pain only | 6% (1–10%) | |
| Imaging findings | Single lesion | 68% (59–76%) |
| Multiple lesions | 32% (23–40%) | |
| Liver lesions | 70% (61–78%) | |
| Spleen lesions | 28% (20–36%) | |
| Liver and spleen lesions | 2% (0–10%) | |
| Liver abscess, right lobe | 71% (59–82%) | |
| Liver abscess, left lobe | 19% (9–29%) | |
| Liver abscess, both lobes | 10% (2–17%) | |
| Liver abscess with calcification | 77% (68–86%) | |
| Spleen abscess with calcification | 26% (11–42%) | |
| Microbiology results | Positive blood culture | 22% (14–31%) |
| Positive pus culture | 17% (8–27%) | |
| Direct serology > 1:160 | 40% (30–50%) | |
| Coombs serology > 1:320 | 96% (91–100%) | |
| Treatment used | Antibiotic only | 47% (38–57%) |
| Antibiotic and surgery | 48% (39–58%) | |
| Antibiotic and percutaneous | 5% (1–9%) | |
| Outcome | Improved | 98% (95–100%) |
| Died | 2% (0–5%) |
Demographic and clinical information.
A total of 57% of the patients were male, 43% female. The median age was 43 years ranging from 3 to 78 years. Only 4 cases were reported in children below the age of 14 years. The majority of cases were from Spain (49%), Turkey (18%), Italy (11%), and France (6%); from other countries only individual cases (less than five) have been reported. The majority of patients (57%) presented with fever and abdominal pain; 37% presented with fever only and 5% reported local symptoms only.
Underlying medical conditions, possibly explaining the development of focal abscesses due to immune suppression or shedding from endocardial infection, were reported rarely: three patients had hematologic disorders (thalassemia,6 monoclonal gammopathy,7 sickle-cell anemia [own]); three patients suffered from cardiac diseases (two endocarditis,8,9 one rheumatic aortic valve replacement10); two patients were pregnant at the time of diagnosis11,12; two patients suffered from diabetes13,14; one patient had hepatitis15 and one patient allergic rhinitis.16 Other patients were described as having no underlying disease or no condition was specified.
Imaging findings.
CT and US imaging studies revealed solitary abscesses in the majority of cases (68%); multiple lesions were seen in 37 cases (32%). Abscesses predominantly involved the liver (70%); 28% of patients presented with lesions in the spleen and 3 (2%) had abscesses in both. Of the 62 patients for whom information on the localization of the liver lesion was reported, the abscesses were localized in the right liver lobe in 44 (71%), in the left liver lobe in 12 (19%), and in 6 (10%) lesions were seen in both lobes. Liver abscesses were significantly more frequently solitary (77%, 95% CI: 68–86%) than those in the spleen (39%, 95% CI: 24–55%; P < 0.001). Liver lesions were larger (mean 6.0 ± 0.3 cm) than lesions in the spleen (mean 2.5 ± 0.3 cm, P < 0.01). When multiple lesions were present, these were significantly smaller than solitary lesions (P < 0.01); cases with multiple abscesses sized 1 cm and smaller were frequently seen.
In the large majority (77%) of the liver abscesses, a central calcification was visible. In splenic abscesses, calcifications were also described, but only in a lower proportion (26%; P < 0.001). In the liver, solitary abscesses more often showed a central calcification (82%, 95% CI: 73–92%) compared with multiple abscesses (43%, 95% CI: 24–70%; P < 0.01). Splenic abscesses contained calcifications less frequently, independent whether solitary (25%, 95% CI: 0–50%) or multiple (24%, 95% CI: 5–42%; P = n.s.).
Only four children were reported with abscesses due to Brucella spp.; in three of them multiple abscesses were seen. In all children the spleen was involved, in two the liver showed additional lesions. None of the pediatric cases showed calcifications, which was statistically significantly different from adults (P < 0.05).
Microbiology results.
In patients for whom isolation of the pathogen from blood cultures was attempted, only 21/90 (23%) grew Brucella spp. Material from the abscess was obtained by aspiration or as part of abdominal surgery in 65 patients; only in 12 (18%) the bacteria could successfully be cultured. In 25 cases, the causative pathogen was further characterized; Brucella melitensis was isolated in 20 (80%), B. abortus in 3 (12%), and B. suis in 2 (8%). The yield of positive blood cultures has increased in recent years, presumably due to improvements in culture technique and, possibly, due to better communication of clinical suspicion to the laboratory. In cases reported in the past 10 years (2005–2015), 11/25 (44%) blood cultures grew the causative organism. Although this is still a low rate, it is significantly higher than that seen in the patients diagnosed before 2005; 10/65 (15%, P < 0.01). Abscess material culture reported between 2005 and 2015 grew organisms in 25% (4/16), whereas in previous years 16% (8/49, P = n.s.) were positive.
In the absence of bacteriologic confirmation, high antibody titers can support the presumptive diagnosis of Brucella infection. A wide range of serologic tests has been used to indirectly diagnose brucellosis, including direct agglutination tests and ELISA. In the reviewed reports, the most widely used assays were serum agglutination tests, that is, Wright's reaction, performed both in tubes or micro-plates, or indirect Coombs test performed in tubes. Direct serology results were reported for 92 patients and the Wright's reaction titers were higher than 1:160 in 37 patients (40%). Indirect Coombs test was performed in 68 patients and was positive (titer > 1:320 or ≥ 1:400 depending on the serum dilution used) in 65 (96%); many patients showed titers of 1:1,280 (1:1,600, respectively) and above. The use of polymerase chain reaction (PCR) was reported from blood samples in seven patients and from pus or biopsy material in seven patients; in all 14 samples Brucella DNA was isolated.17–20
Treatment and outcome.
In 90 cases, the antibiotic regimen was specified; in the remaining “Brucella-specific antibiotic treatment” was reported. In 36 cases (40%), a combination of doxycycline with an aminoglycoside was used, in 27 cases (30%) doxycycline was combined with rifampicin. In 17 patients (19%), a triple combination was used (doxycycline, rifampicin, aminoglycoside in 12; combinations including TMP-SMZ in 5). In some patients, the antibiotics were prescribed for longer than 6 weeks; the combination of doxycycline/rifampicin was given for up to 3–9 months in individual patients.
Successful management by antibiotic treatment was reported for 51 patients (47%). Surgery (splenectomy, hepatic lobectomy) in combination with antibiotic treatment was reported for 52 patients (48%). Antibiotic treatment combined with percutaneous drainage was described in nine patients (8%); four of these patients required surgical intervention later. The indication for surgery was not uniform and depending on the treating physicians; in some cases, surgery was performed as first-line treatment. The efficacy of each strategy can thus not be assessed from the data.
103/105 (98%) patients for whom outcome was reported improved clinically and were eventually cured. This was documented clinically by subsiding fever and/or sonographical shrinking or resolving lesions. Two patients died13,14 amounting to a case-fatality rate of 1.9% (95% CI: 0–4.6%) in this case-report series.
Estimated prevalence of suppurative lesions.
To estimate the proportion of patients with brucellosis who develop abscesses in liver or spleen, five studies reporting on the size of the total patient population, were identified. Two of these studies performed a retrospective review of clinical files of patients diagnosed with brucellosis.2,15 One study assessed patients with the diagnosis of brucellosis prospectively; clinical criteria were used and imaging studies were only performed when the patient showed clinical abnormalities.17 Two studies prospectively screened all brucellosis patients by US for liver and spleen lesions.21,22 The results are summarized in Table 2. From a total of 2,475 patients, 31 cases with hepatosplenic abscesses were identified (1.2% of all patients, 95% CI: 0.8–1.8%). Liver abscesses occurred in 0.8% (95% CI: 0.5–1.3%), spleen abscesses in 0.4% (95% CI: 0.2–0.7%) of patients with brucellosis.
Table 2.
Proportion of patients with brucellosis showing suppurative hepatosplenic abscesses due to Brucella spp.
| N | Liver abscess | % (95% CI) | Spleen abscess | % (95% CI) | All | % (95% CI) | |
|---|---|---|---|---|---|---|---|
| Retrospective* | |||||||
| Reference15 | 905 | 14 | 1.5 (0.8–2.5) | 2 | 0.2 (0.02–0.7) | 16 | 1.7 (1.0–2.8) |
| Reference2 | 530 | 1 | 0.2 (0.01–1.0) | 0 | 0 (0–0.7) | 1 | 0.2 (0.01–1.0) |
| Sub-Total | 1,435 | 15 | 1.0 (0.6–1.7) | 2 | 0.1 (0.01–0.5) | 17 | 1.2 (0.7–1.8) |
| Prospective clinical† | |||||||
| Reference17 | 709 | 4 | 0.6 (0.14–1.5) | 3 | 0.4 (0.09–1.2) | 7 | 1.0 (0.4–2.0) |
| Prospective screening‡ | |||||||
| Reference21 | 80 | 2 | 2.5 (0.3–8.7) | 1 | 1.3 (0.03–6.7) | 3 | 3.8 (0.8–10.6) |
| Reference22 | 251 | 0 | 0 (0–1.4) | 4 | 1.6 (0.4–4.0) | 4 | 1.6 (0.4–4.0) |
| Sub-Total | 331 | 2 | 0.6 (0.1–2.1) | 5 | 1.5 (0.5–3.5) | 7 | 2.1 (0.9–4.3) |
| Total | 2,475 | 21 | 0.8 (0.5–1.3) | 10 | 0.4 (0.2–0.7) | 31 | 1.2 (0.8–1.8) |
CI = confidence interval.
Review of patient's files after discharge.
Patients prospectively screened clinically for signs and symptoms of liver/spleen abscess at the time of diagnosis of brucellosis then imaged.
Patients prospectively screened by ultrasound at the time of diagnosis of brucellosis.
Discussion
Although nonspecific hepatitis and enlargement of liver and spleen occurs frequently in patients with systemic Brucella infection,23 suppurative abscess formation appears to be less common. We found that hepatosplenic abscesses are a characteristic entity with a prevalence of approximately 1% in brucellosis patients and mainly due to B. melitensis. In reviews on clinical manifestations of brucellosis, hepatosplenic abscesses are not represented, probably being subsumed under broader clinical categories such as “hepatitis” or “hepatosplenomegaly.”3 Since the advent of US and CT, the number of cases diagnosed has increased and may increase further with US being now increasingly used in low-resource settings where brucellosis is prevalent.
The imaging findings of hepatosplenic Brucella abscesses are relatively uniform and should raise suspicion even in cases when brucellosis is not yet diagnosed or suspected. On the basis of the data available, we suggest two general imaging patterns: “solitary abscess with calcification” and “miliary.”
“Solitary abscess with calcification” is characterized by a solitary, often large abscess, most frequently affecting the liver, and marked by a central calcification (Figures 1 and 2). It has only been reported in adults and, given the calcifications, which need time to develop, one may surmise a more chronic and smoldering course of the disease. The imaging appears characteristic enough to formulate the diagnosis of brucellosis, especially in an endemic setting. Possible differential diagnoses include hepatocellular carcinoma and metastasis of the liver (both usually without calcification); posttraumatic or postoperative calcified hematomas (in which calcifications are often not central but peripheral), vascular calcifications and hemangiomas (which are usually not calcified). Potential infectious disease differential diagnoses include hydatid cysts (especially type CE 4 and CE 5) caused by Echinococcus granulosus.24 However, calcifications are always peripheral in these lesions.25 Bacterial abscesses usually have a hypo-echoic appearance and may show hyper-echoic areas due to gas collections, which may mimic calcifications and are thus a potential confounder.
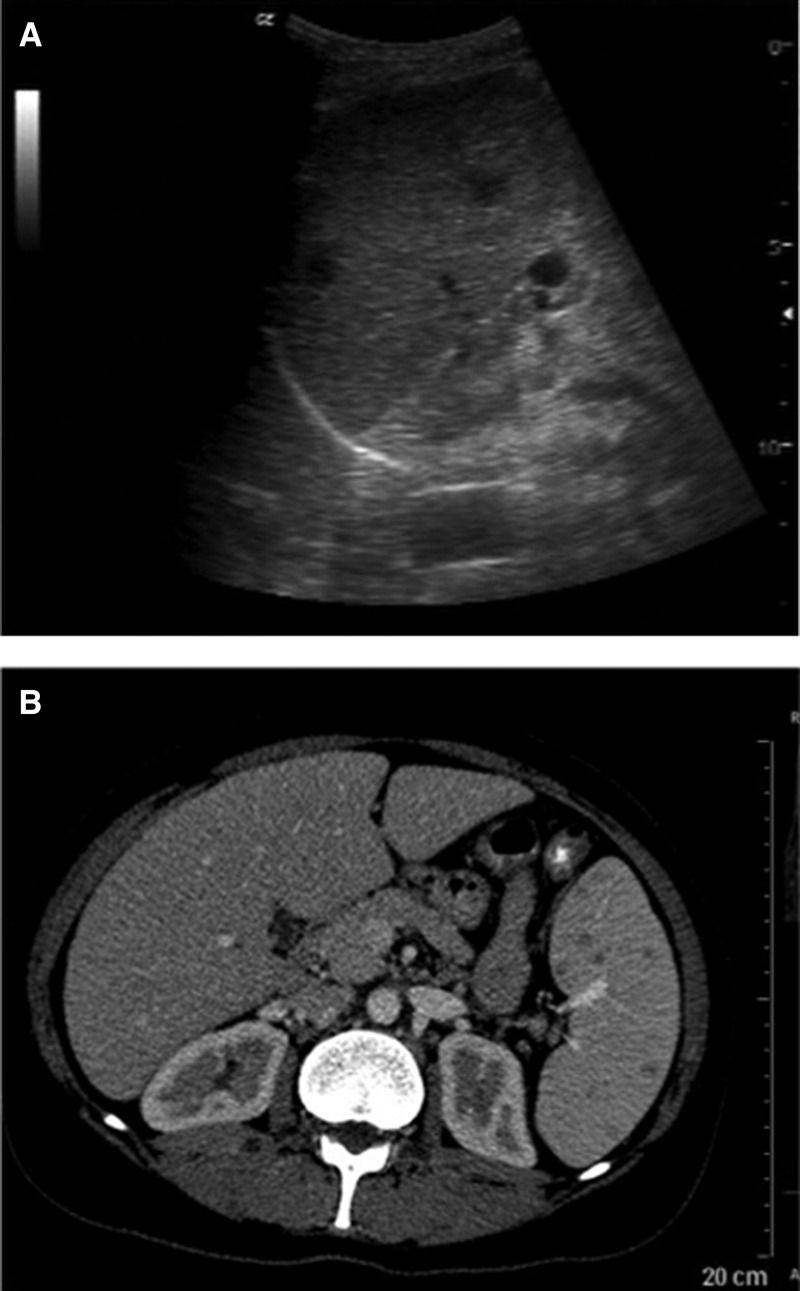
Multiple hypo-echoic, smaller abscesses mark the “miliary” pattern, which predominantly involves the spleen (Figure 3 ). The lesions may be smaller than 1 cm, show few calcifications and have been observed in adults and children. Given the wider spread of the lesions and the fact that the two patients who died due to their brucellosis showed the “miliary” pattern, one may speculate whether it indicates a less favorable balance between host-immune system and the pathogen.
Figure 3.
(A) Ultrasonography of the spleen in a patient with suppurative lesions in the spleen showing two hypo- to anechoic small lesions (diameter approximately 1 cm) (courtesy of D. Kotlar, Jeddah, Saudi Arabia) (B) Computer tomography scan of a patient with Brucella spleen abscesses showing as multiple hypodense lesions in the spleen (courtesy of M. Yilmaz, Istanbul, Turkey).
The “miliary” pattern is less characteristic; the main confounder is disseminated tuberculosis (TB), especially in regions where both diseases are endemic. The pattern of multiple hypo-echoic lesions in the spleen, and less frequently, in the liver, has been described as highly suggestive for disseminated TB and has been included into point-of-care US protocols for extra-pulmonary TB.26 Miliary TB lesions are most frequently seen in immune-compromised HIV patients27 but have also been reported in children without HIV coinfection.28 Infection with Bartonella, for example, HIV-associated bacillary angiomatosis or cat-scratch-disease may cause peliosis29 which is another differential diagnosis of hypo-echoic focal lesions, but peliosis is rare and associated with a positive serology for Bartonella. In Southeast Asian patients, melioidosis, caused by Burkholderia pseudomallei, should be considered as it may present with similar imaging features.30 Disseminated fungal abscesses may also appear similar,29 however, these characteristically show a “wheel-in-wheel” US appearance and are mainly seen in immune-compromised individuals with hematological disorders. Noninfectious differential diagnoses are sarcoidosis, lymphoma, and focal lesions in chronic lymphatic leukemia.
Overall, the two abovementioned patterns of suppurative Brucella lesions should be seen as two ends of a spectrum and a proportion of cases will fall in between and show mixed features.
In all patients suspected of brucellosis, isolation of the pathogen by blood culture, as well as of aspirated material should be attempted, although cultures are only positive in a minority of cases. Even if the diagnostic yield seems to have increased in recent years, a negative culture cannot rule out the diagnosis of brucellosis.
Although rarely reported in the reviewed cases, the development of specific PCR techniques has additionally improved the diagnosis of brucellosis.31 Multiplex PCR has been shown to successfully differentiate between focal complications due to Brucella spp. and Mycobacterium tuberculosis.32 Currently serology is the most commonly used test to support a diagnosis of brucellosis in suppurative lesions; especially indirect Coombs test, which is almost invariably positive, often showing high titers.
No standardized treatment approach can be deduced from the literature, but it seems that antibiotic treatment, for example, with doxycycline and rifampicin, is successful in most cases. If medical treatment is unsuccessful, percutaneous drainage or surgical intervention may be indicated and with these options successful outcome is seen in almost all cases.
In summary, although hepatosplenic abscesses are only seen in a relatively small proportion of patients with Brucella infections, in areas where the disease is prevalent a significant number of patients suffer from them. Imaging findings can be characterized as “solitary abscess with calcification” or “miliary” pattern and clinicians should be aware of these to suspect brucellosis. The use of cross-sectional imaging, especially relatively inexpensive and noninvasive US, should be encouraged in endemic regions to prompt the diagnosis by visualizing the focal complications of Brucella infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Luca Piccoli for help and support in the retrieval of literature. We also thank Mesut Yilmaz, Istanbul, Turkey and Daniel Kotlar, Jeddah, Saudi Arabia for their images of spleen lesions.
Footnotes
Financial support: We did not receive any financial support for this review. Sabine Bélard is a participant in the Charité Clinical Scientist Program founded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health.
Authors' addresses: Tom Heller, Department of Infectious Diseases, Division of Internal Medicine, Center of Tropical Medicine and Travel Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mail: echnatom@web.de. Sabine Bélard, Department of Pediatric Pneumology and Immunology, Charité-Universitätsmedizin, Berlin, Germany, and Department of Infectious Diseases, Division of Internal Medicine, Center of Tropical Medicine and Travel Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mail: sabinebelard@charite.de. Claudia Wallrauch, Helios Klinik Muenchen Perlach, Internal Medicine, Muenchen, Germany, E-mail: claudiawallrauch@web.de. Edoardo Carretto, Arcispedale Santa Maria Nuova, Clinical Microbiology Laboratory, Reggio Emilia, Italy, E-mail: edoardo.carretto@asmn.re.it. Raffaella Lissandrin, Carlo Filice, and Enrico Brunetti, Department of Infectious Diseases, San Matteo Hospital Foundation and University of Pavia, Pavia, Italy, E-mails: raffaella@web.de, carfil@unipv.it, and enrico.brunetti@unipv.it.
References
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Colmenero JD, Reguera JM, Martos F, Sanchez-De-Mora D, Delgado M, Causse M, Martin-Farfan A, Juarez C. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) 1996;75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1929. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyer J, Fawcett J. A case of subdiaphragmatic and hepatic abscess consecutive to Mediteranean fever. Guys Hosp Rep. 1904;59:207–216. [Google Scholar]
- 5.Sisteron O, Souci J, Chevallier P, Cua E, Bruneton JN. Hepatic abscess caused by Brucella US, CT and MRI findings: case report and review of the literature. Clin Imaging. 2002;26:414–417. doi: 10.1016/s0899-7071(02)00507-7. [DOI] [PubMed] [Google Scholar]
- 6.Meloni MF, Andreano A, Laeseke PF, Lee FT, Jr, Sironi S, Filice C, Ferraioli G. Contrast-enhanced ultrasonographic findings in a brucellar hepatic abscess. J Ultrasound Med. 2008;27:1511–1515. doi: 10.7863/jum.2008.27.10.1511. [DOI] [PubMed] [Google Scholar]
- 7.Collazos Gonzalez J, Yanez R, Macarron P, Beneitez Gomez C, Moldenhauer F, Abad JA, Fernandez Guerrero M. Hepatic abscess, osteomyelitis and monoclonal gammapathy in chronic brucellosis. Rev Clin Esp. 1984;174:131–133. [PubMed] [Google Scholar]
- 8.Park SH, Choi YS, Choi YJ, Cho SH, Yoon HJ. Brucella endocarditis with splenic abscess: a report of the first case diagnosed in Korea. Yonsei Med J. 2009;50:142–146. doi: 10.3349/ymj.2009.50.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz MB, Kisacik HL, Korkmaz S. Persisting fever in a patient with Brucella endocarditis: occult splenic abscess. Heart. 2003;89:e20. [PMC free article] [PubMed] [Google Scholar]
- 10.Saadeh AM, Abu-Farsakh NA, Omari HZ. Infective endocarditis and occult splenic abscess caused by Brucella melitensis infection: a case report and review of the literature. Acta Cardiol. 1996;51:279–285. [PubMed] [Google Scholar]
- 11.Naveau S, Poitrine A, Delfraissy JF, Brivet F, Dormont J. Brucellosis hepatic abscesses and pregnancy. Gastroenterology. 1983;84:1643. [PubMed] [Google Scholar]
- 12.Perez-Garcia C, Uribarrena R, Aizcorbe M, Rivero A. Absceso hepatico brucelar y embarazo. Gastroenterol Hepatol. 1987;10:361–362. [Google Scholar]
- 13.Paton NI, Tee NW, Vu CK, Teo TP. Visceral abscesses due to Brucella suis infection in a retired pig farmer. Clin Infect Dis. 2001;32:E129–E130. doi: 10.1086/319748. [DOI] [PubMed] [Google Scholar]
- 14.Park KW, Kim DM, Park CY, Kim HL, Jang SJ, Choi YS, Park MY, Song HJ, Lee SH. Fatal systemic infection with multifocal liver and lung nodules caused by Brucella abortus. Am J Trop Med Hyg. 2007;77:1120–1123. [PubMed] [Google Scholar]
- 15.Ariza J, Pigrau C, Canas C, Marron A, Martinez F, Almirante B, Corredoira JM, Casanova A, Fabregat J, Pahissa A. Current understanding and management of chronic hepatosplenic suppurative brucellosis. Clin Infect Dis. 2001;32:1024–1033. doi: 10.1086/319608. [DOI] [PubMed] [Google Scholar]
- 16.Vallejo JG, Stevens AM, Dutton RV, Kaplan SL. Hepatosplenic abscesses due to Brucella melitensis: report of a case involving a child and review of the literature. Clin Infect Dis. 1996;22:485–489. doi: 10.1093/clinids/22.3.485. [DOI] [PubMed] [Google Scholar]
- 17.Colmenero JD, Queipo-Ortun M, Reguera JM, Suarez-Munoz M, Mart𝚤n-Carballino S, Morata P. Chronic hepatosplenic abscesses in brucellosis. Clinico-therapeutic features and molecular diagnostic approach. Diagn Microbiol Infect Dis. 2002;42:159–167. doi: 10.1016/s0732-8893(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 18.Chourmouzi D, Boulogianni G, Kalomenopoulou M, Kanellos I, Drevelegas A. Brucella liver abscess; imaging approach, differential diagnosis, and therapeutic management: a case report. Cases J. 2009;2:7143. doi: 10.4076/1757-1626-2-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Arco A, De La Torre-Lima J, Prada JL, Aguilar J, Ruiz-Mesa JD, Moreno F. Splenic abscess due to Brucella infection: is the splenectomy necessary? Case report and literature review. Scand J Infect Dis. 2007;39:379–381. doi: 10.1080/00365540600978930. [DOI] [PubMed] [Google Scholar]
- 20.Menendez P, Villarejo P, Cubo T, Padilla D, Gambi D, Menendez JM, Martin J. Hepatic brucelloma: diagnosis and treatment. Gastroenterol Hepatol. 2009;32:291–293. doi: 10.1016/j.gastrohep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Sirmatel O, Yazgan P, Sirmatel F, Ozturk A. Hepatosplenic abscess in brucellosis. Saudi Med J. 2007;28:1613–1615. [PubMed] [Google Scholar]
- 22.Pourbagher MA, Pourbagher A, Savas L, Turunc T, Demiroglu YZ, Erol I, Yalcintas D. Clinical pattern and abdominal sonographic findings in 251 cases of brucellosis in southern Turkey. AJR Am J Roentgenol. 2006;187:W191–W1914. doi: 10.2214/AJR.05.0241. [DOI] [PubMed] [Google Scholar]
- 23.Cervantes F, Bruguera M, Carbonell J, Force L, Webb S. Liver disease in brucellosis. A clinical and pathological study of 40 cases. Postgrad Med J. 1982;58:346–350. doi: 10.1136/pgmj.58.680.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO IWG. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253–261. doi: 10.1016/s0001-706x(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo F, Graffeo M, Brunetti E. Extrahepatic textiloma long misdiagnosed as calcified echinococcal cyst. Case Rep Gastrointest Med. 2013;2013:261685. doi: 10.1155/2013/261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller T, Wallrauch C, Goblirsch S, Brunetti E. Focused assessment with sonography for HIV-associated tuberculosis (FASH): a short protocol and a pictorial review. Crit Ultrasound J. 2012;4:21. doi: 10.1186/2036-7902-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordani MT, Brunetti E, Binazzi R, Benedetti P, Stecca C, Goblirsch S, Heller T. Extrapulmonary mycobacterial infections in a cohort of HIV-positive patients: ultrasound experience from Vicenza, Italy. Infection. 2012;41:409–414. doi: 10.1007/s15010-012-0336-4. [DOI] [PubMed] [Google Scholar]
- 28.Belard S, Heller T, Workman L, Grobusch M, Zar H. Point-of-care ultrasound: improving the diagnosis of childhood tuberculosis. Pediatr Radiol. 2014;44:679–680. doi: 10.1007/s00247-014-2971-7. [DOI] [PubMed] [Google Scholar]
- 29.Paterson A, Frush DP, Donnelly LF, Foss JN, O'Hara SM, Bisset GS., 3rd A pattern-oriented approach to splenic imaging in infants and children. Radiographics. 1999;19:1465–1485. doi: 10.1148/radiographics.19.6.g99no231465. [DOI] [PubMed] [Google Scholar]
- 30.Muttarak M, Peh WC, Euathrongchit J, Lin SE, Tan AG, Lerttumnongtum P, Sivasomboon C. Spectrum of imaging findings in melioidosis. Br J Radiol. 2009;82:514–521. doi: 10.1259/bjr/15785231. [DOI] [PubMed] [Google Scholar]
- 31.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 32.Sanjuan-Jimenez R, Morata P, Bermudez P, Bravo MJ, Colmenero JD. Comparative clinical study of different multiplex real time PCR strategies for the simultaneous differential diagnosis between extrapulmonary tuberculosis and focal complications of brucellosis. PLoS Negl Trop Dis. 2013;7:e2593. doi: 10.1371/journal.pntd.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.