Abstract
Post-kala-azar dermal leishmaniasis (PKDL) is a chronic dermatosis that generally occurs after apparent cure of visceral leishmaniasis caused by Leishmania donovani. In view of the prolonged treatment regimens necessary for PKDL, noncompliance is a major limitation; an optimal regimen is yet to be defined, but 12 weeks of therapy with miltefosine is generally recommended. We performed a single-arm open-label trial of miltefosine administered daily for 16 weeks in 27 patients in Kolkata with PKDL. After 4 weeks of treatment, nine patients were lost to follow-up because of unacceptable side effects, including severe abdominal pain, nausea, and vomiting. Of the 18 remaining patients, seven completed 12 weeks of therapy and 11 completed 16 weeks of therapy. Three of the seven who received 12 weeks of therapy and none of the 11 who received 16 weeks of therapy experienced disease relapse. Our results suggest that a 16-week course of miltefosine is required for reliable cure of PKDL. Further, the study highlighted the urgent need for a multicentric randomized controlled trial of 12 versus 16 weeks of treatment with miltefosine for PKDL so as to achieve the goal of elimination of leishmaniasis in south Asia.
Post-kala-azar dermal leishmaniasis (PKDL), a chronic dermatosis appears in 5–10% and over 60% of apparently cured cases of visceral leishmaniasis (VL or kala-azar) in south Asia and east Africa, respectively. On the basis of their clinical features, patients with PKDL in south Asia can be broadly categorized into two variants namely, polymorphic, where hypopigmented macules, papules/plaques, and/or nodules are present, or macular, where patients primarily present with hypopigmented macules.1 The disease acquires greater clinical relevance in south Asia as, in the absence of a zoonotic transmission, these patients are the proposed reservoirs for VL.2 To achieve the World Health Organization (WHO) target of elimination of VL by 2015 (annual incidence of less than one per 10,000 population) in south Asia, eradication of leishmaniasis has become a national priority (http://www.who.int/tdr/news/2012/vl_elimination/en/). Treatment of PKDL is a challenge for clinicians especially for macular PKDL, since the endpoint of therapy cannot be precisely defined making one dependent on resolution of clinical features.
Sodium antimony gluconate (SAG) was the mainstay of therapy of PKDL; however, the protracted treatment duration along with an increasing incidence of resistance to SAG3 has led to its steady decline. Alternatives include miltefosine and Amphotericin B, the former being recommended since 2012 by the WHO as the first line of therapy, for 12 weeks, the administration being age-dependent dose (Report of a WHO consultative meeting, 2012 http://apps.who.int/iris/bitstream/10665/78608/1/9789241505215_eng.pdf). This recommendation is supported by an open-label, randomized, parallel group multicentric trial with miltefosine (100 mg/day, per oral) for 12 and 8 weeks of therapy that translated into cure rates ranging from 61% to 88% (95% confidence interval [CI]) and from 53% to 90% (95% CI), respectively.4 Furthermore, miltefosine as compared with SAG triggered a more robust Th1 response in Indian PKDL, reinforcing its immunological superiority.5
From 2008 onward, patients (N = 27) who presented with a strong clinical suspicion of PKDL were recruited from the Dermatology Outpatient Department, School of Tropical Medicine, Kolkata and Institute of Postgraduate Medical Education and Research, Kolkata. Diagnosis was based on the presence of symmetrically distributed erythematous papules/plaques/nodules and/or hypopigmented macules mostly occupying the face (muzzle area) followed by trunk and extremities, along with a suggestive history of cured VL. PKDL was confirmed by polymerase chain reaction (PCR) of internal transcribed spacer 1 (ITS1) region of Leishmania sp., rK39 strip test along with the presence of antileishmanial antibodies and Giemsa staining of lesional biopsies.6
Miltefosine (Capsule Impavido® 50 mg; Paladin Therapeutics, Quebec, Canada) was administered orally in a daily single dose of 100 mg/day (body weight ≥ 25 Kg) or 50 mg/day (body weight < 25 Kg) or 2.5 g/kg/day (aged 2–11 years). All patients were treated for 16 weeks unless treatment-emergent side effects limited further therapy; in case of polymorphic PKDL, therapy was terminated earlier, if there was complete resolution of papulo-plaques/nodules (N = 3). Punch biopsies from skin and peripheral blood were collected at disease presentation and thereafter every 4 weeks till completion of treatment; thereafter, biopsies were cultured every 3 months for 1 year (N = 11). The study received approval from the Institutional Ethics Committee of the School of Tropical Medicine, Kolkata, and Institute of Post Graduate Medical Education and Research, Kolkata; all treatment-emergent adverse events were treated.
Lesional parasite load was measured by real-time PCR (RT-PCR) using a standard curve, generated from serially diluted Leishmania donovani promastigotes (ranging from 1 × 105 to 1) added to blood from a healthy control. DNA was extracted using a QIAmp DNA Mini kit (Qiagen, Hilden, Germany) and subjected to RT-PCR using the Applied Biosystem SYBR Green QPCR Master Mix in Applied Biosystem Step One Plus (Applied Biosystems, Foster City, CA). A fragment of 116 bp of L. donovani kDNA was amplified by RT-PCR using a primer set (forward 5′-CCTATTTTACACCAACCCCCAGT-3′ and reverse 5′-GGGTAGGGGCGTTCTGCGAAA-3′).7 Template DNA (5 μL) was added to a 20 μL reaction mixture containing SYBR Green Master mix and 400 nM of each primer. The parasite load was extrapolated from the standard curve and quantified as number of parasites per μg genomic DNA used as template.
All the 27 patients with PKDL were included in this study, their median age being 30.0 (10.0–60.0) years and male female ratio, 24:3; majority (22/27, 81.48%) were polymorphic while five (18.5%) were macular, concordant with published data.1 All patients tested positive for ITS-1 PCR, rK39 strip test, enzyme-linked immunosorbent assay (ELISA) for antileishmanial antibodies, and Leishman–Donovan bodies were identified in Giemsa-stained sections of polymorphic PKDL.
After 4 weeks of treatment, 9/27 were lost to follow-up and were not considered for analysis; eight of them had polymorphic lesions and one had macular lesions (Table 1). They were contacted by telephone and counseled regarding the importance of complete treatment but declined therapy, owing to unacceptable side effects, namely, severe abdominal pain (N = 6), nausea, and vomiting (N = 3, Table 1).
Table 1.
Study population
| No relapse (N = 15) | Relapse/flare-up (N = 3) | Discontinued therapy (N = 9) | |
|---|---|---|---|
| Age, years median (range) | 32.0 (10.0–60.0) | 18.0 (18.0–30.0) | 25 (13.0–47.0) |
| Sex ratio, M:F | 14:1 | 2:1 | 7:2 |
| Lesion type | |||
| Polymorphic | 13 | 3 | 8 |
| Macular | 2 | 0 | 1 |
| Interval between cure of VL and onset of PKDL (median years, range) | 1.0 (0.6–42.0) | 10.0 (1.0–40.0) | 3.0 (0.75–10.0) |
| Disease duration from onset of PKDL till receiving treatment for PKDL (median years, range) | 6.0 (0.5–21.0) | 7.0 (2.0–11.0) | 2.5 (0.25–11.0) |
| Treatment with miltefosine | |||
| 4 weeks | 0 | 0 | 9§ |
| 12 weeks | 4 | 3‡ | 0 |
| 16 weeks | 11 | 0 | 0 |
| Follow-up period (median months, range) | 63.0 (41.0–78.0)* | 17.0 (17.0–39.0)† | Not applicable |
PKDL = Post-kala-azar dermal leishmaniasis; VL = visceral leishmaniasis.
Disease-free period.
Lag period between stoppage of treatment and relapse/flare-up.
Therapy stopped because of cerebrovascular accident (N = 1) or severe abdominal pain/vomiting (N = 2).
Declined therapy due to severe abdominal pain/vomiting.
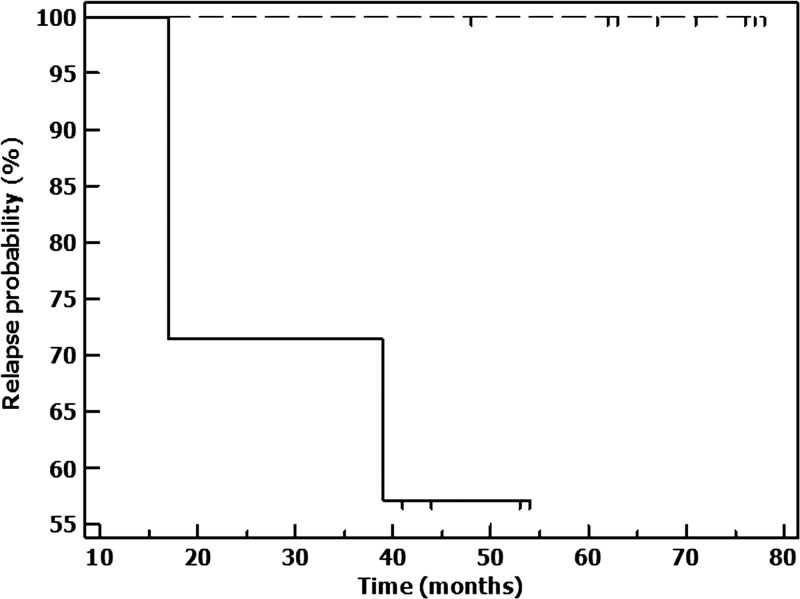
In the 18 patients who were analyzable, 11 completed 16 weeks of therapy that translated into disappearance of papulo-plaques or nodules, whereas seven completed 12 weeks of therapy, of whom, three relapsed (Table 1). Among the 11 who completed 16 weeks of therapy, majority were polymorphic (N = 9) and a minority showed macular lesions (N = 2); till date, none have relapsed (Table 1). Among the seven who completed 12 weeks of therapy, five were polymorphic and two were macular; in the five with polymorphic PKDL, two showed complete resolution of symptoms, hence therapy was terminated; they are yet to show signs of relapse. Among the remaining three patients from this group one developed a cerebrovascular accident (CVA) necessitating termination of treatment; this patient showed a flare-up of existing lesions after a quiescent period of 39 months while in two patients with polymorphic PKDL, therapy was limited to 12 weeks as they developed intractable nausea, vomiting, and upper abdominal pain that could not be managed with proton pump inhibitors, sucralfate, and gastrokinetics. Both relapsed after 17 months characterized by the appearance of widespread hypopigmented macules. Kaplan–Meier survival plots were constructed for the two treatment arms (12 and 16 weeks) and compared by log-rank test; data of subjects (N = 9) who did not complete treatment were censored. There were no relapses with the 16-week treatment, whereas with 12-week treatment, there were three relapses, the median time to relapse exceeding the total observation period using log-rank test. The relapse experience was significantly different (P = 0.019) in favor of 16-week treatment (Figure 1 ).
Figure 1.
Kaplan–Meier survival plots indicating relapse experience in the two treatment arms. There were no relapses in 11 cases that completed 16 weeks of treatment (stippled line) whereas 3/7 cases relapsed after 12 weeks of treatment (solid line).
Antileishmanial antibody titer is known to persist for years after cure and therefore antigen-based studies are accorded greater importance. Among the antigen-based studies, PCR is considered as more sensitive than microscopy.8 At active disease, all the 18 analyzable patients were positive but became negative for ITS1-PCR after 4 weeks of treatment. To validate this, we selected another region (116-bp fragment of the minicircle kDNA) of L. donovani and performed normal PCR and RT-PCR (40 cycles), based on the kDNA copy number being higher in Leishmania parasites, the latter being considered more sensitive than ITS1-PCR8; here too, PCR was negative after 4 weeks of treatment.
The three patients who relapsed after 12 weeks of therapy were diagnosed by PCR and ELISA for antileishmanial response and further treatment is planned with Amphotericin B. Parasite load, that is, number of parasites per microgram of genomic DNA of these three patients at presentation were 10,887, 7,200, and 592 while at the time of relapse, it was 8,505, 2,845, and 36,666, respectively.
This case series highlights the fact that prolonged treatment with miltefosine is associated with gastrointestinal disturbances (abdominal pain, nausea, and vomiting) that led to a substantial number of patients not being accorded 16-week therapy. The CVA occurring in one patient was “possibly” because of miltefosine; causality association being determined by “Naranjo adverse drug reaction probability scale.” This single “severe” incidence (Level 5 on Hartwig's severity assessment scale) should act as a whistle-blower for clinicians to monitor the risk factors for CVA while receiving miltefosine. Our study emphasizes the need to define the end point of therapy, which to date is yet to be precisely defined.9 It strongly advocates the urgent need for a multicentric trial of the existing 12 weeks versus 16 weeks of treatment with miltefosine, so as to achieve the goal of elimination of leishmaniasis in south Asia.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Susmita Ghosh, Shibabrata Mukherjee, Debanjan Mukhopadhyay, Avijit Hazra, and Mitali Chatterjee, Department of Pharmacology, Institute of Postgraduate Medical Education and Research Kolkata, India, E-mails: susmita_ghosh2010@yahoo.co.in, sm.atlantis@gmail.com, debmicro543@gmail.com, blowfans@yahoo.co.in, and ilatim@vsnl.net. Nilay Kanti Das, Department of Dermatology, Medical College and Hospital, Kolkata, India, E-mail: drdasnilay@gmail.com. Jayashree Nath Barbhuiya, Department of Dermatology, School of Tropical Medicine, Kolkata, India, E-mail: jnathbarbhuiya@yahoo.com.
References
- 1.Ganguly S, Das NK, Barbhuiya JN, Chatterjee M. Post-kala-azar dermal leishmaniasis—an overview. Int J Dermatol. 2010;49:921–931. doi: 10.1111/j.1365-4632.2010.04558.x. [DOI] [PubMed] [Google Scholar]
- 2.Mukhopadhyay D, Dalton JE, Kaye PM, Chatterjee M. Post kala-azar dermal leishmaniasis: an unresolved mystery. Trends Parasitol. 2014;30:65–74. doi: 10.1016/j.pt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra J, Madhubala R, Singh S. Visceral and post kala-Azar dermal leishmaniasis isolates show significant difference in their in vitro drug susceptibility pattern. Parasitol Res. 2013;112:1001–1009. doi: 10.1007/s00436-012-3222-1. [DOI] [PubMed] [Google Scholar]
- 4.Sundar S, Sinha P, Jha TK, Chakravarty J, Rai M, Kumar N, Pandey K, Narain MK, Verma N, Das VN, Das P, Berman J, Arana B. Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial. Trop Med Int Health. 2013;18:96–100. doi: 10.1111/tmi.12015. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay D, Das NK, Roy S, Kundu S, Barbhuiya JN, Chatterjee M. Miltefosine effectively modulates the cytokine milieu in Indian post kala-azar dermal leishmaniasis. J Infect Dis. 2011;204:1427–1436. doi: 10.1093/infdis/jir551. [DOI] [PubMed] [Google Scholar]
- 6.Das NK, Singh SK, Ghosh S, Sarkar A, Mukhopadhyay D, Roy S, Ganguly DN, Barbhuiya JN, Saha B, Chatterjee M. Case series of misdiagnosis with rK39 strip test in Indian leishmaniasis. Am J Trop Med Hyg. 2011;84:688–691. doi: 10.4269/ajtmh.2011.10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava A, Sweat JM, Azizan A, Vesely B, Kyle DE. Real-time PCR to quantify Leishmania donovani in hamsters. J Parasitol. 2013;99:145–150. doi: 10.1645/GE-3221.1. [DOI] [PubMed] [Google Scholar]
- 8.Mouttaki T, Morales-Yuste M, Merino-Espinosa G, Chiheb S, Fellah H, Martin-Sanchez J, Riyad M. Molecular diagnosis of cutaneous leishmaniasis and identification of the causative Leishmania species in Morocco by using three PCR-based assays. Parasit Vectors. 2014;7:420. doi: 10.1186/1756-3305-7-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundar S, Singh A, Chakravarty J, Rai M. Efficacy and safety of miltefosine in treatment of post-kala-azar dermal leishmaniasis. ScientificWorldJournal. 2015;2015:414378. doi: 10.1155/2015/414378. [DOI] [PMC free article] [PubMed] [Google Scholar]