Abstract
A 50-year-old male immigrant from Ethiopia presented for consultation after 3 years of hematochezia/melena requiring > 25 units of blood transfusions. Physical examination revealed severe proximal muscle wasting and weakness, central obesity, proptosis, and abdominal striae, accompanied by eosinophilia, elevated hemoglobin A1c, elevated 24-hour urinary cortisol, lack of suppression of 8 am cortisol levels by 1 mg dexamethasone, and inappropriately elevated random adrenocorticotropic hormone (ACTH) level. Histopathological examination of gastrointestinal biopsies showed large numbers of Strongyloides stercoralis, indicating Strongyloides hyperinfection. Treatment with 2 days of ivermectin led to resolution of gastrointestinal bleeding. This syndrome was due to chronic immunosuppression from a pituitary ACTH (corticotroph) microadenoma, of which resection led to gradual normalization of urine cortisol, improved glycemic control, resolution of eosinophilia, and no recurrence of infection.
Introduction
Strongyloidiasis is a major public health issue with increasing global prevalence, especially in certain parts of Europe, North and South America, Asia (southeast Asia and the Indian subcontinent) and Africa.1 In contrast to other nematodes, Strongyloides stercoralis infection is characterized by an autoinfection cycle that typically is chronically controlled and not clinically manifest. However, when the host response fails to control infection, patients become infected with large numbers of parasites (hyperinfection). Hyperinfection is often associated with intestinal damage (including obstruction and perforation), pulmonary hemorrhage, and may lead to bacterial superinfection (accompanied by bacterial sepsis, pneumonia and/or meningitis, and sometimes finding S. stercoralis larvae in bronchoalveolar lavage fluid or cerebrospinal fluid). Hyperinfection typically develops in the context of altered host defenses due to excess corticosteroids (either iatrogenic or endogenous), human T-lymphotropic virus type 1 (HTLV-1) infection, or severe malnutrition. Because symptoms of chronic infection are either absent or nonspecific, diagnosis is often delayed unless suggested by unexplained eosinophilia.
Widespread S. stercoralis larval proliferation and migration can lead to either superinfection (accompanied by bacterial sepsis, pneumonia, and/or bacterial/parasitic meningitis) or hyperinfection, a syndrome preceding superinfection where larger than usual numbers of S. stercoralis cause intestinal damage but do not disseminate (along with intestinal bacteria). Both syndromes are characteristically due to specific forms of immunosuppression, particularly the presence of excessive corticosteroids, whether iatrogenic or endogenous, and coinfection, for example with HTLV-I. Because symptoms of typical chronic infection are either absent or nonspecific, the diagnosis of severe strongyloidiasis may be delayed2 (unless suggested by otherwise unexplained eosinophilia) until complications develop.3–6 Herein, we describe a case of S. stercoralis hyperinfection that led to the diagnosis of underlying Cushing disease.
Case Description
A 50-year-old Ethiopian man with hypertension and poorly controlled diabetes mellitus was referred to infectious disease clinic after 3 years of recurrent gastrointestinal (GI) bleeding and a biopsy suggesting strongyloidiasis. He was born and raised in Addis Ababa, Ethiopia, and had resided in Khartoum, Sudan, before immigrating to the United States 10 years before our evaluation. Previously, he had undergone coronary artery stenting for atherosclerotic disease and received aspirin and clopidogrel for 2 months, when he first developed lightheadedness, weakness, and abdominal discomfort due to GI bleeding. Over the subsequent 3 years, he had intermittent melena and hematochezia complicated by hypotension and anemia, which required multiple hospitalizations and blood transfusions each year.
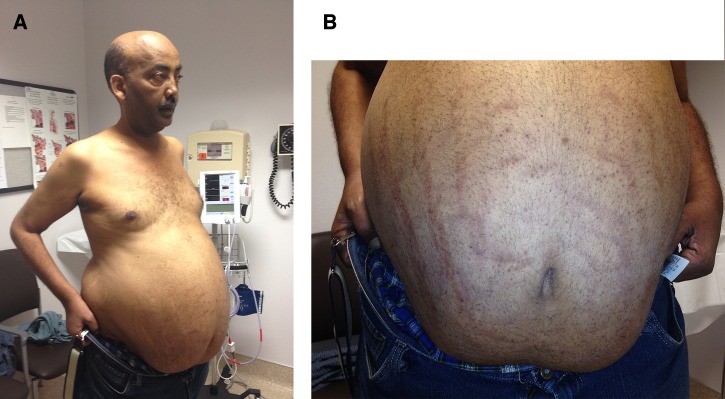
At an infectious disease clinic visit, he reported a 30-pound weight loss, malaise, and severe proximal muscle weakness. He was afebrile (98°F), hypertensive (blood pressure 173/84), and mildly bradycardic (heart rate 58). Physical examination showed proptosis, central obesity with purple abdominal striae, arm and thigh wasting, frail skin, bilateral leg edema, and a positive Gower sign (Figure 1 ; Supplemental Video). Laboratory tests showed hemoglobin 10.9 gm/dL (baseline of 13 gm/dL), white blood cell count 10.9 × 103/mm3, and eosinophilia (18%, normal 1–7%; absolute eosinophil count 1,962/mm3).
Figure 1.
Patient with features of Cushing syndrome including central obesity and wasting of extremities (A) and abdominal striae (B). The patient provided written consent to show his face in this report.
To investigate the etiology of his GI bleeding, multiple laboratory tests, imaging studies, and endoscopic procedures (at least six) were performed from 2010 to 2013. Serum studies were negative for Helicobacter pylori and hepatitis B virus; multiple stool specimens (at least four) were negative for all ova and parasites (including specifically hookworm ova), and for Cryptosporidium spp., Cyclospora cayetanensis, Isospora belli, and Microsporidium spp. Abdominal ultrasound and computerized tomography (CT) scans were noncontributory. Capsule endoscopies and tagged red blood cell scans were unrevealing (capsule endoscopy has been reported to detect hookworms associated with GI hemorrhage7). Enteroscopies revealed Barrett's esophagus, duodenitis, and benign small bowel polyps. Colonoscopies revealed pan-colonic diverticulosis and active bleeding thought to be due to arteriovenous malformations, which were treated with cautery. The patient continued to have hematochezia and melena that required blood transfusion every 3–4 months. In December 2013, upper and lower endoscopies demonstrated grossly similar findings as seen previously but histopathology was formally reported as showing “parasite parts,” subsequently interpreted as S. stercoralis, in the duodenum, jejunum, cecum, and colon (Figure 2 ). These findings prompted referral for infectious diseases outpatient consultation.
Figure 2.
Histopathology demonstrating presence of “parasite parts” in tissue biopsies of (A) duodenum (100× magnification), (B) duodenum (surrounding eosinophilic tissue reaction 400× magnification), (C) jejunum (parasites within glands, surrounding eosinophilic tissue reaction, 400× magnification), (D) descending colon. In (D), parts of a degenerating worm in the descending colonic mucosa with necrosis and eosinophilic infiltration can be seen.
At an endocrinology outpatient consultation, he was noted to be cushingoid, including central obesity and abdominal striae (Figure 1). Endocrinologic evaluations revealed elevated hemoglobin A1c (9.2%), elevated urine cortisol (133 μg/day, normal ≤ 60 μg/day), elevated midnight salivary cortisol (0.397 μg/dL, normal < 0.112 μg/dL), non-suppressed adrenocorticotropic hormone (ACTH; 39 pg/mL, normal 7–69), and abnormal 1-mg overnight dexamethasone suppression test (cortisol at 8 am the following day 22.1 μg/dL, normal < 1.8 μg/dL). These results were consistent with a diagnosis of ACTH-dependent Cushing syndrome.
To define the cause of hypercortisolism, CT scan of the abdomen was performed, which revealed bilateral adrenal adenomas measuring 2.2 cm on the right and 2.7 cm on the left. These findings were attributed to chronic ACTH stimulation rather than a primary source of hypercortisolism.
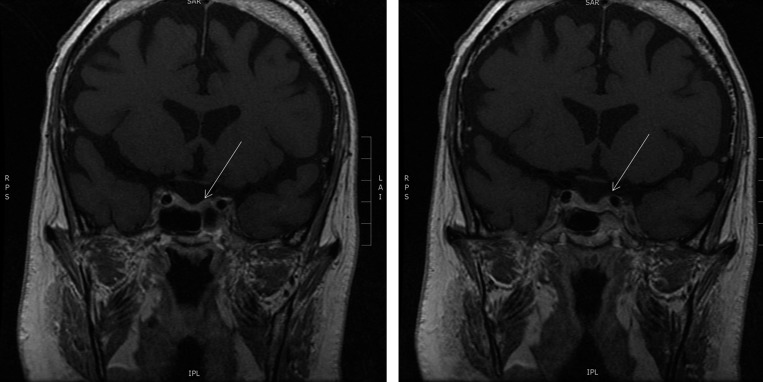
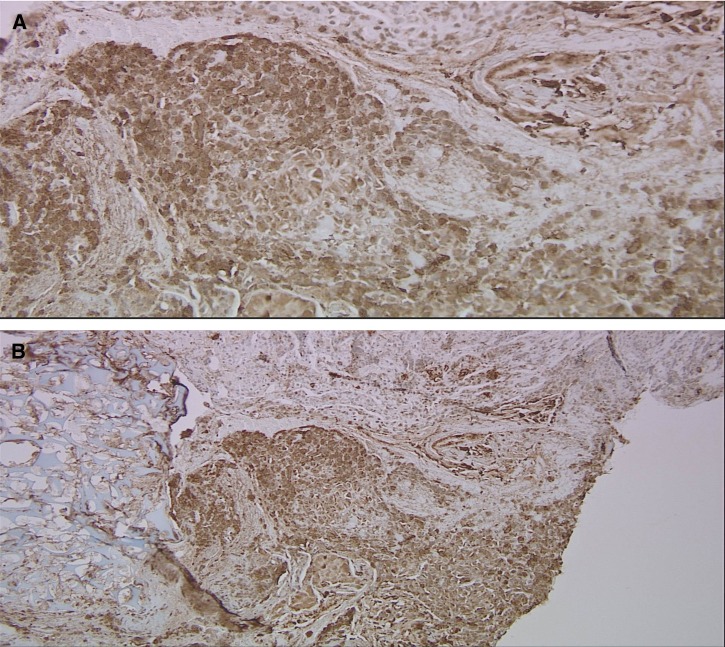
Workup for ectopic ACTH-secreting tumors was performed to evaluate for carcinoid, pheochromocytoma, and small cell lung cancer; octreotide scan and chest magnetic resonance imaging (MRI) were both negative. Pituitary MRI demonstrated a 3-mm non-enhancing lesion on the left side (Figure 3 ), consistent with the source of excess ACTH secretion. Neurosurgery was consulted for transsphenoidal resection. Histopathology demonstrated an ACTH-positive microadenoma with distorted acinar architecture (Figure 4 ).
Figure 3.
A 3-mm hypoenhancing area in the left anterior pituitary seen on dedicated magnetic resonance imaging (2.5 mm coronal cuts post-gadolinium).
Figure 4.
(A) Immunohistochemistry for ACTH appears brown in the specimen, which was only found in the very periphery of the lesion. (B) The normal acinar structure of the pituitary gland is focally disrupted by the unregulated growth of the ACTH-producing microadenoma.
Discussion
The differential diagnosis for this patient's chronic GI bleeding was initially broad and included gastroesophageal reflux disease, Barrett's esophagus, inflammatory bowel disease (IBD), medication-induced colitis, and infectious etiologies. No pathological findings typical of IBD or medication-induced colitis were seen on multiple endoscopic evaluations. Evaluation for bacterial causes of colitis (Campylobacter, Shigella, and Yersinia spp.) and Entamoeba histolytica were negative by culture and serology, respectively. Hookworm infection was excluded given the lack of ova seen on multiple stool examinations,8 as well as the distribution of the parasites in the GI track and the histopathology.8 Given the patient's immigration history, laboratory finding of eosinophilia, and characteristic histopathological findings, S. stercoralis hyperinfection was identified as the cause of his chronic GI bleeding. Serological testing was positive for S. stercoralis (31.9 units/mL, normal < 1.7) further supporting the diagnosis, but negative for Schistosoma mansoni and Schistosoma haematobium (which can cross react with S. stercoralis9). Strongyloides stercoralis hyperinfection was attributed to immunosuppression induced by endogenous hypercortisolemia due to a corticotroph microadenoma.
After ivermectin treatment (200 μg/kg over two consecutive days), GI bleeding and eosinophilia resolved. No other anthelminthic drug was given. At 6-month follow-up, eosinophilia recurred (12%), responding again to the same course of ivermectin. No further GI bleeding occurred. At follow-up, 6 months after re-treatment, the eosinophil count was normal. Resection of the corticotroph microadenoma led to gradual normalization of urine and salivary cortisol levels. Glycemic control improved (hemoglobin A1c 6.8%), but proximal muscle weakness continues.
Herein, we report a case of chronic GI bleeding from S. stercoralis hyperinfection due to unsuspected Cushing disease, a novel presentation that took years to diagnose. This patient's comorbid illnesses (coronary artery disease, aspirin use, diabetes, and Barrett's esophagus) and misinterpreted endoscopic findings (presumed but not confirmed arteriovenous malformations, duodenitis) delayed the diagnosis of strongyloidiasis, which was initially suggested by biopsy and further confirmed by serology.
Two cases of S. stercoralis hyperinfection accompanying Cushing syndrome have been previously reported4,10: one had respiratory symptoms and ectopic ACTH syndrome due to a small cell lung cancer10, the other had abdominal pain and Cushing syndrome of unknown etiology.4 Although this is the third documented case, it is the first to highlight recurrent GI bleeding as the primary presenting symptom of hyperinfection in the setting of Cushing syndrome.
Strongyloides stercoralis is a nematode with the remarkable ability to continue reproducing within a human host via an autoinfection cycle leading to a long-term infection, potentially persisting—in the absence of treatment—for decades.3,5,11 However, individuals with impaired host defense (particularly corticosteroid excess and HTLV-I infection) are at risk for accelerated larval proliferation2,5,12 and high parasite burden leading to S. stercoralis hyperinfection. Severe GI bleeding may occur in hyperinfection due to massive larval invasion of the GI mucosa.3 Widespread, extraintestinal larval invasion beyond the normal pattern of migration and into extraintestinal organs is characteristic of disseminated strongyloidiasis, also known as superinfection, which can lead to serious sequelae such as meningitis, polymicrobial bacteremia, sepsis, and death.1,5,6,10
Strongyloidiasis is typically diagnosed in mild cases when a patient with a relevant travel history presents with eosinophilia, possibly but not always accompanied by abdominal pain or other complaints. Clinical examination, laboratory testing, imaging, and endoscopy1,2 are not specific in the absence of an etiological diagnosis, which can be obtained by visualizing parasites in a stool examination (insensitive), histopathology (invasive), or serology (which can have cross-reactions with other helminths; the negative schistosomiasis serologies helped to rule out that cross-reaction in this case). Our patient underwent multiple hospitalizations, many blood transfusions, and extensive workups with more than 10 endoscopic studies over a 3-year period before histopathologic examination of intestinal biopsies demonstrated parasites. He had many stool samples microscopically negative for ova and parasites even after parasites were found in colonoscopy. Ultimately, the diagnosis was demonstrated by serological testing combined with histopathology of biopsy specimens obtained after additional endoscopy. Endoscopic findings in Strongyloides-related colitis are often nonspecific, ranging from normal to friable, ulcerated, erythematous, or nodular mucosa.1,4 Strongyloidiasis can also be diagnosed with a low yield by straight stool microscopy.13 The sensitivity of stool microscopy improves with serial sampling1 or when combined with serological testing where sensitivity has been reported to be ∼95%, but this requires clinical suspicion, which is not always apparent in industrialized countries.1 Special tests on stools that are more sensitive to detect Strongyloides larvae, including polymerase chain reaction, agar plate culture, and Baermann concentration, were not performed on samples from this patient because there was no suspicion for this infection before the final set of endoscopies and biopsies were performed; such tests are available in specialized laboratories.14,15 Eosinophilia at low or high levels may be the only finding to suggest subclinical Strongyloides infection, but in the presence of corticosteroid excess or HTLV-I coinfection (another risk factor for complicated strongyloidiasis) is distinctly unusual and may well be absent in Strongyloides hyperinfection. The variable presence of eosinophilia in Strongyloides hyperinfection is probably related to bacterial superinfections or unknown drivers of host immune responses.
The nonspecific symptoms of S. stercoralis hyperinfection may delay diagnosis leading to serious sequelae including fibrosis, bowel wall perforation, or gram-negative sepsis.3–6 A key feature illustrated by this case is that the presence of severe strongyloidiasis must lead to suspicion of underlying immunodeficiency. Clinicians must maintain a high index of suspicion when treating patients in developed countries or urban areas where strongyloidiasis infection may be uncommon. Travel and immigration histories are essential for assessing risks of exposure. Strongyloidiasis is endemic in eastern Europe, Latin America, Africa, southeast Asia, and the southern United States,1,3,16,17 warranting a high suspicion for strongyloidiasis in patients from these areas. Although our patient initially presented after having lived in the western United States for about 16 years, his immigration from Ethiopia was a major risk factor for helminthic infection.
According to the World Health Organization guidelines, ivermectin has a cure rate of 82.9% based on a randomized trial performed in children18 but in patients with hyperinfection and/or dissemination, multiple doses may be needed until stool and/or sputum is negative for 2 weeks.19 Typically, serial stool sampling is used to monitor response to treatment. However, our patient never had a positive stool exam despite repetitive stool sampling when parasitic parts were found on colonoscopy. Absence of stool findings in patients with hyperinfection has been previously described.1 Instead, the absolute eosinophil count was used as a biomarker to evaluate parasite clearance. The eosinophil count was normal after each 2-day treatment with ivermectin (200 μg/kg/day), but had increased 6 months after the first treatment, likely related to the corticosteroid-induced defect in host defense. As of this writing, despite his normalized endocrinopathy, proximal myopathy persists. It remains to be seen whether this symptom will gradually resolve as his other chronic conditions are managed.
To our knowledge, this is the first report of profound, chronic GI bleeding as a bellwether of Strongyloides hyperinfection in a patient with Cushing syndrome. The antiplatelet therapy because of coronary artery disease and stent placement contributed to the bleeding. The diagnosis of strongyloidiasis is often delayed because of the lack of specific clinical features or laboratory/imaging findings, combined with low suspicion in industrialized countries. Once the diagnosis of Strongyloides hyperinfection is made, the patient should be evaluated for underlying immunosuppression. Eosinophilia in an immigrant from an endemic area should raise clinical suspicion for helminthic infection even in the absence of symptoms.
Supplementary Material
Disclaimer: This study was presented at Department of Medicine Grand Rounds, University of California San Diego, November 12, 2014.
Footnotes
Authors' addresses: Brittany Yee, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, E-mail: brittany.yee@gmail.com. Nai-Wen Chi, Division of Endocrinology, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, E-mail: nwchi@ucsd.edu. Lawrence A. Hansen, Department of Pathology, University of California San Diego School of Medicine, La Jolla, CA, E-mail: lahansen@ucsd.edu. Roland R. Lee, Department of Radiology, University of California San Diego School of Medicine, La Jolla, CA, E-mail: rrlee@ucsd.edu. Hoi S. U., Department of Neurosurgery, University of California San Diego School of Medicine, La Jolla, CA, E-mail: hoisang@ucsd.edu. Thomas J. Savides, Division of Gastroenterology, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, E-mail: tsavides@ucsd.edu. Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, and Instituto de Medicina Tropical “Alexander von Humboldt” and Departamento de Ciencias Celulares y Moleculares, Facultad de Ciencias y Filosofia, Laboratorio de Investigación y Desarrollo, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mail: jvinetz@ucsd.edu.
References
- 1.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, Chen X. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis. 2014;8:e3018. doi: 10.1371/journal.pntd.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bava AJ, Troncoso AR. Strongyloides stercoralis hyperinfection in a patient with AIDS. J Int Assoc Physicians AIDS Care (Chic) 2009;8:235–238. doi: 10.1177/1545109709336882. [DOI] [PubMed] [Google Scholar]
- 3.Csermely L, Jaafar H, Kristensen J, Castella A, Gorka W, Chebli AA, Trab F, Alizadeh H, Hunyady B. Strongyloides hyper-infection causing life-threatening gastrointestinal bleeding. World J Gastroenterol. 2006;12:6401–6404. doi: 10.3748/wjg.v12.i39.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh SK, Chow PK, Chung AY, Tan BH, Tan PH. Strongyloides colitis in a patient with Cushing's syndrome. Gastrointest Endosc. 2004;59:738–741. doi: 10.1016/s0016-5107(04)00289-5. [DOI] [PubMed] [Google Scholar]
- 5.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roxby AC, Gottlieb GS, Limaye AP. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;49:1411–1423. doi: 10.1086/630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JM, Zhang XM, Wang LJ, Chen Y, Du Q, Cai JT. Overt gastrointestinal bleeding because of hookworm infection. Asian Pac J Trop Med. 2012;5:331–332. doi: 10.1016/S1995-7645(12)60051-0. [DOI] [PubMed] [Google Scholar]
- 8.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 9.Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, Chavez CB, Olortegui MP, Montalvan C, Sanchez GM, Worthen B, Worthen J, Leung F, Ore CV. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:97–102. [PMC free article] [PubMed] [Google Scholar]
- 10.Vadlamudi RS, Van Dort M, Barklow T, Byrd RP, Jr, Moorman JP. Strongyloides hyperinfection syndrome complicating (ectopic) Cushing syndrome. South Med J. 2008;101:750–752. doi: 10.1097/SMJ.0b013e31817a836e. [DOI] [PubMed] [Google Scholar]
- 11.Cox FE. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612. doi: 10.1128/CMR.15.4.595-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummins RO, Suratt PM, Horwitz DA. Disseminated Strongyloides stercoralis infection. Association with ectopic ACTH syndrome and depressed cell-mediated immunity. Arch Intern Med. 1978;138:1005–1006. doi: 10.1001/archinte.138.6.1005. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry U, Choudhry R, Romeo DP, Cammerer RC, Gopalswamy N. Strongyloidiasis: new endoscopic findings. Gastrointest Endosc. 1995;42:170–173. doi: 10.1016/s0016-5107(95)70077-3. [DOI] [PubMed] [Google Scholar]
- 14.Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21:543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, Mohammed AS, Singo R, Benninghoff M, Nsojo AA, Genton B, Daubenberger C. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90:535–545. doi: 10.4269/ajtmh.13-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concha R, Harrington W, Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 18.Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55:477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Parasites: Strongyloides. 2012. http://www.cdc.gov/parasites/strongyloides/health_professionals/ Available at. Accessed January 23, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.