Abstract
Outdoor exposure to mosquitoes is a risk factor for many diseases, including malaria and dengue. We have previously shown that long-lasting permethrin-impregnated clothing protects against tick and chigger bites in a double-blind randomized controlled trial in North Carolina outdoor workers. Here, we evaluated whether this clothing is protective against mosquito bites by measuring changes in antibody titers to mosquito salivary gland extracts. On average, there was a 10-fold increase in titer during the spring and summer when mosquito exposure was likely to be the highest. During the first year of the study, the increase in titer in subjects wearing treated uniforms was 2- to 2.5-fold lower than that of control subjects. This finding suggests that long-lasting permethrin-impregnated clothing provided protection against mosquito bites.
Introduction
Mosquito-borne infectious diseases, such as malaria, dengue, West Nile, and chikunguya fever, affect hundreds of millions of people throughout the world. Long-lasting insecticide-impregnated nets (LLINs) provide protection against anopheline mosquitoes, which are night-time biters.1 However, people spend a substantial amount of time exposed to anophelines during the evening before going to bed under a net.2,3 In addition, many mosquito-borne diseases are spread by day-time biters such as Aedes aegypti and Culex pipiens. Repellents, such as diethyltoluamide, and proper attire provide some protection, but the protection is incomplete. Thus, better personal protective measures against mosquitoes are needed.
Clothing that is impregnated with permethrin by dipping or spraying is protective against mosquitoes in the laboratory, and has been used by the military and in recreational activities for personal protection against bites from arthropods.4–7 Impregnated clothing is typically evaluated either by exposing mosquitoes to clothing and measuring mortality or by exposing individuals wearing impregnated clothing to mosquitoes in the laboratory.8 However, impregnated clothing potentially performs differently under field conditions than in the laboratory.9 There have been few randomized controlled trials (RCTs) under field conditions; because mosquito bites are hard to quantify. Occurrence of disease (malaria) was used as the primary measure of effectiveness. In one large study, impregnated clothing, sheets, and other materials protected against malaria when used for 16 weeks.10 Two other smaller short-term studies were done—one showed an effect11 and one did not.12 Finally, in the only long-term study (13 months), no effect on the incidence of infection was seen.13 The poor performance in the longer trial may have been a consequence of poor compliance with retreatment.
Factory-based permethrin impregnation methods, which yield long-lasting permethrin-impregnated (LLPI) clothing, have been developed to overcome retreatment compliance problems. LLPI clothing exhibits protection and knock-down activity against mosquitoes in the laboratory.5 Many militaries now use LLPI uniforms. Previous studies have measured efficacy by exposing worn fabric to mosquitoes under laboratory conditions.14–16 However, there have been no studies of the efficacy of LLPI clothing in protecting soldiers or outdoor workers from mosquito bites under field conditions.
Our previous studies showed that LLPI clothing protected outdoor workers against self-reported tick bites for 1 year. We first conducted an open-labeled study of 16 North Carolina Division of Water Quality worker subjects, and found a 94% protective efficacy over a 1-year period.17 We then conducted an RCT with 159 North Carolina parks and forestry rangers; the protective efficacy was 83% in the first year, but only 36% in the second year.18 This study suggests that treated clothing had lost its efficacy over a period of 1 year. In annual questionnaires of study participants, fewer subjects in the treatment group reported frequent mosquito bites than in the control group, but the difference was not statistically significant. However, it is likely that study participants could not recall as the focus of the trial was on prevention of tick bites.
During feeding, mosquitoes secrete saliva into host skin to facilitate blood uptake.19 Saliva contains physiologically active chemicals able to induce humoral immune responses that can be measured.20 Previous studies have shown that antibodies against vector salivary proteins can serve as markers for disease risk21–23 and can be used to evaluate the efficacy of vector control efforts.24,25 Our previous studies have shown increased antibody titers to salivary proteins in subjects with malaria or dengue, compared with subjects living in the same region but with no sign of infection.21,22 There have been a number of studies validating the detection of anti-mosquito salivary proteins as biomarkers for mosquito bite exposure.21,24–30 Although the different studies have all used different methods to assess immune response to mosquito salivary proteins, all suggest that antibodies to mosquito salivary proteins are effective biomarkers for exposure.
In this article, we describe efforts to determine whether LLPI uniforms protect against mosquito bites under field conditions. Annual serum samples were obtained from RCT participants and were analyzed for mosquito bite exposure using an enzyme-linked immunosorbent assay (ELISA) that was adapted to detect anti-salivary gland protein antibodies from Aedes albopictus and Aedes atlanticus, two commonly found mosquito species at study participants' exposure sites.31,32
Methods
ELISA assay.
Salivary gland extract (SGE) was prepared from field caught female Ae. albopictus and Ae. atlanticus/tormentor mosquitoes. Mosquitoes were cold anesthetized, washed in 70% ethanol, and placed in phosphate buffered saline (PBS), pH 7.2, for salivary gland dissection. After dissection, salivary glands were placed in a solution of PBS plus proteinase inhibitor (cOmplete ULTRA Tablets, Mini, EDTA-free, EASYpack; Roche Diagnostics, Indianapolis, IN) and were allowed to freeze at −80°C and thaw at 4°C four times to induce cell rupture and release of proteins; the resulting SGE was kept in PBS at −80°C until used. Protein concentration was determined using the Thermo Scientific NanoDrop™ (Thermo Fisher Scientific, Wilmington, DE) and the Bradford method (Bio-Rad Protein Assay; Bio-Rad, Hercules, CA). We prepared a total SGE concentration of 2 mg/mL for Ae. atlanticus/tormentor and 1.2 mg/mL for Ae. albopictus. From these concentrated SGE solutions, a final concentration of 1 μg/mL was prepared in coating buffer (Kierkegaard and Perry Laboratories, Gaithersburg, MD) to coat each ELISA plate.
Working conditions for the ELISA were optimized according to our previous research.21 On the basis of the results from the titration, 96-well ELISA plates (Nunc-Maxisorp; Nalgene Nunc International, Rochester, NY) were coated with 100 μL/well of 1 μg/mL of SGE prepared in coating buffer and incubated overnight at 4°C. After rinsing once with 1× PBS, plates were blocked for 1.5 hours with 5% dry milk in PBS (blocking buffer) (Invitrogen, Carlsbad, CA) at 37°C and incubated with 100 μL/well of 1/100 serum dilution in blocking buffer at 37°C for 2.5 hours. Plates were washed three times with wash solution (1× PBS containing 0.1% Tween) and incubated with 100 μL/well of goat antihuman IgG diluted 1:1,000 horseradish peroxidase (HRP)–conjugated antibodies (Caltag Laboratories, Burlingame, CA) at 37°C for 1.5 hours. Color development was obtained using 100 μL/well tetramethylbenzidine (TMB, one-solution microwell; GenScript, Piscataway, NJ) incubated for 15 minutes at room temperature. The reaction was stopped with 100 μL/well of stop solution (1 M phosphoric acid), and absorbance was measured at 450 nm on an Eon Biotek (BioTek Instruments, Inc., Winooski, VT) using Gen5 v2.0 software. Each sample was tested in duplicate.
Three controls were included in each plate: 1) control blank: two wells without SGE to control for nonspecific induction of color for any of the reagents used in the test; 2) negative control: two wells with SGE but without human serum to control for any nonspecific color induction of the coating antigen; and 3) positive control to correct for plate-to-plate variations. The positive control was a pool of sera from two insectary workers exposed to Aedes spp. mosquito bites on a weekly basis during landing collections made for mosquito surveillance or maintenance of mosquito colonies in an insectary. Positive control optical density (OD) values from each plate were recorded and averaged. This average was then divided by each plate positive control OD value to obtain a “calculation factor” for that respective plate. To correct for plate-to-plate variations, we then multiplied each sample OD value by their respective plate calculation factor to obtain normalized OD's.
Polyacrylamide gel electrophoresis and immunoblotting.
SGE (30 μg) was mixed 1:1 with 2× Laemmli buffer consisting of 65.8 mMTris-HCl, pH 6.8, 2.1% sodium dodecyl sulfate (SDS), 26.3% (w/v) glycerol, 0.01% bromophenol blue, and 5% 2-mercaptoethanol. The SGE mixture was then loaded into each well of a 12% preparative polyacrylamide minigel using tris/glycine/SDS (Bio-Rad) along with 5 μL of a prestained molecular weight marker (Precision Plus Protein™ 10–250 kDa Kaleidoscope™; Bio-Rad) in the designated ladder well and electrophoresed at 90 V for approximately 2.5 hours and subsequently transferred using Trans-Blot® Turbo™ Transfer System to a polyvinylidene fluoride (PVDF) membrane (Mini PVDF Transfer Packs 7 × 8.5 cm PVDF membranes; Bio-Rad) utilizing the 7 minutes “Mixed Molecular Weight” program. Membranes were blocked overnight with blocking buffer and incubated with pooled sera from study participants diluted to 1:100 in blocking buffer for 2 hours at room temperature. Each membrane was washed 5× with wash solution (1× PBS and 0.1% Tween 20; Bio-Rad) and incubated with HRP-conjugated Goat Anti-Human IgG diluted to 1:1,000 in blocking buffer for 1 hour at 37°C. Color development was obtained with the HRP chromogenic substrate TMB (Novex®; Invitrogen). Band corrected density was measured using myImageAnalysis Software version 1.1 (Thermo Fisher Scientific Inc., Rockford, IL). This software uses an algorithm to automatically select and identify lanes and band boundaries for calculation of densitometric values.
Study subjects.
All of the study subjects were enrolled in a previously described double-blind RCT examining effects of permethrin-treated uniforms on the incidence of tick bites in North Carolina outdoor workers. Study participants were recruited from 24 parks, 7 wildlife resource offices, and 21 forestry service offices. In brief, subjects were block-randomized with 1:1 allocation to treatment or control groups. Subjects sent all of their spring and summer work uniforms (shirts, pants, shorts, hats, and socks) directly to the treatment facility where they were either impregnated with permethrin or sham treated.18 Subjects provided serum samples at enrollment, after 1 year and after 2 years. Serum samples were taken in the low-transmission season between October 1 and March 15 starting in October 2010 and ending in March 2013. Of the 159 subjects enrolled, paired serum samples (from two time points, separated by 6–12 months) were only available for 64 subjects. For western blots, four pools of sera were tested: 1) control group year 1; 2) control group year 2; 3) treatment group year 1; and 4) treatment group year 2.
Study participants self-reported mosquito bites during each year of the study. Frequency of mosquito bites was recorded as a three-level categorical variable: never, sometimes, and often. For analysis purposes, we only considered study participants reporting “never” and “often” categories.
Statistical methods.
Change in levels of antibody to mosquito salivary proteins was our primary outcome. Data were entered into Microsoft Excel 2010 (Microsoft, Redmond, WA) and analyzed in SAS, version 9.2.2 (SAS, Cary, NC). Mean antibody levels were calculated. Histograms of the mean values did not approximate normal distributions (either as a whole or in subgroups), so they were log-transformed for further analysis. Differences in the logs of the mean value were calculated for each subject for each species of mosquito and analyzed using the Student t test. An α of 0.05 was used to determine statistically significant associations.
Results
Determination of assay detection limit.
Using a positive control, the ELISAs were linear for both Ae. albopictus and Ae. atlanticus (r2 = 0.95 and 0.97, respectively) with detection limits of 1:1,600 for Ae. atlanticus and 1:800 for Ae. albopictus.
Identification of Ae. atlanticus immunogenic salivary proteins by western blot.
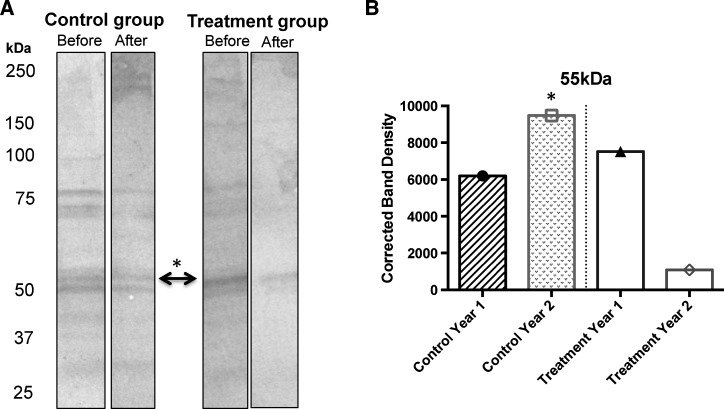
The Ae. atlanticus salivary gland extract contained proteins ranging from approximately 20 kDa to > 250 kDa (Figure 1A ). Western blot analysis showed at least 12 Ae. atlanticus salivary proteins recognized by the pooled sera from study participants. However, one protein of approximately 50 kDa showed noticeable changes in density levels between the control and the treatment group before and after the intervention (Figure 1B).
Figure 1.
(A) Western blot results with pooled sera from participants in the control and treatment group against Aedes atlanticus/tormentor salivary gland extract (SGE). (B) The IgG response against a 55-kDa protein was significantly reduced after implementation of protective clothing in the treatment group.
Antibody to SGE in study subjects.
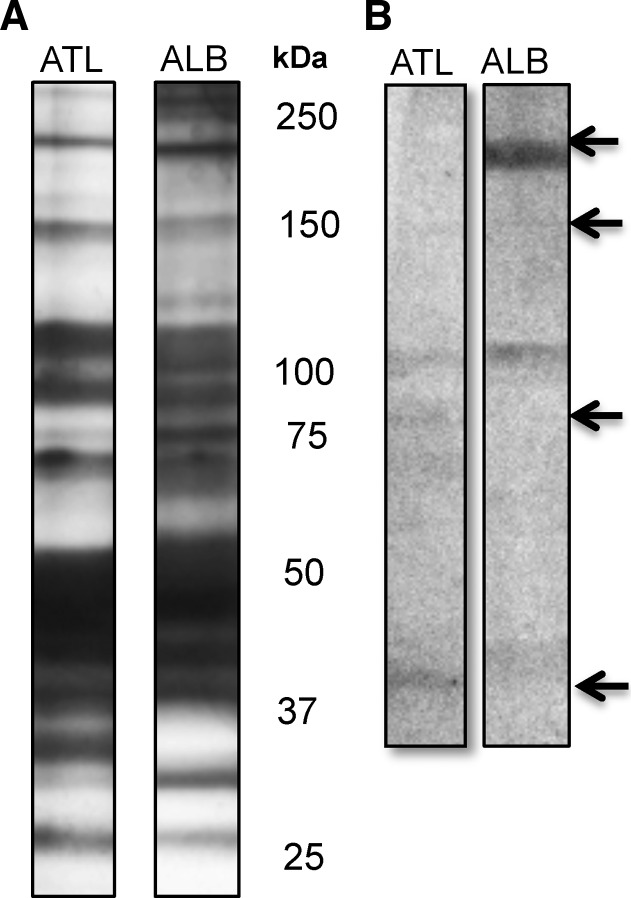
Serum samples were taken from study subjects in the late fall and winter, before and after periods of exposure to mosquito bites. Sixty-four subjects had paired serum samples, as expected, antibody levels to both species of mosquitoes increased substantially. For Ae. atlanticus, 60 of 64 individuals showed increases in antibody levels. The median log increase over a year was 0.99 (SD = 0.729, range = −-0.639–2.90). For Ae. albopictus, 63 of 64 individuals had increased antibody levels. The median log increase was 0.95 (SD = 0.622, range = −0.026–2.867). Thus, the quantity of antibodies that bind to these salivary proteins increased approximately 10-fold during the time the study subjects would have been exposed to mosquito bites. To test for cross-reactivity between Ae. atlanticus and Ae. albopictus, we evaluated the antibodies against specific salivary proteins in the sera of one specific participant in the control group by western blot (Figure 2 ). We found that this participant serum reacted against a 200-kDa protein in the Ae. albopictus SGE, whereas responses to a 100 kDa and an 80 kDa were only visible in the blot of Ae. atlanticus SGE. These results showed the presence of specific proteins with low or no cross-reactivity between these two species. Other bands of approximately 120 kDa and 60 kDa were present in both species.
Figure 2.
Salivary proteins in salivary gland extract (SGE) preparation from Aedes atlanticus/tormentor (ATL) and Aedes albopictus (ALB). (A) Silver stain of salivary proteins. (B) Western blot of protein recognized by serum from a participant in the control group.
Effects of uniform type on anti-SGE antibodies.
Of the subjects, 29 (45%) were in the group wearing LLPI uniforms and 35 (55%) were in the control group (Table 1). The mean change in Ae. atlanticus antibody levels among those in the control group was 1.16 (range = −0.63–2.90, SD = 0.75) and in comparison, changes in antibody levels for the treatment group averaged 0.82 (range = −0.64–2.83, SD = 0.67). The difference trended to statistical significance (t = 1.94, P value = 0.056). The mean change in Ae. albopictus antibody levels among those in the control group was 1.25 (range = 0.22–2.87, SD = 0.66) and 0.84 (range = −0.03–1.83, SD = 0.50) among those in the treatment group. At an α of 0.05, differences between the mean change in Ae. albopictus antibody level was statistically significant between the treatment and control groups (t = 2.86, P value = 0.0058). However, in year 2, these differences were not observed. Differences between control and treatment group in year 1 were roughly 0.3 and 0.4 logs, for Ae. albopictus and Ae. atlanticus, respectively, or the equivalent of 2- to 2.5-fold higher.
Table 1.
Log titers of antibodies to ATL and ALB SGE
| N | ATL | SD | P value | ALB | SD | P value | ||
|---|---|---|---|---|---|---|---|---|
| Overall* | ||||||||
| Control | 35 | 1.16 | 0.75 | 0.056 | 1.25 | 0.66 | 0.0058 | |
| Treated | 29 | 0.82 | 0.67 | 0.84 | 0.5 | |||
| Year 1 | ||||||||
| Control | 11 | 1.74 | 0.66 | 0.007 | 1.37 | 0.74 | 0.09 | |
| Treated | 7 | 0.72 | 0.66 | 0.80 | 0.59 | |||
| Year 2 | ||||||||
| Control | 19 | 0.89 | 0.62 | 0.87 | 1.21 | 0.66 | 0.068 | |
| Treated | 20 | 0.85 | 0.70 | 0.86 | 0.50 | |||
ALB = Aedes albopictus; ATL = Aedes atlanticus; SD = standard deviation; SGE = salivary gland extract.
There were 8 paired samples whose year of study was not recorded.
Antibodies to SGE and self-reported mosquito exposure.
At the end of each season, subjects were asked how often they were bitten by mosquitoes the previous season, and could choose between “never,” “rarely,” “occasionally,” or “frequently.” About one-third of subjects reported being bitten frequently. No association was found between subjects self-categorized in this manner and changes in antibody level. However, no statistically significant association was seen between these self-reported categories and treatment group, either.
Discussion
The goal of this study was to determine whether LLPI clothing protects against mosquito bites using antibody to mosquito SGE as a surrogate marker. Antibody levels to both Ae. albopictus and Ae. atlanticus SGE increased by a log (10-fold) between serum samples taken before and after peak mosquito seasons, as would be expected. These increases differed between study subjects wearing LLPI uniforms and control uniforms. The difference was approximately 0.3–0.4 logs, or 2- to 2.5-fold. This finding suggests that LLPI clothing protected against mosquito bites.
LLPI clothing has the potential to be a sustainable method for the prevention of mosquito-borne diseases, because it does not require constant retreatment. In field studies, self-impregnation of clothing with permethrin was protective against disease in short-term studies,10–12 but not in long term.13 LLPI clothing has been previously shown to be effective in laboratory settings5 and to retain mosquito knock-down efficacy for up to 20 washes.9,33 Here, we find evidence from the field that LLPI clothing protects against mosquito bites for at least 1 year. The absence of significant protection from LLPI in year 2 is consistent with what was observed with tick bites.18 Further studies are planned to determine the duration of bioefficacy.
LLPI clothing is efficacious against other arthropod vectors, such as ticks and chiggers, as has been demonstrated in two U.S. studies17,18 and in a recent German study.6 Work by Wilder-Smith and others indicate that LLPI clothing would be a cost-effective and acceptable means of preventing dengue.34,35
Biomarkers may be the only way to quantitate mosquito bite exposure in field studies. There have been a number of studies on the validity of IgG antibodies against mosquito salivary proteins as biomarkers for mosquito bite exposure.21,24–30 Although the different studies have all used different methods to assess immune response to mosquito salivary proteins, all suggest that antibodies to mosquito salivary proteins are effective biomarkers of exposure. Further research is needed to determine how quickly anti-salivary protein immunity wanes, and exactly how changes in antibody concentration correlate with the frequency of mosquito bites. Nevertheless, the marked increase in anti-SGE titers between samples taken before and after peak exposure season suggests that a strong correlation exists between this change in antibody titer and mosquito exposure.
This study has several limitations. First, less than half of the subjects in the RCT provided paired serum samples, so this could have introduced selection bias. Second, many of the participants could have been bitten by mosquitoes during nonwork hours. Thus, LLPI clothing may have had a larger effect than noted here. Third, pre- and postexposure serum samples were drawn at different intervals for different subjects, since we had to visit workers in sites spread across eastern North Carolina. However, the effect of this variation is likely to be non-differential. Finally, although we had good compliance among outdoor workers18 who wear uniforms, LLPI clothing-based public health interventions might be more difficult to implement among populations who do not wear uniforms.
In summary, we conducted a secondary analysis of samples from subjects in a RCT comparing outdoor workers who wear LLPI uniforms to those who do not. Our results suggest that LLPI clothing provides substantial protection from mosquito bites for a year. Further studies evaluating its protective efficacy against mosquito-borne diseases are warranted.
ACKNOWLEDGMENTS
We would like to thank Insect Shield, LLC, for treatment of study uniforms and Yancy King (NC Parks and Recreation), Karen Patterson (NC Forest Service), and Chuck Stanfill (NC Division of Environment and Natural Resources) for their invaluable assistance.
Footnotes
Financial support: This work was generously supported by NIOSH grant 5R01OH009874-03 and NIH NIAID grant 1K22AI103067-01.
Authors' addresses: Berlin Londono-Renteria and Tonya M. Colpitts, Department of Pathology, Microbiology and Immunology, University of South Carolina School of Medicine, Columbia, SC, E-mails: berlinlondo@yahoo.com and tcolpitt@tulane.edu. Crystal Grippin, Sam B. Jameson, and Dawn M. Wesson, Department of Tropical Medicine, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA, E-mails: cgrippin@tulane.edu, sbishop@tulane.edu, and wesson@tulane.edu. Jaymin Patel, Meagan Vaughn, and Steven R. Meshnick, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, E-mails: jaymin86@email.unc.edu, vaughn.meagan@gmail.com, and meshnick@unc.edu. Sheana Funkhauser, Loganathan Ponnusamy, and Charles Apperson, Department of Entomology, North Carolina State University, Raleigh, NC, E-mails: sheana@nc.rr.com, loganathan_ponnusamy@ncsu.edu, and apperson@ncsu.edu. Christopher N. Mores, Department of Pathobiological Sciences, Louisiana State University, Baton Rouge, LA, E-mail: cmores@lsu.edu.
References
- 1.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, Ricotta E, Hornston S, Berman A, Harvey SA. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar J. 2015;14:35. doi: 10.1186/s12936-015-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangoro O, Kelly AH, Mtali S, Moore SJ. Feasibility of repellent use in a context of increasing outdoor transmission: a qualitative study in rural Tanzania. Malar J. 2014;13:347. doi: 10.1186/1475-2875-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leme TS, Papini S, Vieira E, Luchini LC. Evaluation of personal protective equipment used by malathion sprayers in dengue control in Sao Paulo, Brazil. Cad Saude Publica. 2014;30:567–576. doi: 10.1590/0102-311x00144912. [DOI] [PubMed] [Google Scholar]
- 5.Banks SD, Murray N, Wilder-Smith A, Logan JG. Insecticide-treated clothes for the control of vector-borne diseases: a review on effectiveness and safety. Med Vet Entomol. 2014;28(Suppl 1):14–25. doi: 10.1111/mve.12068. [DOI] [PubMed] [Google Scholar]
- 6.Faulde MK, Rutenfranz M, Keth A, Hepke J, Rogge M, Gorner A. Pilot study assessing the effectiveness of factory-treated, long-lasting permethrin-impregnated clothing for the prevention of tick bites during occupational tick exposure in highly infested military training areas, Germany. Parasitol Res. 2015;114:671–678. doi: 10.1007/s00436-014-4232-y. [DOI] [PubMed] [Google Scholar]
- 7.Schofield S, Crane F, Tepper M. Good interventions that few use: uptake of insect bite precautions in a group of Canadian Forces personnel deployed to Kabul, Afghanistan. Mil Med. 2012;177:209–215. doi: 10.7205/milmed-d-11-00205. [DOI] [PubMed] [Google Scholar]
- 8.Kitau J, Oxborough R, Kaye A, Chen-Hussey V, Isaacs E, Matowo J, Kaur H, Magesa SM, Mosha F, Rowland M, Logan J. Laboratory and experimental hut evaluation of a long-lasting insecticide treated blanket for protection against mosquitoes. Parasit Vectors. 2014;7:129. doi: 10.1186/1756-3305-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimnig JE, Lindblade KA, Mount DL, Atieli FK, Crawford S, Wolkon A, Hawley WA, Dotson EM. Laboratory wash resistance of long-lasting insecticidal nets. Trop Med Int Health. 2005;10:1022–1029. doi: 10.1111/j.1365-3156.2005.01481.x. [DOI] [PubMed] [Google Scholar]
- 10.Rowland M, Durrani N, Hewitt S, Mohammed N, Bouma M, Carneiro I, Rozendaal J, Schapira A. Permethrin-treated chaddars and top-sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans R Soc Trop Med Hyg. 1999;93:465–472. doi: 10.1016/s0035-9203(99)90341-3. [DOI] [PubMed] [Google Scholar]
- 11.Kimani EW, Vulule JM, Kuria IW, Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J. 2006;5:63. doi: 10.1186/1475-2875-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto J, Medina F, Dember N, Berman J. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599–602. doi: 10.1093/clinids/21.3.599. [DOI] [PubMed] [Google Scholar]
- 13.Eamsila C, Frances SP, Strickman D. Evaluation of permethrin-treated military uniforms for personal protection against malaria in northeastern Thailand. J Am Mosq Control Assoc. 1994;10:515–521. [PubMed] [Google Scholar]
- 14.Deparis X, Frere B, Lamizana M, N'Guessan R, Leroux F, Lefevre P, Finot L, Hougard JM, Carnevale P, Gillet P, Baudon D. Efficacy of permethrin-treated uniforms in combination with DEET topical repellent for protection of French military troops in Cote d'Ivoire. J Med Entomol. 2004;41:914–921. doi: 10.1603/0022-2585-41.5.914. [DOI] [PubMed] [Google Scholar]
- 15.Faulde MK, Uedelhoven WM, Malerius M, Robbins RG. Factory-based permethrin impregnation of uniforms: residual activity against Aedes aegypti and Ixodes ricinus in battle dress uniforms worn under field conditions, and cross-contamination during the laundering and storage process. Mil Med. 2006;171:472–477. doi: 10.7205/milmed.171.6.472. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Rutledge LC, Reifenrath WG, Gutierrez GA, Korte DW., Jr Effects of weathering on fabrics treated with permethrin for protection against mosquitoes. J Am Mosq Control Assoc. 1989;5:176–179. [PubMed] [Google Scholar]
- 17.Vaughn MF, Meshnick SR. Pilot study assessing the effectiveness of long-lasting permethrin-impregnated clothing for the prevention of tick bites. Vector Borne Zoonotic Dis. 2011;11:869–875. doi: 10.1089/vbz.2010.0158. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn MF, Funkhouser SW, Lin FC, Fine J, Juliano JJ, Apperson CS, Meshnick SR. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473–480. doi: 10.1016/j.amepre.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro JM, Rossignol PA, Spielman A. Role of mosquito saliva in blood vessel location. J Exp Biol. 1984;108:1–7. doi: 10.1242/jeb.108.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006;98:66–73. doi: 10.1016/j.actatropica.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Londono-Renteria BL, Eisele TP, Keating J, James MA, Wesson DM. Antibody response against Anopheles albimanus (Diptera: Culicidae) salivary protein as a measure of mosquito bite exposure in Haiti. J Med Entomol. 2010;47:1156–1163. doi: 10.1603/me09240. [DOI] [PubMed] [Google Scholar]
- 22.Londono-Renteria B, Cardenas JC, Cardenas LD, Christofferson RC, Chisenhall DM, Wesson DM, McCracken MK, Carvajal D, Mores CN. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS One. 2013;8:e81211. doi: 10.1371/journal.pone.0081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doucoure S, Mouchet F, Cournil A, Le Goff G, Cornelie S, Roca Y, Giraldez MG, Simon ZB, Loayza R, Misse D, Flores JV, Walter A, Rogier C, Herve JP, Remoue F. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: a new biomarker of exposure to dengue vector bites. Am J Trop Med Hyg. 2012;87:504–510. doi: 10.4269/ajtmh.2012.11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drame PM, Poinsignon A, Besnard P, Le Mire J, Dos-Santos MA, Sow CS, Cornelie S, Foumane V, Toto JC, Sembene M, Boulanger D, Simondon F, Fortes F, Carnevale P, Remoue F. Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg. 2010;83:115–121. doi: 10.4269/ajtmh.2010.09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poinsignon A, Samb B, Doucoure S, Drame PM, Sarr JB, Sow C, Cornelie S, Maiga S, Thiam C, Rogerie F, Guindo S, Hermann E, Simondon F, Dia I, Riveau G, Konate L, Remoue F. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop Med Int Health. 2010;15:1198–1203. doi: 10.1111/j.1365-3156.2010.02611.x. [DOI] [PubMed] [Google Scholar]
- 26.Ali ZM, Bakli M, Fontaine A, Bakkali N, Vu Hai V, Audebert S, Boublik Y, Pages F, Remoue F, Rogier C, Fraisier C, Almeras L. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar J. 2012;11:439. doi: 10.1186/1475-2875-11-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badu K, Siangla J, Larbi J, Lawson BW, Afrane Y, Ong'echa J, Remoue F, Zhou G, Githeko AK, Yan G. Variation in exposure to Anopheles gambiae salivary gland peptide (gSG6-P1) across different malaria transmission settings in the western Kenya highlands. Malar J. 2012;11:318. doi: 10.1186/1475-2875-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doucoure S, Mouchet F, Cornelie S, DeHecq JS, Rutee AH, Roca Y, Walter A, Herve JP, Misse D, Favier F, Gasque P, Remoue F. Evaluation of the human IgG antibody response to Aedes albopictus saliva as a new specific biomarker of exposure to vector bites. PLoS Negl Trop Dis. 2012;6:e1487. doi: 10.1371/journal.pntd.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzo C, Ronca R, Lombardo F, Mangano V, Sirima SB, Nebie I, Fiorentino G, Troye-Blomberg M, Modiano D, Arca B. IgG1 and IgG4 antibody responses to the Anopheles gambiae salivary protein gSG6 in the sympatric ethnic groups Mossi and Fulani in a malaria hyperendemic area of Burkina Faso. PLoS One. 2014;9:e96130. doi: 10.1371/journal.pone.0096130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone W, Bousema T, Jones S, Gesase S, Hashim R, Gosling R, Carneiro I, Chandramohan D, Theander T, Ronca R, Modiano D, Arca B, Drakeley C. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS One. 2012;7:e40170. doi: 10.1371/journal.pone.0040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irby WS, Apperson CS. Spatial and temporal distribution of resting female mosquitoes (Diptera: Culicidae) in the coastal plain of North Carolina. J Med Entomol. 1992;29:150–159. doi: 10.1093/jmedent/29.2.150. [DOI] [PubMed] [Google Scholar]
- 32.Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham K, Kayedi MH, Maxwell C, Kaur H, Rehman H, Malima R, Curtis CF, Lines JD, Rowland MW. Multi-country field trials comparing wash-resistance of PermaNet and conventional insecticide-treated nets against anopheline and culicine mosquitoes. Med Vet Entomol. 2005;19:72–83. doi: 10.1111/j.0269-283X.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 34.Murray N, Jansarikij S, Olanratmanee P, Maskhao P, Souares A, Wilder-Smith A, Kittayapong P, Louis VR. Acceptability of impregnated school uniforms for dengue control in Thailand: a mixed methods approach. Glob Health Action. 2014;7:24887. doi: 10.3402/gha.v7.24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tozan Y, Ratanawong P, Louis VR, Kittayapong P, Wilder-Smith A. Use of insecticide-treated school uniforms for prevention of dengue in schoolchildren: a cost-effectiveness analysis. PLoS One. 2014;9:e108017. doi: 10.1371/journal.pone.0108017. [DOI] [PMC free article] [PubMed] [Google Scholar]