Abstract
Purpose
The aim of this study was to investigate the prognostic performance of multiparametric magnetic resonance imaging (mpMRI) and Prostate Imaging Reporting and Data System (PIRADS) score in predicting pathologic features in a cohort of patients eligible for active surveillance who underwent radical prostatectomy.
Methods
A total of 223 patients who fulfilled the criteria for “Prostate Cancer Research International: Active Surveillance”, were included. Mp–1.5 Tesla MRI examination staging with endorectal coil was performed at least 6–8 weeks after TRUS-guided biopsy. In all patients, the likelihood of the presence of cancer was assigned using PIRADS score between 1 and 5. Outcomes of interest were: Gleason score upgrading, extra capsular extension (ECE), unfavorable prognosis (occurrence of both upgrading and ECE), large tumor volume (≥0.5ml), and seminal vesicle invasion (SVI). Receiver Operating Characteristic (ROC) curves and Decision Curve Analyses (DCA) were performed for models with and without inclusion of PIRADS score.
Results
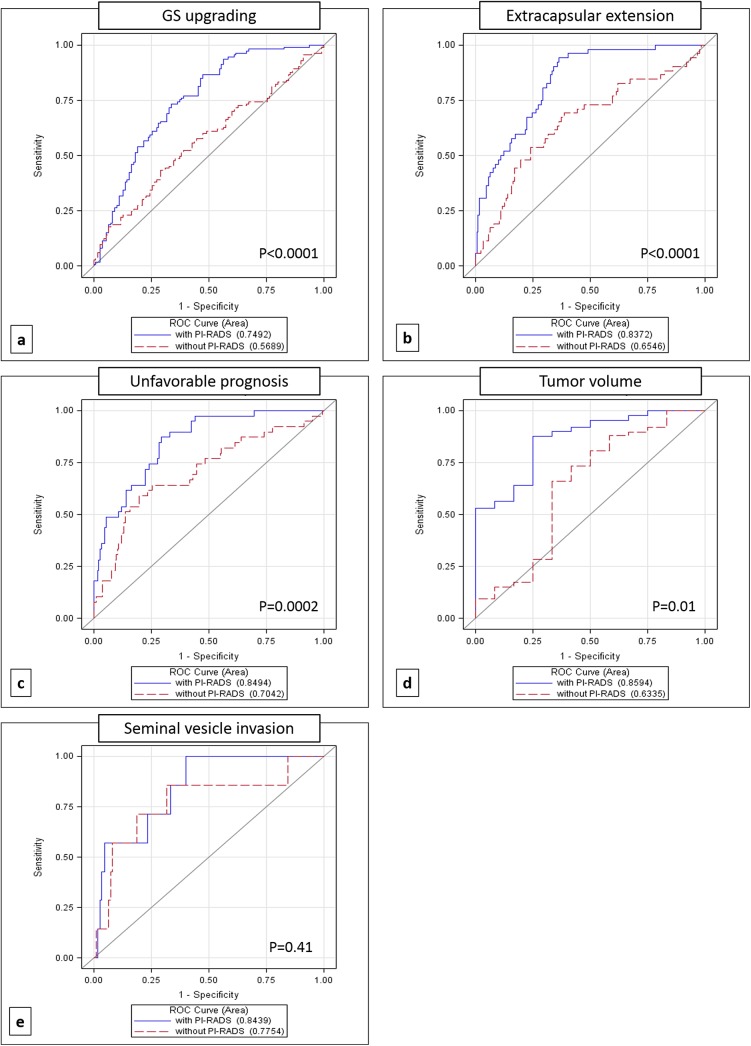
Multivariate analysis demonstrated the association of PIRADS score with upgrading (P<0.0001), ECE (P<0.0001), unfavorable prognosis (P<0.0001), and large tumor volume (P = 0.002). ROC curves and DCA showed that models including PIRADS score resulted in greater net benefit for almost all the outcomes of interest, with the only exception of SVI.
Conclusions
mpMRI and PIRADS scoring are feasible tools in clinical setting and could be used as decision-support systems for a more accurate selection of patients eligible for AS.
Introduction
The use of prostate specific antigen (PSA) testing has recently been criticized for prostate cancer (PCa) screening[1,2], although it continues to be the best biomarker available for early PCa detection. The increasing use of this biomarker in association with several PSA derivatives, such as free to total PSA ratio (%fPSA), PSA density (PSAD), and PSA velocity, has led to frequent detection of small, well differentiated, low-risk PCa without significant decrease in mortality[3]. This fact gives rise to the thought that clinically insignificant disease is being treated excessively and active follow up of these patients should be preferred instead of radical treatment. Active surveillance (AS) is an alternative to initial radical treatment of low-risk PCa, even if the current parameters used for selection and follow up, such as clinical T stage, total PSA, PSA density, Gleason score (GS), and number of positive prostate biopsy cores, incorrectly exclude some patients eligible for AS and misclassify some who actually harbor significant disease[4]. In order to predict the pathologic findings at radical prostatectomy, risk stratification has been improved with validation of several nomograms that aid to reduce the rates of overtreatment in patients with clinically insignificant PCa[5]. Consequently numerous preoperative prognostic tools have analyzed the ability of prostate cancer antigen 3 (PCA3), sarcosine, [–2]proPSA, and Prostate Health Index (PHI) in predicting pathological features at radical prostatectomy[6,7]. Multiparametric magnetic resonance imaging (mpMRI) is increasingly being used in clinical practice to evaluate PCa localization, tumor stage and aggressiveness aiding treatment planning[8]. Although many studies available on the role of mpMRI during PCa-AS have shown the ability to reduce re-biopsies[9,10], not always MRI lesions correspond with guided biopsy or radical prostatectomy (RP) specimen findings[11]. Recently preoperative neural network software including mpMRI variables, PSA level and GS has been reported to predict insignificant prostate cancer, particularly in the context of clinically non-palpable tumors, suggesting a prognostic and pathologic predictive role in clinically very low risk PCa[12]. In this scenario it has been developed a scoring system called Prostate Imaging Reporting and Data System (PIRADS), with the aim to enable elaboration, interpretation, and reporting of prostate mpMRI findings[13]. The aim of this study is to investigate the prognostic performance of MRI and PIRADS score in predicting pathologic features in a cohort of patients eligible for active surveillance who underwent RP.
Patients and Methods
We retrospectively reviewed the medical records of 2,200 patients who underwent robotic RP for PCa between November 2009 and July 2014. None of the patients included in the current study received neoadjuvant androgen-deprivation therapy or drugs that could alter the PSA values. In total 223 patients fulfilled the inclusion criteria for “Prostate Cancer Research International: Active Surveillance”[14] defined as follows: clinical stage T2a or less, PSA<10 ng/ml, 2 or fewer cores involved with cancer after a 12-core biopsy scheme, GS≤6 grade and PSA density<0.2ng/mL/cc. We compared the pathological findings between prostate biopsies and specimens after RP. Specimens were processed and evaluated according to the Stanford protocol[15] by a single, experienced, genitourinary pathologist(G.R.) blinded to index-tests results. After fixing the RP specimens, they were inked and cut at 3-mm intervals perpendicular to the rectal surface. The apical slice was cut para-sagittally at 2-3-mm intervals, and the sections were then divided in halves or quadrants to fit routinely used cassettes for paraffin embedding. The whole prostate was sampled.
This retrospective analysis of prospectively acquired data was approved by the “IRCCS—Istituto Europeo di Oncologia Ethic Committee” who waived the requirement for informed consent specific to the study because all patients provided written informed consent for MR imaging, surgical procedures, and research use of their medical information.
Mp–1.5 Tesla MRI (Avanto; Siemens Medical Solutions, Erlangen, Germany), examination staging with endorectal coil was performed at least 6–8 weeks after TRUS-guided biopsy, in order to avoid distortions and artifacts due to inflammatory process after the bioptic procedure. The following pulse sequences were used: sagittal, coronal, and axial T2-TSE (TR/TE, 831/80 ms), axial Diffusion-Weighted Imaging (DWI) using high b values (b = 800) and ADC maps, axial Dynamic Contrast-Enhanced imaging (DCE) obtained before, during and after injection of gadopentetate dimeglumine (Magnevist; Bayer Healthcare, Berlin, Germany) administered at a dose of 0.1 mmol per kilogram of body weight through a peripheral vein at a flow rate of 3 mL/sec followed by a saline bolus of 10 mL administered at the same flow rate by using a mechanical injector (Spectris MR Injection System; Medrad, Leverkusen, Germany) and axial T1-TSE (TR/TE, 217.8/4.6).
The European Society of Urogenital Radiology (ESUR) in 2012 established clinical guidelines for the acquisition, interpretation, and reporting of mpMRI of the prostate in order to facilitate a greater level of standardisation and consistency[16]. These recommendations, popularly referred to as Prostate Imaging Reporting and Data System (PI-RADS), were based on literature evidence and consensus expert opinion.
One radiologist (G.P.) prospectively read and scored all cases, developing a standardized structured report for each patient. In all patients, the likelihood of the presence of cancer was assigned using PIRADS score (Likert-like scale) between 1 and 5 (1, not suspect; 2, hardly suspect; 3, ambiguous; 4, suspect; 5, highly suspect)[17]. The assigned scores of 3–5 were considered positive, and scores of 1–2 were considered negative for cancer. For patients with more than one region suspected to be cancer, only the region with the highest sum of the PIRADS scores was used for statistical analysis.
Statistical Analysis
Outcomes of interest were: upgrading, extracapsular extension (ECE), unfavorable prognosis (occurrence of both upgrading and ECE), large tumor volume (≥0.5ml) seminal vesicle invasion (SVI). Unfavorable prognosis was also evaluated considering separately unfavorable prognosis with primary GS = 4. Informative parameters for the distribution of continuous variables (age, PSA, PSAD, prostate volume) were calculated and their distributions were tested for normality by the Kolmogorov-Smirnov test. Univariate analyses were performed to evaluate the association of patient and tumor characteristics with upgrading, ECE, unfavorable prognosis, large tumor volume and seminal vesicle invasion. The association for continuous variables was assessed by T-test or non-parametric two-sample Wilcoxon test, as appropriate; the association for categorical variables was assessed by Chi-Square test or Fisher’s Exact Test, as appropriate. Sensitivity, specificity, positive predicted values (PPV) and negative predicted values (NPV) for PIRADS score 3–5 (positive for cancer) versus 1–2 (negative for cancer) were calculated for each outcome of interest. Multivariate unconditional logistic regression models were performed to assess the independent contribution of patient and tumor characteristics in the prediction of upgrading, ECE, unfavorable prognosis, large tumor volume and seminal vesicle invasion; Odds Ratios (OR) and 95% Confidence Intervals (CI) were calculated. Receiver Operating Characteristic (ROC) curves were drawn for models with and without inclusion of PIRADS score, and the corresponding areas under the curve (AUC) of the two models were compared with the De Long test. To graphically evaluate the net benefit for the models with and without inclusion of PIRADS score, a decision-curve analysis (DCA) was performed. DCA expresses the ‘‘net benefit” of a prediction model as the difference between the proportion of patients who are true positive and the proportion who are false positive, the latter weighted by the relative harm of a false–positive and a false–negative result [18].
Statistical significance was defined as p<0.05. Statistical analysis was performed using SAS software, version 9.2. The DCA was performed by using an Excel macro (Microsoft Office Excel 2007).
Results
Table 1 presents the main characteristics of the study population. Sensitivity for MRI in identifying tumors with the most unfavorable prognostic characteristics was extremely high, ranging from 94% for large tumor volume to 100% for cancers with ECE, unfavorable prognosis and SVI (Table 2). MRI presented an excellent ability in ruling out almost all the outcomes of interest: NPV was 94% for upgrading and 100% for ECE, unfavorable prognosis and SVI (Table 2). On the other side, specificity and PPV values were generally low for almost all the outcomes of interest, with the exception of tumor volume, for which we found a PPV = 97%, probably due, however, to the very low number of patients with tumor volume <0.5 ml (Table 2).
Table 1. Patient and tumor characteristics of the study population.
| N (%) | |
|---|---|
| Volume^ | 47.94 (±14.53) |
| Age^ | 62.75 (±8.28) |
| Clinical Stage | |
| cT1c | 191 (85.65%) |
| cT2a | 32 (14.35%) |
| PSA^ | 6.02 (±1.91) |
| PSA Density^ | 0.13 (±0.04) |
| Tumor volume^ | 0.95 (±0.23) |
| Pathological stage | |
| pT2a | 23 (10.31%) |
| pT2b | 3 (1.35%) |
| pT2c | 145 (65.02%) |
| pT3a | 45 (20.18%) |
| pT3b | 7 (3.14%) |
| Positive Cores | |
| 1 | 95 (42.60%) |
| 2 | 128 (57.40%) |
| Pathological Total Gleason Score | |
| 6 | 110 (49.33%) |
| 7 | 110 (49.33%) |
| 8 | 2 (0.90%) |
| 9 | 1 (0.45%) |
| Cancer at MRI | |
| Not visible | 19 (8.52%) |
| Visible | 204 (91.48%) |
| Positive lymph nodes | |
| Yes | 4 (1.79%) |
| No | 219 (98.21%) |
| Seminal vesicle invasion | |
| Yes | 7 (3.15%) |
| No | 215 (96.85%) |
| PIRADS | |
| 1 | 2 (0.91%) |
| 2 | 14 (6.36%) |
| 3 | 58 (26.36%) |
| 4 | 71 (32.27%) |
| 5 | 75 (34.09%) |
^mean (± SD)
Table 2. Sensitivity (SE), specificity (SP), positive predicted values (PPV) and negative predicted values (NPV) with 95%(CI) for 1–2 vs. ≥ 3 PIRADS score.
| SE(CI)* | SP(CI)* | PPV(CI)* | NPV(CI)* | |
|---|---|---|---|---|
| Upgrading | 99 (95–100) | 14 (8–22) | 55 (48–62) | 94 (70–100) |
| Extra capsular extension | 100 (93–100) | 10 (6–15) | 25 (20–32) | 100 (79–100) |
| Unfavorable prognosis | 100 (91–100) | 9 (5–14) | 19 (14–25) | 100 (79–100) |
| Tumor volume | 94 (90–97) | 40 (12–74) | 97 (94–99) | 25 (7–52) |
| Seminal vesicle invasion | 100 (59–100) | 8 (4–12) | 3 (1–7) | 100 (79–100) |
*Percentage
At univariate analysis (Tables 3–7) we found a significant association between PIRADS score and GS upgrading, ECE, unfavorable prognosis and large tumor volume: the probability of each outcome of interest increased with increasing PIRADS score (p<0.0001).The same trend was confirmed when restricting the analysis to patients with unfavorable prognosis and primary GS = 4 (p = 0.01). No significant association was found between PIRADS score and SVI (p = 0.28), although a significant trend for one–unit increase in PIRADS score was observed even for this outcome (p = 0.03). Other possible predictors of unfavorable prognostic characteristics were: age (upgrading, unfavorable prognosis), clinical stage (ECE, unfavorable prognosis, SVI), PSA and PSA density (unfavorable prognosis).
Table 3. Association of patient and tumor characteristics withupgrading: univariate and multivariate analysis.
| Upgrading | Pvalue* | Multivariate Odds Ratio (95%CI) | Pvalue | ||
|---|---|---|---|---|---|
| “Yes” N (%) | “No” N (%) | ||||
| Volume^ | 47.55 (±10.25) | 48.34 (±17.94) | 0.43 | 1.00 (0.98–1.03) 2 | 0.92 |
| Age^ | 63.35 (±9.38) | 62.13 (±6.96) | 0.04 | 1.01 (0.98–1.05) 2 | 0.53 |
| Clinical Stage | 0.34 | 0.59 | |||
| cT1c | 94 (83%) | 97 (88%) | 1.00 (reference) | ||
| cT2a | 19 (17%) | 13 (12%) | 1.27 (0.54–2.97) | ||
| PSA^ | 6.09 (±1.95) | 5.94 (±1.87) | 0.56 | 1.02 (0.86–1.19) 2 | 0.85 |
| PSA Density^ | 0.13 (±0.04) | 0.13 (±0.04) | 0.76 | - 3 | - |
| Positive Cores | 0.28 | 0.55 | |||
| 1 | 44 (39%) | 51 (46%) | 1.00 (reference) | ||
| 2 | 69 (61%) | 59 (54%) | 1.20 (0.66–2.18) | ||
| PIRADS | <0.0001 1 | 2.72 (1.93–3.84) 2 | <0.0001 | ||
| 1 | 0 (0%) | 2 (2%) | |||
| 2 | 1 (1%) | 13 (12%) | |||
| 3 | 17 (15%) | 41 (38%) | |||
| 4 | 40 (35%) | 31 (29%) | |||
| 5 | 55 (49%) | 20 (19%) | |||
| Cancer at MRI | <0.0001 | - 3 | - | ||
| Not visible | 1 (1%) | 18 (16%) | |||
| Visible | 112 (99%) | 92 (84%) | |||
1Mantel-Haenszel p-value for trend = <0.0001
*T test or non parametric two-sample Wilcoxon test for continuous variables, as appropriate; Chi-Square test or Fisher’s Exact Test for categorical variables, as appropriate
2One-unit increase OR
3Not entered in the multivariate model because it is a linear combination of other variables.
Note: significant ORs and p-values are in bold.
Table 7. Association of patient and tumor characteristics with seminal vesicle invasion: univariate and multivariate analysis.
| Seminal vesicle invasion | Pvalue* | Multivariate Odds Ratio (95%CI) | Pvalue | ||
|---|---|---|---|---|---|
| “Yes” (%) | “No” (%) | ||||
| Volume^ | 46.14 (±3.72) | 47.94 (±14.76) | 0.98 | 1.01 (0.92–1.10) 2 | 0.91 |
| Age^ | 66.57 (±5.70) | 62.63 (±8.35) | 0.17 | 1.06 (0.92–1.21) 2 | 0.44 |
| Clinical Stage | 0.01 | 0.02 | |||
| cT1c | 3 (43%) | 187 (87%) | 1.00 (reference) | ||
| cT2a | 4 (57%) | 28 (13%) | 7.78 (1.44–41.93) | ||
| PSA^ | 6.04 (±1.91) | 6.01 (±1.91) | 0.99 | 1.01 (0.63–1.61) 2 | 0.98 |
| PSA Density^ | 0.13 (±0.04) | 0.13 (±0.04) | 0.89 | - 3 | - |
| Positive Cores | 0.70 | 0.88 | |||
| 1 | 2 (29%) | 92 (43%) | 1.00 (reference) | ||
| 2 | 5 (71%) | 123 (57%) | 1.15 (0.19–6.98) | ||
| PIRADS | 0.28 1 | 3.97 (0.92–17.09) 2 | 0.06 | ||
| 1 | 0 (0%) | 2 (1%) | |||
| 2 | 0 (0%) | 14 (7%) | |||
| 3 | 0 (0%) | 58 (27%) | |||
| 4 | 2 (29%) | 68 (32%) | |||
| 5 | 5 (71%) | 70 (33%) | |||
| Cancer at MRI | 1.00 | - 3 | - | ||
| Not visible | 0 (0%) | 19 (9%) | |||
| Visible | 7 (100%) | 196 (91%) | |||
^mean (± SD)
*T test or non parametric two-sample Wilcoxon test for continuous variables, as appropriate; Chi-Square test or Fisher’s Exact Test for categorical variables, as appropriate
1Mantel-Haenszel p-value for trend = 0.03
2 One-unit increase OR
3 Not entered in the multivariate model because it is a linear combination of other variables.
Note: significant ORs and p-values are in bold.
Table 4. Association of patient and tumor characteristics with extra capsular extension: univariate and multivariate analysis.
| Extra capsular extension | Pvalue* | Multivariate Odds Ratio (95%CI) | Pvalue | ||
|---|---|---|---|---|---|
| “Yes” N (%) | “No” N (%) | ||||
| Volume^ | 47.77 (±10.10) | 47.99 (±15.66) | 0.54 | 0.99 (0.96–1.03) 2 | 0.73 |
| Age^ | 63.85 (±8.17) | 62.41 (±8.31) | 0.17 | 0.99 (0.95–1.04) 2 | 0.79 |
| Clinical Stage | 0.02 | 0.02 | |||
| cT1c | 39 (75%) | 152 (89%) | 1.00 (reference) | ||
| cT2a | 13 (25%) | 19 (11%) | 3.19 (1.22–8.35) | ||
| PSA^ | 6.56 (±2.23) | 5.86 (±1.78) | 0.07 | 1.27 (1.03–1.57) 2 | 0.03 |
| PSA Density^ | 0.14 (±0.04) | 0.13 (±0.04) | 0.10 | - 3 | - |
| Positive Cores | 0.34 | 0.68 | |||
| 1 | 19 (37%) | 76 (44%) | 1.00 (reference) | ||
| 2 | 33 (63%) | 95 (56%) | 1.17 (0.55–2.49) | ||
| PIRADS | <0.0001 1 | 5.27 (2.94–9.44) 2 | <0.0001 | ||
| 1 | 0 (0%) | 2 (1%) | |||
| 2 | 0 (0%) | 14 (8%) | |||
| 3 | 1 (2%) | 57 (34%) | |||
| 4 | 15 (29%) | 56 (34%) | |||
| 5 | 36 (69%) | 39 (23%) | |||
| Cancer at MRI | 0.01 | - 3 | - | ||
| Not visible | 0 (0%) | 19 (11%) | |||
| Visible | 52 (100%) | 152 (89%) | |||
1Mantel-Haenszel p-value for trend = <0.0001
*T test or non parametric two-sample Wilcoxon test for continuous variables, as appropriate; Chi-Square test or Fisher’s Exact Test for categorical variables, as appropriate
2One-unit increase OR
3Not entered in the multivariate model because it is a linear combination of other variables.
Note: significant ORs and p-values are in bold.
Table 5. Association of patient and tumor characteristics with unfavorable prognosis: univariate and multivariate analysis.
| Unfavorable prognosis N (%) | Favorable prognosis N (%) | Pvalue* | Multivariate Odds Ratio (95%CI) | Pvalue | |
|---|---|---|---|---|---|
| Volume^ | 49.08 (±10.01) | 47.70 (±15.33) | 0.20 | 1.01 (0.97–1.05) 2 | 0.74 |
| Age^ | 65.36 (±7.80) | 62.19 (±8.29) | 0.01 | 1.04 (0.98–1.10) 2 | 0.26 |
| Clinical Stage | 0.03 | 0.04 | |||
| cT1c | 29 (74%) | 162 (88%) | 1.00 (reference) | ||
| cT2a | 10 (26%) | 22 (12%) | 2.96 (1.06–8.22) | ||
| PSA^ | 6.87 (±2.08) | 5.84 (±1.83) | 0.002 | 1.36 (1.08–1.72) 2 | 0.01 |
| PSA Density^ | 0.14 (±0.04) | 0.13 (±0.04) | 0.04 | - 3 | - |
| Positive Cores | 0.38 | 0.98 | |||
| 1 | 14 (36%) | 81 (44%) | 1.00 (reference) | ||
| 2 | 25 (67%) | 103 (56%) | 1.01 (0.43–2.37) | ||
| PIRADS | <0.0001 1 | 5.42 (2.74–10.70) 2 | <0.0001 | ||
| 1 | 0 (0%) | 2 (1%) | |||
| 2 | 0 (0%) | 14 (8%) | |||
| 3 | 0 (0%) | 58 (32%) | |||
| 4 | 11 (28%) | 60 (33%) | |||
| 5 | 28 (72%) | 47 (26%) | |||
| Cancer at MRI | 0.05 | - 3 | - | ||
| Not visible | 0 (0%) | 19 (10%) | |||
| Visible | 39 (100%) | 165 (90%) |
1Mantel-Haenszel p-value for trend = <0.0001
*T test or non parametric two-sample Wilcoxon test for continuous variables, as appropriate; Chi-Square test or Fisher’s Exact Test for categorical variables, as appropriate
2One-unit increase OR
3Not entered in the multivariate model because it is a linear combination of other variables.
Note: significant ORs and p-values are in bold.
Table 6. Association of patient and tumor characteristics with tumor volume: univariate and multivariate analysis.
| Tumor volume | Pvalue* | Multivariate Odds Ratio (95%CI) | Pvalue | |||
|---|---|---|---|---|---|---|
| ≥0.5 ml N (%) | <0.5 ml N (%) | |||||
| Volume^ | 47.52 (±11.25) | 55.25 (±42.24) | 0.30 | 0.99 (0.96–1.01) 2 | 0.31 | |
| Age^ | 62.66 (±8.39) | 64.27 (±5.96) | 0.53 | 0.97 (0.87–1.08) 2 | 0.58 | |
| Clinical Stage | 0.68 | 0.47 | ||||
| cT1c | 181 (86%) | 10 (83%) | 1.00 (reference) | |||
| cT2a | 30 (14%) | 2 (17%) | 0.49 (0.07–3.42) | |||
| PSA^ | 6.05 (±1.92) | 5.41 (±1.68) | 0.26 | 1.33 (0.89–1.97) 2 | 0.17 | |
| PSA Density^ | 0.13 (±0.04) | 0.12 (±0.05) | 0.39 | - 3 | - | |
| Positive Cores | 0.26 | 0.62 | ||||
| 1 | 88 (42%) | 7 (58%) | 1.00 (reference) | |||
| 2 | 123 (58%) | 5 (42%) | 1.41 (0.37–5.43) | |||
| PIRADS | <0.0001 1 | 3.43 (1.56–7.65) 2 | 0.002 | |||
| 1 | 2 (1%) | 0 (0%) | ||||
| 2 | 10 (5%) | 4 (40%) | ||||
| 3 | 53 (25%) | 5 (50%) | ||||
| 4 | 71 (34%) | 0 (0%) | ||||
| 5 | 74 (35%) | 1 (10%) | ||||
| Cancer at MRI | <0.0001 | - 3 | - | |||
| Not visible | 13 (6%) | 6 (50%) | ||||
| Visible | 198 (94%) | 6 (50%) | ||||
1Mantel-Haenszel p-value for trend = 0.0002
*T test or non parametric two-sample Wilcoxon test for continuous variables, as appropriate; Chi-Square test or Fisher’s Exact Test for categorical variables, as appropriate
2One-unit increase OR
3Not entered in the multivariate model because it is a linear combination of other variables.
Note: significant ORs and p-values are in bold.
At multivariate analysis (Table 3) the association of PIRADS score with upgrading, ECE, unfavorable prognosis and large tumor volume was confirmed. The risk of having unfavorable prognosis was more than quintupled for every unit increase of PIRADS score. Clinical stage cT2a was a significant independent predictor of ECE, unfavorable prognosis and SVI, while PSA was a significant independent predictor of ECE and unfavorable prognosis.
Fig 1 shows the ROC curves comparing models with and without PIRADS score. The differences between the correspondent AUC were statistically significant for upgrading (p<0.0001), ECE (p<0.0001), unfavorable prognosis (p = 0.0002), and tumor volume (p = 0.01), whereas it was not significant for SVI (p = 0.41) probably due to the very low number of patients with SVI.
Fig 1. ROC Curves comparing models with and without inclusion of PIRADS score for a) Gleason score (GS) upgrading, b) extra capsular extension, c) unfavorable prognosis, d) tumor volume and e) seminal vesicle invasion.
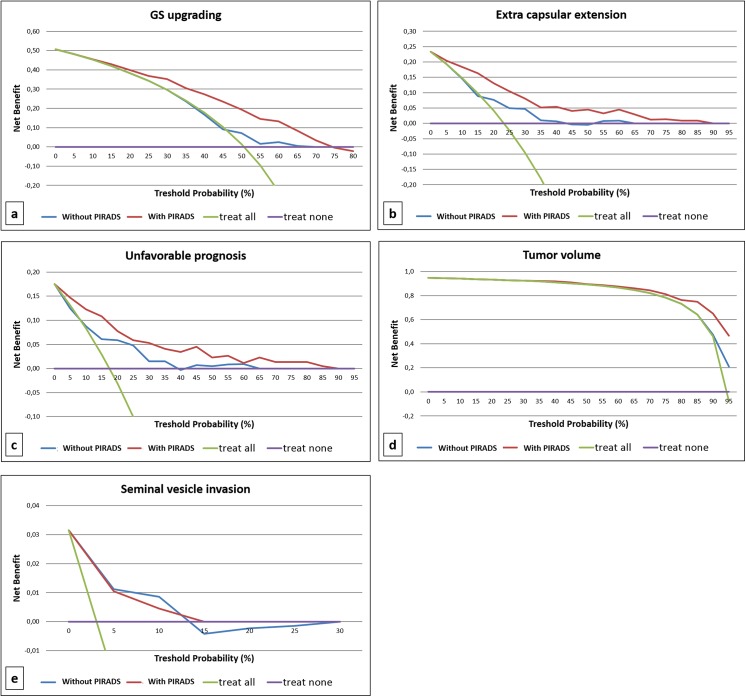
Fig 2 presents the decision curves for the multivariable models presented in Table 2 and Fig 1. Models including PIRADS score resulted in greater net benefit for almost all the outcomes of interest if compared with models without the inclusion of PIRADS score, again with the only exception of SVI. Inclusion of PIRADS score in prediction tools may therefore increase the net benefit over almost all the range of probabilities when the outcome of interest is upgrading, upstaging or their combination (unfavorable prognosis), while it results in increased net benefit only at a threshold probability>80% when the outcome of interest is tumor volume.
Fig 2. Decision curve analysis of the effect of prediction models on the detection of a) upgrading, b) extra capsular extension, c) unfavorable prognosis, d) tumor volume and e) seminal vesicle invasion.
Model with PIRADS score (red line) is plotted against treat none (violet line), treat all (green line) and model without PIRADS score (blue line).
Discussion
The proportion of men with low-risk PCa ranged from 16% in 2000 to 21% in 2006, showing an increasing of 'watchful waiting option' from 0% to 39% over the same period[19]. These data confirm the favorable outcomes of watchful waiting reported in the PIVOT study[20]. Thus the goal of PCa care is to identify and treat only men with clinically significant disease. In this setting, AS aims to avoid unnecessary treatment in men with slow-growing PCa, although current risk stratification schemes misclassify some patients. Selvadurai et al observed that about one-third of those men undergoing deferred RP had adverse features at the time of surgery, such as extracapsular extension, high-grade disease, or positive margins[21]. Circulating biomarkers represent a promising approach to identify men with apparently low-risk biopsy pathology, but who harbor potentially aggressive tumors unsuitable for AS[22,23]. Recently van den Bergh et al. provided a summary of the current studies examining imaging and novel biomarkers in AS for PCa, emphasizing their burden role of monitoring during AS[4]. Several studies have suggested the benefit of early repeat biopsy or more extended biopsy to reduce the risk of unfavorable disease on RP specimens regardless of how AS criteria are defined[24,25]. Kuru et al in a retrospective evaluation of the PIRADS in mpMRI based on single cores and single-core histology, confirmed a significant correlation between this decision-support scoring system and histopathology[26]. The adding performance of MRI to the initial clinical evaluation of men with clinically low risk PCa helped prediction, showing that an overall PIRADS score of 5 had a high sensitivity for GS upgrading on confirmatory biopsy, and suggesting a potential role in patients’ selection for AS[27]..Recently, Abdi et al demonstrated on multivariate analysis an increased rate of AS termination for patients with PIRADS score 4 or 5 (vs 3) undergoing MRI fusion technology during transrectal ultrasound-guided biopsy[28]. Bittencourt et al in 133 consecutive PCa patients, who underwent prostatectomy, showed moderate overall accuracy of ESUR/PIRADS criteria in the prediction of EPE in a subpopulation with intermediate to high-risk disease and large-volume tumors [29].
Other authors[30,31] showed that MRI does not improve the prediction of high-risk and/or non organ-confined disease in a RP specimen.
According to previous reports [32,33], our study supports the prognostic accuracy of MRI and PIRADS score in predicting pathological features such as GS upgrading, ECE, unfavorable prognosis and large tumor volume in a cohort of patients eligible for AS. Particularly, considering the multivariable model for predicting unfavorable prognosis, we found a strong association with one unit increase PIRADS score as well as with one unit increase PSA and clinical stage cT2a compared with cT1c. DCA further confirmed the benefit given by using a model including PIRADS score when compared with the decision of treating all patients or treating none, as well as compared with a model that do not include this scoring system. The inclusion of PIRADS score in prediction tools may increase the net benefit over almost all the range of probabilities when the outcome of interest is GS upgrading, ECE or their combination. In particular, we found that the PIRADS score for detecting cancer was highly sensitive for both ECE and seminal vesicle invasion, although we did not use PIRADS-specific scores in order to assess these variables. Also, it results in increased net benefit at a threshold probability>80% when the outcome of interest was tumor volume.
Conclusions
Our findings show that mpMRI and PIRADS scoring are feasible tools in clinical setting and could be used as decision-support systems for a more accurate selection of patients eligible for AS. ROC curves and DCA showed the higher accuracy of the models including PIRADS score in predicting GS upgrading, ECE, unfavorable prognosis and tumor volume at final histology.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157(2):120 10.7326/0003-4819-157-2-201207170-00459 [DOI] [PubMed] [Google Scholar]
- 2. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR,et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104(2):125 10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vickers AJ, Wolters T, Savage CJ, Cronin AM, O'Brien MF, Pettersson K et al. Prostate-specific antigen velocity for early detection of prostate cancer: result from a large, representative, population-based cohort. Eur Urol. 2009;56(5):753 10.1016/j.eururo.2009.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Bergh RC, Ahmed HU, Bangma CH, Cooperberg MR, Villers A, Parker CC., et al. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014;65(6):1023 10.1016/j.eururo.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 5. Loeb S1, Bruinsma SM2, Nicholson J3, Briganti A4, Pickles T5, Kakehi Y6 et al. Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur Urol. 2015;67(4):619 10.1016/j.eururo.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cantiello F, Russo GI, Ferro M, Cicione A, Cimino S, Favilla V et al. Prognostic accuracy of Prostate Health Index and urinary Prostate Cancer Antigen 3 in predicting pathologic features after radical prostatectomy. Urol Oncol. 2015. January 6. pii: S1078-1439(14)00448-7. [DOI] [PubMed] [Google Scholar]
- 7. Ferro M, Lucarelli G, Bruzzese D, Perdonà S, Mazzarella C, Perruolo G, et al. Improving the prediction of pathologic outcomes in patients undergoing radical prostatectomy: the value of prostate cancer antigen 3 (PCA3), prostate health index (phi) and sarcosine. Anticancer Res. 2015. February;35(2):1017 [PubMed] [Google Scholar]
- 8. Feng TS, Sharif-Afshar AR, Smith S2, Miller J, Nguyen C, Li Q, et al. Multiparametric magnetic resonance imaging localizes established extracapsular extension of prostate cancer. Urol Oncol. 2015. March;33(3):109.e15. [DOI] [PubMed] [Google Scholar]
- 9. Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, et al. Clinical Implications of a Multiparametric Magnetic Resonance Imaging Based Nomogram Applied to Prostate Cancer Active Surveillance. J Urol. 2015. January 26. pii: S0022-5347(15)00178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015. March 5. pii: S1078-1439(15)00057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullins JK, Bonekamp D, Landis P, Begum H, Partin AW, Epstein JI, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013. June;111(7):1037 10.1111/j.1464-410X.2012.11641.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shukla-Dave A, Hricak H, Akin O, Yu C, Zakian KL, Udo K, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012. May;109(9):1315 10.1111/j.1464-410X.2011.10612.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barentsz JO1, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012. April;22(4):746 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bangma CH, Bul M, Roobol M. The Prostate cancer Research International: Active Surveillance study. Curr Opin Urol. 2012. May;22(3):216 10.1097/MOU.0b013e328351dcc7 [DOI] [PubMed] [Google Scholar]
- 15. van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol. 2011;24(1):16 10.1038/modpathol.2010.156 [DOI] [PubMed] [Google Scholar]
- 16. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012.Eur Radiol 2012;22:746e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Portalez D, Mozer P, Cornud F, Renard-Penna R, Misrai V, Thoulouzan M,et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol. 2012;62(6):986 10.1016/j.eururo.2012.06.044 [DOI] [PubMed] [Google Scholar]
- 18. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making; 26(6):565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McVey GP, McPhail S, Fowler S, McIntosh G, Gillatt D, Parker CC Initial management of low-risk localized prostate cancer in the UK: analysis of the British Association of Urological Surgeons Cancer Registry. BJU Int. 2010;106(8):1161 10.1111/j.1464-410X.2010.09288.x [DOI] [PubMed] [Google Scholar]
- 20. Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203 10.1056/NEJMoa1113162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selvadurai ED, Singhera M, Thomas K, Mohammed K, Woode-Amissah R, Horwich A, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64(6):981 10.1016/j.eururo.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 22. Tosoian JJ, Loeb S, Feng Z, Isharwal S, Landis P, Elliot DJ et al. Association of [–2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol. 2012;188(4):1131 10.1016/j.juro.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isharwal S, Makarov DV, Sokoll LJ, Landis P, Marlow C, Epstein JI, et al. ProPSA and diagnostic biopsy tissue DNA content combination improves accuracy to predict need for prostate cancer treatment among men enrolled in an active surveillance program. Urology. 2011;77(3):763e1. 10.1016/j.urology.2010.07.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ploussard G, Xylinas E, Salomon L, Allory Y, Vordos D, Hoznek A, et al. The role of biopsy core number in selecting prostate cancer patients for active surveillance. Eur Urol. 2009;56(6):891 10.1016/j.eururo.2009.07.053 [DOI] [PubMed] [Google Scholar]
- 25. Ploussard G, de la Taille A, Terry S, Allory Y, Ouzaïd I, Vacherot F, et al. Detailed biopsy pathologic features as predictive factors for initial reclassification in prostate cancer patients eligible for active surveillance. Urol Oncol. 2013;31(7):1060 10.1016/j.urolonc.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 26. Kuru TH, Roethke MC, Rieker P, Roth W, Fenchel M, Hohenfellner M, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int. 2013;112(8):1080 10.1111/bju.12259 [DOI] [PubMed] [Google Scholar]
- 27. Vargas HA1, Akin O, Afaq A, Goldman D, Zheng J, Moskowitz CS, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188(5):1732 10.1016/j.juro.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdi H, Pourmalek F, Zargar H, Walshe T, Harris AC, Chang SD, et al. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology. 2015; 85(2):423 10.1016/j.urology.2014.09.060 [DOI] [PubMed] [Google Scholar]
- 29. Kayat Bittencourt L, Litjens G, Hulsbergen-van de Kaa CA, Turkbey B, Gasparetto EL, Barentsz JO, et al. Prostate Cancer: The European Society of Urogenital Radiology Prostate Imaging Reporting and Data System Criteria for Predicting Extraprostatic Extension by Using 3-T Multiparametric MR Imaging. Radiology. 2015. April 3:141412. [DOI] [PubMed] [Google Scholar]
- 30. Lee DH, Koo KC, Lee SH, Rha KH, Choi YD, Hong SJ et al. Tumor lesion diameter on diffusion weighted magnetic resonance imaging could help predict insignificant prostate cancer in patients eligible for active surveillance: preliminary analysis. J Urol. 2013. October;190(4):1213–7. 10.1016/j.juro.2013.03.127 [DOI] [PubMed] [Google Scholar]
- 31. Guzzo TJ, Resnick MJ, Canter DJ, Bivalacqua TJ, Rosen MA, Bergey MR et al. Endorectal T2-weighted MRI does not differentiate between favorable and adverse pathologic features in men with prostate cancer who would qualify for active surveillance. Urol Oncol. 2012. May-Jun;30(3):301–5. 10.1016/j.urolonc.2010.08.023 [DOI] [PubMed] [Google Scholar]
- 32. Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013. July;268(1):144–52. 10.1148/radiol.13121325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ploussard G, Xylinas E, Durand X, Ouzaïd I, Allory Y, Bouanane M et al. Magnetic resonance imaging does not improve the prediction of misclassification of prostate cancer patients eligible for active surveillance when the most stringent selection criteria are based on the saturation biopsy scheme. BJU Int. 2011. August;108(4):513–7. 10.1111/j.1464-410X.2010.09974.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.