Pain is the most important osteoarthritis (OA) symptom; however, it is poorly understood and markers of disease severity cannot explain pain variability. With no cure for OA, the authors recognized the need to identify modifiable factors to decrease pain and increase physical function. This study examined factors that characterize OA patients experiencing different levels of pain and investigated the relationships among these factors and OA pain.
Keywords: Exercise, Knee osteoarthritis, Medication, Pain, Supplements, WOMAC
Abstract
BACKGROUND:
With no cure or effective treatments for osteoarthritis (OA), the need to identify modifiable factors to decrease pain and increase physical function is well recognized.
OBJECTIVE:
To examine factors that characterize OA patients at different levels of pain, and to investigate the relationships among these factors and pain.
METHODS:
Details of OA characteristics and lifestyle factors were collected from interviews with healthy adults with knee OA (n=197). The Western Ontario and McMaster Universities Osteoarthritis Index was used to assess pain. Factors were summarized across three pain score categories, and χ2 and Kruskal-Wallis tests were used to examine differences. Multiple linear regression analysis using a stepwise selection procedure was used to examine associations between lifestyle factors and pain.
RESULTS:
Multiple linear regression analysis indicated that pain was significantly higher with the use of OA medications and higher body mass index category, and significantly lower with the use of supplements and meeting physical activity guidelines (≥150 min/week). Stiffness and physical function scores, bilateral knee OA, body mass index category and OA medication use were significantly higher with increasing pain, whereas self-reported health, servings of fruit, supplement use and meeting physical activity guidelines significantly lower. No significant differences across pain categories were found for sex, age, number of diseases, duration of OA, ever smoked, alcoholic drinks/week, over-the-counter pain medication use, OA supplement use, physical therapy use, servings of vegetables or minutes walked/week.
CONCLUSIONS:
Healthy weight maintenance, exercise for at least 150 min/week and appropriate use of medications and supplements represent important modifiable factors related to lower knee OA pain.
Abstract
HISTORIQUE :
Puisqu’il n’existe ni traitement curatif ni traitement efficace de l’arthrose, il est bien établi qu’il faut déterminer les facteurs modifiables pour atténuer la douleur et accroître la fonction physique.
OBJECTIF :
Examiner les facteurs qui caractérisent les patients atteints d’arthrose à différents niveaux de douleur et étudier le lien entre ces facteurs et la douleur.
MÉTHODOLOGIE :
Les chercheurs ont colligé les caractéristiques détaillées de l’arthrose et les facteurs liés au mode de vie s’y rapportant à partir d’entrevues auprès d’adultes en santé atteints d’arthrose du genou (n=197). Ils ont utilisé l’indice d’arthrose des universités Western Ontario et McMaster pour évaluer la douleur. Ils ont résumé les facteurs dans trois catégories de scores de la douleur et utilisé le test du chi carré et le test de Kruskal-Wallis pour examiner les différences. Ils se sont servis de l’analyse de régression linéaire multiple au moyen d’une sélection graduelle pour évaluer les associations entre les facteurs liés au mode de vie et la douleur.
RÉSULTATS :
L’analyse de régression multiple indiquait que la douleur était beaucoup plus importante avec l’utilisation de médicaments contre l’arthrose et un indice de masse corporelle plus élevé et beaucoup plus faible avec l’utilisation de suppléments et le respect des directives en matière d’activité physique (≥150 minutes par semaine). La raideur et les scores de fonction physique, l’arthrose bilatérale du genou, la catégorie d’indice de masse corporelle et l’utilisation de médicaments contre l’arthrose étaient beaucoup plus élevés entre les catégories de douleur (avec l’augmentation de la douleur), tandis que la santé autodéclarée, les portions de fruits, l’utilisation de suppléments et le respect des directives en matière d’activité physique étaient beaucoup plus faibles. Les chercheurs n’ont constaté aucune différence importante entre les catégories de douleur en fonction du sexe, de l’âge, du nombre de maladies, de la durée de l’arthrose, du fait d’avoir déjà fumé, du nombre de consommations d’alcool par semaine, de l’utilisation de médicaments en vente libre, de l’utilisation de suppléments contre l’arthrose, du recours à la physiothérapie, des portions de légumes ou du nombre de minutes de marche par semaine.
CONCLUSIONS :
Le maintien d’un poids santé, l’exercice pendant au moins 150 minutes par semaine et le bon usage des médicaments et des suppléments représentent des facteurs modifiables importants liés à la diminution de la douleur causée par l’arthrose du genou.
Knee osteoarthritis (OA) is the most common cause of knee pain and lower limb disability in older adults (1). Approximately 4.4 million Canadians have OA; due to increased longevity, reduced physical activity and increased obesity, this number is expected to increase to 10 million within 30 years (2). Pain is the most important OA symptom among affected individuals and contributes to disability, fatigue and decreased quality of life (3,4). Pain is also what drives health care use in OA, with direct and indirect health care costs of $27 billion in Canada in 2010 (2). In general, pain is poorly understood and, in OA specifically, much of its variability cannot be explained by markers of disease severity (5–7). Many modifiable factors, including psychological and physical health, social support, coping behaviours and self-efficacy, have been found to influence OA pain (8).
With no cure available, management of OA focuses on analgesia and maintenance of physical function (9). International evidence-based guidelines suggest that OA management begin with nonpharmacological options, including weight loss or maintenance, moderate exercise and physical activity, physical therapies and assistive devices, followed by pharmacological interventions, starting with over-the-counter (OTC) pain medications such as acetaminophen (10–12). However, nonpharmacological approaches are infrequently recommended by clinicians and used by patients (9,13). Individuals with OA also tend to use pain medications incorrectly, and many live in pain and seek to manage it by limiting activities and movement (14,15). With the lack of successful treatment options, expected rise in OA cases and the increasing burden on the health care system, there is significant need to revisit OA management strategies and to focus on modifiable lifestyle changes to decrease pain and increase or maintain physical function (9,16). Modifiable factors that may affect pain in OA include oral management techniques (eg, OTC pain medications, supplements), use of physical therapies (eg, physiotherapy, chiropractor), assistive devices (eg, walking aids, braces), home treatment methods (eg, heat, ice), exercise and diet.
The most important modifiable risk factor for knee OA is body weight. Excess body weight is the strongest and most consistent risk factor for the onset and progression of knee OA (9,17–19). The excessive loads to the knee, inflammation and associated inactivity among overweight and obese individuals contributes to knee OA pathogenesis and pain (9,20).
With the exception of diet-induced weight loss, the literature examining the effect of diet on knee OA is mixed. Epidemiological studies have shown an increased risk for disease progression with low intake of antioxidant micronutrients (ie, vitamin C, vitamin E, beta-carotene), while most intervention studies report no significant effects of these micronutrients on the progression of knee OA (21–26). It is possible that antioxidant vitamins only exert an influence on knee OA when they are in their original food matrix; however, the association of fruit and vegetable intake with OA has not been investigated.
The important role of exercise and physical activity in the development and management of knee OA is recognized, but it is complex and not well understood. OA clinical guidelines also recognize regular physical activity as pivotal in OA management (9,10). A modest amount of low-impact exercise can strengthen the muscular support around the joints, help to avoid obesity, increase mood and psychological health, and help to preserve function and delay disability (3,8). Exercise intervention studies consistently show that exercise decreases pain and improves function in adults with OA (27–30). However, no association between physical activity level and knee pain was observed in a large prospective cohort study (31).
Although OA pain is poorly understood, it is the leading complaint by individuals with OA and is influenced by many factors. The objective of the present study was to examine factors that characterize OA patients at different levels of pain, and to investigate the relationships among those factors and OA pain.
METHODS
The present study is based on cross-sectional data collected as part of a screening interview for a nutrition intervention clinical trial (n=157) (32), with additional interviews (n=40) completed to increase the sample size to a total of 200 participants, based on previous cross-sectional analyses (33–37).
For the intervention and additional interviews, men and women ≥18 years of age with self-reported physician-diagnosed knee OA were recruited from the Guelph area using posters and newspaper advertisements. Exclusion criteria were: other systemic or rheumatic arthritis; concomitant inflammatory processes; upcoming knee replacement surgery; clinically significant, uncontrolled cardiovascular, hepatic or renal disorders; and any serious medical condition within six months such as myocardial infarction, stroke, cancer or diabetes. The study was conducted at the Human Nutraceutical Research Unit at the University of Guelph (Guelph, Ontario) and was approved by the University of Guelph Human Research Ethics Board (REB#13OC002); all participants provided written consent.
Study procedures
Trained study coordinators conducted the interviews, which included a questionnaire developed for the study with health history and lifestyle questions regarding OA diagnosis, general health, smoking history, alcohol consumption, body weight, height, medication and supplement intake, comorbidities, exercise habits and use of physical therapies. Participants were asked how many minutes per week they participated in weight training/resistance exercise, aerobic exercise, aquatic exercise or other types of exercise (eg, walking, sport participation, mixed fitness classes). Body mass index (BMI) was calculated from self-reported height and body weight and categorized into a BMI category. Participants were asked to bring all medications and supplements to the interview, which were grouped into medications (any prescription and OTC), medications for OA (prescriptions and OTC medications taken specifically for OA pain), prescription medications for OA and OTC pain medications. Supplements were grouped as OA specific and non-OA specific. Participants were considered to use physical therapy if they currently visited a practitioner. The time spent performing different types of exercise was summed to a total number of minutes of exercise per week, and the time spent walking was summed to a total number of walking minutes per week. A participant was considered meeting physical activity guidelines when their total number of minutes of exercise per week met or exceeded 150 min (38).
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) has three subscales to evaluate pain, stiffness and physical function associated with OA (39). The 100 mm visual analogue scale version of the questionnaire was completed in the presence of a study coordinator and scored according to the WOMAC Osteoarthritis Index User Guide IX (40). If a participant had OA in both knees, they were instructed to choose the more painful knee as the signal joint. Higher scores indicate greater pain and stiffness, and worse physical function.
A 24 h dietary recall was conducted by a trained study coordinator and involved leading the participant through a series of questions regarding all food and beverage intake in the previous 24 h. Detailed questions were asked during the recall to ensure specific amounts, preparation methods, brand names, and complete information about foods and beverages were recorded. All were entered into ESHA Food Processor software version 9.12 (USA). Foods were classified into one of the four main food groups (ie, fruits and vegetables, grain products, milk and alternatives, and meat and alternatives) according to the current Canadian Food Guide to Healthy Eating (41). Fruit and vegetable servings were also calculated and analyzed separately. Foods not included in these groups were classified as ‘other’. Based on previous nutrition research, mixed meals were broken down into their component foods appropriate to the serving sizes. Juice was classified as a fruit or vegetable depending on content (eg, orange versus carrot), and potatoes and French fries were classified as vegetables (42).
Statistics
All analyses were performed using SAS version 9.3 (SAS Inc, USA); P≤0.05 was considered to be statistically significant. Continuous variables are presented as mean ± SD. These data were not normally distributed, as determined by the Shapiro-Wilk test. Dichotomous and categorical variables are presented as n (%).
To examine health history and lifestyle factors at different levels of pain, WOMAC pain scores were grouped into four categories including mild (0 to 125), moderate (126 to 250), severe (251 to 375) and extreme (376 to 500). Although there is no standardized method of dividing WOMAC scores, categories were devised similar to previous work by Blamely et al (37) and Kim et al (43). Only three participants reported a pain score >375 (378, 398 and 442) and were included in the severe category, leaving three pain groups (mild, moderate and severe). Health history and lifestyle factors were summarized for each pain category; differences were examined using χ2 tests for dichotomous and categorical variables, and Kruskal-Wallis tests for continuous variables. Linear regression analysis using continuous WOMAC pain scores was conducted using a stepwise regression to determine the best predictors of pain, setting probability to enter the model at P<0.15. Examination of residual plots revealed that the data were homoscedastic, the relationship was linear and the distribution of the residuals was normal.
RESULTS
Participant characteristics
Participant characteristics are presented in Table 1. There were 197 adults (69% women) with a mean age of 63 years. The mean duration of disease was 98 months; 59% of individuals had OA in both knees. Fifty-one percent of participants reported “good” health and the mean number of diseases or conditions, including OA, was three. The most commonly reported comorbidities were cardiovascular diseases, depression, hypertension, high cholesterol, hyperthyroidism and cancer (data not shown). Eighty-one percent of the participants reported using at least one medication and 69% used at least one medication for OA. A high percentage of participants (88%) took supplements, although only 37% took an OA-specific supplement. The most commonly consumed supplements were vitamin D, multivitamin and minerals, calcium, omega-3/fish oil, glucosamine and vitamin C. The most commonly reported OA-specific supplement taken was a glucosamine product (glucosamine or a combination of glucosamine and chondroitin or methylsulfonylmethane). Only 33% of participants reported current use of physical therapy, with physiotherapy, acupuncture and chiropractic being the most common. Forty-eight percent of participants met the physical activity guidelines of 150 min exercise/week, with walking being the most frequently reported activity. Walking minutes were highly variable (mean 58 min/week, median 40 min/week). Overall, average WOMAC scores revealed a mild to moderate level of pain, stiffness and physical function in the study participants.
TABLE 1.
Summary of participant characteristics (n=197)
| Characteristic | |
|---|---|
| Female sex | 136 (69) |
| Age, years, mean ± SD | 63±10.9 |
| Disease duration, months, mean ± SD | 98.1±95.6 |
| Bilateral OA | 117 (59) |
| Number of diseases, mean ± SD | 3.0±1.5 |
| Self-reported health | |
| Poor | 6 (3) |
| Good | 100 (51) |
| Very good | 79 (40) |
| Excellent | 12 (6) |
| Ever smoked | 94 (48) |
| Number of alcoholic drinks/week, mean ± SD | 3.4±4.5 |
| Medication and supplement use | |
| Medications | 159 (81) |
| Medications for OA | 136 (69) |
| Prescription medications for OA | 53 (27) |
| Over-the-counter pain medication for OA | 116 (59) |
| Supplements | 173 (88) |
| Supplements for OA | 73 (37) |
| Participates in physical therapy | 65 (33) |
| Met physical activity guidelines | 95 (48) |
| Walking minutes, mean ± SD | 58.7±71.6 |
| WOMAC subscale scores, mean ± SD | |
| Pain score (maximum 500) | 159.1±92.9 |
| Stiffness score (maximum 200) | 100.6±50.1 |
| Physical disability score (maximum 1700) | 578.6±347.8 |
Data presented as n (%) unless otherwise indicated. OA Osteoarthritis; WOMAC Western Ontario and McMaster Universities Osteoarthritis Index
Factors by pain categories
Table 2 summarizes the health history and lifestyle factors for participants across the three pain categories. There were no significant differences across the pain categories in terms of distributions of sex, age, number of diseases, duration of OA, ever smoked, number of alcoholic drinks/week, use of OTC pain medication, use of OA supplements, use of physical therapy, servings of vegetables or minutes walked/week.
TABLE 2.
Health history and lifestyle factors across mild, moderate and severe Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain categories
| Mild pain (n=83) | Moderate pain (n=79) | Severe pain (n=35) | P | |
|---|---|---|---|---|
| Age, years, mean ± SD | 64.9±11.8 | 63.0±9.7 | 60.2±10.8 | 0.14 |
| OA duration, months, mean ± SD | 90.1±83.9 | 103.3±106.0 | 105.2±98.3 | 0.86 |
| Number of diseases, mean ± SD | 2.9±1.4 | 3.0±1.6 | 3.2±1.7 | 0.79 |
| WOMAC scores, mean ± SD | ||||
|
| ||||
| Stiffness score | 68.3±45.1 | 117.6±39.9 | 139.3±33.0 | <0.0001 |
| Disability score | 304.4±208.0 | 689.2±249.0 | 977.8±267.0 | <0.0001 |
| Sex | ||||
|
| ||||
| Female | 57 (69) | 53 (67) | 26 (74) | 0.74 |
| Male | 26 (31) | 26 (33) | 9 (26) | |
| Unilateral or bilateral OA | ||||
|
| ||||
| Unilateral | 44 (53) | 27 (34) | 9 (26) | 0.002 |
| Bilateral | 39 (47) | 52 (66) | 26 (74) | |
| BMI category* | ||||
|
| ||||
| Underweight | 1 (1) | 0 (0) | 0 (0) | 0.008 |
| Normal weight | 25 (31) | 12 (16) | 5 (14) | |
| Overweight | 34 (41) | 29 (38) | 8 (23) | |
| Obese | 22 (27) | 36 (46) | 22 (63) | |
| Self-reported health | ||||
|
| ||||
| Poor | 0 (0) | 1 (1) | 5 (14) | 0.001 |
| Good | 40 (48) | 46 (58) | 14 (40) | |
| Very good | 37 (45) | 27 (34) | 15 (43) | |
| Excellent | 6 (7) | 5 (6) | 1 (3) | |
| Smoking history and alcohol consumption | ||||
|
| ||||
| Ever smoke | 36 (44) | 37 (47) | 21 (60) | 0.25 |
| Alcoholic drinks/week, mean ± SD | 3.1±3.8 | 3.8±5.3 | 3.3±3.9 | 0.86 |
| Medication, supplement and alternative therapy use | ||||
|
| ||||
| OA medications | 44 (53) | 59 (75) | 33 (94) | <0.0001 |
| OA prescriptions | 14 (17) | 25 (32) | 15 (43) | 0.009 |
| OTC pain | 43 (52) | 49 (62) | 25 (71) | 0.12 |
| Supplements | 78 (94) | 69 (87) | 26 (74) | 0.01 |
| OA supplements | 37 (45) | 22 (28) | 14 (40) | 0.08 |
| Physical therapy | 28 (34) | 23 (29) | 14 (40) | 0.71 |
| Serving of fruit and vegetables, mean ± SD | ||||
|
| ||||
| Fruits | 2.1±1.5 | 1.6±1.4 | 1.5±1.4 | 0.03 |
| Vegetables | 2.5±1.3 | 2.7±1.4 | 2.4±1.4 | 0.49 |
| Physical activity | ||||
|
| ||||
| Meet guidelines† | 53 (64) | 34 (43) | 8 (23) | 0.0001 |
| Walking minutes, mean ± SD | 67.1±78.0 | 59.6±67.4 | 38.6±61.7 | 0.09 |
Data presented as n (%) unless otherwise specified.
Underweight, body mass index (BMI) <18.5 kg/m2; normal weight, BMI 18.5–24.9 kg/m2; overweight, BMI 25.0–29.9 kg/m2; obese, BMI >30.0 kg/m2);
Physical activity guidelines of ≥150 min/week of exercise (Public Health Agency of Canada, 2012 [38]). OA Osteoarthritis; OTC Over-the-counter
WOMAC stiffness and physical function scores were different across the pain categories, and increased with increasing WOMAC pain score (P<0.0001).
Having OA in only one knee was most common in the mild pain category (53%) (P=0.008) and this proportion decreased across pain categories. Having OA in both knees was more common in the moderate (66%) and severe pain (74%) categories than the mild pain category (47%) (P=0.008).
The proportion of participants in each BMI category differed across pain categories (P=0.008). In the mild pain category, the majority of participants were in the normal weight (31%) and overweight (41%) categories, and the percent of normal weight participants was lower in the moderate (16%) and the severe (14%) pain category. The percent of participants in the obese category was higher with increasing pain level (Figure 1A).
Figure 1).
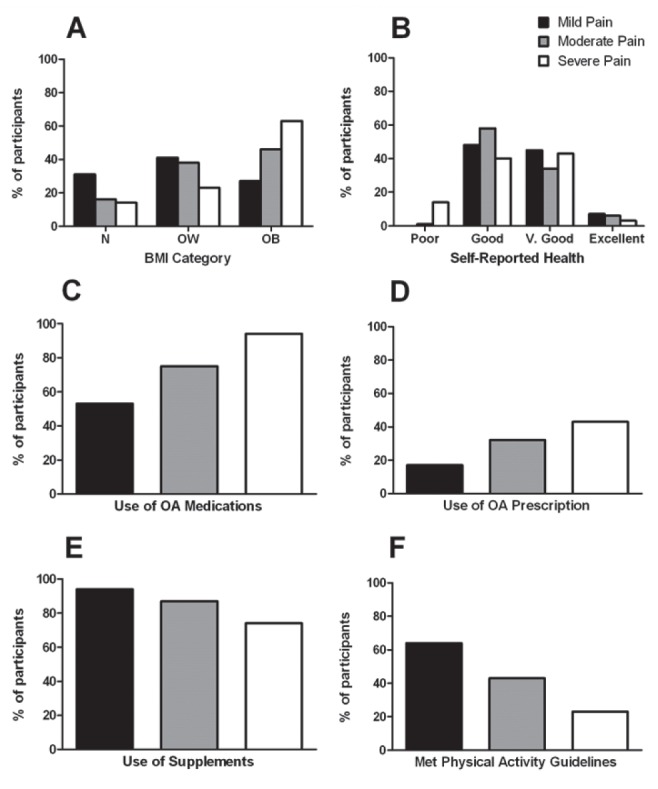
χ2 analysis revealed significant differences in the percent of participants in the mild pain (0 to 125), moderate pain (126 to 250) and severe pain (251 to 500) categories in terms of body mass index (BMI) category (normal weight [N], overweight [OW] or obese [OB]) (A), self-reported health (B), use of medications for osteoarthritis (OA) (C), use of prescription medications for OA (D), use of supplements (E) and met physical activity guidelines (F). V Very
Self-reported health categories were also different among pain categories, with fewer participants reporting poor health in the mild (0%) and moderate (1%) versus severe (14%) pain categories, and more participants reporting very good health in the mild (45%) versus moderate (34%) pain categories (P=0.001) (Figure 1B).
Servings of fruit were highest in the mild pain category (2.1±1.5), which was significantly higher than the moderate (1.6±1.4) and severe (1.5±1.4) pain categories (P=0.03).
The proportion of participants who used a medication for OA increased across pain categories (P<0.001) (Figure 1C), with a similar pattern observed for prescription medications (P=0.009) (Figure 1D). The opposite trend was observed with supplements, with participants in the mild pain category reporting the highest proportion of supplement use and decreasing supplement use across pain categories (P=0.003) (Figure 1E).
Participating in exercise for ≥150 min/week was most common in the mild pain category (64%); this percentage decreased as the pain category increased (P=0.0001) (Figure 1F).
Regression analysis
Multiple linear regression using a stepwise selection procedure revealed that BMI category, use of supplements, use of OA medications and meeting physical activity guidelines was the best combination of factors to explain the variation in pain score, with the model accounting for 28.4% (P<0.0001) (Table 3). The model revealed that pain score increased with use of OA medications and increasing BMI category, and decreased with use of supplements and meeting physical activity guidelines. Factors that were not included in the model included age, sex, duration of OA, ever smoked, number of alcoholic drinks/week, number of diseases, use of OA supplements, use of OA prescriptions, use of OTC pain, use of physical therapy, fruit consumption, vegetable consumption and walking min/week.
TABLE 3.
Stepwise multiple regression examining factors related to pain score in adults with knee osteoarthritis
| Variable | Regression coefficient | SE | P |
|---|---|---|---|
| Body mass index category | 19.7 | 7.6 | 0.02 |
| Use of osteoarthritis medications | 60.1 | 13.1 | <0.001 |
| Meet physical activity guidelines | −33.7 | 12.4 | 0.007 |
| Use of supplements | −35.3 | 17.8 | 0.05 |
| Self-reported health | −16.4 | 9.1 | 0.07 |
| Use of medications | 26.4 | 15.0 | 0.08 |
| Bilateral knee osteoarthritis | 11.8 | 7.7 | 0.13 |
P<0.15 was used as cut-off for inclusion in the model
DISCUSSION
The present study examined modifiable lifestyle factors and their associations with OA pain in a sample of adults with knee OA. Sixty-nine percent of participants in the present study were women, with a mean age of 63 years and the majority of BMIs in the overweight and obese categories. This sex distribution, age and overweight status is reflective of Canadian OA patients (2). The majority of participants had mild (WOMAC pain score 0 to 125; n=83) or moderate (WOMAC pain score 125 to 250; n=79) pain, providing an opportunity to characterize modifiable lifestyle factors in adults with relatively low OA knee pain. Several lifestyle factors (stiffness score, physical function score, bilateral knee OA, BMI category, OA medication use, self-reported health, servings of fruit, supplement use and meeting physical activity guidelines) were significantly different among pain categories. Additionally, linear regression analysis revealed that BMI, meeting physical activity guidelines, OA medication use and supplement use accounted for 28.4% of the variance in pain score. The unexplained variability is potentially attributable to structural, psychological and socioeconomic factors not measured in the present study.
Use of medications was reported by 81% of participants, which may reflect that participants also reported an average of three diseases or conditions. Use of OA medications was a significant predictor for higher pain score and, although not included in the regression model, the use of any medication and OA prescription significantly increased as pain category increased. Use of OA medications has previously been associated with higher levels of pain (44,45), which could indicate that only individuals with high pain levels take medications, that current OA pain medications are not effective at relieving pain or that adults with OA are not using medications correctly. It has been found that individuals with OA use pain medications at lower doses and less frequently than recommended (14,46,47). Pharmaceutical pain management is often seen by OA sufferers as undesirable and a ‘last resort’, due to concerns about side effects, addiction, tolerance, and general dislike for taking pills (14,46). Furthermore, patient knowledge and beliefs about self-management with medication is poor (47,48). OA patients may benefit from education on the correct use of OA medications.
Use of supplements was significant in the regression model for pain, and 94% of individuals in the mild pain category reported using a supplement. Overall, supplement use was high in the present study (88%), which may have been influenced by the fact that screening data from a nutrition intervention study was used and interviews were conducted in a nutrition research unit. Estimates of supplement use in adults with OA vary from 34% to 90% based on geography, population and the definition of supplement (44). The most commonly consumed supplements in the present study were vitamin D, multivitamin, calcium, omega-3/ fish oil, glucosamine products and vitamin C. As discussed previously, research investigating the effects of vitamin intake on OA is mixed. It has been suggested that in individuals with vitamin deficiencies, supplements may be beneficial in preventing OA progression (49). In addition, higher dietary intakes of vitamin C and beta-carotene were associated with lower OA pain in adults with knee OA (21). The same trends of lower OA pain were observed with serum levels of vitamin D (50) and with supplementation with vitamin E (51). Vitamin D may reduce inflammatory pain through regulation of cytokines and macrophage activity, although more studies are warranted (52). Intervention studies with omega-3 rich oils in adults with OA have demonstrated significantly reduced pain (53,54), ostensibly based on anti-inflammatory mechanisms. Although not investigated in the present study, it is possible that individuals with higher pain may have taken more medications to manage the pain and been less likely to take supplements for fear of interactions or general dislike of taking multiple pills (46,47).
Increasing BMI category was a significant predictor of higher pain scores in the present study. The association of BMI with pain is well established (17,55,56) and reducing body weight has been related to dose-dependent decreases in pain and improvements in physical function (18).
Not meeting physical activity guidelines was a significant predictor of higher pain score and, as pain category increased, the percent of participants meeting physical activity guidelines decreased. Similarly, walking minutes/week decreased as pain category increased, although this was highly variable and not statistically significant (P=0.09). In exercise intervention studies, pain decreases with increased activity levels (27–30); however, the monitored exercise regimes used in interventions are not replicated in everyday life. Indeed, physical activity levels quantified with the Physical Activity Scale for the Elderly questionnaire did not correlate to WOMAC pain scores in a large, prospective cohort study in adults with or at risk for knee OA (31). Participants in the present study were active, with 48% meeting the Canadian physical activity guidelines of ≥150 min/week. Previous reports in adults with OA are highly variable, ranging from 10% (57), to 30% to 40% (58–60) and as high as 55.9% (61). Different assessment techniques could explain this variation, including accelerometers (20,57), questionnaires (56,60) and self-reporting of activities (59,62), as well as different parameters, including distance walked/ week (62), years of sporting participation (56), categories of questionnaire scores (56,60) and percent of participants meeting physical activity guidelines (57,61). Exploring the role of exercise and physical activity in OA management would benefit from standardized methods and outcome measures. It is also possible that individuals with severe pain in the present study did not feel that they could exercise. However, there are reports of individuals with knee OA walking at a high cadence or velocity with low pain levels (27,63). Further, after total knee replacement, improvements in pain are reported, but are not associated with increases in physical activity (64), supporting the argument that pain is not the limiting factor to exercise in knee OA. Similar to the general population, time, effort, scheduling and commitment have been reported as barriers to exercise in individuals with OA (46,63). Exercise is a key modifiable factor to manage pain in OA as well as to decrease risk of other chronic diseases, which were common in the present study (83%). Interestingly, all-cause mortality is higher in adults with OA versus without OA and the increased risk for death is predicted by walking disability, highlighting the importance of physical activity and exercise in this population (65).
In the present study, physical function score significantly increased across pain category, indicating worse physical functioning with higher pain. The strong correlation commonly observed between the pain and physical function scales on the WOMAC is suggested by Terwee et al (34) to indicate that self-reported physical function assessments innately measure both pain and exertion, in addition to functioning. In the Short Form-36 questionnaire, pain questions are placed after the function questions, and there is lower correlation observed between scales compared with the WOMAC, where the pain questions are asked before the physical function questions (34). Similarly, Stratford and Kennedy (66) found that correlations between self-reported physical function and performance-based physical function tests were strengthened when patient-rated pain and exertion of the performance-based test was combined into a composite score. In addition, adults with painful knee OA report keeping still and limiting movement as a way to manage pain. This lack of activity could contribute to deconditioning and reduced functioning (14,47). Another factor that significantly increased across pain categories was the percentage of adults with bilateral knee OA. This has previously been associated with higher pain scores, which may reflect the presence of additional tissue degradation and inflammation (66,68).
Daily fruit servings reported by participants were significantly higher in the mild versus moderate and severe pain categories. Of note, regardless of pain category, the number of daily fruit servings (ie, 1.5 to 2.6 servings) was below recommended levels. Fruits are high in antioxidants and polyphenols; fruit consumption has been associated with lower musculoskeletal pain in adults (69), and berries, tart cherries, blueberries and Concord grapes, specifically, have been found to have antinociceptive effects in rats (70). Healthy eating patterns are also associated with better self-reported health, more time in exercise and less depressive symptoms, which have all been associated with lower pain in OA (20). Although the 24 h diet recall is not the most robust method of dietary assessment, increasing fruit consumption and improving diet quality are important modifiable factors that warrant continued investigation for the potential to reduce pain in adults with knee OA.
In the present study, self-reported health status was significantly different across pain categories, although most participants reported good or very good health. Self-reported health has been shown to be reliable in OA populations (71), although associations with pain have not always been consistent. Reichmann et al (72) found that lower income, greater number of comorbidities and greater functional limitations, but not pain, were associated with self-reported health status in adults with knee OA. In contrast, Allen et al (73) found that lower self-reported health was associated with higher pain scores in adults with hip or knee OA. In the present study, 91% of participants reported “good” or “very good” health, which could be related to the fact that 82% of participants were in the mild or moderate pain categories.
Several factors were not significantly different among pain categories or significant in the regression analysis. For example, age showed a nonsignificant protective directional trend, with increasing age being associated with decreased pain, consistent with previous OA research (67). Surprisingly, sex was not a significant factor in the regression model and there was no difference among pain categories. Women frequently report higher pain than men in OA (17,35,74), but no differences in pain have been reported between the sexes in some studies (75,76). There were more women in the severe (74%) compared with the mild and moderate (approximately 68%) categories, and more men in the mild and moderate (approximately 32%) than the severe (26%) categories. There were also no significant differences in duration of OA across pain category. Although Rosemann et al (77) reported that the duration of OA was a predictor of pain, several studies have shown that OA can remain stable for years (78). The number of diseases reported did not differ across pain category, consistent with a previous report (79). However, the relationship between comorbidities in OA and pain is mixed, and examination of specific diseases may reveal that different comorbidities impact OA pain in different ways. For example, strong links between depression and pain have been found in several studies investigating adults with OA (3,77). OA supplement use was not significantly related to pain. Glucosamine products were almost exclusively used in this category, and evidence for its effect on pain is limited due to high variation among studies (10,80). Physical therapy was only used by 33% of adults in the present study and its use did not differ across the pain categories. A systematic review and meta-analysis of physical therapies for OA concluded that only a few physical therapy interventions were effective (81), but it is possible that effects are not seen in intervention studies due to the fact that a true placebo group does not exist for any of the physical therapies.
Limitations of the present cross-sectional study include causality of the factors studied could not be determined, and the data were self-reported. In addition, several factors that have been shown to affect pain were not measured in the present study, including psychological variables, self-efficacy, education level, socioeconomic data and fatigue (3,36,37,82). Selection bias toward adults with less severe OA is also a possibility because participants were required to be mobile enough to travel to the research centre for interviews. However, this gave the opportunity to characterize individuals with lower pain, as some studies investigating factors of OA pain examine patients waiting for knee replacement, thereby representing a much different population and more extreme level of pain than is representative of many people with knee OA (78). Finally, BMI calculations for the present study were based on self-reported heights and weights, which has also been used in previous OA research (55,60,73,83). Given the potential for inaccuracies, BMI categories were used.
CONCLUSION
Several modifiable factors were associated with OA pain including BMI, meeting physical activity guidelines, OA medication use and supplement use. Having a BMI in the healthy range is associated with lower pain levels, as well as being physically active for at least 150 min/week. Exercise plus a diet high in fruits and vegetables is important in weight maintenance, and increasing fruit consumption could be important in this study population who are consuming low levels. Supplement use was associated with lower pain, and use of OA medication was associated with higher pain. Patients should be educated on proper use of medications to manage pain in OA. Research efforts and clinical practice should continue to focus on modifiable lifestyle changes to decrease pain in OA.
Acknowledgments
The authors thank all the study participants, as well as Natasha Sheikh, Svitlana Yurchenko, Kate Faughnan and Bonnie Huang for their assistance in data collection.
Footnotes
GRANT: The research was sponsored by the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA #200121).
REFERENCES
- 1.Jordan KP, Wilkie R, Muller S, Myers H, Nicholls E. Measurement of change in function and disability in osteoarthritis: Current approaches and future challenges; Arthritis Research Campaign National Primary Care Centre. Curr Opin Rheumatol. 2009;21:525–30. doi: 10.1097/BOR.0b013e32832e45fc. [DOI] [PubMed] [Google Scholar]
- 2.Bombardier C, Hawker G, Mosher D. The impact of arthritis in Canada: Today and over the next 30 years. Arthritis Alliance of Canada. 2012 [Google Scholar]
- 3.Hawker GA, Gignac MAM, Badley E, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthrit Care Res. 2011;63:1382–90. doi: 10.1002/acr.20298. [DOI] [PubMed] [Google Scholar]
- 4.Briggs A, Scott E, Steele K. Impact of osteoarthritis and analgesic treatment on quality of life of an elderly population. Ann Pharmacother. 1999;33:1154–9. doi: 10.1345/aph.18411. [DOI] [PubMed] [Google Scholar]
- 5.Van Spill WE, DeGrootz J, Lemsx WF, Oostveenk JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip osteoarthritis: A systematic review applying the consensus BIPED criteria. Osteoarthr Cartilage. 2010;18:605–12. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskeletal Disorders. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson DT. The current and future status of biomarkers in osteoarthritis. J Rheumatol. 2014;41:834–6. doi: 10.3899/jrheum.140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawker G. The challenge of pain for patients with OA. HSSJ. 2012;8:42–44. doi: 10.1007/s11420-011-9254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter D. Focusing osteoarthritis management on modifiable risk factors and future therapeutic prospects. Ther Adv Musculoskel Dis. 2009;1:35–47. doi: 10.1177/1759720X09342132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartilage. 2014;22:363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. European League Against Rheumatism (EULAR) Ann Rheum Dis. 2013;72:1125–35. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan A, Mather RC, III, Karas V, et al. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthroscopy. 2014;30:65–71. doi: 10.1016/j.arthro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sale JE, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2006;55:272–8. doi: 10.1002/art.21853. [DOI] [PubMed] [Google Scholar]
- 15.Merkle D, McDonald DD. Use of recommended osteoarthritis pain treatment by older adults. J Adv Nurs. 2009;65:828–35. doi: 10.1111/j.1365-2648.2008.04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkiewic A, Petersson IF, Bjork J, et al. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr Cartilage. 2014;22:1826–32. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Elbaz A, Debbi EM, Segal G, et al. Sex and body mass index correlate with Western Ontario and McMaster Universities Osteoarthritis Index and quality of life scores in knee osteoarthritis. Arch Phys Med Rehabil. 2011;92:1618–23. doi: 10.1016/j.apmr.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: A cohort study. Arthritis Care Res (Hoboken) 2013;65:15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Song J, Hootman JM, et al. Obesity and other modifiable factors for physical inactivity measured by accelerometer in adults with knee osteoarthritis. Arthrit Care Res. 2013;65:53–61. doi: 10.1002/acr.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;30:648–56. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 22.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin d to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 23.McAlindon TE, Felson DT. Nutrition: Risk factors for osteoarthritis. Ann Rheum Dis. 1997;56:397–407. doi: 10.1136/ard.56.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand C, Snaddon J, Bailey M, Cicuttini FM. Vitamin E is ineffective for symptomatic relief of knee osteoarthritis: A six month double blind, randomized, placebo controlled study. Ann Rheum Dis. 2001;60:946–9. doi: 10.1136/ard.60.10.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wluka AE, Stuckey S, Brand C, et al. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: A 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29:2585–91. [PubMed] [Google Scholar]
- 26.McAlindon TE, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA. 2013;309:155–62. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 29.Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: A randomized controlled trial. J Rheumatol. 2001;8:1655–65. [PubMed] [Google Scholar]
- 30.Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis. 2005;64:544–8. doi: 10.1136/ard.2004.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansournia MA, Danaei G, Forouzanfar MH, et al. Effect of physical activity on functional performance and knee pain in patients with osteoarthritis: Analysis with marginal structural models. Epidemiology. 2012;23:631–40. doi: 10.1097/EDE.0b013e31824cc1c3. [DOI] [PubMed] [Google Scholar]
- 32.Connelly AE, Tucker AJ, Tulk H, et al. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J Med Food. 2014;17:1361–7. doi: 10.1089/jmf.2013.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goncalves RS, Pinheirp JP, Cabri J. Evaluation of potentially modifable physical factors as predictors of health status in knee osteoarthritis patients referred for physical therapy. Knee. 2012;19:373–9. doi: 10.1016/j.knee.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Terwee CB, van der Slikke RM, van Lummel RC, Benink RJ, Meijers WG, de Vet HC. Self-reported physical functioning was more influenced by pain than performance-based physical functioning in knee-osteoarthritis patients. J Clin Epidemiol. 2006;59:724–31. doi: 10.1016/j.jclinepi.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Tonelli SM, Rakel BM, Cooper NA, Angstom WL, Sluka KA. Women with knee osteoarthritis have more pain and poorer function than men, but similar physical activity prior to total knee replacement. Biol Sex Differ. 2011;2:12. doi: 10.1186/2042-6410-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz-Almedia Y, King CD, Goodin BR, et al. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:1786–94. doi: 10.1002/acr.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blamely R, Jolly K, Greenfield S, Jobanputra P. Patterns of analgesic use, pain and self-efficacy: A cross-sectional study of patients attending a hospital rheumatology clinic. BMC Musculoskel Dis. 2009;10:137. doi: 10.1186/1471-2474-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Public Health Agency of Canada, Physical Activity Guidelines < http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/pa-ap/03paap-eng.php> (Accessed November 15, 2014).
- 39.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 40.Bellamy N. WOMAC osteoarthritis index: User guide X. 10th edn. Queensland, Australia: 2011. [Google Scholar]
- 41.Health Canada Eating Well with Canada’s Food Guide. < www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/food-guide-aliment/view_eatwell_vue_bienmang-eng.pdf> (Accessed November 15, 2014).
- 42.Attorp A, Scott JE, Yew AC, Rhones RE, Barr SI, Naylor J. Associations between socioeconomic, parental and home environment factors and fruit and vegetable consumption of children in grades five and six in British Columbia, Canada. BMC Public Health. 2014;14:150. doi: 10.1186/1471-2458-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim IJ, Kim DH, Jung JY, et al. Association between bone marrow lesions detected by magnetic resonance imaging and knee pain in community residents in Korea. Osteoarthr Cartilage. 2013:1207–13. doi: 10.1016/j.joca.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Lapane KL, Sands MR, Yang S, McAlindon TE, Eaton CB. Use of complementary and alternative medicine among patients with radiographic-confirmed knee osteoarthritis. Osteoarthr Cartilage. 2012;20:22–8. doi: 10.1016/j.joca.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher JE, Ballantyne PJ, Hawker GA. Older adults living with osteoarthritis: Examining the relationship of age and gender to medicine use. Can J Aging. 2012;31:323–33. doi: 10.1017/S0714980812000256. [DOI] [PubMed] [Google Scholar]
- 46.Ross MM, Carswell A, Hing M, Hollingworth G, Dalziel WB. Seniors’ decision making about pain management. J Adv Nurs. 2001;35:442–51. doi: 10.1046/j.1365-2648.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 47.Davis GC, Hiemenz ML, White TL. Barriers to managing chronic pain of older adults with arthritis. 2002. J Nurs Scholarship. 2002;2:121–6. doi: 10.1111/j.1547-5069.2002.00121.x. [DOI] [PubMed] [Google Scholar]
- 48.Hill J, Bird H. Patient knowledge and misconceptions of osteoarthritis assessed by a validated self-completed knowledge questionnaire (PKQ-OA) Rheumatology. 2007;46:796–800. doi: 10.1093/rheumatology/kel407. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Prentice LF, Vitetta L, Wluka AE, Cicuttini FM. The effect of nutritional supplements on osteoarthritis. Altern Med Rev. 2004;9:275–96. [PubMed] [Google Scholar]
- 50.Muraki S, Dennison E, Jameson K, et al. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthr Cartilage. 2011;19:1301–6. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya I, Saxena R, Gupta V. Efficacy of vitamin E in knee osteoarthritis management of North Indian geriatric population. Ther Adv Musculoskelet Dis. 2012;4:11–9. doi: 10.1177/1759720X11424458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: A systematic review. Rheumatology. 2013;52:1323–34. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 53.Zawadzi M, Janosch C, Szechinski J. Perna canaliculus lipid complex PCSO-524TM demonstrated pain relief for osteoarthritis patients benchmarked against fish oil, a randomized trial, without placebo control. Drugs. 2013;11:1920–35. doi: 10.3390/md11061920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deutsch L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr. 2007;26:39–48. doi: 10.1080/07315724.2007.10719584. [DOI] [PubMed] [Google Scholar]
- 55.Goulston LM, Kiran A, Javaid MK, et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res (Hoboken) 2011;63:1398–406. doi: 10.1002/acr.20546. [DOI] [PubMed] [Google Scholar]
- 56.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 57.Dunlop DD, Song J, Semanik PA, et al. Objective physical activity measurement in the osteoarthritis initiative: Are guidelines being met? Arthritis Rheum. 2011;63:3372–82. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farr JN, Going SB, Lohman TG, et al. Physical activity levels in patients with early knee osteoarthritis measured by accelerometry. Arthritis Rheum. 2008;15:1229–36. doi: 10.1002/art.24007. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holden MA, Nicholls EE, Young J, Hay EM, Foster NE. Exercise and physical activity in older adults with knee pain: A mixed methods study. Rheumatology. 2015;54:413–23. doi: 10.1093/rheumatology/keu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fontaine KR, Heo M, Bathon J. Are US adults with arthritis meeting public health recommendations for physical activity? Arthritis Rheum. 2004;50:624–8. doi: 10.1002/art.20057. [DOI] [PubMed] [Google Scholar]
- 61.Barbour KE, Hootman JM, Helmick CG, et al. Meeting physical activity guidelines and the risk of incident knee osteoarthritis: A population-based prospective cohort study. Arthritis Care Res (Hoboken) 2014;66:139–46. doi: 10.1002/acr.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felson DT, Niu J, Clancy M, Sack B, Alibadi P, Zhnag Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: The Framingham Study. Arthritis Rheum. 2007;57:6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 63.White DK, Tudor-Locke C, Felson DT, et al. Do radiographic disease and pain account for why people with or at high risk of knee osteoarthritis do not meet physical activity guidelines? Arthritis Rheum. 2013;65:139–47. doi: 10.1002/art.37748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res. 2014;5:1502–11. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ. 2011;8:342. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stratford PW, Kennedy DM. Performance measures were necessary to obtain a complete picture of osteoarthritic patients. J Clin Epidemiol. 2006;59:160–7. doi: 10.1016/j.jclinepi.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Schiphof D, Kerkhof HJ, Damen J, et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:695–702. doi: 10.1002/acr.21886. [DOI] [PubMed] [Google Scholar]
- 68.Adegoke BO, Babatunde FO, Oyeyemo AL. Pain, balance, self-reported function and physical function in individuals with knee osteoarthritis. Physiother Theory Pract. 2012;1:32–40. doi: 10.3109/09593985.2011.570858. [DOI] [PubMed] [Google Scholar]
- 69.Høstmark AT, Haug A, Holmboe-Ottesen Musculoskeletal pain as related to some diet items and fatty acids in the cross-sectional Oslo Health Study. J Musculoskelet Pain. 2014;22:365–72. [Google Scholar]
- 70.Jensen GS, Ager DM, Redman KA, Mitzner MA, Benson KF, Schauss AG. Pain reduction and improvement in range of motion after daily consumption of an Acai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J Med Food. 2011;14:702–11. doi: 10.1089/jmf.2010.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: The Framingham Study. Am J Med. 1999;106:151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 72.Reichmann WM, Katz JN, Kessler CL, Jordan JM, Losina E. Determinants of self-reported health status in a population-based sample of persons with radiographic knee osteoarthritis. Arthritis Rheum. 2009;61:1046–53. doi: 10.1002/art.24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: Potential explanatory factors. Osteoarthr Cartilage. 2010;18:160–7. doi: 10.1016/j.joca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Glass N, Segal NA, Sluka KA, et al. Examining sex differences in knee pain: The Multicenter Osteoarthritis Study. Osteoarthr Cartilage. 2014;22:1100–6. doi: 10.1016/j.joca.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Debi R, Mor A, Segal O, et al. Differences in gait patterns, pain, function and quality of life between males and females with knee osteoarthritis: A clinical trial. BMC Musculoskelet Disord. 2009;13:127. doi: 10.1186/1471-2474-10-127. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–9. [PubMed] [Google Scholar]
- 77.Rosemann T, Laux G, Szecenyi J. Osteoarthritis: Quality of life, comorbidities, medication, and health service utilization assessed in a large sample of primary care patients. J Orthop Surg Res. 2007;2:12. doi: 10.1186/1749-799X-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 79.Leite AA, Costa AJ, Lima Bde A, Padilha AV, Albuquerque EC, Marques CD. Comorbidities in patients with osteoarthritis: Frequency and impact on pain and physical function. Rev Bras Reumatol. 2011;51:118–23. [PubMed] [Google Scholar]
- 80.Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: Why do trial results differ? Arthritis Rheum. 2007;56:2267–77. doi: 10.1002/art.22728. [DOI] [PubMed] [Google Scholar]
- 81.Wang SY, Olson-Kellogg B, Shamliyan TA, Choi JY, Ramakrishnan R, Kane RL. Physical therapy interventions for knee pain secondary to osteoarthritis: A systematic review. Ann Intern Med. 2012;157:632–44. doi: 10.7326/0003-4819-157-9-201211060-00007. [DOI] [PubMed] [Google Scholar]
- 82.Cleveland RJ, Luong MN, Knight JB, et al. Independent associations of socioeconomic factors with disability and pain in adults with knee osteoarthritis. BMC Musculoskelet Disord. 2013;14:297. doi: 10.1186/1471-2474-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thiem U, Lamsfuß R, Gunther S, et al. Prevalence of self-reported pain, joint complaints and knee or hip complaints in adults aged 40 years: A cross-sectional survey in Herne, Germany. PLoS ONE. 2013;8:e60753. doi: 10.1371/journal.pone.0060753. [DOI] [PMC free article] [PubMed] [Google Scholar]


