Abstract
The formation of the pericentriolar matrix (PCM) and a fully functional centrosome in syncytial Drosophila melanogaster embryos requires the rapid transport of Cnn during initiation of the centrosome replication cycle. We show a Cnn and Polo kinase interaction is apparently required during embryogenesis and involves the exon 1A-initiating coding exon, suggesting a subset of Cnn splice variants is regulated by Polo kinase. During PCM formation exon 1A Cnn-Long Form proteins likely bind Polo kinase before phosphorylation by Polo for Cnn transport to the centrosome. Loss of either of these interactions in a portion of the total Cnn protein pool is sufficient to remove native Cnn from the pool, thereby altering the normal localization dynamics of Cnn to the PCM. Additionally, Cnn-Short Form proteins are required for polar body formation, a process known to require Polo kinase after the completion of meiosis. Exon 1A Cnn-LF and Cnn-SF proteins, in conjunction with Polo kinase, are required at the completion of meiosis and for the formation of functional centrosomes during early embryogenesis.
Keywords: Drosophila, centrosomin (cnn), Polo kinase, meiosis, cleavage mitosis
THE animal centrosome is the dominant microtubule-organizing center (MTOC) in cells and is composed of a centrally located pair of centrioles surrounded by the pericentriolar matrix (PCM). In Drosophila melanogaster, Centrosomin (Cnn) is the most abundant protein in the PCM (Lange et al. 2000) and is required for the localization of many other PCM components during mitosis (Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999). The cnn gene is transcriptionally complex, using three promoters with unique initiating coding exons associated with multiple splice variants making up two protein families (Eisman et al. 2009), contrary to its frequent depiction as a single-protein gene. Much of the work on Cnn has been done on the Cnn-Long Form (Cnn-LF) protein family, containing a highly conserved KFC eukaryotic motif required for γ-tubulin localization (Fu and Glover 2012) and three insect-specific conserved motifs (Eisman and Kaufman 2013). The gene also utilizes two of its promoters to produce several splice variants of the Cnn-Short Form (Cnn-SF) protein family, which contain the conserved KFC motif (Eisman et al. 2009) and a rapidly evolving coiled-coil carboxy terminus (Eisman and Kaufman 2013). While all members of either protein family are similar, their amino termini vary with respect to the promoter utilized during transcription.
Many studies have shown Cnn-LF proteins are the predominant component of the PCM at mitotic centrosomes, based on immunostaining and the live localization of ectopically expressed GFP::Cnn-PA fusion proteins. In syncytial embryos Cnn-LF protein is always present at centrosomes, whereas in cells the protein is detectable only during mitosis. Two studies have shown Cnn-LF localizes to the centrosome during the peak of flare formation, which occurs at the onset of centrosome replication and continues during S phase and prophase (Megraw et al. 2002; Conduit et al. 2010). Additionally, the size of mature centrosomes is directly proportional to the amount of cytoplasmic Cnn-LF present during this localization phase (Conduit et al. 2010).
The molecular characterization of Cnn-SF proteins has been minimal. However, Cnn-SF antibodies initially localize to polar bodies and the male aster during syngamy at the initiation of development (Eisman et al. 2009). This same study also showed Cnn-SF antibodies localize to mitotic centrosomes in syncytial embryos concurrent with Cnn-LF localization during centrosome replication. Unlike Cnn-LF protein, Cnn-SF cycles off centrosomes during each cell cycle, as the protein is no longer detectable by the onset of anaphase.
Genetically cnn is essential only during the rapid syncytial cleavage divisions and gametogenesis, as the maternal supply is sufficient for the development of morphologically normal sterile adult flies (Megraw et al. 2001). Although cells lacking Cnn fail to form a functional PCM during mitosis (Megraw et al. 2001; Dobbelaere et al. 2008), these cells accurately segregate their chromosomes, consistent with the finding that centrioles and centrosomes are not required for division in D. melanogaster (Dinkel et al. 2011). In these cells microtubules (MTs) are nucleated from within the spindle, utilizing existing pathways that normally increase the rate of spindle assembly (Luders and Stearns 2007; O’Connell and Khodjakov 2007). These mechanisms exist in syncytial embryos but are not sufficient for division, as all mutations in cnn result in aneuploidy, disorganized actin and MT cytoskeletons, and embryonic failure prior to cellularization (Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999; Eisman et al. 2009).
Unfortunately, the gross morphological similarities among cnn mutants have led to the assumption that all mutations are equal and all phenotypes are due to the loss of Cnn-LF protein function. We have previously shown mutations resulting in truncated forms of Cnn-LF are less severe than the cnn null phenotype, which eliminates both Cnn-LF and Cnn-SF proteins (Eisman et al. 2009). This work found two critical differences between Cnn-LF truncation mutations and the null phenotype. First, truncated Cnn-LF proteins are weak hypomorphs that accumulate in the spindle and Cnn-SF protein fails to cycle, remaining at spindle poles during multiple rounds of nuclear divisions. Second, embryogenesis and gametogenesis fail much earlier in cnn null embryos compared to truncation mutants. This argues strongly that both Cnn-LF and Cnn-SF proteins play a necessary role during development and may both be important during formation of the PCM.
Knowledge of the Cnn protein state as well as the transcriptional complexity of the gene and its splice variants is critical for understanding cnn function. Cnn centrosomal function is likely to be complex, as the proteins are required for the initial formation of the PCM and subsequent centrosome maturation events throughout mitosis. A primary regulatory mechanism for Cnn during mitosis is likely phosphorylation, as several global mass spectrometry (MS) studies of the D. melanogaster phosphoproteome have identified 16 phosphorylated serine/threonine residues in Cnn-LF proteins (Diella et al. 2004, 2008; Bodenmiller et al. 2007, 2008; Zhai et al. 2008; Dinkel et al. 2011). Furthermore, two-dimensional (2D) Western analyses have shown the protein exists as a pool with up to nine phosphate groups per Cnn-LF molecule in a variety of cell types (Eisman et al. 2009 and this study). For example, the initial localization of Cnn-LF protein at the onset of centrosome replication may be regulated by Polo kinase in some cell types (Dobbelaere et al. 2008). An RNAi screen of S2 cells covering 92% of the D. melanogaster genome found the localization of Polo and Cnn-LF is codependent, suggesting an interaction between these proteins. Additionally, chemical inhibition of Polo kinase in cells completely blocks localization of Cnn-LF to the centrosome at the onset of mitosis (Fu and Glover 2012). Since S2 cells are embryonic, it seems reasonable Polo kinase may also be required for Cnn-LF localization in syncytial embryos. However, this interaction may be important only for a subset of Cnn splice variants, as Cnn localization does not require Polo kinase in larval neuroblasts (Donaldson et al. 2001b).
Additionally, Polo is required for centrosome maturation (Donaldson et al. 2001a; Diella et al. 2004, 2008; Bodenmiller et al. 2008) and progression through mitosis (Logarinho and Sunkel 1998). Polo activity is also required for polar body formation in embryos, as loss of Polo kinase results in the repeated replication and division of all four female meiotic products (Riparbelli et al. 2000). The dynamic pattern of Polo kinase in D. melanogaster embryos is consistent with many target substrates (Moutinho-Santos et al. 1999). Several Polo kinase substrates have been identified in D. melanogaster (Bodenmiller et al. 2007; Zhai et al. 2008; Santamaria et al. 2011; Habermann et al. 2012) but most targets remain unknown. In mammals, two consensus phosphorylation sequences have been described for the orthologous Polo-like Kinase1 (Plk1). Moreover, Plk1 will phosphorylate many degenerate sequences in vitro, albeit at a much lower efficiency than the consensus sequence (Nakajima et al. 2003; Santamaria et al. 2011). Thus, since Polo may phosphorylate degenerate sequences in vitro, sequence alone is not necessarily an accurate predictor for identifying in vivo target substrates.
Prior to substrate phosphorylation, Polo typically binds to a phosphorylated polo-binding motif via the Polo-Box domain (PBD) located at the carboxy terminus of the kinase (reviewed in Williams et al. 1999). The PBD recognizes a core sequence of S−1-(pS/pT)0-(P/X)+1, where X can be any residue and the central S/T is phosphorylated (Elia et al. 2003a) by the priming kinase CDK1 (Neef et al. 2007). High-affinity binding sites have a proline at the +1 position but an in-depth analysis of Plk1 target substrates found 30% of targets had low-affinity sites, suggesting the binding sites were primed by a kinase other than CDK1 or did not require phosphopriming (Santamaria et al. 2011). Additionally, the Polo inhibitor Map205 binds to Polo at the PBD but does not require phosphopriming (Archambault et al. 2008) and the encoded inhibitor Matrimony binds to Polo via a noncanonical mechanism (Bonner et al. 2013). Clearly Polo can interact with target substrates by more than one mechanism. The binding of Polo is thought to be critical to localize the kinase for interactions with phosphorylation target substrates. Following Polo binding, the kinase typically phosphorylates the protein to which it is bound (processive phosphorylation), although there is evidence Polo may phosphorylate a different substrate in trans (distributive phosphorylation) (Williams et al. 1999; Archambault et al. 2008). If Cnn and Polo interact directly, cnn should encode a Polo phosphorylation target sequence associated with a polo-binding motif, and these two peptide sequences should be conserved within the genus Drosophila.
We applied the modENCODE RNA-seq data (Graveley et al. 2011; Brown et al. 2014) and a genomic Cnn BAC to identify the most likely candidate cnn splice variants involved in a potential Cnn–Polo interaction during embryogenesis. This analysis shows exon 1A splice variants contain the only consensus Polo target sequence encoded by cnn, as well as an adjacent polo-binding motif, and deletion of this exon from the genomic BAC results in a polo-like phenotype. This work also revealed a novel function for Cnn-SF proteins, which block the polo-like phenotype, suggesting they are required for polar body formation. Additionally, we have used the Gal4/UAS system to express Cnn-LF mutant proteins in different cnn mutant backgrounds to analyze the effect of potential loss of Polo binding, Polo phosphorylation of Cnn, and a combination of loss of both during syncytial development. We propose a model for Cnn–Polo interactions that are required for the initiation of Cnn transport to the centrosome during formation of the PCM. We also demonstrate the importance of identifying which Cnn splice variants are present in different tissues throughout development and reemphasize the importance of genotype when investigating Cnn function in D. melanogaster.
Materials and Methods
Growth conditions and stocks
All flies used in this study were grown on standard cornmeal agar medium at 25° unless otherwise noted. Wild-type flies were Oregon R. The following mutant alleles were used: cnnhk21 (FBal0012091), cnnmfs7 (FBal0098270), cnnmfs3 (FBal0089468), and the deficiency Df(2R)cnn (FBab0024394) (Heuer et al. 1995). To express the eGFP::Cnn-tagged proteins created in this study (see below) during embryogenesis w; P{w+mC=pUASP-eGFP-cnnx} were crossed to a nanos::Gal4 line (P{GAL4::VP16-nos.UTR}CG6325MVD1) (FBti0012410) (Van Doren et al. 1998). Embryos produced by P{GAL4::VP16-nos.UTR}CG6325MVD1; P{w+mC=pUASP-eGFP-cnnx} mothers were collected and observed live or stained for the presence of GFP to determine the pattern of accumulation of the chimeric protein. The nanos::Gal4 (driver) and P{w+mC=pUASP-eGFP-cnnx} (responder) lines were also crossed into cnn mutant and Df(2R)cnn backgrounds by standard genetic crosses. Crosses of driver and responder lines with these backgrounds were then crossed to obtain P{GAL4::VP16-nos.UTR}CG6325MVD1; cnnx/Df(2R)cnn; P{w+mC=pUASP-eGFP-cnnx} females, which were crossed to Ore R males. The genotypes of these females are listed in Supporting Information, Table S1. Transformed lines carrying the Bac duplications created in this study (see below) were also crossed into cnn mutant and Df(2R)cnn backgrounds. These were then crossed together to recover cnnx/Df(2R)cnn; Dp(2; 3)BAC•cnnx females. The genotypes of these females are listed in Table S1. The GFP-tagged Polo stock was obtained from Mark Peifer (Rusan and Peifer 2007). The original transgenic lines were made in the Sunkel laboratory (Moutinho-Santos et al. 1999) and the stock used here contains two P-element transgenic inserts, one on the second chromosome (P{GFP-polo}p2; FBti0016200) and the other on the third chromosome (P{GFP-polo}p31; FBti0016201).
cnn expression data from modENCODE/FlyBase
Using the modENCODE RNA-Seq transcriptome data (Graveley et al. 2011; Brown et al. 2014) posted in FlyBase (Gelbart and Emmert 2013; St. Pierre et al. 2014), we extracted the log2-transformed Reads Per Kilobase of transcript per Million mapped reads (RPKM) values for each annotated exon of cnn. The values recovered correspond to the embryonic, larval, pupal, and adult male and female stages plus those for ovaries and testes. The values for the 12 embryonic, 6 larval, 6 pupal, and 3 adult ages were averaged for each stage group, as were the two stages of adult ovary. Only a single value for adult testes was used. These values were graphically represented in relation to the gene models available in FlyBase (St. Pierre et al. 2014).
Sequence analyses to find PO4 sites
To identify putative phosphorylation sites we submitted D. melanogaster protein sequences representing all known cnn coding exons to NetPhos 2.0 (Blom et al. 1999). Additionally, we created protein subsequences for the Polo kinase consensus and degenerate target sites and Polo kinase binding sites, using MacVector 12.7.5 (MacVector, Inc., 2012), and queried the same Cnn protein sequences. To reduce the number of putative phosphorylation sites (>140), we aligned previously described Cnn protein splice variants from 14 Drosophila species (Eisman and Kaufman 2013) and identified the highly conserved sites within the genus Drosophila.
Embryo and ovary collection, fixation, and staining (antibodies)
Embryos (0–2 hr) were collected, dechorionated, and fixed in 50% heptane:50% MeOH/EGTA as previously described (Eisman et al. 2006). Ovaries were dissected in Robb’s medium and fixed as previously described (Eisman et al. 2009). Embryos and ovaries were immunostained as previously described (Gorman and Kaufman 1995). Specimens were mounted on Superfrost glass slides (Fisher Scientific, Pittsburgh) in 90% glycerol:10% PBS with 2 mM n-propylgallate (Sigma, St. Louis).
The α-Cnn-Short Form antibody was previously described (Eisman et al. 2009) and the Melon Gel-purified (Melon Gel IgG Purification Kit; Thermo Scientific/Pierce, catalog no. 45212) Rat126 antibody was produced by Cocalico Biologicals and used at 1:50. Additional antibodies used in this study were Melon Gel-purified rabbit α-Cnn-LongForm at 1:300 (Heuer et al. 1995), mouse monoclonal E7 α-γ-tubulin (Hybridoma Bank) at 1:1000, and rabbit α-GFP (Torrey Pines) at 1:50. DNA was stained with TOTO3 (Molecular Probes, Eugene, OR) at a final concentration of 1:1000 following an ON treatment with 0.15 μg/μl RNaseA (QIAGEN, Valencia, CA). All fluorescent secondary antibodies were used at 1:200 (Jackson ImmunoResearch Laboratories).
Recombineering the cnn BAC
The cnn BAC used in this study is CH322-189K22 from the CHORI BacPac library collection (https://bacpac.chori.org). First, 50-bp homology arms were added to GalK, using PCR, and the product was inserted into cnn immediately upstream of the initiating methionine in exon 1A following the National Cancer Research Institute (NCRI) protocol (Warming et al. 2005) widely distributed via the internet (http://ncifrederick.cancer.gov/research/brb/protocol.aspx). The eGFP tag at exon 1A of cnn was created by adding 50-bp homology arms to eGFP, using PCR, and this product replaced the existing GalK fragment. To create the deletion of exon 1A a 100-mer was synthesized with 50-bp homology to cbs and 50-bp homology to cnn immediately downstream of the last codon in exon 1A. The replacement of GalK with this 100-mer results in the removal of the nontranscribed region between cbs and cnn, the 5′-UTR, and the entire coding sequence of exon 1A. All oligos were synthesized by Sigma, GalK source DNA was supplied by the NCRI, and eGFP source DNA was previously described (Eisman et al. 2009). All clones were sequenced by the Indiana Molecular Institute according to their protocols (http://imbi.bio.indiana.edu/index.php?page_id=DNA%20Sequencing%20Facilities&features=1).
Cloning
To construct the pUASp point mutations at serine 22 and threonine 27 we used a previously described P{UASp-GFP-cnn.PA} (FBtp0056358) fusion construct (Eisman et al. 2009). To change the native residues to alanine we used the Stratagene Site-Directed Mutagenesis kit (catalog no. 200518) and followed manufacturer’s protocols. Clones were sequenced as described above and correct clones were purified for injection.
Transgenesis
All P-element (pUASp constructs) injections were done by Genetic Services and surviving larvae were allowed to eclose and crossed to a w1 stock. All w+ transgenic progeny were mapped and a single homozygous viable and fertile line on chromosome III was used to generate the genotypes described in this study. The nomenclature used to designate these clones is presented in Table S2. Transgenic lines with the cnn BAC were generated as previously described (Venken et al. 2010), using the VK00033 stock carrying a ϕC31 insertion site on chromosome III (y1M{vas-int.Dm}ZH-2A w*; PBac{y+-attP-3B}VK00033)(FBti0099694; FBti0076453) available from the Bloomington Drosophila Stock Center (FBst0024871). The nomenclature used to designate these clones and their molecular characteristics are shown in Table S3.
Western blots
Embryos (0–2 hr) were collected and dechorionated in 50% bleach:50% NaCl (0.2%) and Triton X-100 (0.02%) and washed with NaCl/Triton X-100 wash. Embryos were immediately lysed in urea lysis buffer as previously described (Eisman et al. 2006). Ovaries were dissected in Robb’s medium, washed with PBS, and immediately lysed in urea lysis buffer. We followed the Western protocol outlined previously (Eisman et al. 2006), with the following modifications: (a) Samples were never washed or stored in alcohol; (b) samples were ground in lysis buffer immediately after collection and either loaded directly on a gel or stored in an ultrafreezer for <5 days before loading to a gel; and (c) loading buffer did not include addition of 10% Beta-MercaptoEthanol BME, which appears to destabilize Cnn protein. These modifications seemed to produce cleaner, more reproducible results for Western blot analysis. The primary antibodies used were Melon Gel-purified α-Cnn (Heuer et al. 1995) at 1:100 dilution, α-Tub 84B (Matthews et al. 1989) at 1:1000 dilution, and mouse α-Polo MA294 (Llamazares et al. 1991) at 1:50 (a gift from Claudio Sunkel, Intituto de Biologia Molecular e Celular Universidade do Porto Rua do Campo Alegre 823 Porto, Portugal); secondary α-rabbit antibodies were used at 1:20,000 dilution (Jackson ImmunoResearch Laboratories), detected with Clarity Western ECL substrate [Bio-Rad Laboratories (Hercules, CA) no. 170-5061].
Co-immunoprecipitation
Initial co-immunoprecipitation attempts followed published protocols (Neumuller et al. 2012), including a preclear step using underivatized agarose beads [Pierce (Rockford, IL) product no. 26150]. Results indicated the need to use magnetic beads (Chromotek GFP-Trap_M) and buffer optimization to maintain GFP (native lysis buffer: 25 mM Tris, 192 mM glycine) and appropriate wash conditions (500 mM NaCl added to native lysis buffer (“high salt wash buffer”) in comparison to Chromotek Protocol wash buffer [10 mM Tris-Cl (pH 7.5), 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40]. Finally, we used Protein A Magnetic Beads (Pierce product no. 88845), following manufacturer’s protocol with 10 µg each of the following rabbit antibodies for binding: α-Cnn (previously described); α-Plk1, which recognizes activated Plk1 phosphorylated at Thr210 (Thermo product no. PA1-126); and α-GFP (Life Technologies product no. A11122). These antibodies were incubated at 4° overnight with 2 mg total embryonic protein (0–2 hr, unfrozen) ground in binding buffer (Thermo product no. 87788) with fresh addition of protease and phosphatase inhibitors [Thermo product no. 78444; 0.5 mg/ml each (1000×) of Leupeptin, Pepstatin A, and Aprotonin]. Subsequent Western blots were stained with rat-13 α-Cnn whole sera (a parting gift from Tim Megraw, Biomedical Sciences Florida State University College of Medicine Tallahassee, FL) at 1:500 and mouse α-Polo MA294 (previously described) at 1:50.
Microscopy and Imaging
All images were captured on a Leica TCS confocal microscope with a 63× HCX Plan Apo oil immersion objective, using SP2 software. Images are all Z-stacks that range from 5 to 30 μm thick, composed of 0.4- to 0.9-μm-thick sections. Projected images were further processed and assembled into figures with Adobe Photoshop Creative Suite version 5.0.
Data availability
Drosophila stocks and clones created in this report are available upon request. Table S1 contains the genotypes of transformed animals used in this study. Table S2 lists the contents and names of the UAS P-element clones used to make the transgenic lines used in this study. Table S3 lists the names and content of the BAC clones used in this study. Figure S1 shows confocal micrographs and western blots of two cnn insertion mutations demonstrating that they are not null mutations. Figure S2 shows 2D gel western blot analyses of tissues and tissue culture lines demonstrating that Cnn is phosphorylated multiple times and in different patterns depending on tissue, time and cell line. Figure S3 shows the demonstrated sites of phosphorylation in Cnn protein and the potential kinase enzymes responsible. Figure S4 shows western blots of immune-precipitation controls demonstrating that two different bead types non-specifically bind to Cnn.
Results
Cnn expression: modENCODE/FlyBase data
We have previously described the transcriptional and translational complexity of centrosomin (cnn), which uses three promoters and initiation coding exons termed 1A, 1B, and 1C (Figure 1) (Eisman et al. 2009). The RNA-Seq data from the modENCODE project (Graveley et al. 2011; Brown et al. 2014) show exons 1A and 1B are utilized throughout development in both males and females in somatic tissue, testes, and ovaries, albeit exon 1B transcripts are not detected in 0- to 2-hr embryos. Exon 1C expression is restricted to the late larval and pupal stages as well as adult males and their testes. We conclude this expression is likely from the male animals in these mixed-sex preparations and from the developing testes at these stages. These data describe three unique families of transcripts encoding a mix of Cnn-Long Form (Cnn-LF) and Cnn-Short Form (Cnn-SF) proteins differing at their carboxy-terminal halves (Figure 1). Cnn-LF proteins include sequences encoded by exons 5, 6, and 7 from the 3′ end of the gene and are approximately twice the size of Cnn-SF proteins. The carboxy termini of Cnn-SF proteins are encoded by several internal exons alternatively spliced by an intron retention/exclusion mechanism (Eisman et al. 2009). Cnn-SF proteins are not included in transcripts driven from the 1B exon. The 1C male/testes-specific promoter produces most of the Cnn-SF transcripts, with a low level of Cnn-SF transcripts from the 1A promoter, shown by the low but finite level of signal associated with these small exons seen in embryonic and adult female stages (Figure 1).
Figure 1.
The transcriptional complexity and expression of cnn splice variants. The known cnn splice variants are shown at the top, grouped by the exon 1A, 1B, and 1C promoters and initiating coding exons. The exons encoding the carboxy termini of Cnn-SF proteins are in the boxed region and the positions of premature stop codons for cnnhk21, cnnmfs7, and cnnmfs3 mutations are indicated at the top. Bar graphs below represent the average RPKM values determined by the modENCODE study for each coding exon throughout embryogenesis, larval stages, pupal stages, in whole males (red) and whole females (blue), and in testes (red) and ovaries (blue). The initiating exons 1A and 1B are utilized throughout development in both sexes with exon 1A being the most abundant transcript in all stages except pupal development. The exon 1C initiating exon is not detected in embryos, whole females, and ovaries, suggesting this promoter is male specific. Transcripts encoding Cnn-SF proteins are present at low levels in embryos and moderate levels in larvae and are abundant in pupae, whole males, and testes. The Cnn-SF transcripts data in females and ovaries are consistent with cnn-RK being a rare transcript at the start of embryogenesis.
The phenotypic differences associated with cnn mutations are important features of the complexity of cnn. To date, all known nonsense mutations in cnn except cnnhk21 result in hypomorphic or loss-of-Cnn-LF function alleles, but do not affect Cnn-SF protein function (Eisman et al. 2009). We find cnnhk21 the only true cnn null allele, a nonsense mutation in exon 3 that eliminates all functional Cnn proteins (Figure 1). We have also tested cnne00441 (FBti0046435) and cnn04547 (FBti0051837) piggyBac insertion mutations and found neither to be Cnn-LF or Cnn-SF null alleles based on immunostaining of syncytial embryos and Western blotting (Figure S1). Only the cnnmfs3 mutation accumulates enough protein to be readily detected in ovaries and embryos by Western analyses, employing the protein isolation, gel, and Western conditions used in this study (Figure 2).
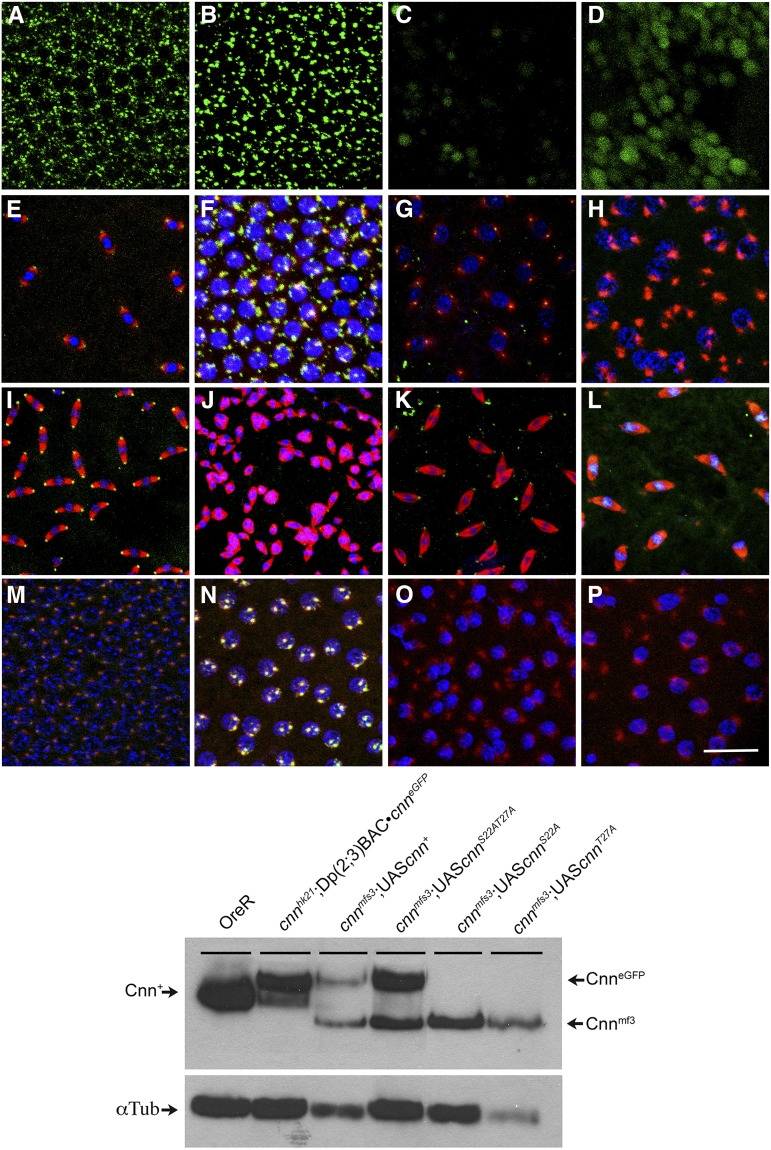
Figure 2.
Ovarian and embryonic Cnn protein accumulation in cnn mutant backgrounds. Western analyses of Cnn-LF protein in ovaries (right panels) and embryos (left panels) show the shift associated with the eGFP fusion protein expressed from the modified BAC [Dp(2; 3)Bac•cnneGFP] compared to native Cnn (OreR) (leftmost lanes in both panels) and protein accumulation in Dp(2; 3)Bac•cnnΔEx1A and the three cnn mutant backgrounds used in this study. Only cnnmfs3 mutant protein accumulates to readily detectable levels in ovaries and embryos, and this truncated protein migrates faster than native Cnn protein (OreR). After overnight exposure low levels of cnnmfs7 protein are detectable (not shown) whereas the cnnhk21 mutant protein is never detectable.
While these data do not unequivocally show which promoter expresses the predominant centrosomal proteins, they do eliminate the exon 1C transcripts from the maternally provided embryonic pool. Additionally, the undetectable levels of exon 1B transcripts in 0- to 2-hr embryos and the exon 1A Cnn-PA protein rescue of cnn mutants that retain Cnn-SF function (Eisman et al. 2009) suggest exon 1A transcripts, including cnn-RA, cnn-RL, and cnn-RK (St. Pierre et al. 2014), encode the primary syncytial pool of Cnn proteins.
Mapping of Cnn phosphorylation sites reveals only exon 1A has a consensus Polo target site
Previous 2D Western analysis of syncytial embryos has shown Cnn is phosphorylated at multiple residues and phosphatase treatment results in a single spot on a 2D gel (Eisman et al. 2009). Similar analyses of several cell lines reveal Cnn is also phosphorylated at multiple sites, although no two cell lines have a similar pattern and none are identical to embryos (Figure S2). In D. melanogaster, in silico analysis identifies >140 potential kinase consensus sites in the Cnn-PA splice variant, targeted by several of the major kinases in Drosophila. To reduce the number of potential candidate sites we used the rapid evolution of Cnn proteins (Eisman and Kaufman 2013) and compared Cnn-PA from 12 sequenced genomes of Drosophila species as well as a few additional species with RNA-Seq data. Our comparison showed that 10 of the predicted sites are highly conserved within the genus. MS analyses of the phosphoproteome in D. melanogaster have now identified 16 serine and threonine residues that are phosphorylated and include peptides containing 7 of the highly conserved residues (Diella et al. 2004, 2008; Bodenmiller et al. 2007, 2008; Zhai et al. 2008; Dinkel et al. 2011). Of the remaining phosphorylated residues, some are conserved only within the melanogaster group while others are conserved in the subgenus Sophophora. The MS data suggest Cnn-PA is a target substrate for several kinases and several of the phosphorylated residues are potential targets for more than one kinase (Figure S3). Taken together, it is clear the regulation of Cnn by phosphorylation throughout development is complex.
Our foci in this analysis are a potential Polo kinase-binding motif and a consensus Polo target site along with encoded peptide sequences in exon 1A, which are highly conserved within the genus Drosophila (Figure 3). Polo kinase or Cnn loss in mitotic cells has been shown to block formation of the pericentriolar matrix (Dobbelaere et al. 2008) and similar to Cnn, Polo localizes to the centrosome from prophase to early anaphase (Logarinho and Sunkel 1998). In Cnn exon 1A, Polo kinase potentially binds to the three-residue motif centered on Ser22, a dual protein kinase C (PKC)/casein kinase II (CKII) consensus target site (Figure 3). Although the Cnn Polo-binding motif is a low-affinity site, lacking four residues upstream of a longer Polo-binding consensus motif (Elia et al. 2003a,b), and is not a CDK1 target, these sites are sufficient for Polo binding. Similar low-affinity sites have been shown to bind Plk1 (Kang et al. 2006; Neef et al. 2007; Burkard et al. 2009; Wolfe et al. 2009; Santamaria et al. 2011) and may or may not require phosphopriming prior to Plk1 binding (Santamaria et al. 2011). Since Cnn proteins lack additional high-affinity Polo-binding motifs throughout the protein in the Drosophila species analyzed (Figure 3), Ser22 could serve as a motif for Polo binding especially if it is itself phosphorylated and thus converted to a high-affinity site.
Figure 3.
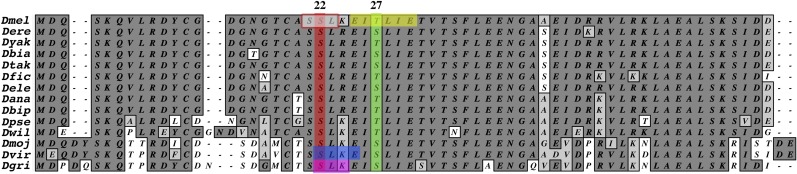
The conservation of exon 1A in the genus Drosophila. The coding region of exon 1A is highly conserved within the genus Drosophila, including a potential Polo kinase binding site (red box at top) centered on serine 22 (highlighted in red) and the only consensus Polo kinase target sequence encoded by cnn (yellow box at top), which is predicted to be phosphorylated at threonine 27 (highlighted in green). Prior to Polo kinase binding, serine 22 would be phosphorylated by either PKC (magenta box at bottom) or CKII (blue box at bottom). The conservation of this dual kinase site implicates its importance in tissue-specific mitotic Cnn and Polo interactions.
Two consensus sequences for Plk1 phosphorylation have been shown to be (D/E)X(pS/pT)ΦX(D/E) (Nakajima et al. 2003) or L(Φ)(E/N/D(Q))X(pS/pT)L(Φ) (Santamaria et al. 2011), where X is any residue and Φ denotes a hydrophobic residue. Based on both consensus sequences, an analysis of all known cnn-coding exons identifies a single consensus sequence, which is encoded by exon 1A and would be phosphorylated at Thr27 in D. melanogaster (Figure 3). If a single mismatch is allowed at constrained positions, there are an additional 13 sites in the Cnn-PA splice variant (see Figure S3). While Plk1 will phosphorylate peptides in vitro with single nonconsensus changes at these constrained sites, the kinase has a much lower affinity for the substrates and phosphorylation is significantly reduced or eliminated (Nakajima et al. 2003). Moreover, none of these less-constrained positions are associated with a conserved high- or low-affinity PBD.
Based on these results, we have identified a possible PBD motif centered on a highly conserved dual PKC/CKII phosphorylation site and a closely linked consensus Polo kinase phosphorylation sequence in cnn exon 1A. A recent study of a Cnn peptide containing three nonconsensus Polo sites found human Plk1 would phosphorylate one of these sites in vitro and concluded this site was responsible for all Cnn phosphorylation (Conduit et al. 2014). However, both biochemical and genetic data argue against the conclusions of the study and suggest the identified serine is not a bona fide Polo target site (see Discussion). For this reason we have investigated the single consensus Polo kinase target sequence encoded by exon 1A and the associated PBD motif. As these motifs are unique to exon 1A encoded peptides, the three isoforms encoded by this initiating exon may be the only isoforms regulated by Polo at the centrosome.
BAC-supplied eGFP exon 1A fusion proteins localize to the centrosome and rescue cnn mutant phenotypes
Based on the modENCODE expression data, the most likely Cnn proteins at the early embryonic centrosome should be isoforms encoded by the family of RNAs expressed from the exon 1A start site. We used recombineering to fuse eGFP to the initiating methionine of exon 1A in a genomic BAC [Dp(2; 3)BAC•cnneGFP::Ex1A] and created transgenic lines that were otherwise null for cnn (see Materials and Methods). We also generated a similar line with an untagged version of the BAC [Dp(2; 3)BAC•cnn+] as a control. Both the tagged and untagged BACs rescue the cnnhk21 null phenotype, with hatching rates of 51.5% and 72.5%, respectively, and restore fertility to both males and females.
To compare the pattern of the eGFP fusion protein to known patterns for antibodies to Cnn-LF (Heuer et al. 1995), we immunostained embryos. In syncytial embryos α-GFP staining is localized to the centrosome at all stages of mitosis with a reduction noted at prophase (Figure 4, A–C). α-Cnn-LF staining of embryos expressing untagged Cnn from the BAC is similar to the tagged version (Figure 4, D–F), consistent with the transcriptional expression data indicating that exon 1A proteins are the major maternally supplied products. Unfortunately α-Cnn-LF and α-Cnn-SF staining shows the two isoforms colocalize during cleavage, masking differentiation between the two eGFP-tagged splice variants.
Figure 4.
Characterization of eGFP-tagged Cnn exon 1A BAC [Dp(2; 3)Bac•cnneGFP] proteins in embryos. Immunostained embryos with the BAC [Dp(2; 3)Bac•cnneGFP] (A–C) show GFP localizes weakly to prophase centrosomes (A) and is abundant at metaphase (B) and replicating telophase (C) centrosomes. (D–F) In embryos with the untagged BAC, Cnn-LF antibody is abundant at prophase (D), metaphase (E), and telophase (F) centrosomes similar to what is seen in wild-type embryos. The only significant difference observed between GFP and Cnn-LF antibodies is during prophase (A and D). (A–F) Tubulin is in red and DNA in blue. Bars, 25 μm for A–F.
Loss of cnn exon 1A transcripts results in a polo-like phenotype
To test the potential effects of the loss of only exon 1A splice variants we used recombineering to delete the entire exon 1A coding region, the 5′-UTR, and an additional 150 bp upstream of the transcription start site in the cnn BAC. This construct [Dp(2; 3)BAC•cnnΔEx1A] should result in the loss of the three exon 1A transcripts with little or no effect on the downstream gene products expressed from exons 1B and 1C. We questioned whether the loss of exon 1A proteins in a cnn null background would show a complete loss of Cnn function or a novel phenotype.
There are significant differences among cnn mutations during gametogenesis and embryogenesis due to the severity of Cnn-LF protein truncation and the presence or absence of Cnn-SF proteins (Eisman et al. 2009). Others have reported that meiosis completes in cnn null embryos, despite frequent misaligned anaphase II spindles and size reduction of the male pronuclear-associated aster (Riparbelli and Callaini 2005). However, there are no reports on development following the completion of meiosis. Since a complete loss of cnn (both LF and SF) has a dramatic effect on oogenesis (Eisman et al. 2009) and many studies analyze loss of cnn in trans-heterozygous mutants that retain Cnn-SF proteins, the characterization of embryogenesis in a complete cnn null embryo seemed appropriate.
We find that cnn null embryos have a persistent meiotic-like spindle when development is detectable. In 34% of cnnhk21/Df(2R)Cnn embryos examined (n = 250) we detect a variety of spindle shapes located at the cortex (Figure 5, A–D) and often observed only a few nuclei deep in the cytoplasm of the embryo. Approximately 7% of the embryos examined have multiple nuclei at the cortex but lack deep divisions and an obvious remnant of the meiotic spindle (Figure 5A). The final class of embryos that develop (32%) have a mix of cortical and deep divisions and a persistent large spindle associated with the cortex. All other embryos examined (27%) either lacked any detectable signs of development or were necrotic. Interestingly, we never observe polar body (PB) formation in either a haploid/diploid or a triploid configuration, suggesting Cnn may have a function during the termination of meiosis prior to and/or following syngamy.
Figure 5.
The difference between the hk21 and exon 1A deletion phenotypes. In most embryos produced by cnnhk21 mutant females, meiotic-like spindles persist at the cortex and polar bodies fail to form (top, a–d and B). Spindles are scattered in space and are relatively rare and nuclear fusions are common (A). Embryos typically fail early, probably due to aneuploidy and failure to form centrosomes. Unlike the cnnhk21 phenotype, embryos produced by Dp(2; 3)BAC•cnn ΔEx1A undergo many rounds of division and nuclei cover the cortex (C–E). Tubulin foci are present at prophase (C) and metaphase (D) but do not appear to organize the meiotic-like spindle poles. Initially nuclei appear to be haploid but as numbers increase fusions occur during metaphase and anaphase (F–H). Polar bodies and persistent meiotic spindles are not observed in these animals similar to the loss of Polo kinase phenotype. Cnn-LF protein is not detected in either embryonic genotype. Cnn-LF is shown in green, tubulin in red, and DNA in blue. For Western analyses of these genotypes see Figure 2.
Based on transcription data and mutant analyses, we expected a cnn null phenotype in embryos carrying Dp(2; 3)BAC•cnn ΔEx1A in an otherwise null background. While we see a similar percentage of embryos that lack any obvious divisions, the remaining embryos show multiple divisions and frequent anastral spindles cover the cortex, yet these embryos fail to cellularize. Similar to 7% of cnn null embryos, PBs do not form, nor do meiotic-like spindles persist. The meiotic products appear to divide repeatedly, progressing from a few cortical nuclei to many nuclei covering the entire cortex (Figure 5, C–E). We do not observe any nuclei or spindles deep within the cytoplasm of the embryo. The cortical spindles lack astral microtubules and tend to be more synchronized than is typical of cnn mutant embryos. While many nuclei appear to be haploid, nuclear fusions (Figure 5, F–H) similar to those seen in other cnn mutants are present. Although these embryos are similar to the 7% of the cnn null embryos described above, the frequency of this class of animals is nearly 60% and there are many more rounds of division in cnnhk21/Df(2R)Cnn;Dp(2; 3)BAC•cnn ΔEx1A embryos compared to cnnhk21/Df(2R)Cnn embryos without the transgenic duplication.
Transcription products from the exon 1B and 1C promoters could explain the phenotypic variance in the embryos carrying Dp(2; 3)BAC•cnn ΔEx1A, but we did not observe protein accumulation in ovaries or embryos based on Western analyses or immunostaining (Figure 2 and Figure 5). While proteins are not detected by Western analyses, the change in frequency from 7% in cnn null embryos to 60% in Dp(2; 3)BAC•cnn ΔEx1A embryos suggests oogenesis is improved in duplication-carrying females. Egg production does not decline rapidly over time as it does in cnnhk21/Df(2R)Cnn null females (Eisman et al. 2009). Interestingly, the embryonic phenotype is essentially identical to the loss of Polo kinase at these stages in the weak hypomorphic polo1 allele (Riparbelli et al. 2000), suggesting Cnn and Polo may interact to block polar nuclear divisions.
Exon 1A Cnn-SF protein is required for polar body formation
The exon 1A-encoded Cnn-SF has been shown to localize to the core of the PB following meiosis (Eisman et al. 2009). The above results suggest Cnn proteins play a role in forming PBs and the fusion of the three polar nuclei at the end of female meiosis. The complete loss of cnn results in a more severe phenotype than the loss of exon 1A isoforms only. To test the role of the exon 1A-encoded Cnn-SF protein, we combined Dp(2; 3)BAC•cnn ΔEx1A with the cnnmfs7 and cnnmfs3 mutant alleles. The resident cnn locus in females of these genotypes either lacks Cnn-LF function (cnnmfs7) or expresses a truncated potentially hypomorphic Cnn-LF protein that fails to localize to centrosomes (cnnmfs3) (Eisman et al. 2009). Since the causative stop codons in these mutant alleles reside in exon 6 (see Figure 1), they still have the potential to express exon 1A-driven Cnn-SF protein, which the transgenic BAC cannot.
Embryos produced by cnnmfs3or7/Df(2R)Cnn; Dp(2; 3)BAC•cnn ΔEx1A females exhibit the “classic” cnn mutant embryonic phenotype (Megraw et al. 1999), having multiple nuclei/spindles scattered throughout the embryo (deep and cortical) and frequent nuclear fusions. Most importantly, these embryos produce normal triploid polar bodies following the completion of meiosis (Figure 6, A and C). In cnnmfs3 and cnnmfs7 embryos carrying Dp(2; 3)BAC•cnn ΔEx1A early spindles frequently have an obvious spindle pole (Figure 6, A and C) but the poles are lost as development proceeds and barrel-shaped anastral spindles accumulate, accompanied by nuclear fusions (Figure 6, B and D). Consistent with previous results (Eisman et al. 2009), microtubule (Figure 6, E and G) and Cnn-SF (Figure 6, F and H) antibodies localize around the fused chromosomes of haploid/diploid and triploid PBs. Additionally, in wild-type embryos Cnn-SF staining appears at prophase centrosomes (Figure 6, I and J), increases slightly at metaphase centrosomes (Figure 6, K and L), and is undetectable by midanaphase, whereas in mutant embryos staining persists throughout mitosis at spindle poles (Eisman et al. 2009). While similar results are seen in cnnmfs3 and cnnmfs7 embryos, in the latter case spindles are almost always anastral and fusions occur earlier in development (data not shown).
Figure 6.
Cnn-SF protein is required for polar body formation in embryos. In cnnmfs7 and cnnmfs3 embryos with Dp(2; 3)BAC•cnn ΔEx1A Cnn-SF protein can be transcribed from the endogenous chromosome. In these embryos polar bodies form and block the Polo-like phenotype (arrowheads, A and C), thereby allowing deep divisions to proliferate (B and D). (A–D) Cnn-LF is in green, tubulin in red, and DNA in blue. In mutant and wild-type (E–H and I–L) embryos Cnn-SF antibody (green in E–L) localizes to the central region of haploid and diploid polar bodies (E and F) and the terminal triploid polar body (G and H). This staining is decreased or absent after multiple rounds of zygotic division. Cnn-SF antibody also localizes to the center of prophase centrosomes (I and J) and in metaphase at centrosomes and the tip of the spindle (K and L). This staining is no longer detectable prior to the start of anaphase B. (E–I and K) Tubulin is in red and DNA in blue. Bars, 25 μm for A–H and 30 × 30 μm for I–L.
The significant difference between these embryos and embryos produced by females carrying Dp(2; 3)BAC•cnn ΔEx1A in an otherwise cnn null background is the presence of Cnn-SF protein. Considering the transcriptional expression data, it is likely the exon 1A short form transcript encodes this protein. The normal PBs formed in these embryos suggest this splice variant is required to block the polo-like phenotype that may require an early Polo–Cnn interaction during embryogenesis.
Failure to phosphorylate exon 1A blocks centrosome localization of native Cnn in wild-type and hemizygous embryos
We used the Gal4=>UAS bipartite system (Brand and Perrimon 1993) to test the effects of mutations in the conserved PKC/CKII phosphorylation site and potential Polo-binding motif (S22) and the potential Polo kinase target site (T27) and compared any associated defects with those seen with the deletion of exon 1A. We used P{GAL4::VP16-nos.UTR}CG6325MVD1 located on the X chromosome as the driver construct. Since the Cnn-PA splice variant appears to be the predominant protein at mitotic embryonic centrosomes, we created P{UAS::eGFP::cnn-RA-P} (hereafter UAScnn-P) transgenes in which the serine and threonine residues were changed to alanine to block phosphorylation. To determine whether one or both sites were important, transgenic fly lines were created that carried either a single mutation (S22A or T27A) or the double mutation (S22A+T27A).
As an initial test, the three UAScnn−P and a control UAScnn+ transgene (or transgenes) were expressed in a cnn+ background at 18°, 25°, and 29° to decrease or increase expression levels of the transgenes, respectively. We also crossed the four UAS transgenic constructs into Df(2R)Cnn/cnn+ backgrounds to assess the effect of reduction of the pool of Cnn protein from the resident allele. Further, localization of native and transgenic Cnn-LF was analyzed for effects of the transgene on normal Cnn and centrosome function during syncytial development.
The transgenically encoded eGFP::Cnn-PA+ (hereafter Cnn+) protein has no obvious effect on localization of Cnn (Figure 7A) and microtubules (Figure 7E) during prophase and metaphase (Figure 7I). In striking contrast, the eGFP::Cnn-PAS22AT27A (CnnS22AT27A), eGFP::Cnn-PAS22A (CnnS22A), and eGFP::Cnn-PAT27A (CnnT27A) cnn−P mutants all block the normal localization of both fusion and native Cnn protein during prophase, as the majority of Cnn staining appears cytoplasmic (Figure 7, B–D). In both CnnS22AT27A and CnnS22A embryos, MTs form distinct foci that lack Cnn-LF protein (Figure 7, F and G), whereas the MT foci in CnnT27A embryos are not well organized, and replication and separation of MT foci appear to be delayed (Figure 7H). However, by metaphase, Cnn staining is localized to the centrosome and the majority of spindles have normal morphology, although nuclear and centrosomal defects are common in the CnnS22AT27A embryos (Figure 7J). Cytoplasmic Cnn staining remains throughout mitosis in both the CnnS22AT27A and CnnS22A embryos (Figure 7, J and K), but is absent by metaphase in CnnT27A embryos, suggesting phosphorylation of S22 may be necessary for the initial transport of Cnn to the centrosome. While the above results are seen at 29°, similar but less dramatic dominant negative effects are also seen at 18° (data not shown).
Figure 7.
(Top) (A–D) The S22 and T27 residues in exon 1A are required for normal Cnn centrosomal localization during prophase in wild-type and hemizygous embryos. Localization of Cnn-LF antibody (green) reveals the dominant negative effect from the ectopic expression at 29° of the eGFP::Cnn-RAS22AT27A , eGFP::Cnn-RAS22A, and eGFP::Cnn-RAT27A transgenes in wild-type embryos. During prophase Cnn localization is relatively normal with the eGFP::Cnn-RA+ transgene (A) but both transgenic and native Cnn protein is cytoplasmic and fails to localize to prophase centrosomes with the eGFP::Cnn-RAS22AT27A (B), eGFP::Cnn-RAS22A (C), and eGFP::Cnn-RAT27A (D) transgenes. Enlarged images (boxed areas in A–D) show tubulin (red) localizes to well-defined foci in the presence of eGFP::Cnn-RA+ (E), eGFP::Cnn-RAS22AT27A (F), and eGFP::Cnn-RAS22A (G) proteins, but is diffuse in the presence of eGFP::Cnn-RAT27A (H). By metaphase Cnn-LF protein localizes to centrosomes, normal spindles form (I–L), and most of these embryos complete development. When these transgenes are expressed in a hemizygous background at 25°, Cnn-LF localization to prophase centrosomes is normal in the presence of eGFP::Cnn-RA+ (M) protein and reduced in the presence of eGFP::Cnn-RA22AT27A (N) and eGFP::Cnn-RAS22A (O) proteins. In striking contrast the eGFP::Cnn-RAT27A (P) protein completely blocks initial localization of all Cnn-LF protein during prophase. Consistent with Cnn-LF staining tubulin localization in these embryos (boxed areas in M–P) is normal for eGFP::Cnn-RA+ (Q) embryos, slightly disorganized in eGFP::Cnn-RAS22AT27A (R) embryos, and reduced in eGFP::Cnn-RAS22A (S) embryos. In eGFP::Cnn-RAT27A (T) embryos, tubulin fails to localize to prophase centrosomes. As in the wild-type embryos native Cnn-LF protein eventually forms a functional centrosome and development proceeds, although fertility is decreased compared to that in wild-type embryos. DNA is in blue in all images. Bars, 25 μm for A–D and M–P and 30 × 30 μm for enlarged areas in I–L and Q–T. (Bottom) Western analyses of total embryonic protein extracts from OreR and wild-type embryos grown at 25° expressing the four transgenic constructs show the effect of the transgenic protein on native protein. In the presence of the eGFP::Cnn-RA+ and eGFP::Cnn-RAS22AT27A proteins both native and eGFP fusion proteins are readily detectable. In the presence of eGFP::Cnn-RAS22A protein, native Cnn is slightly reduced and the mutant fusion protein is barely detectable. The eGFP::Cnn-RAT27A protein is not detectable and the pool of native Cnn-LF is significantly reduced, demonstrating the global effect of the transgenic proteins. For both eGFP::Cnn-RAS22A and eGFP::Cnn-RAT27A tubulin is more abundant than the other three genotypes. The tubulin staining for this blot is shown at the bottom.
The ability of the cnn−P mutant transgenes to differentially affect the native pool of Cnn (depending on levels of expression) suggests stoichiometric amounts of transgenic and native Cnn protein might be important. We repeated the above experiment in a cnn hemizygous [cnn+/Df(2R)Cnn] background at 25°. As expected, the Cnn+ control protein has no obvious effect on prophase (Figure 7, M and Q) or throughout mitosis. Unlike the control, the three cnn−P mutant transgenes each have a somewhat unique effect on the centrosome during prophase. In CnnS22AT27A embryos, Cnn localizes to the centrosome (Figure 7N) but MTs are poorly focused and centrosome separation is impaired (Figure 7R). In CnnS22A embryos, Cnn localizes to the prophase centrosome and accumulates in smaller cytoplasmic aggregates around the centrosome (Figure 7O). At these centrosomes microtubules are typically present as distinct foci but the amount of MT staining is reduced compared to that in animals expressing Cnn+ (Figure 7S). The CnnT27A embryos uniquely show Cnn localization during prophase is significantly reduced or undetectable (Figure 7P) and microtubule staining is primarily cytoplasmic (Figure 7T). As in the transgenic UAScnn+ background, by metaphase the Cnn and spindle staining in these hemizygous embryos is relatively normal, but the number of division defects in each embryo is increased relative to that seen in the cnn+/cnn+ backgrounds. While the hemizygous results are similar to our cnn+/cnn+ results at 18°, the frequency of cleavage division defects seen is increased in the hemizygous embryos.
Consistent with the immunostaining results in hemizygous embryos, Western analyses show the single-point mutations have a significant effect on native Cnn protein pools. Both the Cnn+ and CnnS22AT27A transgene-encoded protein products are readily detectable on Western blots as are approximately equal amounts of native Cnn protein (Figure 7, bottom). Native Cnn protein is also abundant in the presence of the UAScnnS22A transgene, although obvious accumulation of the encoded protein is not detected under similar parameters (Figure 7, bottom). In contrast to these results, expression of the UAScnnT27A transgene is associated with an apparent reduction in native protein accumulation despite undetectable mutant protein concentrations under the conditions used (Figure 7, bottom). This clearly shows accumulation of sufficient mutant protein to affect the native protein pool. Taken together these results suggest that loss of the putative Polo kinase target sites has a significant effect on both mutant and native Cnn protein pools.
In cnn null embryos Cnn-PA wild-type and mutant proteins result in a polo-like phenotype
In light of the cnnhk21/Df(2R)Cnn; Dp(2; 3)BAC•cnn ΔEx1A results, we tested the effect of the UAS::cnn+ control transgene and the three UAS::cnn−P transgenes in a cnnhk21/Df(2R)Cnn null background. We have previously shown the UAScnn+ control transgene fails to rescue cnnhk21/Df(2R)Cnn null embryos (Eisman et al. 2009), but reported an improvement only in the number of mitotic divisions. In these tests we wanted to know whether any of the four transgenically encoded proteins accumulated in embryos, whether the majority of nuclear divisions are diploid following syngamy, whether there are polo-like haploid divisions, and whether PBs form.
The results obtained with all four transgenes are similar albeit with key differences, depending on the transgene. The most common nuclear type in prophase stage embryos appears to be haploid with most nuclei located at the cortex (Figure 8, A–D), but we do observe deep divisions in a low percentage (≤5%) in the four embryonic genotypes. Additionally, we do not detect PB formation or the persistent meiotic-like spindle observed in cnnhk21/Df(2R)Cnn null embryos, suggesting all four genotypes have a polo-like phenotype. Finally, none of the transgenes rescue the mutant phenotype and development fails well before cellularization.
Figure 8.
UAS wild-type (WT) and UAScnn−P mutant proteins are sufficient for repeated divisions of meiotic nuclei but not for centrosome formation in a cnn null background. Ectopic expression of the four transgenes at 25° in a cnnhk21 null background fails to rescue the mutant phenotype but is sufficient to switch most embryos to the Polo-like phenotype. (A) In all these animals nuclei are at the cortex and only the eGFP::Cnn-RAT27A protein is not detectable and the pool of native Cnn-LF is significantly reduced, with defined tubulin foci associated with nuclei. (B and C) In both eGFP::Cnn-RAS22AT27A (B) and eGFP::Cnn-RAS22A (C) embryos tubulin foci form in the cytoplasm but these do not appear to be functional centrosomes. In these three genotypes, low levels of punctate Cnn-LF protein are detectable but are not associated with nuclei. In the eGFP::Cnn-RAT27A (D) embryos Cnn-LF protein is absent and tubulin is typically not detectable during prophase. (E–H) During metaphase some spindles have defined tubulin poles in the eGFP::Cnn-RA+ embryos (E), whereas spindles in eGFP::Cnn-RAS22AT27A (F), eGFP::Cnn-RAS22A (G), and eGFP::Cnn-RAT27A (H) embryos lack defined poles. None of the embryos from these four genotypes ever reach cellularization. Cnn-LF is in green, tubulin in red, and DNA in blue. Bars, 25 μm for A–H.
Interesting differences in the organization of centrosomal and spindle MTs at prophase and metaphase are seen among the transgenes. Nuclei in embryos expressing Cnn+ frequently have two defined MT foci at opposite poles during prophase (Figure 8A) and poles are present in metaphase spindles (Figure 8E). Punctate Cnn is present in the cytoplasm, but Cnn+ protein fails to localize to the MT foci or any other tubulin structures. The CnnS22AT27A mutant protein also accumulates at low levels in the cytoplasm during prophase (Figure 8B) and very low levels of protein are detected at some poles of metaphase spindles (Figure 8F). However, unlike Cnn+, CnnS22AT27A-expressing animals do not form defined MT foci during prophase and metaphase spindles are aberrant. In CnnS22A-expressing embryos, MT foci are present at prophase throughout the cytoplasm but most are unassociated with nuclei (Figure 8C), and the resultant metaphase spindles are barrel shaped and frequently fuse (Figure 8G). Low levels of the mutant CnnS22A protein can be detected at metaphase chromosomes in some nuclei. Conversely, CnnT27A-expressing animals do not organize MTs during prophase (Figure 8D) and metaphase spindles are all aberrant (Figure 8H), exhibiting the fewest divisions of the four transgenes.
The phenotypes associated with each transgene are consistent with the effects of the transgenes on the pool of native Cnn protein. The Cnn+ protein improves division but does not support development. The CnnS22A mutant peptide allows the formation of MT foci but these either form in the cytoplasm or rapidly dissociate from nuclei, whereas CnnT27A mutant animals never organize MT foci and spindles are poorly organized. In both of the single-mutant phenotypes, the mutant protein is barely detectable throughout the embryo, although CnnS22A accumulates at some nuclei. The CnnS22AT27A mutant animals form weak MT foci during prophase. While some mutant protein accumulates at poles of metaphase spindles, these spindles are not discernably different from the spindles seen in CnnS22A-expressing animals.
Truncated Cnn-LF protein reveals the requirement for phosphorylation of exon 1A
These results suggest the UAS transgene-encoded protein products interact with the pool of Cnn-LF proteins and may form some type of heterologous multimeric protein complex. Additionally, it appears that Cnn-SF protein is required for the completion of female meiosis and PB formation, thereby blocking the polo-like phenotype. Unfortunately these results provide few clues regarding the role of phosphorylation of exon 1A Cnn-LF proteins, as a single copy of native cnn is sufficient to rescue function during each division cycle and the complete loss of cnn results in the polo-like phenotype. We therefore expressed the four UAS transgenes in a cnnmfs3/Df(2R)Cnn hypomorphic background. In addition to normal Cnn-SF protein, the cnnmfs3 allele makes a stable phosphorylated truncated Cnn-LF protein that mislocalizes to chromosomes during syncytial divisions (Eisman et al. 2009).
We began with rescue experiments, defining rescue as the number of adults that eclose from the total number of eggs oviposited by cnnmfs3 sterile mutant phenotype females crossed to Oregon R males. The UAScnn+ transgene rescues 32% of the eggs (n = 1432), UAScnnS22AT27A rescues 14% (n = 2127), and UAScnnS22A and UAScnnT27A transgenes each rescue 1% of the eggs (n = 837 and n = 574, respectively). In cnnmfs7 mutant embryos the UAScnn+ and UAScnnS22AT27A transgenes have similar rescue rates, the UAScnnS22A transgene rescues 0.5%, and the UAScnnT27A transgene fails to rescue. It is important to note these transgenes have to rescue only the first 10–12 division cycles and supply enough normal diploid nuclei to support development once zygotic expression of the paternally supplied cnn+ is initiated.
To characterize the rescue phenotypes we first looked at the accumulation of GFP in live embryos during syncytial development. The Cnn+ protein initially accumulates in multiple aggregates surrounding prophase nuclei (Figure 9A), which resolve into two primary foci with a haze surrounding nuclei. Similar to the Cnn+ protein, the CnnS22AT27A protein forms aggregates surrounding prophase nuclei but these aggregates are larger and have more amorphic shapes (Figure 9B). However, both the CnnS22A (Figure 9C) and CnnT27A (Figure 9D) proteins fail to form dense foci and instead accumulate in spheres of various sizes. These GFP-positive spheres are at or near the embryonic cortex and do not form a pattern that can be correlated with centrosomal or nuclear behavior during division. While the total number and size of spheres are variable among embryos, the CnnT27A protein tends to form larger and more abundant spheres compared to the CnnS22A protein. We do not observe these spheres in embryos expressing the Cnn+ or CnnS22AT27A proteins.
Figure 9.
(Top) Embryonic phenotypes suggest Cnn phosphomutant and Cnnmfs3 hypomorphic proteins may interact during mitosis. (A–D) Live imaging shows both the eGFP::Cnn-RA+ (A) and eGFP::Cnn-RAS22AT27A (B) fusion proteins accumulate at the centrosome and in multiple aggregates surrounding nuclei. The intensity of the eGFP::Cnn-RAS22A (C) and eGFP::Cnn-RAT27A (D) fusion proteins is always reduced and is localized to spheres of various sizes scattered throughout the embryo. (E–H) In immunostained embryos during prophase, Cnn-LF antibody (green) localizes exclusively to the centrosome in eGFP::Cnn-RA+ (E) embryos, to the centrosome and assorted aggregates in eGFP::Cnn-RAS22AT27A (F) embryos, and weakly to the centrosomes and cytoplasmic puncta in eGFP::Cnn-RAS22A (G) embryos and is not detected in the eGFP::Cnn-RAT27A (H) embryos even though large tubulin foci are present. We do not see staining of aggregates in eGFP::Cnn-RA+ embryos or the spheres in eGFP::Cnn-RAS22A and eGFP::Cnn-RAT27A embryos. (I) At metaphase many embryos have normal spindles with Cnn-LF at the centrosomes in eGFP::Cnn-RA+ embryos, although only one-third of these embryos will hatch. (J–L) In contrast, metaphase in the majority of the eGFP::Cnn-RAS22AT27A (J), eGFP::Cnn-RAS22A (K), and eGFP::Cnn-RAT27A (L) embryos resembles the phenotype described for cnnmfs3 mutant embryos. (M and N) γ-Tubulin (green) localization at prophase centrosomes is normal in eGFP::Cnn-RA+ (M) embryos and excessive in eGFP::Cnn-RAS22AT27A (N) embryos, which may cause the spindle problems seen at metaphase (J). (O and P) Even though tubulin foci are associated with prophase nuclei in eGFP::Cnn-RAS22A (O) and eGFP::Cnn-RAT27A (P) embryos, γ-tubulin is barely detectable at a few foci and absent at most, indicating these are not fully functional centrosomes. These prophase tubulin foci are not common in cnnmfs3 mutant embryos. Tubulin is shown in red and DNA in blue in all images. Bars, 25 μm for A–P. (Bottom) Western analysis of the four transgenes in a cnnmf3 hypomorphic background is similar to Western analysis in a cnn wild-type background. The first four lanes (left to right) show native Cnn-LF from OreR and the eGFP fusion protein from the BAC [Dp(2; 3)Bac•cnneGFP]. The next four lanes show truncated Cnnmfs3 protein accumulates in all four transgenic embryos, but only the eGFP::Cnn-RA+ and eGFP::Cnn-RAS22AT27A fusion proteins are detected after a short exposure. After an overnight exposure (not shown) eGFP::Cnn-RAS22A protein and very low levels of eGFP::Cnn-RAT27A are detected. α-Tubulin is shown at the bottom.
Although live imaging is informative with respect to the pool of transgenic protein, it provides no information about the behavior of MT structures, nuclei, or mutant Cnn protein produced by the cnnmfs3 allele during mitosis. Immunostaining results do not recapitulate the patterns observed for eGFP in live embryos. In fixed embryos expressing the UAScnn+ transgene, Cnn-LF antibody localizes to prophase (Figure 9E) and metaphase (Figure 9I) centrosomes but never localizes to multiple aggregates. Additionally, GFP antibody staining is similar to Cnn-LF staining (data not shown), suggesting the aggregates are not due to the accumulation of GFP that has been cleaved from the fusion protein. Cnn-LF antibody does localize to both prophase centrosomes and aggregates in embryos with the CnnS22AT27A protein (Figure 9F), although aggregate staining is not as abundant as that seen in live embryos. During metaphase, the majority of CnnS22AT27A-expressing embryos show Cnn-LF antibody is barely detectable or absent at spindle poles, suggesting accumulated prophase protein may not be fully functional and is degraded or dissociated during metaphase. In CnnS22A-expressing embryos Cnn-LF antibody does localize weakly to MT foci during prophase (Figure 9G) and to spindle poles during metaphase (Figure 9K) in a few embryos, but the majority of embryos have no significant development. In contrast to the CnnS22A mutation, Cnn-LF antibody is barely detectable during prophase in CnnT27A-expressing embryos (Figure 9H) and is localized around the chromosomes during metaphase (Figure 9L), similar to what is observed in cnnmfs3 embryos without the transgene. As with the CnnS22A-expressing embryos, most of the CnnT27A-expressing animals fail to develop. There is cytoplasmic staining of small aggregates in CnnS22A embryos and diffuse cytoplasmic staining during metaphase in CnnT27A embryos, but there is no evidence of the spheres observed in live embryos.
Cnn-LF is required for the localization of γ-tubulin to mitotic centrosomes (Megraw et al. 1999; Megraw and Kaufman 2000). In the four genotypes investigated, there are usually two MT foci at nuclei despite variable accumulation of Cnn-LF at these foci. In the presence of Cnn+ protein, small distinct foci of γ-tubulin are present at prophase MT foci (Figure 9M), similar to results in wild-type embryos. Surprisingly, the presence of UAS-driven CnnS22AT27A protein shows excessive accumulation of γ-tubulin at prophase centrosomes (Figure 9N), frequently forming foci equivalent in size to Cnn foci. Consistent with results presented above, γ-tubulin does not localize to MT foci in the presence of either CnnS22A protein (Figure 9O) or CnnT27A protein (Figure 9P), suggesting these MT foci are not functionally normal centrosomes.
Consistent with both the live imaging and immunostaining data, we can readily detect the Cnn+ and CnnS22AT27A transgenically encoded proteins on Western blots, as well as the truncated Cnn-LFmfs3 protein in all four genotypes (Figure 9, bottom). In contrast, CnnS22A protein is detectable at low levels after an overnight exposure, whereas the CnnT27A protein is not (not shown), even though the live imaging suggests there is abundant protein present. While the truncated Cnn-LFmfs3 protein is detected by Western blots in all four genotypes, the immunostaining pattern described for this protein in embryos (Eisman et al. 2009) is observed only in the CnnT27A-expressing embryos.
These data indicate the importance of stoichiometry of Cnn splice variants during syncytial development as even the transgenic Cnn+ protein rescues only one-third of the eggs produced by mutant females. Additionally, phosphorylation of the S22 and T27 residues during cleavage mitoses is likely to be important for normal cycling of Cnn during division. Finally, in the presence of truncated Cnn-LFmfs3 protein, the CnnS22AT27A protein has a less severe effect on division than either the S22A or T27A mutations alone. As with the effects seen when a wild-type Cnn protein expressed from the resident cnn gene is present, the protein produced by the cnnmfs3 allele may interact directly with the transgenically encoded proteins. Rather than the proteins having a dominant negative effect on wild-type Cnn, the truncated Cnn-LFmfs3 protein restores partial function to the cnn-P mutant proteins, and the cnn−P mutant proteins may restore a functional carboxy terminus to the heterologous multimer.
Cnn-LF proteins directly interact with activated Polo kinase during mitosis
These results suggest a direct interaction between Cnn-LF protein and Polo kinase. To test for an interaction we initially tried co-immunoprecipitation (co-IP), using GFP nanobodies conjugated to agarose beads (Neumuller et al. 2012), but found both native Cnn-LF and Polo kinase precipitated with underivitized agarose beads. Cnn-LF protein binding apparently requires the carboxy terminus as truncated Cnnmfs3 protein fails to bind agarose, whereas Polo binds even in the absence of Cnn (Figure S4). In a further attempt we found that native Cnn-LF protein also efficiently binds to GFP nanobodies conjugated with magnetic beads, suggesting any charged surface may be sufficient for nonspecific Cnn binding (Figure S4). The majority of Cnn-LF elutes from both substrates only when samples are boiled, although some Cnn-LF will elute from beads without boiling under gentle wash conditions or when the amount of total protein loaded on the beads exceeds 3 mg. These results suggest this is an important control when immune precipitating Cnn-LF protein from complex extracts and raise questions about numerous results reporting co-IP findings that include Cnn.
Subsequently, we found that immunoprecipitation was possible using Protein A conjugated to magnetic beads coupled with antibodies to Cnn, GFP, and a phosphorylated form of Plk1 (see Materials and Methods). Based on our genetic and confocal results, we hoped to determine whether Cnn-LF and Polo interact directly during mitosis and wanted to investigate the formation of Cnn-LF heteromultimers. To test the latter possibility, we used a Dp(2; 3)Bac•cnneGFP::Ex1A line that expresses a copy of native cnn, produced by a gene conversion event at the cnnhk21 locus (Coveny et al. 2002; Osada and Innan 2008; Casola et al. 2010). In the genotypes tested, the Cnn-LF and GFP antibodies precipitate only Cnn-LF or GFP-tagged proteins, respectively. In contrast, the antibody against activated Plk1, phosphorylated at Thr210 in Plk1 (Archambault and Carmena 2012; Bruinsma et al. 2012; Xu et al. 2013), which is equivalent to Thr182 in D. melanogaster Polo (Carmena et al. 2012, 2014; Kachaner et al. 2014), efficiently co-IPs both Cnn-LF and Polo proteins (Figure 10). The GFP antibody precipitates GFP-tagged Polo and co-IPs both GFP-tagged and native Cnn-LF protein, but does not precipitate Cnn in the absence of GFP-tagged Cnn protein (Figure 10). This latter result is evidence for the formation of heteromultimers between Cnn-LF molecules during mitosis. Additionally, these results suggest only activated Polo kinase is associated with Cnn-LF at mitosis and the majority of Cnn-LF and Polo proteins are not associated in total protein extracts.
Figure 10.
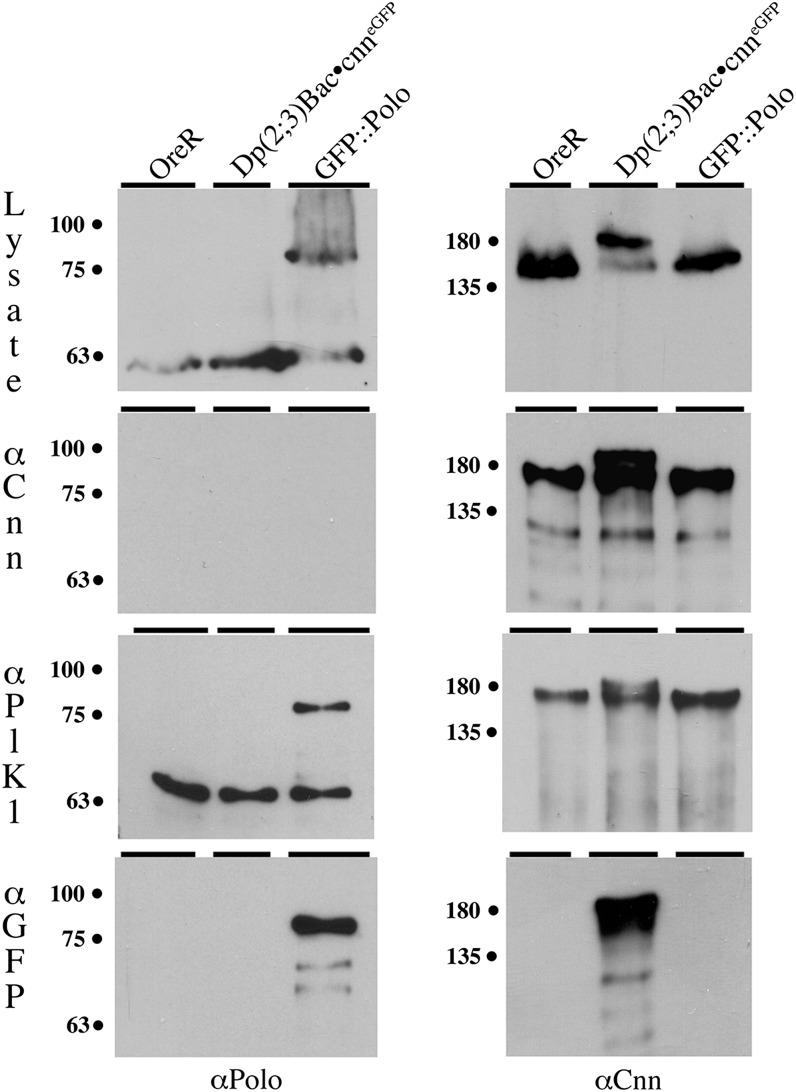
Only mitotically active Polo kinase interacts with Cnn-LF protein. Western blots stained with either Polo (left) or Cnn (right) antibodies (bottom labels) show the results for IP experiments of three genotypes (top labels) precipitated with three different antibodies (left-hand labels). Due to a gene conversion event in the Dp(2; 3)Bac•cnn+eGFP::Ex1A line this strain now expresses native Cnn-LF protein in addition to the GFP fusion BAC protein. Western blots show Cnn-LF and Polo native and GFP fusion proteins are present in the lysate (top row). Immunoprecipitation results show the Cnn antibody IPs only Cnn-LF native and GFP fusion proteins (second row). In contrast, the Plk1 antibody co-IPs both Polo and Cnn-LF native and GFP fusion proteins (third row), demonstrating a direct interaction between mitotically active Polo kinase and Cnn-LF proteins. The GFP antibody IPs just the Polo GFP fusion protein (left, bottom row), and IPs both Cnn-LF native and GFP fusion proteins in the BAC line (center lane, right, bottom row). Since the GFP antibody (bottom) does not IP native Cnn-LF from the OreR or GFP::Polo embryonic extracts, this result demonstrates the formation of Cnn-LF hetero-multimers during mitosis.
While Polo kinase clearly is associated with Cnn-LF protein, these data do not provide any insights into the Cnn residues involved. However, if this interaction involves S22 or T27, the antibody to activated Polo should co-IP the UAScnn+ encoded protein but not the UAScnnS22AT27A encoded protein when expressed with native Cnn-LF. Western analysis of the IP experiments shows the UAS proteins are present at low levels in the lysate compared to native Cnn (Figure 11). Consistent with the above co-IP results, activated Polo kinase co-IPs with native Cnn-LF, GFP-tagged BAC, and the UASCnn+ proteins, but not with the UASCnnS22AT27A protein (Figure 11). Although additional biochemical analyses are required, this result suggests residues encoded by exon 1A are required for the Cnn–Polo kinase interaction. While these IP experiments investigate the simultaneous loss of S22 and T27, the negative effect on native Cnn-LF when either residue is mutated, coupled with their mimic of Polo kinase deficiency, makes it likely both residues are necessary for this interaction.
Figure 11.
The Cnn-LF-Polo kinase interaction requires cnn exon 1A S22 and T27 residues. Western blots stained with Polo (left) or Cnn (center and right) antibodies (bottom labels) show the results for the co-IP of proteins from the Dp(2; 3)Bac••cnn+eGFP::Ex1A line used in Figure 10 and the UAScnn+ (center lanes) or UAScnnS22AT27A (right lanes) lines expressed in a hemizygous cnn background. Blots show Polo (left panel), native Cnn-LF (center panel), and GFP-tagged Cnn-LF proteins (right panel) are present in the lysate. Antibody against mitotically active Polo kinase IPs Polo (bottom left panel), although the amount of activated Polo is less in the UAS lines even though Polo is present in nearly equal amounts in the lysate. Activated Polo co-IPs with native Cnn-LF in all three lines (bottom, center and right panels) and GFP-tagged BAC Cnn-LF proteins after 30-sec (center panel) and 10-min exposure times (right panel) of the same blot. The UAScnn+ is detectable on the longer exposure of the blot (center lane, right panel), but activated Polo does not co-IP the UAScnnS22AT27A protein, suggesting these residues are required for the Cnn–Polo protein interaction. These Western results are consistent with immunostaining results of centrosomes in syncytial embryos.
Discussion
D. melanogaster Cnn is necessary for the formation of the centrosomal PCM during embryogenesis (Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999; Eisman et al. 2009), gametogenesis (Li et al. 1998; Yamashita et al. 2003; Eisman et al. 2009), asymmetric larval neuroblast divisions, and mitosis in tissue culture cells (Megraw and Kaufman 2000; Dobbelaere et al. 2008). Additionally, Cnn is required to maintain the centriole pair at the center of the PCM (Lucas and Raff 2007) and is an important determinant of centrosome size at mitosis (Conduit et al. 2010; Gopalakrishnan et al. 2012). However, Cnn has been shown to be essential only for early embryogenesis and spermatogenesis as nucleation of MTs by chromatin and spindle-associated mechanisms found in many eukaryotes (Luders et al. 2006; Luders and Stearns 2007; O’Connell and Khodjakov 2007) are sufficient for the development of sterile adult flies (Megraw et al. 2001). The complexity of cnn and its differential requirement(s) during development require an assessment of cnn as a single gene encoding a single, one-dimensional protein. Careful attention must be paid to the genotype used, the splice variants present, and the post-translational modification of cnn’s encoded proteins.
As previously reported (Eisman et al. 2009) and revisited in this study, only the cnnhk21 allele is a verified protein null. In all non-hk21 alleles, the mutations produce low levels of truncated Cnn-LF protein and fully functional Cnn-SF protein. The cnne00441 allele produces an aberrant Cnn-SF protein that remains cytoplasmic during embryogenesis, as well as low levels of full-length Cnn-LF protein (Figure S1). While Western analyses show truncated Cnn-LF protein does not accumulate to easily detectable levels in all but cnnmfs3 mutant embryos, immunostaining reveals low levels of protein associated with spindles and chromosomes. Since these truncation mutants retain one to three of the highly conserved motifs present in all Cnn-LF splice variants (Eisman and Kaufman 2013), it is possible they retain partial Cnn function. Additionally, Cnn-SF is functional in all but the cnne00441 allele and localizes to spindle poles during syncytial development. In wild-type embryos Cnn-SF localizes to polar bodies and cycles onto the centrosome at late telophase and prophase and off by anaphase during each cleavage division (Eisman et al. 2009). In Cnn-LF truncation mutants, Cnn-SF protein localizes to well-focused spindle poles that decrease in number as development proceeds and the protein fails to cycle off during division cycles. Since Cnn-SF proteins contain the highly conserved KFC motif and persist at poles throughout syncytial cell cycles, in the absence of normal Cnn-LF the short form proteins may provide a redundant MT nucleation function. Additionally, development aborts much earlier in cnnhk21 mutant embryos than in all the other mutant alleles, including cnne00441. Including this study, the accumulated data for cnn mutations show the presence or absence of Cnn-SF proteins and the severity of the Cnn-LF truncations are important considerations when choosing cnn genotypes for experimental analyses.
The role of cnn transcription presents another confounding factor. Recent modENCODE RNA-Seq data (Graveley et al. 2011; Brown et al. 2014) have provided new insights into promoter usage during development, making it possible to predict the proteins present based on initiating coding exons. Our analysis of these data shows that the exon 1A promoter is used throughout development and in adults (Figure 1). Moreover, it is the only detected maternal promoter in the oocyte transcript pool. Additionally, the primary transcript in this pool encodes the Cnn-PA long form protein, as the unique exons encoding Cnn-SF proteins are present only at very low levels in early embryos. As syncytial development completes and zygotic expression begins, the exon 1B promoter is active in addition to the exon 1A promoter, and both pools of transcripts are present in embryos and adult tissues. The exon 1C promoter is initially active in late third instar larvae and is present in pupae, adult males, and testes, but is absent in adult females and ovaries, suggesting this promoter is male specific and likely testes and potentially germline specific. Different development stages and tissues may have unique pools of Cnn proteins with unique amino termini and potentially very different post-translational regulatory sites. Our own comparative 2D Western analyses of the previously described pool of embryonic Cnn-LF phosphoproteins (Eisman et al. 2009) in several tissues and tissue culture cell lines are consistent with this prediction. These analyses show no two tissue culture cell types have the same pattern of modification, more than one splice variant may be present, and the pI values of the expressed pool in the adult head suggest that exon 1B-encoded proteins may accumulate exclusively (Figure S2). These results are consistent with several global phosphoproteomic studies in D. melanogaster that have to date identified 16 phosphorylated residues in Cnn-LF proteins (Figure S3). The identified residues strongly suggest Cnn is a substrate for several kinases, demonstrating the complexity of the post-translational regulation by phosphorylation of Cnn during development.
We focused primarily on exon 1A transcripts, as they appear to make up nearly the entire maternal load in oocytes. Exon 1A is conserved within the genus Drosophila and contains a dual PKC/CKII phosphorylation site immediately upstream of the only Polo consensus phosphorylation site encoded by the cnn gene. The phosphorylated serine in the PKC/CKII site is also centered on the core consensus sequence of a low-affinity Polo-binding motif. Cnn-LF proteins are reportedly phosphorylated by a Polo-dependent reaction in S2R+ tissue culture cells (Dobbelaere et al. 2008). However, that study identified just two major Cnn foci on 2D Western blots, which were interpreted as phosphorylated and nonphosphorylated forms of Cnn-LF. Paradoxically the pI shift between the foci appears to be much larger than expected for the addition of a single phosphate group. In contrast, our own analysis of S2R+ cells identified multiple Cnn-LF foci with an average pI shift between foci of ∼0.2, consistent with the addition of multiple single phosphate groups to the Cnn-PA splice variant, precisely as predicted by ProMoST (Bonner et al. 2013) (http://proteomics.mcw.edu/promost.html) (also see Figure S2). While we cannot explain the significant differences between these two studies, they may be due to inherent disparities between the two S2R+ cell lines used and the six other cell lines we investigated (Figure S2). Additionally, while the earlier study found the appearance of the more acidic Cnn focus in S2R+ cells was Polo dependent, the large shift in pI between foci suggests a protein modification other than the simple addition of a single phosphate group to Cnn-LF proteins.
A recent study claims to have identified a serine residue in a nonconsensus Polo recognition sequence that is phosphorylated by Polo, based on the finding that Plk1 phosphorylates this residue in vitro (Conduit et al. 2014) (for the peptide used see Figure S3). In embryos lacking native Cnn-LF, localization of mutant Cnn-LF proteins with alanine in place of this serine is reduced by only 20% compared to that in control embryos. In fact, even when 10 serine and threonine residues are mutated simultaneously to alanine, localization of the mutant protein is reduced by 50% and partially rescues the mutant phenotype. These in vivo results are in striking disagreement with the findings that RNAi depletion of Polo (Dobbelaere et al. 2008) and inhibition of Polo (Fu and Glover 2012) completely block localization of Cnn to mitotic centrosomes. Additionally, 8 of these residues are covered by a peptide recovered by MS analyses of the phosphoproteome but none were found to be phosphorylated in vivo. Since the peptide region investigated contains a leucine zipper, a structural region conserved in the Insecta (Eisman and Kaufman 2013), it seems more likely multiple mutations have a deleterious effect on Cnn-LF protein folding rather than phosphorylation by Polo.
To determine the localization pattern of exon 1A-encoded proteins in embryos, we tagged the exon with eGFP in a cnn-containing BAC and maintained a transgenic BAC copy in a cnnhk21 null background. The fusion protein or proteins localize to the centrosome throughout syncytial development and at mitotic centrosomes in cellular embryos. Although additional analyses will be necessary to know the full complement of Cnn PCM proteins in all cell types, the potential Polo-interacting exon 1A proteins are likely to be in the PCM during mitosis in many cell types.
The phenotype associated with the loss of just exon 1A-encoded proteins should be the same as that seen in cnnhk21 null embryos. When there is development, the cnnhk21 embryonic phenotype is variable with few deep and/or cortical nuclear divisions, no polar bodies are formed, and persistent meiotic-like spindles are found at the cortex. Surprisingly, the cnnhk21 embryos carrying Dp(2; 3)BAC•cnn ΔEx1A show two distinct phenotypes. The first one is seen in embryos, which show no development, similar to cnnhk21 animals. However, unlike cnnhk21 animals the majority of embryos (>60%) undergo several to many cortical divisions and lack detectable deep divisions. The Dp(2; 3)BAC•cnn ΔEx1A embryos lack polar bodies but do not exhibit the persistent meiotic-like spindles found in many cnnhk21 embryos. The spindle morphology, initial nuclear size, location at the cortex, and absence of polar bodies all suggest the observed mitotic divisions are the product of repeated nuclear division cycles of the haploid female meiotic products. This is identical to the phenotype described for the polo1 kinase mutant allele (Riparbelli et al. 2000), implying a Polo–Cnn interaction is required to block meiotic product nuclear division after meiosis II completes. A similar but less dramatic phenotype is observed in 7% of the cnnhk21 mutant embryos, indicating the addition of the Dp(2; 3)BAC•cnn ΔEx1A to this background changes oocyte quality. Some property or encoded product of Dp(2; 3)BAC•cnn ΔEx1A appears to block the formation and/or proliferation of zygotic nuclei while enhancing the division of haploid meiotic nuclei. Consideration of a potential Cnn contribution to the Polo-like phenotype leads us to conclude that the most likely candidate for blocking meiotic product divisions is the exon 1A Cnn-SF protein, as antibodies to the protein localize to polar bodies. We generated embryos carrying the Dp(2; 3)BAC•cnn ΔEx1A in cnnmfs3 and cnnmfs7 backgrounds. These mutant alleles produce hypomorphic and loss-of-function Cnn-LF protein, respectively, but produce fully functional Cnn-SF protein. Polar bodies form, deep zygotic divisions occur, and eventually these embryos fail with the classic cnn mutant phenotype. These results implicate a Polo-independent requirement for Cnn-LF proteins during the completion of meiosis, since all genotypes with some form of Cnn-LF protein complete meiosis normally, unlike the cnnhk21 null embryos. Following meiosis, Cnn-SF proteins are required for the formation of polar bodies, a Polo kinase-dependent function. While future studies will be necessary to determine a direct or indirect Polo–Cnn interaction, we favor a model in which Polo directly phosphorylates exon 1A Cnn-SF during polar body formation.
If exon 1A is a substrate for potential Polo binding and/or phosphorylation by Polo, elimination of these sites in Cnn-LF protein should affect the function of these proteins in embryos. We generated UAS eGFP::Cnn-PA transgenes that mimic wild type or block phosphorylation at the PBD motif, at the Polo target site, or at the PBD and Polo target site together. These transgenes were then ectopically expressed using the nanos::Gal4 driver in cnn wild-type, hemizygous, cnnhk21, cnnmfs3, and cnnmfs7 backgrounds. These experiments provided new insight into interactions between Cnn-LF proteins and why both Polo and Cnn are required for the initial formation of the PCM at mitotic centrosomes.
We initially intended to use the UAS transgenes in wild-type embryos to determine localization patterns of the fusion proteins and potential effects on native Cnn and the centrosome. The experiments expressing fusion protein in embryos were done at three temperatures to increase and decrease transcriptional activation by GAL4. The Cnn+-encoding transgene in wild-type embryos at all three temperatures had no obvious effect on the centrosome or nuclear division, consistent with this transgene rescuing all cnn mutants except cnnhk21. Surprisingly, potentially blocking Polo binding and phosphorylation of Cnn by Polo (CnnS22AT27A), Polo binding (CnnS22A), or phosphorylation of Cnn by Polo (CnnT27A) all impede localization of native Cnn to the prophase centrosome. During this stage of the cell cycle Cnn flares move in and out of the centrosome (Megraw et al. 2002) and the amount of Cnn in replicated centrosomes increases (Conduit and Raff 2010). Formation and separation of MT foci at apparent centrosomes is relatively unaffected by either the CnnS22AT27A- or the CnnS22A-encoding transgenes, whereas the CnnT27A-encoding transgene disrupts the organization of MT foci and delays separation of these structures. These effects delay prophase but eventually native Cnn accumulates at centrosomes and most metaphase spindles appear to be normal. Most of these embryos develop into adults, indicating the defects observed are temporary during each cell cycle. The dominant negative effects of the transgenes are observed at all three temperatures but are less severe when levels of the transgenic products are reduced.
Our results indicate the stoichiometry of transgene protein and native Cnn is important. To verify the dominant negative effects found at 29° we repeated these experiments at 25° in hemizygous embryos carrying a single copy of native cnn. As with the above tests the Cnn+ protein has no deleterious effect on native Cnn and the rapid cleavage divisions. However, under these conditions PCM-associated Cnn at prophase is variable in CnnS22AT27A embryos, reduced in CnnS22A embryos, and undetectable at most centrosomes in CnnT27A embryos. Additionally, centrosomal MT foci are variable and poorly formed in CnnS22AT27A embryos, reduced in size in CnnS22A embryos, and completely disorganized in the CnnT27A embryos. The apparent defects in MT organization are temporary, as native Cnn eventually accumulates at metaphase centrosomes, and many hemizygous embryos hatch, although fertility is reduced compared to wild type. To our knowledge, loss of several gene products causes a significant reduction in the amount of Cnn at the PCM, but only the loss or inhibition of Polo kinase completely blocks the localization of native Cnn to the PCM (Dobbelaere et al. 2008; Fu and Glover 2012).
These results provide new evidence for several aspects of Cnn regulation during the cell and centrosome cycle in embryos. The initial step in Cnn localization to the centrosome occurs in the cytoplasm or a subcellular compartment such as the endoplasmic reticulum and/or the Golgi. Based on our mutations to putative phosphorylation sites, the dual S22 PKC/CKII site in Cnn exon 1A is likely phosphorylated, although phosphorylation may not be required at low-affinity Polo-binding motifs (Santamaria et al. 2011) and is not required for the interaction between Map205 and Polo (Archambault et al. 2008). Following the putative binding of Polo to this site, Polo must phosphorylate Cnn at T27. Because the effects on Cnn are similar for the three cnn−P mutant proteins, these events are likely to occur in rapid succession. Although nothing is known about the oligomerization state of Cnn, these results imply Cnn proteins form some type of multimer as the cnn−P mutant proteins effectively block the normal processing of native Cnn protein. Perhaps most importantly these effects are observed for the CnnS22A and CnnT27A proteins that are not detectable by Western analysis or immunostaining. While it is possible very low levels are sufficient to indirectly block this process, the observed reduction of native Cnn in these embryos suggests rapid degradation of hetero-multimers containing mutant and native Cnn, possibly due to incorrect protein folding. These predictions are supported by our results from Western analyses of the single-site mutations presenting a much greater effect on protein stability than the CnnS22AT27A double mutant where phosphorylation is blocked. If hetero-multimers form, the sequential phosphorylation and binding steps required for Cnn localization occur via the native Cnn proteins and prevent the degradation of Cnn proteins. We tested these possibilities by cnn−P mutant transgene expression in cnnhk21 and cnnmfs3 mutant embryos.
Expression of the four transgenes in cnnhk21 null embryos results in the Polo-like phenotype. With the exception of the Cnn+ protein, most embryos have fewer divisions and nuclei are poorly organized compared to Dp(2; 3)BAC•cnn ΔEx1A embryos. Polar bodies fail to form and the defects associated with nontransgenic cnnhk21 null embryos are absent. This is consistent with our prediction that any form of Cnn-LF protein is sufficient to improve the completion of meiosis, and in the absence of Cnn-SF protein meiotic nuclei replicate and divide repeatedly. Although the phenotype associated with each transgene is similar, key differences among genotypes indicate the functionality and stability of each protein. The Cnn+, CnnS22AT27A, and CnnS22A proteins accumulate at low levels in the cytoplasm without evidence of centrosomal function, even though CnnS22AT27A does aggregate near some metaphase spindle poles. In contrast to these peptides, the CnnT27A protein is not detectable at prophase, consistent with the instability of the mutant protein. Interestingly, the Cnn+ embryos form MT foci at prophase nuclei and metaphase spindle poles even though no Cnn is detected. While the four transgenes transform embryos from the cnnhk21 phenotype to the Polo-like phenotype, none of these proteins rescue embryogenesis. This failure to rescue will be the subject of future investigations, but the absence of Cnn-SF protein and defects in oogenesis are likely candidates.
The cnnhk21 null background is informative but limited, since the repeated divisions of all female meiotic products essentially block the proliferation of any deep nuclei provided by the male at fertilization or produced by syngamy. In the absence of any Cnn-LF protein, it is impossible to test our hypothesis that hetero-multimers form between native and cnn−P mutant proteins. The cnnmfs3 mutant allele is unique in that it produces a stable truncated Cnn-LF protein that is post-translationally modified, but fails to localize to the centrosome (Eisman et al. 2009). Our previous experiments showed the Cnn+ protein would rescue the embryonic phenotype. The cnn−P mutant proteins should not, unless the Cnnmfs3 protein provided the two phosphorylation sites in exon 1A and the cnn−P mutant protein provided a functional carboxy terminus required for centrosomal localization (i.e., there was complementation between the two defective peptides) in a trans-heterodimer.
The Cnn+ protein rescued the expected one-third of the embryos and, somewhat surprisingly, the CnnS22AT27A protein rescued 14% of the embryos. Live analyses of these two proteins revealed multiple GFP aggregates surrounding nuclei during late telophase to early prophase, when Cnn normally localizes to the replicated centrosomes. Although these live GFP aggregates are abundant in embryos at this stage, immunostaining for GFP and Cnn-LF shows centrosomal staining but fails to detect aggregates in the Cnn+ embryos with the exception of a small number in the CnnS22AT27A embryos. This failure to stain the cytoplasmic aggregates may be due to the rapid degradation of aggregates during our fixation process, or possibly the protein epitopes are masked by lysosomal vesicles or aberrant protein folding.
Consistent with all the experiments in this study, the CnnS22A and CnnT27A single mutants fail to accumulate to significant levels in embryos, with the CnnS22A protein detected by Western analysis only after overnight exposures. Additionally, the CnnT27A protein causes a reduction in the amount of Cnnmfs3 protein in the total embryonic pool, suggesting an interaction between the two proteins. In most CnnS22A and CnnT27A fixed embryos, Cnn is not detectable and mitosis is aberrant, most likely because Cnn is sequestered in the vesicles present in live GFP images, which do not stain with our antibodies. However, in a small percentage of embryos (1%) low levels of Cnn-LF protein are detected at prophase centrosomes and the MT foci surrounding centrioles are nearly normal in both genotypes. Since the Cnnmfs3 protein alone fails to localize to centrosomes, the Cnn present at these centrosomes is likely to be hetero-multimers with a stoichiometric combination of Cnnmfs3 protein and cnn−P mutant protein that restores partial function and avoids the degradation pathway. In the few embryos (1%) where this occurs, enough diploid nuclei are produced during the early cleavage divisions to support development after zygotic expression of the paternally supplied wild-type cnn begins. We feel the results of these experiments in cnnmfs3 embryos argue strongly for the formation of Cnn hetero-multimers.
The γ-tubulin staining at centrosomes in cnnmfs3; UAS::cnn-P embryos strengthens the hetero-multimer hypothesis. Normal localization of γ-tubulin to the centrosome requires functional Cnn-LF protein (Megraw et al. 1999; Vaizel-Ohayon and Schejter 1999) and the amount of γ-tubulin in the PCM is proportional to the amount of Cnn-LF protein in the PCM (Conduit and Raff 2010). As expected, localization of γ-tubulin in Cnn+ embryos is normal but is not detected at centrosomes in the CnnS22A and CnnT27A embryos. In striking contrast to these results, γ-tubulin is in excess at the centrosomes of CnnS22AT27A embryos, which must be due to the presence of the double cnn−P mutant protein. Since this protein perturbs localization of native Cnn in wild-type embryos and fails to localize in cnnhk21 embryos, the most likely mechanism for localization is to form hetero-multimers with Cnnmfs3 protein. This result also suggests phosphorylation at Ser22 and Thr27 and possibly the subsequent activity of phosphatases are required to regulate the amount of γ-tubulin at the mitotic centrosome.
The IP experiments further support the formation of Cnn-LF hetero-multimers, as GFP antibody co-IPs both native and GFP-tagged Cnn from embryonic extracts expressing both protein forms. When coupled with the apparent functional trans-complementation when Cnn−P proteins are expressed with cnnmfs alleles, this demonstrates the importance of genotype when investigating Cnn function. Our IP results also show antibodies against mitotically active Polo kinase co-IP Cnn and Polo, whereas antibodies against Cnn and GFP efficiently IP their respective target proteins but do not co-IP the binding partners. This suggests only a small portion of the Cnn-LF and Polo protein pools interact during mitosis and this interaction is probably transient at the onset of the centrosome cycle when Cnn rapidly localizes to replicating centrosomes. The finding that mitotically active Polo kinase also co-IPs with UAScnn+-encoded protein, but not with UAScnnS22AT27A-encoded protein, supports a direct interaction between Polo and cnn exon 1A-encoded residues. While considerable biochemistry remains to be done to unequivocally identify which residues are responsible for the interaction, the genetic and molecular data support an interaction involving both S22 and T27. The loss of either residue is sufficient to perturb normal dynamics of native Cnn-LF protein, resulting in a phenotype that mimics loss of Polo kinase function at mitotic centrosomes. We propose cnn exon 1A splice variants are the best candidate proteins for future studies of the Cnn–Polo kinase interaction during formation of the centrosomal PCM.
We feel the combined data from this work strongly argue the exon 1A-containing Cnn peptides mediate the interdependent relationship of Cnn and Polo kinase for formation of the PCM at mitotic centrosomes in embryos. The conservation of the exon within the genus Drosophila suggests this interaction is conserved within the genus. Based on these data we propose a model for the Polo–Cnn interaction diagrammed in Figure 12. Ser22 is required for Polo binding and is probably phosphorylated by a kinase prior to Polo binding. In silico analysis predicts PKC and CKII are putative priming kinases, although the reality in flies may be different. This phosphorylation event creates a core sequence capable of binding Polo kinase (Elia et al. 2003a), albeit a low-affinity binding site, but one similar to known binding sites (Kang et al. 2006; Neef et al. 2007; Burkard et al. 2009; Wolfe et al. 2009; Santamaria et al. 2011). Following Polo binding at Ser22, Polo phosphorylates Cnn at Thr27, which is essential for localization of Cnn to the centrosome. These events are initiated away from the centrosome and coincide with the timing of increased Polo localization to the centrosome (Moutinho-Santos et al. 1999). When one or both of these sites cannot be phosphorylated in the presence of native Cnn-LF protein, hetero-multimers form and the cnn−P mutant proteins initially delay the normal localization of Cnn and in the case of the single mutants cause the degradation of mutant and native Cnn. When these same cnn−P mutant proteins form hetero-multimers with truncated Cnnmfs3 protein, the single-site mutations lead to the degradation of Cnn in most embryos, whereas the CnnS22AT27A restores function to at least a portion of the multimers. The finding that Cnn−P single-mutant proteins fail to localize to centrosomes and block normal localization of native Cnn-LF is in complete agreement with data showing Polo kinase is required for Cnn centrosomal localization (Dobbelaere et al. 2008; Fu and Glover 2012). In contrast, the previous report of a Polo target site in the middle of Cnn found loss of three nonconsensus Polo sites and seven other Ser/Thr residues reduces Cnn at the centrosome by only 50% in embryos (Conduit et al. 2014). Although additional biochemistry must be performed to verify our model, the genetics support it.
Figure 12.
Model for the formation of Cnn hetero-multimers and the effect of putative loss of Polo binding and phosphorylation on the Cnn protein pool. (Top) Hetero-multimers between native Cnn-PA and eGFP::Cnn-PA UASp fusion proteins (left) or UASp eGFP::Cnn-PA fusion proteins and native Cnnmfs3 mutant proteins (right), depicted as dimers although the actual oligomerization state of Cnn is unknown. The wild-type UASp transgene functions normally in the presence of wild-type native Cnn and rescues one-third of the embryos in the presence of cnn truncation mutants. (Middle) Schematic representations of the three phosphomutant UASp transgenes and the predicted effect of the mutation on Cnn–Polo kinase interactions. The single amino acid changes have a strong negative influence on the function of native Cnn and perturb the normal transport of Cnn to replicating centrosomes during prophase. The effect of the double mutant (CnnS22AT27A) is more benign and rescues a small percentage of the cnn truncation mutants. (Bottom) A table with a brief description of the immunostaining (IS) and Western (W) results for the three phosphomutant transgenic proteins in cnn wild-type, null, and truncation mutant backgrounds.
Our results indicate some isoform of Cnn-LF protein must mediate a currently unknown function required for the completion of meiosis. Following the completion of meiosis, Cnn-SF protein must be present and presumably phosphorylated by Polo by the mechanism described above for the formation of polar bodies. Provided these events are normal, exon 1A Cnn-LF proteins and activated Polo interact at each embryonic centrosome replication cycle to form the PCM. Of future interest will be the determination of which Cnn splice variants are at the centrosome in different cell types. For example, Polo is not required for Cnn centrosomal localization in larval neuroblasts (Donaldson et al. 2001b), suggesting the exon 1A promoter may not encode this splice variant. Our 2D Western analysis of adult heads found the two isoforms present had pI values similar to those predicted for exon 1B-encoded proteins. When coupled with the modENCODE data it is likely in some cell types Cnn will not be regulated by Polo. In addition to providing new insight into the regulation of Cnn at embryonic centrosomes, it is our hope that this article demonstrates the importance of genotype and some knowledge of which Cnn isoforms may be present in an experimental system. Clearly cnn is complex and any monolithic view of the gene or its protein products should be avoided.
Supplementary Material
Acknowledgments
The authors thank Koen Venken and Hugo Bellen for introducing us to the joys of recombineering and making the ΨC31 recipient stocks and the BAC library. We also appreciate the efforts of Roger Hoskins and Karen Schultze for their alignment and characterization of the BAC library. Indeed we are indebted to the members of the Bellen laboratory for their contributions to the advancement of numerous technological innovations that have made life easier for us all. We thank Claudio Sunkel for the Polo monoclonal antibody and Mark Peifer for supplying the flies containing the P{GFP-polo} inserts made in the Sunkel laboratory. Alisa Veverka and Stacy Holtzman provided excellent technical help with the BAC injections and husbandry of the stocks recovered. Amelia Tomlinson demystified the vagaries and inscrutabilities of the microbial world. Victor Strelets helped with the extraction of the modENCODE data from FlyBase. The Bloomington Drosophila Stock Center was the source of Drosophila stocks not created for this investigation. This work was supported by funds from Indiana Genomics Initiative INGEN and Indiana Metabolomics and Cytomics Initiative METACyt.
Footnotes
Communicating editor: B. Sullivan
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.181842/-/DC1.
Literature Cited
- Archambault V., Carmena M., 2012. Polo-like kinase-activating kinases: Aurora A, Aurora B and what else? Cell Cycle 11: 1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault V., D’Avino P. P., Deery M. J., Lilley K. S., Glover D. M., 2008. Sequestration of Polo kinase to microtubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev. 22: 2707–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N., Gammeltoft S., Brunak S., 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294: 1351–1362. [DOI] [PubMed] [Google Scholar]
- Bodenmiller B., Malmstrom J., Gerrits B., Campbell D., Lam H., et al. , 2007. PhosphoPep—a phosphoproteome resource for systems biology research in Drosophila Kc167 cells. Mol. Syst. Biol. 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B., Campbell D., Gerrits B., Lam H., Jovanovic M., et al. , 2008. PhosphoPep—a database of protein phosphorylation sites in model organisms. Nat. Biotechnol. 26: 1339–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner A. M., Hughes S. E., Chisholm J. A., Smith S. K., Slaughter B. D., et al. , 2013. Binding of Drosophila Polo kinase to its regulator Matrimony is noncanonical and involves two separate functional domains. Proc. Natl. Acad. Sci. USA 110: E1222–E1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Brown J. B., Boley N., Eisman R., May G. E., Stoiber M. H., et al. , 2014. Diversity and dynamics of the Drosophila transcriptome. Nature 512: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma W., Raaijmakers J. A., Medema R. H., 2012. Switching Polo-like kinase-1 on and off in time and space. Trends Biochem. Sci. 37: 534–542. [DOI] [PubMed] [Google Scholar]
- Burkard M. E., Maciejowski J., Rodriguez-Bravo V., Repka M., Lowery D. M., et al. , 2009. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 7: e1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M., Pinson X., Platani M., Salloum Z., Xu Z., et al. , 2012. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol. 10: e1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M., Lombardia M. O., Ogawa H., Earnshaw W. C., 2014. Polo kinase regulates the localization and activity of the chromosomal passenger complex in meiosis and mitosis in Drosophila melanogaster. Open Biol. 4: 140162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola C., Ganote C. L., Hahn M. W., 2010. Nonallelic gene conversion in the genus Drosophila. Genetics 185: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conduit P. T., Raff J. W., 2010. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr. Biol. 20: 2187–2192. [DOI] [PubMed] [Google Scholar]
- Conduit P. T., Brunk K., Dobbelaere J., Dix C. I., Lucas E. P., et al. , 2010. Centrioles regulate centrosome size by controlling the rate of Cnn incorporation into the PCM. Curr. Biol. 20: 2178–2186. [DOI] [PubMed] [Google Scholar]
- Conduit P. T., Feng Z., Richens J. H., Baumbach J., Wainman A., et al. , 2014. The centrosome-specific phosphorylation of Cnn by Polo/Plk1 drives Cnn scaffold assembly and centrosome maturation. Dev. Cell 28: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveny A. M., Dray T., Gloor G. B., 2002. The effect of heterologous insertions on gene conversion in mitotically dividing cells in Drosophila melanogaster. Genetics 161: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diella F., Cameron S., Gemund C., Linding R., Via A., et al. , 2004. Phospho.ELM: a database of experimentally verified phosphorylation sites in eukaryotic proteins. BMC Bioinformatics 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diella F., Gould C. M., Chica C., Via A., Gibson T. J., 2008. Phospho.ELM: a database of phosphorylation sites–update 2008. Nucleic Acids Res. 36: D240–D244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel H., Chica C., Via A., Gould C. M., Jensen L. J., et al. , 2011. Phospho.ELM: a database of phosphorylation sites–update 2011. Nucleic Acids Res. 39: D261–D267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J., Josue F., Suijkerbuijk S., Baum B., Tapon N., et al. , 2008. A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS Biol. 6: e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson M. M., Tavares A. A., Hagan I. M., Nigg E. A., Glover D. M., 2001a The mitotic roles of Polo-like kinase. J. Cell Sci. 114: 2357–2358. [DOI] [PubMed] [Google Scholar]
- Donaldson M. M., Tavares A. A., Ohkura H., Deak P., Glover D. M., 2001b Metaphase arrest with centromere separation in polo mutants of Drosophila. J. Cell Biol. 153: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman R. C., Kaufman T. C., 2013. Probing the boundaries of orthology: the unanticipated rapid evolution of Drosophila centrosomin. Genetics 194: 903–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman R. C., Stewart N., Miller D., Kaufman T. C., 2006. Centrosomin’s beautiful sister (cbs) encodes a GRIP-domain protein that marks Golgi inheritance and functions in the centrosome cycle in Drosophila. J. Cell Sci. 119: 3399–3412. [DOI] [PubMed] [Google Scholar]
- Eisman R. C., Phelps M. A., Kaufman T. C., 2009. Centrosomin: a complex mix of long and short isoforms is required for centrosome function during early development in Drosophila melanogaster. Genetics 182: 979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A. E., Cantley L. C., Yaffe M. B., 2003a Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299: 1228–1231. [DOI] [PubMed] [Google Scholar]
- Elia A. E., Rellos P., Haire L. F., Chao J. W., Ivins F. J., et al. , 2003b The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115: 83–95. [DOI] [PubMed] [Google Scholar]
- Fu J., Glover D. M., 2012. Structured illumination of the interface between centriole and peri-centriolar material. Open Biol. 2: 120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart, W. M., and D. B. Emmert, 2013 FlyBase high throughput expression pattern data. Available at: http://flybase.org/reports/FBrf0221009.html.
- Gopalakrishnan J., Chim Y. C., Ha A., Basiri M. L., Lerit D. A., et al. , 2012. Tubulin nucleotide status controls Sas-4-dependent pericentriolar material recruitment. Nat. Cell Biol. 14: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman M. J., Kaufman T. C., 1995. Genetic analysis of embryonic cis-acting regulatory elements of the Drosophila homeotic gene sex combs reduced. Genetics 140: 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann K., Mirgorodskaya E., Gobom J., Lehmann V., Muller H., et al. , 2012. Functional analysis of centrosomal kinase substrates in Drosophila melanogaster reveals a new function of the nuclear envelope component otefin in cell cycle progression. Mol. Cell. Biol. 32: 3554–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer J. G., Li K., Kaufman T. C., 1995. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development 121: 3861–3876. [DOI] [PubMed] [Google Scholar]
- Kachaner D., Pinson X., El Kadhi K. B., Normandin K., Talje L., et al. , 2014. Interdomain allosteric regulation of Polo kinase by Aurora B and Map205 is required for cytokinesis. J. Cell Biol. 207: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. H., Park J. E., Yu L. R., Soung N. K., Yun S. M., et al. , 2006. Self-regulated Plk1 recruitment to kinetochores by the Plk1-PBIP1 interaction is critical for proper chromosome segregation. Mol. Cell 24: 409–422. [DOI] [PubMed] [Google Scholar]
- Lange B. M., Bachi A., Wilm M., Gonzalez C., 2000. Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO J. 19: 1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Xu E. Y., Cecil J. K., Turner F. R., Megraw T. L., et al. , 1998. Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J. Cell Biol. 141: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., et al. , 1991. Polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 5: 2153–2165. [DOI] [PubMed] [Google Scholar]
- Logarinho E., Sunkel C. E., 1998. The Drosophila POLO kinase localises to multiple compartments of the mitotic apparatus and is required for the phosphorylation of MPM2 reactive epitopes. J. Cell Sci. 111(Pt 19): 2897–2909. [DOI] [PubMed] [Google Scholar]
- Lucas E. P., Raff J. W., 2007. Maintaining the proper connection between the centrioles and the pericentriolar matrix requires Drosophila centrosomin. J. Cell Biol. 178: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J., Stearns T., 2007. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8: 161–167. [DOI] [PubMed] [Google Scholar]
- Luders J., Patel U. K., Stearns T., 2006. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8: 137–147. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F., Kaufman T. C., 1989. Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev. Biol. 132: 45–61. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Kaufman T. C., 2000. The centrosome in Drosophila oocyte development. Curr. Top. Dev. Biol. 49: 385–407. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Li K., Kao L. R., Kaufman T. C., 1999. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development 126: 2829–2839. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Kao L. R., Kaufman T. C., 2001. Zygotic development without functional mitotic centrosomes. Curr. Biol. 11: 116–120. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Kilaru S., Turner F. R., Kaufman T. C., 2002. The centrosome is a dynamic structure that ejects PCM flares. J. Cell Sci. 115: 4707–4718. [DOI] [PubMed] [Google Scholar]
- Moutinho-Santos T., Sampaio P., Amorim I., Costa M., Sunkel C. E., 1999. In vivo localisation of the mitotic POLO kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis in Drosophila. Biol. Cell 91: 585–596. [PubMed] [Google Scholar]
- Nakajima H., Toyoshima-Morimoto F., Taniguchi E., Nishida E., 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278: 25277–25280. [DOI] [PubMed] [Google Scholar]
- Neef R., Gruneberg U., Kopajtich R., Li X., Nigg E. A., et al. , 2007. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat. Cell Biol. 9: 436–444. [DOI] [PubMed] [Google Scholar]
- Neumuller R. A., Wirtz-Peitz F., Lee S., Kwon Y., Buckner M., et al. , 2012. Stringent analysis of gene function and protein-protein interactions using fluorescently tagged genes. Genetics 190: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C. B., Khodjakov A. L., 2007. Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 120: 1717–1722. [DOI] [PubMed] [Google Scholar]
- Osada N., Innan H., 2008. Duplication and gene conversion in the Drosophila melanogaster genome. PLoS Genet. 4: e1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M. G., Callaini G., 2005. The meiotic spindle of the Drosophila oocyte: the role of centrosomin and the central aster. J. Cell Sci. 118: 2827–2836. [DOI] [PubMed] [Google Scholar]
- Riparbelli M. G., Callaini G., Glover D. M., 2000. Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J. Cell Sci. 113(Pt 18): 3341–3350. [DOI] [PubMed] [Google Scholar]
- Rusan N. M., Peifer M., 2007. A role for a novel centrosome cycle in asymmetric cell division. J. Cell Biol. 177: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria, A., B. Wang, S. Elowe, R. Malik, F. Zhang et al., 2011 The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol. Cell Proteomics 10: M110.004457. [DOI] [PMC free article] [PubMed]
- St. Pierre S., Ponting L., Stefancsik R., McQuilton P., Consortium F., 2014. FlyBase 102 - advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42: D780–D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaizel-Ohayon D., Schejter E. D., 1999. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 9: 889–898. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A., Lehmann R., 1998. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8: 243–246. [DOI] [PubMed] [Google Scholar]
- Venken K. J., Popodi E., Holtzman S. L., Schulze K. L., Park S., et al. , 2010. A molecularly defined duplication set for the X chromosome of Drosophila melanogaster. Genetics 186: 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S., Costantino N., Court D. L., Jenkins N. A., Copeland N. G., 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. D., Marin O., Pinna L. A., Proud C. G., 1999. Phosphorylated seryl and threonyl, but not tyrosyl, residues are efficient specificity determinants for GSK-3beta and Shaggy. FEBS Lett. 448: 86–90. [DOI] [PubMed] [Google Scholar]
- Wolfe B. A., Takaki T., Petronczki M., Glotzer M., 2009. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 7: e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Shen C., Wang T., Quan J., 2013. Structural basis for the inhibition of Polo-like kinase 1. Nat. Struct. Mol. Biol. 20: 1047–1053. [DOI] [PubMed] [Google Scholar]
- Yamashita Y. M., Jones D. L., Fuller M. T., 2003. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301: 1547–1550. [DOI] [PubMed] [Google Scholar]
- Zhai B., Villen J., Beausoleil S. A., Mintseris J., Gygi S. P., 2008. Phosphoproteome analysis of Drosophila melanogaster embryos. J. Proteome Res. 7: 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drosophila stocks and clones created in this report are available upon request. Table S1 contains the genotypes of transformed animals used in this study. Table S2 lists the contents and names of the UAS P-element clones used to make the transgenic lines used in this study. Table S3 lists the names and content of the BAC clones used in this study. Figure S1 shows confocal micrographs and western blots of two cnn insertion mutations demonstrating that they are not null mutations. Figure S2 shows 2D gel western blot analyses of tissues and tissue culture lines demonstrating that Cnn is phosphorylated multiple times and in different patterns depending on tissue, time and cell line. Figure S3 shows the demonstrated sites of phosphorylation in Cnn protein and the potential kinase enzymes responsible. Figure S4 shows western blots of immune-precipitation controls demonstrating that two different bead types non-specifically bind to Cnn.