Abstract
The use of genetic polymorphism data to understand the dynamics of adaptation and identify the loci that are involved has become a major pursuit of modern evolutionary genetics. In addition to the classical “hard sweep” hitchhiking model, recent research has drawn attention to the fact that the dynamics of adaptation can play out in a variety of different ways and that the specific signatures left behind in population genetic data may depend somewhat strongly on these dynamics. One particular model for which a large number of empirical examples are already known is that in which a single derived mutation arises and drifts to some low frequency before an environmental change causes the allele to become beneficial and sweeps to fixation. Here, we pursue an analytical investigation of this model, bolstered and extended via simulation study. We use coalescent theory to develop an analytical approximation for the effect of a sweep from standing variation on the genealogy at the locus of the selected allele and sites tightly linked to it. We show that the distribution of haplotypes that the selected allele is present on at the time of the environmental change can be approximated by considering recombinant haplotypes as alleles in the infinite-alleles model. We show that this approximation can be leveraged to make accurate predictions regarding patterns of genetic polymorphism following such a sweep. We then use simulations to highlight which sources of haplotypic information are likely to be most useful in distinguishing this model from neutrality, as well as from other sweep models, such as the classic hard sweep and multiple-mutation soft sweeps. We find that in general, adaptation from a unique standing variant will likely be difficult to detect on the basis of genetic polymorphism data from a single population time point alone, and when it can be detected, it will be difficult to distinguish from other varieties of selective sweeps. Samples from multiple populations and/or time points have the potential to ease this difficulty.
Keywords: coalescent theory, genetic hitchhiking, natural selection, soft sweep, standing variation
IN recent decades, an understanding of how positive directional selection and the associated hitchhiking effect influence patterns of genetic variation has become a valuable tool for evolutionary geneticists. The reductions in genetic diversity and long extended haplotypes that are characteristic of a recent selective sweep can allow for both the identification of individual genes that have contributed to recent adaptation within a population (i.e., hitchhiking mapping) and understanding the rate and dynamics of adaptation at a genome-wide level (Wiehe and Stephan 1993; Andolfatto 2007; Eyre-Walker and Keightley 2009; Elyashiv et al. 2014).
While the contribution of many different modes to the adaptive process has long been recognized, early work on the hitchhiking effect focused largely on the scenario where a single codominant mutation arose and was immediately beneficial, rapidly sweeping to fixation (Maynard Smith and Haigh 1974; Kaplan et al. 1989). Both simulation studies and analytical explorations during the last decade, however, have drawn attention to models in which adaptation proceeds from alleles present in the standing variation or arising via recurrent mutation once the sweep has already begun (Innan and Kim 2004; Przeworski et al. 2005; Hermisson and Pennings 2005; Pennings and Hermisson 2006a,b; Barrett and Schluter 2008; Hermisson and Pfaffelhuber 2008; Ralph and Coop 2010; Pokalyuk 2012; Roesti et al. 2014; Wilson et al. 2014). Collectively, these phenomena have come to be known as “soft sweeps,” a term originally coined by Hermisson and Pennings (2005) and now often used as a catchall phrase to refer to any sweep for which the most recent common ancestor at the locus of the beneficial allele(s) predates the onset of positive selection (Messer and Petrov 2013).
Empirical work occurring largely in parallel with the theory discussed above suggests that soft sweeps of one variety or another likely make a substantial contribution to adaptation. For example, many freshwater stickleback populations have independently lost the bony plating of their marine ancestors due to repeated selection on an ancient standing variant at the Eda gene (Colosimo 2005), and a substantial fraction of the increased apical dominance in maize relative to teosinte can be traced to a standing variant that predates domestication by at least 10,000 years (Studer et al. 2011). Additional examples of adaptation from standing variation have been documented in Drosophila (Magwire et al. 2011), Peromyscus (Domingues et al. 2012), and humans (Peter et al. 2012), among others. Adaptations involving simultaneous selection on multiple alleles of independent origin at the same locus have also been documented across a wide array of species (Menozzi et al. 2004; Nair et al. 2006; Karasov et al. 2010; Salgueiro et al. 2010; Schmidt et al. 2010; Jones et al. 2013). Nonetheless, the general importance of soft sweeps for the adaptive process remains somewhat contentious (see, e.g., Jensen 2014; Schrider et al. 2015).
While models of the hitchhiking effect under soft sweeps involving multiple independent mutations have received a fair amount of analytical attention (Pennings and Hermisson 2006a,b; Hermisson and Pfaffelhuber 2008; Pokalyuk 2012; Wilson et al. 2014), the model of a uniquely derived mutation that segregates as a standing variant before sweeping in response to an environmental change is less well characterized. Present understanding of the hitchhiking effect in a single population under this model comes primarily from two sources. The first one is a pair of simulation studies (Innan and Kim 2004; Przeworski et al. 2005), which focused largely on simple summaries of diversity and the allele frequency spectrum, and the second one is the general verbal intuition that, similar to the multiple-mutation case, the beneficial allele should be found on “multiple haplotypes.” In contrast to the multiple-mutation case, these additional haplotypes are created as a result of recombination events during the period before the sweep when the allele was present in the standing variation, rather than due to recurrent mutations on different ancestral haplotypes (Barrett and Schluter 2008; Messer and Petrov 2013).
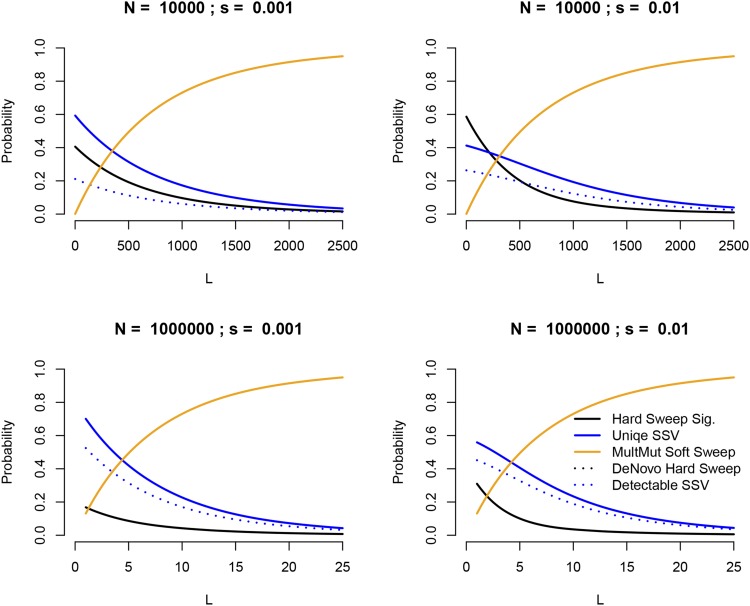
Before we turn to the coalescent for sweeps from uniquely derived standing variation, it is worth first asking under what circumstances we might expect such sweeps. To illustrate this we consider a single-locus model in which a population that was previously at mutation–drift equilibrium adapts in response to an environmental change, either by drawing on material from the standing variation or from new mutations that occur after the environmental change. In particular, we are interested in exploring the relationship between the source of genetic material the population uses to adapt and the specific signature left behind in genetic polymorphism data at the conclusion of the event. If adaptation proceeds entirely from de novo mutation, the signature will be that of either a classic hard sweep or a multiple-mutation soft sweep. However, if the population adapts at least partially from standing variation, a broad range of possible signatures are possible. First, the population may use more than one allele present in the standing variation, in which case we again have a multiple-mutation soft sweep. Alternately, if only a single allele from the standing variation is used, a range of signatures are possible. If the allele was at a frequency at the moment of the environmental change, then a hard sweep signature is produced because, conditional on escaping loss due to drift and eventually reaching fixation, the allele must have rapidly increased in frequency even before it became beneficial. If adaptation proceeds via an allele that was at some low frequency greater than , an altered signature is produced (e.g., Przeworski et al. 2005, and a model for generating that pattern is the primary focus of this article), whereas adaptation from a single high-frequency derived allele leaves essentially no detectable signature in polymorphism data. Drawing on results from a number of previously published studies (Hermisson and Pennings 2005; Przeworski et al. 2005; Pennings and Hermisson 2006a) in the Appendix we calculate the probability of observing each of these different signatures for this model of a sharp transition from drift–mutation equilibrium to positive selection as a function of the population size, strength of selection, and time since the environmental change and present the results in Figure 1. These calculations reveal that, under this model, all of these signatures are of importance at least in some parameter regimes and in particular suggest that a sweep of a uniquely derived allele from the standing variation should constitute a nonnegligible proportion of all sweeps that begin from mutation–drift equilibrium. Our model also applies to sweeps of alleles that previously exhibited long-term asymmetric balancing selection, which represent an unknown fraction of adaptive alleles.
Figure 1.
The probability of observing a number of different sweep signatures in a sample of 20 chromosomes, assuming a model in which an allele that was previously neutral suddenly becomes beneficial in response to an environmental change. Calculations are given in the Appendix. Results are displayed for a range of population sizes (N), selection coefficients (s), and mutational target sizes (L) and assuming 1000 generations since the environmental change. In general, we see that selective sweeps in which adaptation proceeds from a uniquely derived allele represent a nontrivial proportion of all sweeps under this model, provided that the mutational target size is not large and that Ns is not too small. A hard sweep signature is left by any sweep for which a single allele sweeps from a frequency of , while a unique sweep from standing variation (SSV) corresponds to any sweep in which a single allele sweeps from a frequency greater than this value. Multiple-mutation soft sweeps refer to the variety described in Pennings and Hermisson (2006a,b). De novo hard sweep refers to sweeps in which the beneficial allele did not arise until after the environmental change (corresponding to the model originally studied by Maynard Smith and Haigh 1974), while detectable SSVs are sweeps of a single unique allele that was present at a frequency and may therefore plausibly be distinguished from both the hard sweep model and the neutral model.
In this article, we present an analytical treatment of the model in which an allele with a single mutational origin segregates at a low frequency (either neutrally or under the influence of balancing selection with asymmetric heterozygote advantage) and then sweeps to fixation after a change in the environment. The central observation is that, with some simplifying assumptions, the recombination events that are responsible for the multiple haplotypes on which the beneficial allele is found have a close analogy to mutations in the infinite-alleles model, and we can therefore leverage the Ewens sampling formula (Ewens 1972) to obtain an analytical description for the genealogical history of a neutral locus linked to the beneficial allele. We then show that this model can be used to obtain a highly accurate approximation for the expected deviation in the frequency spectrum at a given genetic distance, as well as to shed light on how the expected patterns of haplotype structure differ between the multiple recurrent mutation and sweep from standing variation cases. We conclude with a brief simulation study examining the order statistics of the haplotype frequency spectrum under the classic hard sweep, multiple-mutation soft sweep, and standing sweep models, with the aim of demonstrating how future methods to identify and classify sweeps can best make use of this information.
Model
We consider two linked loci separated on the chromosome by a recombination distance r. At one of these loci a new allele, B, arises in a background of ancestral b alleles. This allele segregates at low frequency for some period of time (either due to neutral fluctuations or because it is a balanced polymorphism), before a change in the environment causes it to become beneficial and sweep to fixation. A schematic depiction of the model is given in Figure 2. Our aim is to describe some features of genealogies both at the locus of the B allele and at nearby linked sites and to use this understanding to build intuition regarding the process of a sweep from standing variation, as well as to derive the patterns of DNA sequence variation we expect to observe near a recently completed sweep from standing variation. For the most part, we focus on describing the pattern of recombination events that (backward in time) move lineages from the B background onto the b background, with nucleotide diversity generated via ancestral diversity that enters via these recombination events. Where necessary, we develop additional approximations to include the effect of new mutations occurring on the B background.
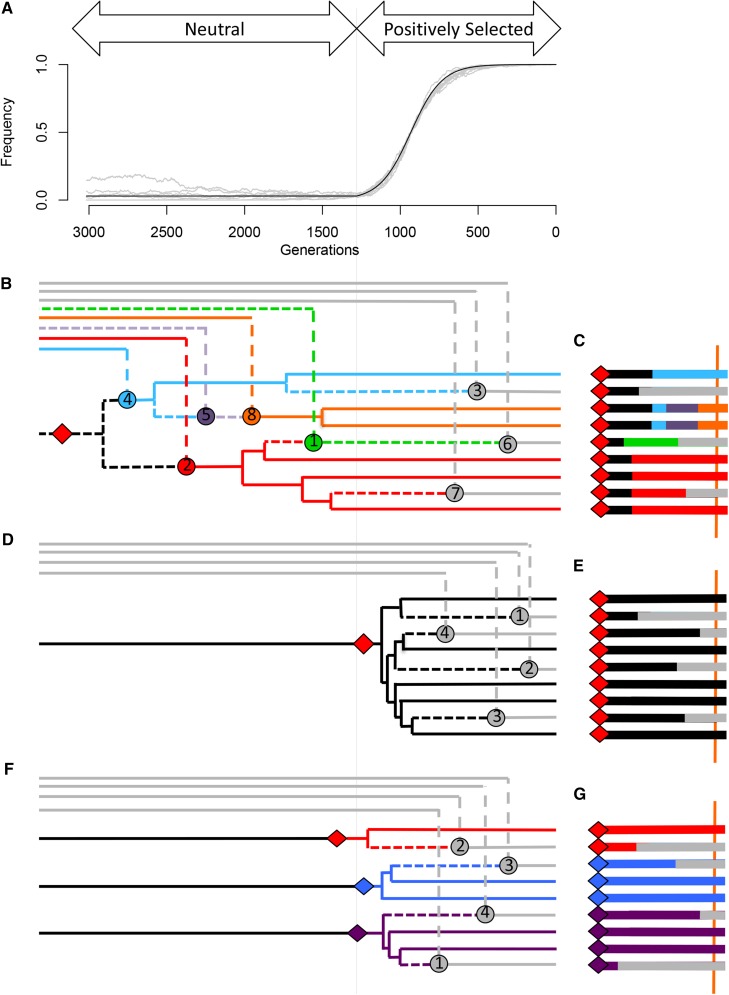
Figure 2.
A schematic depiction of our model along with two other common sweep models. (A) The frequency trajectory of alleles in the sweep from the standing variation model. Gray lines depict 10 simulated sweeps with and in a population of The solid black line represents the frequency trajectory assumed for our analytical calculations for a sweep with those parameters. (B and C) The genealogy, history of recombination events, and sequence associated with a sample of nine chromosomes taken at the moment of fixation under the sweep from standing variation model. The red diamond (on both the genealogy and the sequence) represents the mutation responsible for the beneficial allele. The tree subtending this mutation in B is the genealogy at the locus of this mutation. Solid lines represent the genealogy experienced by a neutral site located at the position of the vertical orange bar in C, with lineages that escape coalescence under the red mutation coalescing on a longer timescale off the left side. Circles on the genealogy in B represent the recombination events falling between the beneficial mutation and the orange bar in C and are responsible for changes in haplotype identity (color) along the sequence. Short dashed lines represent components of the ancestral recombination graph between the red mutation and the orange bar that are not a part of the local genealogy at the position of the orange bar. Long dashed lines represent movement from the selected to the nonselected background via recombination. At the distance marked by the orange bar, there are three sweep phase recombinants, and the remaining six sequences are partitioned into three haplotypes of frequencies three, two, and one, according to the infinite-alleles process described in the main text. (D and E) Genealogy, recombination history, and sequence associated with a standard hard sweep. Here, the beneficial mutation generally occurs after the onset of positive selection, and most recombination events occur as singletons during the middle of the sweep. The sweep signature therefore consists chiefly of a single core haplotype that is slowly whittled down by singleton recombinants. (F and G) Genealogy, recombination history, and sequence associated with a multiple-mutation soft sweep. Here, the beneficial mutations all generally occur around the time of the onset of position selection, creating multiple core haplotypes, which are each subsequently whittled down by recombination events during the course of the sweep.
Our general approach is to break the history of the standing sweep into two periods, the first one being the time during which the B allele is selectively favored and rising in frequency (we refer to this as the sweep phase) and the second one being the period after the mutation has arisen but before the environmental shift causes it to become beneficial (we refer to this as the standing phase). We assume that the frequency trajectory of the allele is logistic during the sweep phase and that selection is sufficiently strong relative to the sample size such that only recombination (i.e., no coalescence) occurs during this phase. We approximate the standing phase by assuming that the frequency of the B allele is held at some constant value f infinitely far into the past prior to the onset of selection. While this is obviously a coarse approximation to the true history of a low-frequency allele, it is nonetheless accurate enough for our purposes and enjoys some theoretical justification, as we discuss below. The key advantage to using this approximation is that it allows us to model the genealogy of the B alleles as a standard neutral coalescent (rescaled by a factor f) and therefore to treat recombination events moving away from the selected locus in a manor analogous to that for mutations in the standard infinite-alleles model. This allows us to use a version of the Ewens sampling formula to calculate a number of summaries of sequence diversity and to build intuition for how patterns of haplotype diversity should change in regions surrounding a standing sweep.
Data availability
All custom simulation code was written by J.J. Berg in R and is provided in File S1. We also made use of the as yet unpublished program mssel, written by Dick Hudson, a copy of which is also provided in File S1.
Analysis and Results
Sweep phase
Looking backward in time, let be the frequency of the B allele at time t in the past, where is the moment of fixation [i.e., ]. If we consider a neutral locus a genetic distance r away from the beneficial allele, the probability that it fails to recombine off of the selected background in generation t, given that it has not done so already, is . If we let be the generation in which the environmental change occurred, marking the boundary between the sweep phase and the standing phase [i.e., ], then the probability that a single lineage fails to recombine off the selected background at any point during the course of the sweep phase is given by
| (1) |
for If the effect of our beneficial allele on relative fitness is strictly additive, such that heterozygotes enjoy a selective advantage of s and homozygotes an advantage of , then the trajectory of the beneficial allele through the population can be approximated deterministically by the logistic function, and the integral in the exponential in Equation 1 can be approximated as , yielding
| (2) |
We assume selection is strong, such that there is not enough time for a significant amount of coalescence during the sweep phase. Therefore, each lineage either recombines off the beneficial background or fails to do so, independently of all other lineages. The probability that i of n lineages fail to escape off the sweeping background is then
| (3) |
This binomial approximation has been made by a number of authors in the context of hard sweeps (e.g., Maynard Smith and Haigh 1974; Fay and Wu 2000; McVean 2006; Coop and Ralph 2012), but better approximations do exist (Barton 1998; Durrett and Schweinsberg 2004, 2005; Schweinsberg and Durrett 2005; Etheridge et al. 2006; Messer and Neher 2012). Under the hard sweep model, most of the error of the binomial approximation arises due to coalescent events during the earliest phase of the sweep. Because this phase is replaced in our model by the standing phase described below, the binomial approximation is a better fit for our use than in the classic hard sweep case.
Standing phase
Looking backward in time, having originally sampled n lineages at , we arrive at the beginning of the standing phase at time with i lineages still linked to the beneficial background, the other having recombined into the nonbeneficial background during the sweep.
We apply a separation of timescales argument, noting that coalescence of the i lineages that fail to recombine off the B background during the sweep will occur much faster than coalescence of the lineages that do recombine during the sweep. We therefore assume that nothing happens to lineages on the b background until all lineages have escaped the B background via either mutation or recombination, at which point b lineages follow the standard neutral coalescent.
The coalescent process of the B alleles:
A number of previous studies have examined the behavior of this process (Rannala 1997; Griffiths and Tavare 1998, 1999; Wiuf and Donnelly 1999; Wiuf 2000; Griffiths 2003; Patterson 2005), conditional on the frequency of the allele either in a sample or in the population. Wiuf (2000) has shown that the expected time to the first coalescent event is in the absence of other information, e.g., as to whether the allele is ancestral or derived. However, the distribution of coalescence times is no longer exponential. The variance of the time between coalescent events is increased relative to the exponential as a direct result of the fact that the frequency may increase or decrease from f before a given coalescent event is reached. Further, in contrast to the standard coalescent, there is nonzero covariance between subsequent coalescent intervals, as a result of the information they contain about how the frequency of the allele has changed and thus about the rate at which subsequent coalescent events occur. Finally, if the allele is known to be either derived or ancestral, the expected coalescent times have a more complicated expression, as the allele is in expectation either decreasing or increasing in frequency backward in time due to the conditioning on derived or ancestral status, respectively.
Despite these complications, we have found that assuming that all pairs of lineages coalesce at a constant rate and that coalescent time intervals are independent (in other words, that the allele frequency does not drift from f) is not a bad approximation when , even when we condition on the allele being derived (Supporting Information, Figure S1, Figure S2, and Figure S3).
The main reason for using this approximation is that, in conjunction with the separation of timescales, it allows us to work with a simple, well-understood caricature of the true process (i.e., the neutral coalescent) that still describes the genealogy at the selected site with reasonable accuracy. Given this simplified coalescent process, we can study the recombination events occurring between the beneficial and neutral loci to understand the properties of the genetic variation at the neutral locus that will hitchhike along with the B allele once the sweep phase begins.
Recombination events occurring during the standing phase:
We again rely on the condition that and assume that any lineage at the neutral locus that recombines off of the background of our beneficial allele will not recombine back into that background before it is removed by mutation. Under these assumptions, recombination events that move lineages at the neutral locus from the B background onto the b background can be viewed simply as events on the genealogy at the beneficial locus that occur at rate for each lineage independently. Rescaling time by , an understanding of the genealogy at the neutral locus can therefore be found by considering the competing Poisson processes of coalescence at rate 1 per pair of lineages and recombination at rate per lineage.
We are interested in the number and size of different recombinant clades at a given genetic distance from the selected site (colored clades in Figure 2B, which give rise to colored haplotypes in Figure 2C). Under our approximate model for the history of coalescence and recombination at these sites, this a direct analogy of the infinite-alleles model (Kimura and Crow 1964; Watterson 1984). In the normal infinite-alleles process, we imagine simulating from the coalescent, scattering mutations down on the genealogy, and then assigning each lineage to be of a type corresponding to the mutation that sits lowest above it in the genealogy. Alternately, we can create a sample from the infinite-alleles model by simulating the mutational and coalescent processes simultaneously: coalescing lineages together as we move backward in time, “killing” lineages whenever they first encounter a mutation, and assigning all tips sitting below the mutation to be of the same allelic type (Griffiths 1980).
Given the direct analogy to the infinite-alleles model under our set of approximations, the number and frequency of the various recombinant lineage classes at a given distance from the selected site can be found using the Ewens sampling formula (ESF). The population-scaled mutation rate in the infinitely many alleles model () is replaced in our model by the rate of recombination out of the selected class []. If i lineages sampled at the moment of fixation fail to recombine off of the beneficial background during the course of the sweep, then the probability that these i lineages coalesce into a set of k recombinant lineages is
| (4) |
where is an unsigned Stirling number of the first kind,
| (5) |
These recombinant lineages partition our sample up between themselves, such that each lineage has some number of descendants in our present-day sample , where . Conditional on k, the probability of a given sample configuration is
| (6) |
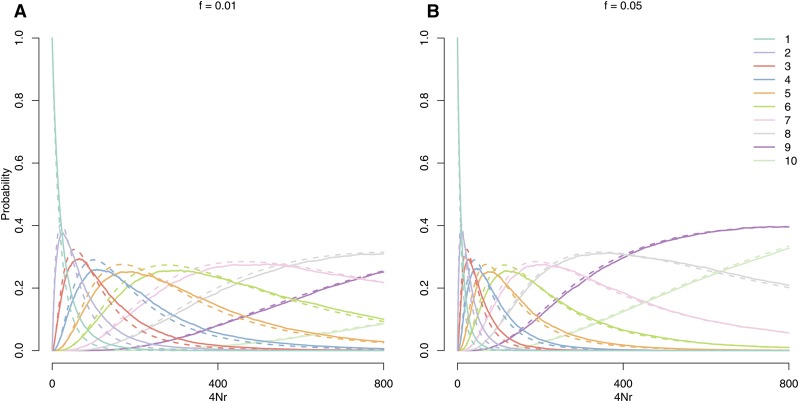
Note that this does not depend on , which gives the classic result that the number of alleles is a sufficient statistic for (i.e., the partition is not needed to estimate ). Figure 3 shows a comparison of this approximation to simulations for the number of distinct coalescent families at a given distance from the focal site.
Figure 3.
The probability that a sample of 10 lineages taken on the background of an allele at frequency 1% (A) or 5% (B) coalesce into k families before exiting the background, as a function of population-scaled genetic distance () from the conditioned site. The effective population size in the simulations is The solid lines give the proportion of 1000 coalescent simulations, with an explicit stochastic frequency trajectory (as described in Simulation details), in which k families of lineages recombined off of the sweep at distance . The dotted lines give our approximation under the ESF (Equation 4) with .
We are usually interested in the case where and thus . As such, the properties of the standing part of the sweep are well captured by the population sized-scaled compound parameter , the number of individuals who carry the selected allele when the sweeps begins. This means that the effect of standing variation on sweep patterns depends critically on the effective population size. A sweep from a variant at frequency would for all intents and purposes be a hard sweep in humans, where the historical effective population size is ∼10,000, but would result in quite different patterns in Drosophila melanogaster, whose long-term effective population size is closer to 1 million.
Patterns of neutral diversity surrounding standing sweeps
This approximate model of the coalescent for a sweep from standing variation allows us to calculate a number of basic summaries of sequence variation in the region surrounding the sweep. For now we neglect mutations that occur over the timescale of our shrunken coalescent tree and assume that all diversity comes from mutations that occurred prior to the sweep or equivalently that this part of the genealogy contributes negligibly to the total time. This corresponds to an assumption that , in line with our previous assumption that As long as this assumption holds, we can consider patterns of diversity in our sample at a given site simply by considering properties of the recombinant lineages in our sample, which correspond to alleles drawn independently from a neutral population prior to the start of our sweep. We partially relax this assumption in the Appendix for those of our calculations where it substantially affects the fit to simulation data.
Reduction in pairwise diversity:
The expected reduction in pairwise diversity following a standing sweep relative to neutral expectation is given by the probability that at least one lineage in a sample of two manages to recombine off of the B background (during either the sweep phase or the standing phase) before the coalescent event during the standing phase
| (7) |
(Figure 4). Given the exponential form of and the fact that can be approximated as for small values of , we can further approximate (7) as . Recalling that the reduction in diversity for a classic hard sweep with strong selection can be approximated as (Durrett and Schweinsberg 2004; Pennings and Hermisson 2006b), it is tempting to suppose that there may exist a choice of , an “effective” selection coefficient, for which the classic hard sweep model produces a reduction in diversity over the same scale as the standing sweep model. While it is simple to set the terms in the exponentials equal to one another and solve for the appropriate value of (see Appendix), it turns out that for all choices of N, s, and f for which our model applies, . In other words, the reduction in diversity caused by a sweep from standing variation cannot be caused by a hard sweep in which standard strong selection approximations apply, which means that care should be taken when interpreting estimates of the rate of adaptation that depend solely on classic hard sweep–strong selection approximations (Elyashiv et al. 2014). No doubt there is a choice of selection coefficient under which a weakly selected allele will produce a similar reduction in diversity, but there are no adequate approximations available under this model, and we do not pursue it further here.
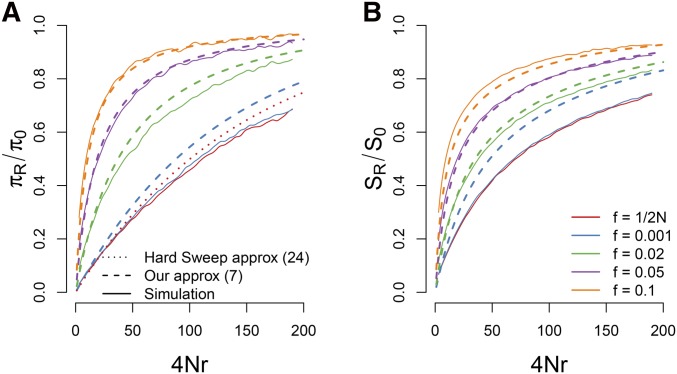
Figure 4.
A comparison of our approximations for the reduction in (A) pairwise diversity and (B) the number of segregating sites for a sweep with and starting from a variety of different frequencies. For pairwise diversity we also include the hard sweep approximation given in Equation A1. Our approximations are generally accurate as long as the sweep begins from a frequency .
Number of segregating sites:
We can also use our approximation to calculate the total time in the genealogy at a given distance from the selected site, which allows us to calculate the expected number of segregating sites. Conditional on m independent lineages escaping the sweep, the expected total time in the genealogy is , the standard result for a neutral coalescent with m lineages (Watterson 1975). For a moment conditioning on no recombination during the sweep phase (i.e., ), the probability that k independent lineages escape during the standing phase, given that there are at least two (otherwise all coalescence occurs on the B background and the total time in the genealogy, ), is
| (8) |
Conditional on no recombination during the sweep phase, the expected time in the genealogy is
| (9) |
When we allow for all possible numbers of singleton recombinants during the sweep phase, the expected total time in the genealogy is
| (10) |
[note that we have taken and ; whereas it is typically impossible to obtain a sample with zero alleles, in our case we must define these probabilities to accommodate the case in which all n lineages recombine out during the sweep phase] and the expected number of segregating sites can be found by multiplying this quantity by the mutation rate (Figure 4).
The frequency spectrum:
Finally, we can use our approximation to obtain an expression for the full frequency spectrum at sites surrounding a sweep from standing variation. To break the problem into approachable components, we first consider the frequency spectrum of an allele that is polymorphic within the set of lineages that do not recombine during the sweep (i.e., ignoring sweep phase events for now), and we condition on a fixed number k recombinant families from the standing phase. Borrowing from Pennings and Hermisson (2006b) (equation 14 of their article), if we condition on j of these k recombinant lineages carrying a derived allele, then we can obtain the probability that l of the i sampled lineages carry the derived allele by summing over all possible partitions of the i lineages into k families such that the j recombinant ancestors carrying the derived mutation have exactly l descendants in the present day:
| (11) |
Next, we write to denote the number of polymorphic mutations that were present j times among the k ancestral lineages that escape the standing phase. For our purposes, we assume this follows the standard neutral coalescent expectation
| (12) |
although an empirical frequency spectrum measured from genome-wide data, as in Nielsen et al. (2005), could also be used. The expected number of derived alleles that are present in l of i sampled lineages, conditional on there having been k recombinant families, is then
| (13) |
Summing over the distribution of k given by (4), we obtain an expression for the frequency spectrum within the set of i lineages that do not recombine during the sweep as
| (14) |
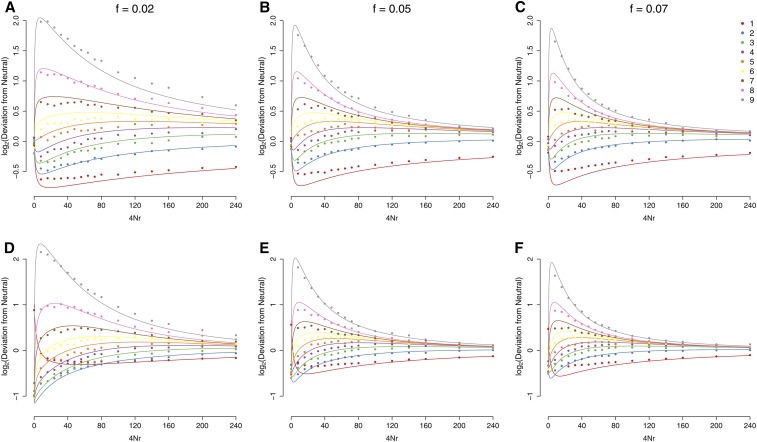
This expression is essentially identical to the one presented in equation 15 of Pennings and Hermisson (2006b). The only difference is that the Ewens clustering parameter in their model is given by the beneficial mutation rate and holds only for sites fully linked to the selected loci, whereas in our model it is a linear function of the genetic distance from the selected site. In terms of accurately describing observed patterns of polymorphism, this approximation is highly accurate for loci that are distant from the focal site, but breaks down for loci that are tightly linked. The reason for this is that very near the focal site, it is actually very unlikely that there have been any recombination events at all, and so while polymorphism is rare, when it is present it is likely to have arisen due to new mutations on the genealogy of the B allele (in which case their distribution is that of the standard neutral frequency spectrum), rather than ancestrally. While a full accounting for the contribution of all new mutations under this model is beyond our scope, we can develop an ad hoc approximation by assuming that mutations are new if there have not been any recombination events and are old if there has been at least one recombination (see Appendix). This approximation is quite accurate, especially when the focal allele is at low frequency (Figure 5).
Figure 5.
The frequency spectrum, in a sample of in a population of In A–C, we take a sample on the background of a focal allele at the end of the standing phase, but before the sweep phase. In D–F, we take a sample from the full population immediately after fixation of the beneficial allele. Results are shown as the log ratio of the normalized frequency relative to its expectation under the standard neutral coalescent. s = 0.05 for the postfixation case. Solid circles give simulations, while solid lines give the theoretical result of Equation A6.
When we allow for recombination during the sweep, the expression becomes more complex, as we must take into account the fact that a mutation may be polymorphic after the sweep even if it is either absent or fixed in the set of lineages that hitchhike. Nonetheless, we obtain an expression for the frequency spectrum of ancestral polymorphism as
| (15) |
where
| (16) |
gives the probability that g of a total of j derived alleles that existed before the sweep are found on singleton recombinants created during the sweep, given that there are singletons, and k recombinant families created during the standing phase.
In words, lineages recombine out during the selected phase, while the remaining i lineages are partitioned into k families at frequencies due to recombination and coalescence in the standing phase. Of the singleton lineages, g of them carry the derived allele, while the remaining copies of the derived allele give rise to derived alleles due to coalescence during the standing phase, and we take the sum over all possible combinations of these values that result in a final frequency of in the present-day sample.
Once again, this expression is accurate far from the selected site, but in error at closely linked sites due to the contribution of new neutral mutations on the background of the B allele. Again, we can develop an ad hoc approximation by allowing for new mutations during the sweep phase on any lineage that does not recombine during that phase and on the i lineages that reach the standing phase, provided there are no recombination events during that phase (see Appendix and Figure 5). New mutations during the standing phase are ignored once there has been at least one recombination event during that phase. This approximation is quite accurate at all distances (especially when the sweep comes from relatively low frequency) and highlights the fact that sweeps from standing variation are characterized by an excess of derived mutations at a range of frequencies >40–50%, in contrast to hard sweeps, which exhibit a much stronger skew toward extremely low- or high-frequency alleles (Przeworski et al. 2005).
Patterns of haplotype variation and routes to inference
To this point, we have focused on an analytical description for the effect of a sweep from standing variation on a single tightly linked neutral locus on the same chromosome. It is also of value to consider the effect of a sweep as a process that occurs along the sequence, as this gives some perspective into how haplotype structure unfolds in the region surrounding a standing sweep. Efforts to identify standing sweeps via polymorphism data hinge on identifying recombination events that occurred during the standing phase by recognizing the way in which they break a single core haplotype down into a succession of coupled samples from the Ewens distribution with progressively larger clustering parameters. We first describe some properties of pairs of sequences, before considering larger samples.
For any pair of sequences, recombination events from the sweep phase are encountered at rate , while events from the standing phase are encountered on average at rate . A simple measure of the relative importance of the two phases for patterns of haplotype structure and linkage disequilibrium can be found by competing Poisson processes. Using the expectation of the pairwise coalescence time from the standing phase, the probability that the first recombination encountered traces its history to the standing phase is approximately
| (17) |
and in general events occurring during the standing phase will dominate the haplotype partition when , while those from the sweep phase will dominate when .
Next consider that the lower bound on the frequency from which a sweep can start and still conform to our model is (Przeworski et al. 2005). Below this frequency, the effect of conditioning on fixation creates a more complicated set of dynamics that skew the shape of the genealogy away from that expected under our model. As either the selection coefficient or the population size increases, this stochastic threshold gets pushed down into the high-density region of the neutral frequency spectrum, with the result that the genealogy for a larger and larger proportion of all single-mutation sweeps can be described by our model. However, for standing sweeps from low frequency, it will tend to be the case that most of the recombination events encountered trace their history to the sweep phase as opposed to the standing phase.
Therefore, while increases in result in an increased probability of sweeps conforming to our model relative to the classic hard sweep model (Figure 1), these sweeps will be difficult to distinguish from classic hard sweeps because most of the recombination events will occur during the sweep phase, where the two models are identical.
The practical task of identifying a sweep from standing variation requires more extensive knowledge about the haplotype partition from a larger sample. The necessary task is to identify recombination events from the standing phase as they unfold along the sequence (see Figure 2C). Unfortunately, explicit analytical expressions for these haplotype partition transitions are unavailable under any sweep model, and ours is no exception (although Innan and Nordborg 2003 have provided some results regarding our standing phase). Nonetheless, we gain a few simple insights from a description of the process, and this description motivates some further simulations.
If we consider the best case for identifying a sweep from standing variation and distinguishing it from a classic hard sweep, this should occur in the parameter regime , where events from the sweep phase tend to happen much more distantly than those from the standing phase, but the decay in haplotype structure attributable to events occurring during the standing phase still occurs gradually enough that it can be distinguished from neutrality.
In this limit, the sweep happens instantaneously, and the total time in the tree is equal to the total time from the standing phase . The distance to the first recombination is , where gives the recombination rate per base pair. Using the standard approximation for the total time in the tree, the expected length scale over which a single haplotype should persist away from the selected site is (and twice this distance if we consider both sides of the sweep). This recombination partitions the haplotypes according to the standard neutral frequency spectrum (e.g., the green recombinant moving to the left in Figure 2C). Moving down the sequence we then generate the next distance to a recombination, again from . We again uniformly simulate a position on the tree for this new recombination; however, this time only a recombination on some of the branches would result in a new haplotype being introduced into the sample (e.g., the red recombinant in Figure 2B is responsible for the second transition in the haplotype partition scheme in Figure 2C). If the recombination falls in a place that does not alter the configuration, we ignore it and simulate another distance from this new position. Otherwise, we keep the recombination event and split the sample configuration again. (For example, the orange recombinant in Figure 2C does not alter the status of identity-by-descent relationships with respect to the sweep and therefore does not result in an increase in the number of haplotypes under our convention.) We iterate this procedure, moving away from the selected site, generating exponential distances to the next recombination, placing the recombination down, and updating the configuration if needed, until we reach the point that every colored haplotype is a singleton. We then repeat this procedure on the other side of the selected site, using the same underlying genealogy.
An equivalent way to describe this process is to simulate distances to the next recombination that alters the configuration, given the tree and the previous recombinations. To do this we consider the total time in the tree where a recombination would alter the configuration. Numbering these recombinations out from the selected site, we start at the selected site , with , and generate a distance to the first recombination, . We place the recombination on the tree and then prune the tree of branches where no further change in sample configuration could result in a new colored haplotype. We then set to the total time in these pruned subtrees, place the next recombination uniformly on the pruned branches at an distance, and carry on this process until we have pruned the entire tree such that all lineages reach their own unique recombination event before coalescing with any other lineage.
The result of this process is a series of coupled partitions and breakpoints between them, where the units of the breakpoints are on the same scale as the clustering parameter of the ESF ( in our case). As laid out above, any marginal slice through the outcome of the process is a valid sample from the ESF with the clustering parameter equal to the distance from the focal site at which the slice is taken, as measured in units of . Progress toward analytical results regarding the set of coupled partitions and their breakpoints created by this process is not likely to come easily (J. Hermisson, personal communication), but would represent significant developments in relating the infinite-sites and infinite-alleles models, and given the general interest in the ESF motivated by exchangeable partitions and clustering algorithms, would likely be of wider interest.
Routes to inference:
Any effort to identify and distinguish between different varieties of sweeps is necessarily an attempt to determine the shape and size of the genealogy during the earliest phase of the selected allele’s history. One approach to building inference machines to distinguish these different varieties may be to build upon recent developments in coalescent hidden Markov models (Li and Durbin 2011; Paul et al. 2011; Sheehan et al. 2013; Rasmussen et al. 2014) that provide efficient algorithms to explore the space of gene trees consistent with the sequence data and leverage these algorithms to explicitly model the effect of selective sweeps on the ancestral recombination graph. To do this effectively, we need a way to evaluate the likelihood of a particular pattern of coalescence under a variety of sweep models. Our results suggest a way to accomplish this effectively for sweeps from standing variation, and recent works on both hard and soft sweeps (Barton 1998; Durrett and Schweinsberg 2004, 2005; Schweinsberg and Durrett 2005; Etheridge et al. 2006; Hermisson and Pfaffelhuber 2008; Messer and Neher 2012) provide a route to doing so under these models.
Alternately, it will likely be fruitful to continue along the lines of popular sweep-finding approaches implemented to date and define summary statistics that can effectively distinguish between different models. Below, we use simulations to illustrate how different features of the haplotype frequency spectrum can be informative for distinguishing between different varieties of sweeps and draw attention to which features of the underlying genealogy are indicated by certain patterns in the haplotype frequency spectrum.
Observed haplotype frequency spectrum
To this point, our discussions of haplotype variation have focused on haplotypes defined via identity-by-descent, which cannot be observed directly. It is useful to consider how the understanding gained here can be leveraged to improve our ability to identify standing sweeps. To do this we turn to the ordered haplotype frequency spectrum.
For a window of size L, we define the ordered haplotype frequency spectrum (OHFS) as , where gives the sample frequency of the most common haplotype and there are a total of distinct haplotypes within the window. Coarse summaries of the OHFS have been a popular vehicle for sweep-finding methods [e.g., EHH, iHS, and H12 (Sabeti et al. 2002; Voight et al. 2006; Garud et al. 2015; Garud and Rosenberg 2015)]. We focus on identifying which aspects of the OHFS should be most informative about the size as well as the shape of the genealogy at the focal site.
Specifically, we conducted coalescent simulations with a sample size of chromosomes under four different models of sequence evolution (hard sweeps, standing sweeps from , soft sweeps conditional on three origins of the beneficial mutation, and neutral), with all sweep simulations set to For the standing sweeps, this corresponds to a situation in which the signature of the standing phase is largely visible, but partially obscured by the sweep phase.
One simple prediction on the basis of our analytical investigation above is that, because the genealogy under a standing sweep is generally larger than that under a hard sweep, recombination events out of the sweep should accumulate more quickly along the sequence and therefore the total number of haplotypes in a window of a given size should be larger for a standing sweep than for a hard sweep. In Figure 6 we show from simulations over a range of L for one-sided windows extending away from the selected site. As expected, we see that the number of haplotypes increases more quickly with distance from the selected site for a standing sweep than for a hard sweep. Unfortunately, as we alluded to above, this signal is largely confounded by the fact that one can also obtain a similarly rapid increase in the number of haplotypes from a hard sweep with a slightly weaker selection coefficient.
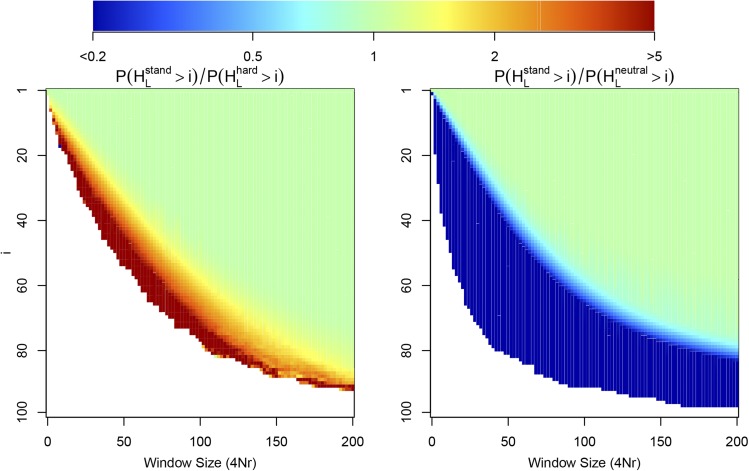
Figure 6.
The ratio of the probability that there are at least i haplotypes in a one-sided window extending away from the selected site for the standing sweep model relative to the hard sweep model (left) and the neutral model (right). For all simulations , , and we simulate a chromosomal segment with total length divided into discrete loci, with for the whole segment. Probabilities are calculated using the basis of 5000 simulations under each model.
Alternately, we may want to use the OHFS to obtain information about the shape of the genealogy at the focal site, which should hopefully be less confounded by a change in selection coefficient. This information is found in differences in the relative frequencies of certain haplotypes between the different models, rather than in the total number of haplotypes. Specifically, if there are multiple haplotypes present close to the selected site, they should be more common under the sweeps from the standing variation model than under the full sweep model. In other words, there should be a window near the selected site where for , due to recombinations occurring on internal branches of the genealogy from the standing phase.
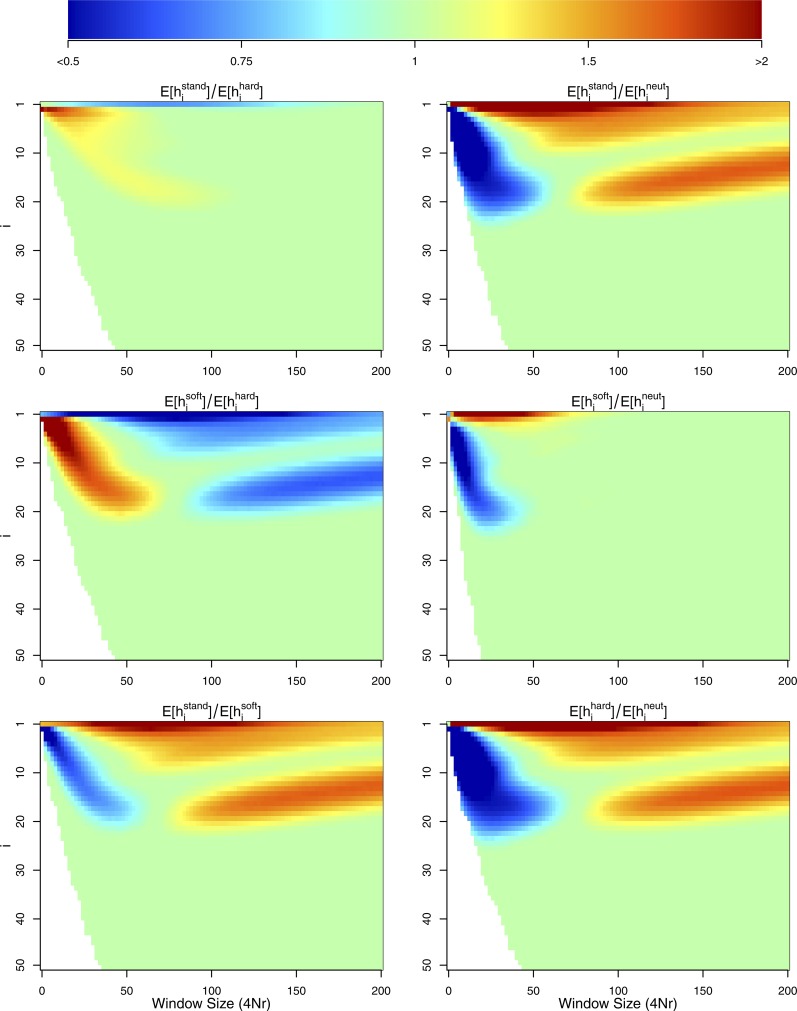
In Figure 7, we show , where and denote different models of sequence evolution (i.e., standing, hard, or soft sweep or neutral). In particular, we want to draw attention to the fact that, similar to the multiple-mutation model, most of the useful information within the OHFS for distinguishing standing sweeps from hard sweeps lies within a small window near the selected site and comes in the form of a decrease in the relative frequency of the core haplotype and corresponding overabundance of the next few most common haplotypes. In contrast to the multiple-mutation case, the enrichment of moderate-frequency haplotypes for the standing case is relatively subtle, and beyond moderate recombination distances, there is little information to distinguish a standing sweep from a hard sweep. We also observe that, contrary to the multiple-mutation soft sweep model, far away from the selected site, standing sweeps resemble hard sweeps across the majority of the OHFS. This is in line with expectations from our results above, in that close to the selected site the haplotype partition is dominated by events occurring during the standing phase, while far from the selected site it is dominated by events occurring during the sweep phase.
Figure 7.
The ratio of expected sample frequency of the most common haplotype, conditional the haplotype existing in our sample, between two different models and , i.e., .. This is shown as a function of the recombination distance from the selected site. Green indicates that the frequency of the most common haplotype is similar in the two models, blue that it has lower frequency under model , and red that it has lower frequency in We simulated coalescent histories for a sample size of chromosomes under four different models of sequence evolution, hard sweeps, standing sweeps from , soft sweeps conditional on three origins of the beneficial mutation, and a neutral model, with all sweep simulations using a selection coefficient of For all simulations , and we simulate a chromosomal segment with total length divided into discrete loci, with for the whole segment. Expectations are taken over 5000 simulations of each model.
On the basis of these simulation results, we suspect that future methods for identifying and distinguishing different varieties of sweeps will see benefits from incorporating haplotypic information over a range of window sizes surrounding the focal site and from pushing deeper into the haplotype frequency spectrum, particularly when large samples are available.
Discussion
An accurate portrait of the patterns of sequence diversity expected in the presence of recent or ongoing positive selection has proved to be vital for the identification of adaptive loci. Recent theoretical and empirical work has drawn attention to the fact that adaptation from standing variation may be relatively common and that patterns of sequence variation produced in such scenarios may differ markedly from those produced under the classical hard sweep model. In this article, we have focused on developing a tractable model of strong positive selection on a single mutation that was previously segregating (or balanced) at low frequency. We have shown that many aspects of the presweep standing phase of the mutation’s history on posthitchhiking patterns variation can be approximated via an application of the ESF. This provides a way to build intuition for the process and obtain various analytical approximations for patterns of variation following a sweep from standing variation.
Our results can be understood within the context of a number of recent approximations for different sweep phenomena, which divide the sweep into distinct phases (see, e.g., Barton 1998; Etheridge et al. 2006). Because rates of coalescence and recombination vary across these phases, different sections of the sequence surrounding a sweep will convey information about different phases, with sites distant from the selected locus generally conveying information about the late stages of the sweep and sites close to the selected locus conveying information about the early stages of the sweep. In our model, the late phase corresponds largely to what we have called the sweep phase, while the early phase corresponds to our standing phase. In general, the major differences between different sweep phenomena occur during the earliest phases, and thus the information to distinguish them is found near the selected site, while extra information about the strength of the sweep can be found at sites that are more distant.
It is worth noting, however, that all of our results are obtained for populations with equilibrium demographics. If population size is variable, particularly over the course of the standing phase, then the ESF fails to accurately describe recombinations during this phase, just as it fails to accurately describe the infinite-alleles model with nonequilibrium demographics. Inference methods based on our analytical calculations would likely be inaccurate in these situations (Bank et al. 2014). Nonetheless, the general insight remains that, holding demography equal, sweeps from standing variation will generate genealogies with longer internal branches than classic hard sweeps and will therefore be characterized by more intermediate-frequency haplotypes.
Although we do not pursue it, essentially all of our results also likely apply to fully recessive sweeps from de novo mutation. This is because a recessive beneficial mutation is effectively neutral until it reaches sufficient frequency for homozygotes to be formed at appreciable enough rates to feel the effects of selection. The result is that recessive sweeps should be fairly well approximated by setting in our model for the standing phase and taking the value of for the latter phase to be
| (18) |
where is Green’s function for a recessive allele under positive selection. This conclusion is foreseen by Ewing et al. (2011), who suggested just this sort of approximation for the reduction in diversity following a recessive sweep. The result is that it is likely to be extremely difficult to distinguish between a sweep from previously neutral or balanced standing variation and a recessive sweep without additional biological information about dominance relationships at the locus of interest.
It is also worth addressing the fact that our model relates to standing variation that is either neutral or balanced prior to the onset of positive selection, while many sweeps from standing variation may in fact proceed from alleles that were previously deleterious (Orr and Betancourt 2001; Hermisson and Pennings 2005). Exactly to what extent this is true is an empirical question that remains largely unanswered, but there is at least some support in the empirical literature for both conditional neutrality and antagonistic pleiotropy (e.g., Anderson et al. 2013). Our model therefore represents one bound of what is effectively a continuum of possible histories for the beneficial allele prior to the onset of positive selection. When alleles are neutral or balanced prior to the onset of positive selection, the distribution on the number and size of recombinant families at linked sites is approximately given by our singleton inflated ESF. As the selection coefficient experienced by the standing variant prior to the environmental change becomes more negative, this distribution shifts toward fewer independent recombinant families, and the largest family comes to dominate the distribution as the genealogy at the selected locus becomes more and more star-like.
While we do not pursue this model in detail, consider a few simple observations. Conditional on being derived and being found at frequency f at the time of the environmental change, the trajectory that an allele with a deleterious selection coefficient of some value took to get there is precisely the same trajectory that would be taken by a beneficial allele with a selection coefficient of equal magnitude but opposite sign (Nagasawa and Maruyama 1979). If the environmental switch merely caused a change in the sign of the selection coefficient, but no change in magnitude, then this sweep from standing variation would be impossible to distinguish from a classic hard sweep with a constant beneficial selection coefficient, as the trajectories are exactly identical. If, however, the magnitude of the selection coefficient were to change as well, then one might in principle be able to determine that the sweep came from standing variation by spotting the fact that the selection coefficient implied by the changes in the partition scheme occurring close to the selected site, which reflect the earliest portion of the sweep’s history, and the period when deleterious selection would have been operating are inconsistent with the selection coefficient implied by the distances at which singleton recombination events are observed, which largely reflect the later portion of the frequency trajectory, when the allele was beneficially selected. To what extent this task can be accomplished in practice requires further investigation.
Unfortunately, our work largely confirms the intuition and existing results indicating that standing sweeps are likely to be rather difficult to identify, and characterize, on the basis of genetic data from a single population time point, and when they can be identified, they may be difficult to distinguish from classic hard sweeps (Peter et al. 2012; Schrider et al. 2015). This can be understood from first principles by recognizing that the identification of a standing sweep amounts to recognizing that a particular region of the genome effectively experienced a reduction in effective population size by a factor of f, followed by a period of rapid growth back up to the experienced by the bulk of the genome. This task is made difficult by the fact that one has effectively only a single genealogy with which to make this inference and rather imperfect information about its shape and size and that it shares features both with genealogies expected under a classic hard sweep and with those under neutrality. As discussed above, this problem becomes even more challenging when the allele was previously deleterious, as the genealogy prior to the environmental change will be even more similar to that experienced in a classic hard sweep.
As a result, we suspect that efforts to detect selection from standing variation will continue to be most effective when additional data are available from populations where the allele was not favored or failed to spread for some other reason (Innan and Kim 2008; Chen et al. 2010; Roesti et al. 2014). If we have good evidence that the allele has spread rapidly, e.g., if the populations are very closely related, then evidence that it is a sweep from standing variation could be gained from demonstrating that the genomic width of the sweep was much smaller than expected and there are too many intermediate-frequency haplotypes, given how quickly it would have to have transited through the population. Ancient DNA is also likely to be of value, as we may similarly be able to identify alleles that were at low frequency too recently in the past given observed present-day patterns of genetic variation.
Simulation details
To check our analytical results, we wrote a program to simulate allele frequency trajectories under our model and then either ran custom-written structured coalescent simulations (Figure 3, Figure S1, Figure S2, and Figure S3) or submitted these trajectories to the program mssel to generate sequence data (Figure 4, Figure 5, Figure 6, and Figure 7).
Frequency trajectories
We simulate frequency trajectories under a similar discretized approximation to the diffusion to that used by Przeworski et al. (2005). To simulate trajectories conditional on selection having begun when the allele was at frequency f, we set and simulate allele frequency change forward in time according to
| (19) |
where
| (20) |
| (21) |
and we take so that one time step is equivalent to the duration of one generation in the discrete-time Wright–Fisher model, and we have conditioned on the eventual fixation of the allele. To simulate the neutral portion of the frequency trajectory prior to the onset of selection, conditional on the allele having been derived, we take advantage of the time reversibility property of the diffusion process, which dictates that the distribution on the prior history of an allele conditional on being derived and being found at frequency f is the same as the future trajectory of an allele that is at frequency f and destined to be lost from the population. This allows us to simulate from
| (22) |
where
| (23) |
We simply then paste these two trajectories together to give a frequency trajectory that is conditioned on a sweep beginning when the allele is at frequency f without any unnatural conditioning on the sweep beginning the first time the allele reaches frequency f. For simulations intended to check our standing phase calculation independent of the sweep phase, we simply discard the sweep portion of the simulation and retain only the neutral trajectory.
Genealogy and recombination histories
We simulate the genealogy backward in time at the locus of the beneficial allele by allowing a coalescent event to occur between two randomly chosen lineages in generation t with probability , where gives the number of lineages existing in generation t. Coalescent times obtained from these simulations are then used to generate Figure S1, Figure S2, and Figure S3. We then simulate the history of recombination events that move lineages off of the beneficial background as follows.
We calculate the total time in the genealogy, T, and simulate an distance to the first recombination event. A position on the tree (i.e., a branch and a specific generation for the event to occur in) is chosen uniformly at random. This event is accepted with probability , where gives the generation in which the event occurred; otherwise it is ignored. This process is repeated outward away from the focal site until the end of the sequence is reached, and the physical position along the sequence, the branch, and the generation of each recombination event are recorded.
We then generate haplotype identities (i.e., the colors in Figure 2) as follows. We begin at the root of the tree and assign each sequence to have the same identity over its entire length. We then move forward in time, from one recombination event to the next, and for each recombination event we assign the chromosomes that subtend it a unique new identity extending from the position where that event occurred out the end of the sequence, overwriting whatever identity previously existed there. We iterate this procedure all the way until the present day. An equivalent method would be to simply assign each portion of sequence an identity corresponding to the most recent recombination event that falls between it and the focal site. These haplotype identities are what we use to generate Figure 3.
Simulated sequence data
To simulate sequence data, we simply submit the trajectories simulated as described above to the program mssel (developed by R. R. Hudson, compiled code is included in File S1). Whereas our simulations of haplotype identity above still represent a somewhat heuristic approximation to the true process, in that they ignore recombination events that do not result in transitions to the alternate background, these simulations of sequence data are exact under the structured coalescent with recombination for our model up to the discretization of the diffusion process used for the selected allele. These simulations are used to generate Figure 4, Figure 5, Figure 6, and Figure 7.
Supplementary Material
Acknowledgments
We thank Simon Aeschbacher, Gideon Bradburd, Ivan Juric, Kristin Lee, Alisa Sedghifar, Chenling Xu, and members of the Ross-Ibarra and Schmitt laboratories at University of California, Davis, as well as John Wakeley and two anonymous reviewers for helpful feedback on the work described in this article. This work was supported by the National Science Foundation (NSF) GRFP under award 1148897, by the NSF under grant 1353380 to John Willis and G. Coop, and by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under awards NIH R01GM83098 and R01GM107374 to G. Coop.
Appendix
Incompatibility of Standing Sweep and Classic Strong Selection Models
Consider the small r approximation for the recovery of diversity under our standing sweep model and the approximation
| (A1) |
for the hard sweep model. We can set these two expressions equal to one another and solve for , yielding
| (A2) |
where is Lambert’s W function. This function evaluates to approximately zero for all sensible combinations of parameters under our model, and this fact is responsible for the inability to map the effect of sweeps under the standing sweep model to the strong selection–hard sweep model.
Inclusion of New Mutations in the Frequency Spectrum
In the main text, we defined as the expected number of segregating mutations present on j of k ancestral lineages. Here, we introduce a subscript and then define
| (A3) |
We can then give an improvement upon Equation 14 as
| (A4) |
and obtain the normalized version by dividing by their sum.
We can make an improvement upon Equation 15 in a similar manner. We first redefine
| (A5) |
In other words, we allow new mutations to occur during the standing phase provided that there have been no recombination events during this phase, and we also allow new mutations during the sweep phase on all lineages that do not recombine during that phase. Obtaining an improvement over Equation 15 is then once again simply a matter of adding the term for new mutations into the previous expression,
| (A6) |
When Does This Model Apply?
From Hermisson and Pennings (2005), given that one or more adaptive alleles are present in the population and either fixed or destined for fixation G generations after an environmental change, and that these alleles were neutral prior to the environmental change, the probability that a population uses material from the standing variation is approximately
| (A7) |
If the population uses material from the standing variation, the probability of finding a single uniquely derived copy of the allele in a sample of n lineages is approximately
| (A8) |
following from the work of Pennings and Hermisson (2006a). If this allele was at a frequency at the moment of the environmental change, the signature left in polymorphism data will be that of a hard sweep (see Przeworski et al. 2005). The probability density function for the frequency f of a derived allele at the moment of the environmental change, conditional on its eventual fixation, is
| (A9) |
such that we can define the probability that our sweep from standing variation comes from between frequencies a and b as
| (A10) |
The probability that a sweep of a uniquely derived allele from the standing variation will leave a classic hard sweep signature is therefore
| (A11) |
If we take as an approximate upper bound on the frequency from which a uniquely derived sweep from standing variation can be successfully detected, then the probability of such an event is
| (A12) |
whereas the probability that adaptation proceeds from a uniquely derived standing variant but is essentially undetectable is
| (A13) |
On the other hand, the probability of obtaining a classic hard sweep signature via a new mutation that occurs after the environmental change is
| (A14) |
while the probability of a multiple-mutation soft sweep, regardless of whether it comes from standing variation or de novo mutation, is
| (A15) |
Footnotes
Communicating editor: J. Wakeley
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.178962/-/DC1.
Literature Cited
- Anderson, J. T., C.-R. Lee, C. A. Rushworth, R. I. Colautti, and T. Mitchell-Olds, 2013. Genetic trade-offs and conditional neutrality contribute to local adaptation. Mol. Ecol. 22(3): 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfatto P., 2007. Hitchhiking effects of recurrent beneficial amino acid substitutions in the Drosophila melanogaster genome. Genome Res. 17(12): 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank C., Ewing G. B., Ferrer-Admettla A., Foll M., Jensen J. D., 2014. Thinking too positive? Revisiting current methods of population genetic selection inference. Trends Genet. 30(12): 540–546. [DOI] [PubMed] [Google Scholar]
- Barrett R. D. H., Schluter D., 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23(1): 38–44. [DOI] [PubMed] [Google Scholar]
- Barton N. H., 1998. The effect of hitch-hiking on neutral genealogies. Genet. Res. 72: 123–133. [Google Scholar]
- Chen H., Patterson N., Reich D., 2010. Population differentiation as a test for selective sweeps. Genome Res. 20(3): 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo P. F., 2005. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307(5717): 1928–1933. [DOI] [PubMed] [Google Scholar]
- Coop G., Ralph P., 2012. Patterns of neutral diversity under general models of selective sweeps. Genetics 192: 205–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues V. S., Poh Y.-P., Peterson B. K., Pennings P. S., Jensen J. D., et al. , 2012. Evidence of adaptation from ancestral variation in young populations of beach mice. Evolution 66(10): 3209–3223. [DOI] [PubMed] [Google Scholar]
- Durrett R., Schweinsberg J., 2004. Approximating selective sweeps. Theor. Popul. Biol. 66(2): 129–138. [DOI] [PubMed] [Google Scholar]
- Durrett R., Schweinsberg J., 2005. A coalescent model for the effect of advantageous mutations on the genealogy of a population. Stoch. Proc. Appl. 115(10): 1628–1657. [Google Scholar]
- Elyashiv, E., S. Sattath, T. T. Hu, A. Strustovsky, G. McVicker et al., 2014 A genomic map of the effects of linked selection in Drosophila. arXiv:1408.5461: 1–100. [DOI] [PMC free article] [PubMed]
- Etheridge A., Pfaffelhuber P., Wakolbinger A., 2006. An approximate sampling formula under genetic hitchhiking. Ann. Appl. Probab. 16(2): 685–729. [Google Scholar]
- Ewens W. J., 1972. The sampling theory of selectively neutral alleles. Theor. Popul. Biol. 112: 87–112. [DOI] [PubMed] [Google Scholar]
- Ewing G., Hermisson J., Pfaffelhuber P., Rudolf J., 2011. Selective sweeps for recessive alleles and for other modes of dominance. J. Math. Biol. 63(3): 399–431. [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A., Keightley P. D., 2009. Estimating the rate of adaptive molecular evolution in the presence of slightly deleterious mutations and population size change. Mol. Biol. Evol. 26(9): 2097–2108. [DOI] [PubMed] [Google Scholar]
- Fay J. C., Wu C. I., 2000. Hitchhiking under positive Darwinian selection. Genetics 155: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud N. R., Rosenberg N. A., 2015. Enhancing the mathematical properties of new haplotype homozygosity statistics for the detection of selective sweeps. Theor. Popul. Biol. 92: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud N. R., Messer P. W., Buzbas E. O., Petrov D. A., 2015. Recent selective sweeps in North American Drosophila melanogaster show signatures of soft sweeps. PLoS Genet. 11(2): e1005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. C., 1980. Lines of descent in the diffusion approximation of neutral Wright-Fisher models. Theor. Popul. Biol. 17(1): 37–50. [DOI] [PubMed] [Google Scholar]
- Griffiths R. C., 2003. The frequency spectrum of a mutation, and its age, in a general diffusion model. Theor. Popul. Biol. 64(2): 241–251. [DOI] [PubMed] [Google Scholar]
- Griffiths R. C., Tavare S., 1998. The age of a mutation in a general coalescent tree. Stoch. Models 14(1–2): 273–295. [Google Scholar]
- Griffiths R. C., Tavare S., 1999. The ages of mutations in gene trees. Ann. Appl. Probab. 9: 567–590. [Google Scholar]
- Hermisson J., Pennings P. S., 2005. Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169: 2335–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson J., Pfaffelhuber P., 2008. The pattern of genetic hitchhiking under recurrent mutation. Electron. J. Probab. 13: 2069–2106. [Google Scholar]
- Innan H., Kim Y., 2004. Pattern of polymorphism after strong artificial selection in a domestication event. Proc. Natl. Acad. Sci. USA 101(29): 10667–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan H., Kim Y., 2008. Detecting local adaptation using the joint sampling of polymorphism data in the parental and derived populations. Genetics 179: 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan H., Nordborg M., 2003. The extent of linkage disequilibrium and haplotype sharing around a polymorphic site. Genetics 165: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. D., 2014. On the unfounded enthusiasm for soft selective sweeps. Nat. Commun. 5: 5281. [DOI] [PubMed] [Google Scholar]
- Jones B. L., Raga T. O., Liebert A., Zmarz P., Bekele E., et al. , 2013. Diversity of lactase persistence alleles in Ethiopia: signature of a soft selective sweep. Am. J. Hum. Genet. 93(3): 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N. L., Hudson R. R., Langley C. H., 1989. The “hitchhiking effect” revisited. Genetics 123: 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov T., Messer P. W., Petrov D. A., 2010. Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genet. 6(6): e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Crow J. F., 1964. The number of alleles that can be maintained in a finite population. Genetics 49: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R., 2011. Inference of human population history from individual whole-genome sequences. Nature 475(7357): 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwire M. M., Bayer F., Webster C. L., Cao C., Jiggins F. M., 2011. Successive increases in the resistance of Drosophila to viral infection through a transposon insertion followed by a duplication. PLoS Genet. 7(10): e1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J., Haigh J., 1974. The hitch-hiking effect of a favourable gene. Genet. Res. 23(1): 23–35. [PubMed] [Google Scholar]
- McVean G., 2006. The structure of linkage disequilibrium around a selective sweep. Genetics 175: 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi P., Shi M. A., Lougarre A., Tang Z. H., Fournier D., 2004. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer P. W., Neher R. A., 2012. Estimating the strength of selective sweeps from deep population diversity data. Genetics 191(2): 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer P. W., Petrov D. A., 2013. Population genomics of rapid adaptation by soft selective sweeps. Trends Ecol. Evol. 28(11): 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M., Maruyama T., 1979. An application of time reversal of Markov processes to a problem of population genetics. Adv. Appl. Probab. 11(3): 457–478. [Google Scholar]
- Nair S., Nash D., Sudimack D., Jaidee A., Barends M., et al. , 2006. Recurrent gene amplification and soft selective sweeps during evolution of multidrug resistance in malaria parasites. Mol. Biol. Evol. 24(2): 562–573. [DOI] [PubMed] [Google Scholar]
- Nielsen R., Williamson S., Kim Y., Hubisz M. J., Clark A. G., et al. , 2005. Genomic scans for selective sweeps using SNP data. Genome Res. 15(11): 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A. and A. J. Betancourt, 2001 Haldane’s sieve and adaptation from the standing genetic variation. Genetics 157: 875–884. [DOI] [PMC free article] [PubMed]
- Patterson N. J., 2005. How old is the most recent ancestor of two copies of an allele? Genetics 169: 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. S., Steinrücken M., Song Y. S., 2011. An accurate sequentially Markov conditional sampling distribution for the coalescent with recombination. Genetics 187: 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings P. S., Hermisson J., 2006a Soft sweeps II–molecular population genetics of adaptation from recurrent mutation or migration. Mol. Biol. Evol. 23(5): 1076–1084. [DOI] [PubMed] [Google Scholar]
- Pennings P. S., Hermisson J., 2006b Soft sweeps III: the signature of positive selection from recurrent mutation. PLoS Genet. 2(12): e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B. M., Huerta-Sanchez E., Nielsen R., 2012. Distinguishing between selective sweeps from standing variation and from a de novo mutation. PLoS Genet. 8(10): e1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokalyuk C., 2012. The effect of recurrent mutation on the linkage disequilibrium under a selective sweep. J. Math. Biol. 64(1–2): 291–317. [DOI] [PubMed] [Google Scholar]
- Przeworski M., Coop G., Wall J. D., 2005. The signature of positive selection on standing genetic variation. Evolution 59(11): 2312–2323. [PubMed] [Google Scholar]
- Ralph P., Coop G., 2010. Parallel adaptation: One or many waves of advance of an advantageous allele? Genetics 186: 647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala B., 1997. On the genealogy of a rare allele. Theor. Popul. Biol. 52(3): 216–223. [DOI] [PubMed] [Google Scholar]
- Rasmussen M. D., Hubisz M. J., Gronau I., Siepel A., 2014. Genome-wide inference of ancestral recombination graphs. PLoS Genet. 10(5): e1004342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesti M., Gavrilets S., Hendry A. P., Salzburger W., Berner D., 2014. The genomic signature of parallel adaptation from shared genetic variation. Mol. Ecol. 23(16): 3944–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti P. C., Reich D. E., Higgins J. M., Levine H. Z. P., Richter D. J., et al. , 2002. Detecting recent positive selection in the human genome from haplotype structure. Nature 419(6909): 832–837. [DOI] [PubMed] [Google Scholar]
- Salgueiro P., Vicente J. L., Ferreira C., Teófilo V., Galvão A., et al. , 2010. Tracing the origins and signatures of selection of antifolate resistance in island populations of Plasmodium falciparum. BMC Infect. Dis. 10(1): 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Good R. T., Appleton B., Sherrard J., Raymant G. C., et al. , 2010. Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet. 6(6): e1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrider D. R., Mendes F. K., Hahn M. W., Kern A. D., 2015. Soft shoulders ahead: spurious signatures of soft and partial selective sweeps result from linked hard sweeps. Genetics 200: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsberg J., Durrett R., 2005. Random partitions approximating the coalescence of lineages during a selective sweep. Ann. Appl. Probab. 15(3): 1591–1651. [Google Scholar]
- Sheehan S., Harris K., Song Y. S., 2013. Estimating variable effective population sizes from multiple genomes: a sequentially Markov conditional sampling distribution approach. Genetics 194: 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer A., Zhao Q., Ross-Ibarra J., Doebley J., 2011. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 43(11): 1160–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight B. F., Kudaravalli S., Wen X., Pritchard J. K., 2006. A map of recent positive selection in the human genome. PLoS Biol. 4(3): e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson G. A., 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7(2): 256–276. [DOI] [PubMed] [Google Scholar]
- Watterson G. A., 1984. Lines of descent and the coalescent. Theor. Popul. Biol. 26(1): 77–92. [Google Scholar]
- Wiehe T. H., Stephan W., 1993. Analysis of a genetic hitchhiking model, and its application to DNA polymorphism data from Drosophila melanogaster. Mol. Biol. Evol. 10(4): 842–854. [DOI] [PubMed] [Google Scholar]
- Wilson B. A., Petrov D. A., Messer P. W., 2014. Soft selective sweeps in complex demographic scenarios. Genetics 198: 669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiuf C., 2000. On the genealogy of a sample of neutral rare alleles. Theor. Popul. Biol. 58(1): 61–75. [DOI] [PubMed] [Google Scholar]
- Wiuf C., Donnelly P., 1999. Conditional genealogies and the age of a neutral mutant. Theor. Popul. Biol. 56(2): 183–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All custom simulation code was written by J.J. Berg in R and is provided in File S1. We also made use of the as yet unpublished program mssel, written by Dick Hudson, a copy of which is also provided in File S1.