Abstract
Genetic heterogeneity occurs when individuals express similar phenotypes as a result of different underlying mechanisms. Although such heterogeneity is known to be a potential source of unexplained heritability in genetic mapping studies, its prevalence and molecular basis are not fully understood. Here we show that substantial genetic heterogeneity underlies a model phenotype—the ability to grow invasively—in a cross of two Saccharomyces cerevisiae strains. The heterogeneous basis of this trait across genotypes and environments makes it difficult to detect causal loci with standard genetic mapping techniques. However, using selective genotyping in the original cross, as well as in targeted backcrosses, we detected four loci that contribute to differences in the ability to grow invasively. Identification of causal genes at these loci suggests that they act by changing the underlying regulatory architecture of invasion. We verified this point by deleting many of the known transcriptional activators of invasion, as well as the gene encoding the cell surface protein Flo11 from five relevant segregants and showing that these individuals differ in the genes they require for invasion. Our work illustrates the extensive genetic heterogeneity that can underlie a trait and suggests that regulatory rewiring is a basic mechanism that gives rise to this heterogeneity.
Keywords: complex traits, genetic mapping, invasive growth, regulatory networks, yeast
GENETIC studies in humans and model organisms have reported unexplained heritability for many traits (Manolio et al. 2009). A possible contributor to this “missing” heritability is genetic heterogeneity—individuals exhibiting similar phenotypes owing to different genetic and molecular mechanisms (Risch 2000; McClellan and King 2010; Wray and Maier 2014). Genetic heterogeneity can reduce the statistical power of mapping studies (Manchia et al. 2013; Wray and Maier 2014) and may involve multiple variants segregating in the same gene (allelic heterogeneity) or different genes (nonallelic heterogeneity) (Risch 2000). Work to date has shown that allelic heterogeneity is widespread (e.g., McClellan and King 2010; Ehrenreich et al. 2012; Long et al. 2014) and often involves two or more null or partial loss-of-function variants segregating in a single phenotypically important gene (e.g., Nogee et al. 2000; Sutcliffe et al. 2005; Will et al. 2010). However, the prominence and underlying mechanisms of nonallelic heterogeneity are less understood.
In this paper we describe an example of nonallelic heterogeneity using heritable variation in the ability of Saccharomyces cerevisiae strains to undergo haploid invasive growth as our model. Invasive growth is a phenotype that is triggered by low carbon or nitrogen availability and is thought to be an adaptive response that allows yeast cells to adhere to and penetrate surfaces (Cullen and Sprague 2000). Invasion typically requires expression of FLO11, which encodes a cell surface glycoprotein that facilitates cell-cell and cell-surface adhesion (Lo and Dranginis 1998; Rupp et al. 1999). In addition to FLO11, S. cerevisiae possesses other cell surface proteins that can contribute to adhesion-related traits [as described in Guo et al. (2000) and Halme et al. (2004) and elsewhere]. In some cases, these cell surface proteins are regulated by multiple signaling cascades (Bruckner and Mosch 2012), potentially providing an opportunity for genetic variants in different pathways to have similar effects on invasion.
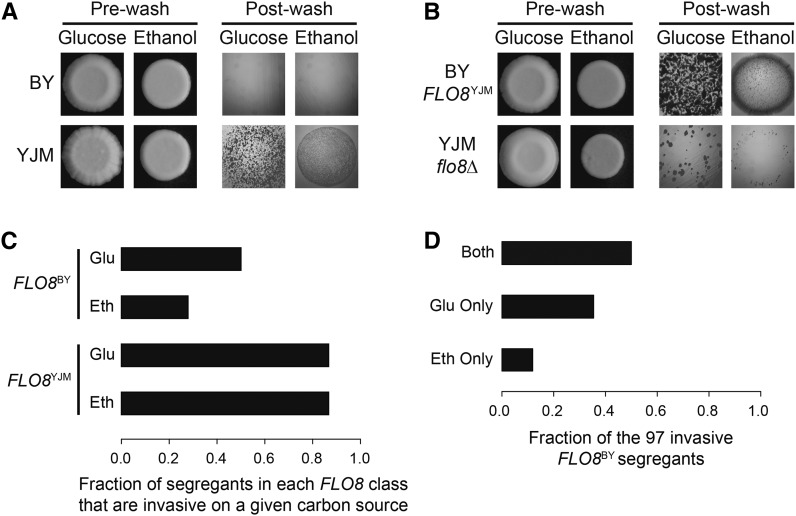
Here we examine the genetic basis of variation in the ability to invade on two carbon sources—glucose and ethanol—in a cross of the laboratory strain BY4716 (BY) and the clinical isolate YJM789 (YJM) (Liti et al. 2009). YJM is highly invasive on both carbon sources (Figure 1A). In contrast, BY cannot grow invasively on either carbon source (Figure 1A). This is because BY carries a nonsense allele of FLO8 (Figure 1B; see also Materials and Methods), which encodes a transcriptional activator that is regulated by the Ras-cAMP-PKA pathway. Flo8 is typically required for invasive growth in both S. cerevisiae (Liu et al. 1996) and Candida albicans (Cao et al. 2006). Consistent with the importance of FLO8 for invasion, deletion of this gene from YJM significantly reduces its invasive growth on both carbon sources (Figure 1B; see also Materials and Methods).
Figure 1.
Effects of FLO8 on ability to invade. (A) BY and YJM were grown for 5 days on YPD or YPE plates at 30°. Colonies were then washed off the plates using water and examined for invasion. (B) Comparison of BY with a functional allele of FLO8 and YJM flo8Δ. (C) Fraction of the initial mapping population of 127 F2 BYxYJM segregants that shows invasion on glucose (“glu”) or ethanol (“eth”) in each FLO8 genotype class. (D) Fraction of the 97 invasive FLO8BY segregants that shows invasion on glucose, ethanol, or both carbon sources (“both”).
While screening BYxYJM segregants for invasion on the two carbon sources, we found that many individuals exhibit invasion even though they possess the FLO8BY nonsense allele, a result that also was recently reported by Song et al. (2014). We show that this FLO8-independent growth has a heterogeneous genetic basis that reflects the presence of multiple distinct regulatory architectures that enable FLO8-independent invasion. Most of these regulatory architectures are FLO11 dependent but require different transcriptional activators; however, we also provide evidence for an architecture that is FLO11 independent. Our results suggest that regulatory rewiring is an important source of nonallelic genetic heterogeneity and illustrate how studying the causes of phenotypic similarities among genetically distinct individuals can advance our understanding of complex traits.
Materials and Methods
Generation of initial mapping population
We used the synthetic genetic array marker system (Tong et al. 2001) to generate recombinant BYxYJM MATa segregants. The BY parent of our cross was MATα can1∆::STE2pr-SpHIS5 lyp1∆ his3∆, while the YJM parent was MATa his3∆::natMX ho::kanMX. We mated these BY and YJM haploids to produce the diploid progenitor of our cross, which was sporulated using standard techniques (Sherman 1991). MATa segregants were obtained using random spore plating on minimal medium containing canavanine, as described previously (Ehrenreich et al. 2010; Taylor and Ehrenreich 2014).
Phenotyping for invasive growth
Strains were phenotyped for invasive growth on 2% agar plates containing yeast extract and peptone (YP) with either 2% glucose (dextrose) or 2% ethanol as the carbon source (YPD and YPE, respectively). Prior to pinning onto the agar plates, strains were grown overnight to stationary phase in liquid YPD. After this culturing step, strains were then pinned onto agar plates and allowed to grow for 5 days. Following this incubation period, we screened for invasive growth by applying water to the agar plates, manually scrubbing colonies, and decanting the mixture of water and cells. Presence or absence of invasion was scored by eye under a light microscope. Each segregant was phenotyped three independent times, and the median phenotype was used in analyses (Supporting Information, Table S1).
Genotyping by sequencing
Segregants were genotyped by Illumina sequencing. Whole genome libraries were constructed using the Illumina Nextera XT DNA Library Preparation Kit. These libraries then were sequenced in multiplex to at least five times genomic coverage on either an Illumina HiSeq 2000 or an Illumina NextSeq 500 with 100 basepair (bp) × 100 bp reads. We also sequenced BY and YJM to ∼100 times genomic coverage and used the data to identify 57,402 high-confidence SNPs. Reads for segregants were mapped to the BY genome using a Burrows-Wheeler Aligner (BWA) (Li and Durbin 2009) and SAMtools (Li et al. 2009). We called genotypes for each individual by taking the base calls at the SNPs and employing a hidden Markov model by chromosome using the HMM package in R, as described by Taylor and Ehrenreich (2014).
Data availability
The sequence data from our experiments is available from the NCBI Sequence Read Archive under accession numbers SRR2039809–SRR2039935, SRR2039936–SRR2039992, SRR2040045–SRR2040076, SRR2040023–SRR2040044, and SRR2039993–SRR2040022 (Table S1, Table S2, Table S3, Table S4, Table S5, and Table S6). All other data from the paper are provided in the Supplement or are available by request from the authors.
Detection of loci influencing ability to invade
Allele frequency analyses were computed using the genotype data of all individuals from a particular mapping population that exhibited the same phenotype. To determine the intervals of the identified causal loci, we identified regions where the alleles were either fixed or at a frequency of 95% or higher.
Genetic engineering
Knockouts were generated by PCR amplifying the CORE cassette with homology-tailed primers and then selecting for transformants on G418 (Storici et al. 2001). NEB Phusion high-fidelity DNA polymerase was used for PCR under the recommended reaction conditions with 35 cycles and an extension time of 30 s per kilobase. The entire coding region of target genes was deleted in these strains. Correct integration of the CORE cassette was checked for each deletion strain using PCR. Allele replacement strains were constructed using the cotransformation of two partially overlapping PCR products (Figure S1), similar to the work of Erdeniz et al. (1997). One product contained the promoter and coding region of the gene to be replaced, while the other included (in order) 60 bp of overlap with the 3′ end of the gene PCR product, kanMX or natMX, and 30–50 bp of the genomic region immediately downstream of the transcribed portion of the gene. Replacement of a gene was verified using Sanger sequencing.
Generation of backcross segregants
Backcrosses were conducted by mating a BYxYJM segregant to a MATα his3∆ version of BY or YJM. Sporulation and selection for MATa backcross segregants were performed as described for the initial mapping population.
Screening for mating type and nongenetic effects
To induce mating-type switching in our MATa segregants, we first deleted URA3 from these individuals using the hphMX cassette with homology-tailed primers, as described earlier. Correct integration of the cassette was verified using PCR and further checked by plating the ura3Δ strains onto 5-FOA plates. Next, mating-type switching was performed using the pGAL-HO plasmid, as described previously (Herskowitz and Jensen 1991). Otherwise, isogenic MATa and MATα individuals were mated to produce homozygous diploids. These individuals were sporulated as described earlier, and standard microdissection techniques were used to obtain spores from the homozygous diploids. Tetrads from which all four spores were recovered were then grown on glucose and ethanol and checked for the ability to invade (Table S7).
Amplification of the FLO11 coding region
The entire FLO11 coding region was PCR amplified using 5′-GGAAGAGCGAGTAGCAACCA as the forward primer and 5′-TTGTAGGCCTCAAAAATCCA as the reverse primer. The sizes of the BY and YJM alleles were compared on a 2% agarose gel.
Results
Many BYxYJM segregants show invasion that is independent of FLO8
We examined a population of 127 genotyped BYxYJM MATa segregants for the ability to invade on two carbon sources—glucose and ethanol (see Materials and Methods). Despite the major role of FLO8 in the invasion phenotypes of BY and YJM (Figure 1, A and B), we unexpectedly found that a large fraction (52%) of segregants with the FLO8BY nonsense allele were capable of invading in at least one condition (Figure 1C). A possible explanation for these individuals’ phenotypes is that FLO8BY is partially functional in some genetic backgrounds. Flo8 is comprised of a LisH domain (amino acids 72–105) that is involved in physical interactions with the transcription factor Mss11 and a transcriptional activation domain (amino acids 701–799) that is necessary for DNA binding (Kim et al. 2014). The nonsense polymorphism in FLO8BY occurs after the LisH domain at amino acid 142, suggesting that the truncated Flo8 may retain some functionality. We tested for partial functionality of FLO8BY by deleting the entire coding portion of FLO8 from multiple invasive FLO8BY segregants and phenotyping them for invasive growth on glucose and ethanol (see Materials and Methods). Complete deletion of FLO8 had no effect on invasion, suggesting that other mechanisms enable these individuals to grow invasively.
Initial effort to identify loci underlying FLO8-independent invasion
As a first step in identifying the genetic basis of FLO8-independent invasion, we screened 384 additional F2 segregants for invasion on glucose and ethanol. We obtained 55 invasive FLO8BY individuals from this experiment, bringing the total number of invasive FLO8BY individuals to 97. Among these 97 individuals, 50% were invasive on both glucose and ethanol, 37% were invasive only on glucose, and 12% were invasive only on ethanol (Figure 1D). We genotyped the 55 new individuals using low-coverage genome sequencing and attempted to detect enriched alleles among the larger set of 97 genotyped FLO8BY strains that were capable of invasion (see Materials and Methods). Although our past work suggested that such selective genotyping should have high statistical power (Ehrenreich et al. 2010), even in the presence of complex nonadditive genetic effects (Taylor and Ehrenreich 2014), we failed to detect any loci using this strategy (Figure S2A).
FLO8-independent invasion in glucose-only individuals depends on the MAPK cascade
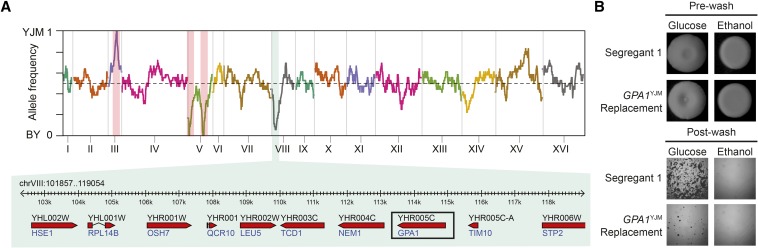
We hypothesized that FLO8-independent invasion is genetically heterogeneous in the BYxYJM cross, reducing the statistical power of our genetic mapping effort. To mitigate this potential problem, we attempted to identify causal loci by focusing on different classes of FLO8BY segregants. We first looked at FLO8BY individuals that showed invasion on both glucose and ethanol, but this analysis did not identify any loci (Figure S2B). We next examined individuals that invaded in only one condition, under the assumption that different mechanisms might underlie condition-specific invasion. Among the segregants showing FLO8-independent invasion only on glucose (n = 36), nearly all these individuals carried the BY allele of a locus on chromosome VIII, which we were able to delimit to 10 genes (Figure 2A; see also Materials and Methods).
Figure 2.
Genetic dissection of FLO8-independent glucose-only invasion. (A) Genome-wide relative allele frequency plot of glucose-only FLO8BY BYxYJM segregants. FLO8 and the markers used to generate haploid progeny are highlighted by red vertical bars, while the strongly enriched locus on chromosome VIII, which was nearly fixed for the BY allele, is highlighted by a green vertical bar. The genomic interval underlying the chromosome VIII peak is also provided. (B) Comparison of Segregant 1, a glucose-only FLO8BY individual, and the GPA1YJM Segregant 1 supports GPA1 as the causal gene underlying the chromosome VIII locus.
To determine the causal gene(s) at the chromosome VIII locus, we replaced the BY allele of each gene in this interval with the YJM allele in a FLO8BY segregant that was invasive only on glucose (Segregant 1; see also Materials and Methods). Each replacement spanned the promoter, coding region, and part of the downstream region of the tested gene (Figure S1). The only replacement that had an effect was GPA1, a subunit of the G-protein-coupled receptor involved in the mitogen-activated protein kinase (MAPK) cascade pheromone response (Fujimura 1989). Converting Segregant 1’s GPA1 allele to the YJM version rendered the strain nearly incapable of invading on glucose and had no effect on ethanol (Figure 2B). BY is known to possess a laboratory-derived amino acid variant (S469I) in GPA1 that causes a large number of gene expression changes specifically in glucose (Yvert et al. 2003; Smith and Kruglyak 2008). This amino acid substitution also may be the causal variant in our study.
Multiple architectures of FLO8-independent invasion in ethanol-only individuals
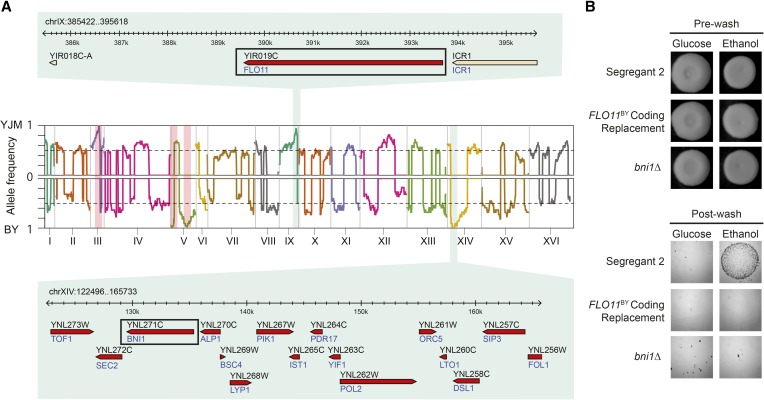
We next studied FLO8BY individuals that were invasive only on ethanol. Because our sample size for this group was small (n = 12), we generated backcross populations in a manner similar to Taylor and Ehrenreich (2014) and used these populations to identify loci that influence invasive growth in a single segregant (Segregant 2; see Materials and Methods). In the backcross to BY, we screened 192 segregants and found that 16% were invasive only on ethanol. Among these individuals (n = 30), we identified a single locus that was nearly fixed for the YJM allele (Figure 3A, top), which was located on chromosome IX and overlapped FLO11. FLO11 is known to harbor extensive functional variation across yeast isolates in both its coding and noncoding regions (Fidalgo et al. 2006, 2008). To test for functional variation at FLO11 in the BYxYJM cross, we separately replaced the coding and noncoding regions of FLO11 in Segregant 2 with the BY alleles (Figure S1; see also Materials and Methods). We found that replacement of the FLO11 coding region caused a loss of invasion on ethanol (Figure 3B), while replacement of the noncoding region had no effect. A number of amino acid differences, as well as an ∼700-bp length difference, distinguish the BY and YJM alleles of FLO11 (Figure S3 and Figure S4), making it difficult to determine the causal variant.
Figure 3.
Genetic dissection of ethanol-only invasion by backcrossing Segregant 2 to BY and YJM. (A) Genome-wide relative allele frequency plots for the BY and YJM backcrosses are shown on the top and bottom, respectively. FLO8 and the markers used to generate haploid progeny are highlighted with red vertical bars, while the strongly enriched intervals on chromosomes IX and XIV are highlighted with green vertical bars. The genomic intervals underlying the chromosome IX and XIV loci are also provided. (B) Comparison of Segregant 2, an ethanol-only FLO8BY individual, to FLO11YJM replacement and BNI1 deletion strains in the Segregant 2 background supports FLO11 and BNI1 as the causal genes underlying the chromosome IX and XIV loci, respectively.
In the backcross of Segregant 2 to YJM, we also screened 192 segregants and found that 11% were invasive only on ethanol. Among these individuals (n = 22), we identified a single locus on chromosome XIV that was fixed for the BY allele. Based on the genotype data, we delimited this interval to 16 candidate genes (Figure 3A, bottom; see also Materials and Methods). We tested every gene in this interval for an effect on Segregant 2’s ability to invade using gene knockouts and found that only deletion of BNI1 resulted in a loss of invasion (Figure 3B; see also Materials and Methods). The BY and YJM alleles of BNI1 possess 31 coding SNPs, 7 of which are nonsynonymous, as well as 3 SNPs upstream of the gene (Figure S5). Bni1, which has been shown previously to affect invasive growth (Mosch and Fink 1997; Kang and Jiang 2005), is involved in the assembly of actin cables (Sagot et al. 2002) and physically interacts with multiple components of the MAPK cascade involved in pheromone response (Chen and Thorner 2007).
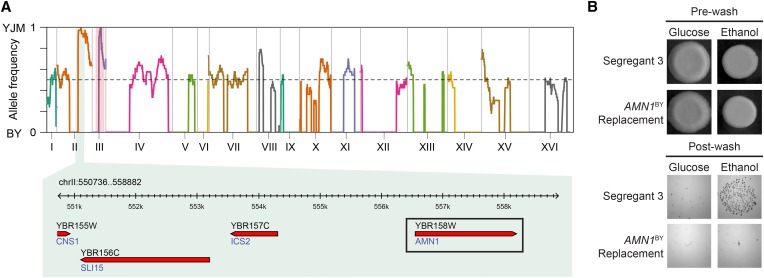
Although the FLO11YJM coding region contributes to invasion on ethanol, not all the ethanol-only segregants possessed this allele. Among the 12 individuals that were invasive only on ethanol in our genotyped F2 population, two carried FLO11BY. To determine the mechanism that allows these individuals to invade only on ethanol, we backcrossed one relevant segregant (Segregant 3) to BY and YJM. The YJM backcross exhibited very low sporulation; for this reason, we were only able to perform genetic mapping in the BY backcross. We screened 192 segregants and found 32 individuals (17%) that grew invasively only on ethanol. We performed genetic mapping to look for enriched alleles and identified a single locus on chromosome II, at which individuals were fixed for the YJM allele (Figure 4A). This locus was detected at a resolution of four genes, of which only AMN1 had an effect when deleted. To verify that the BY and YJM alleles functionally differ, we replaced Segregant 3’s AMN1YJM with AMN1BY and found that this resulted in a loss of invasion (Figure 4B and Figure S1; see also Materials and Methods). An amino acid variant (D368V) in AMN1, which plays a role in daughter cell separation and exit from mitosis (Wang et al. 2003), has been implicated as a major determinant of FLO11-independent cell clumping in multiple studies (Yvert et al. 2003; Li et al. 2013) and also may be the causal variant in our study.
Figure 4.
Genetic dissection of FLO11-independent ethanol-only invasion by backcrossing of Segregant 3 to BY. (A) Genome-wide relative allele frequency plot of ethanol-only invasion in the backcross of Segregant 3 to BY. The marker used to generate haploid progeny is highlighted with a red vertical bar, while the enriched locus on chromosome II is highlighted with a green vertical bar. The genomic interval underlying the chromosome II locus is also provided. (B) Comparison of Segregant 3, a FLO11-independent ethanol-only FLO8BY individual, to AMN1BY replacement strains in the Segregant 3 background supports AMN1 as the causal gene underlying the chromosome II locus.
Testing for effects of mating type and nongenetic factors on FLO8-independent invasion
Nongenetic factors are known to influence the expression of traits in yeast crosses (e.g., Sirr et al. 2015) and also may contribute to FLO8-independent invasion. Additionally, because our experiments were conducted exclusively in MATa haploids, some of the FLO8-independent invasion may be mating-type dependent. To test both these possibilities, we generated and sporulated homozygous diploid versions of Segregants 1, 2, and 3 (see Materials and Methods). From each individual we obtained 7–10 four-spore tetrads. Only mating type and nongenetic factors should segregate among these spores (see Materials and Methods). If we have identified loci that depend on mating type, then invasion should cosegregate 2:2 with mating type. Alternatively, if nongenetic factors contribute to FLO8-independent invasion, then less than 100% of the examined spores should show the same phenotype as their progenitor.
The effects of mating type and nongenetic factors varied among the tested segregants. For Segregants 2 and 3, which only invade on ethanol, all the haploid spores also showed ethanol-only invasion (Table S7). This indicates that mating type and nongenetic factors likely do not influence the phenotypes of these individuals. In contrast, Segregant 1, which only invades on glucose, provided evidence for both mating-type and nongenetic effects. Among the 40 tested spores from this individual, 16 of 20 MATa spores showed glucose-only invasion, while none of the 20 MATα spores exhibited invasion (Table S7). This suggests that Segregant 1’s phenotype is mating-type dependent and also may have a nongenetic component.
Segregants that invade in a FLO8-independent manner require different transcription factors and cell surface proteins
Our results to this point indicate that FLO8-independent invasion has a heterogeneous basis that is largely genetic. This genetic heterogeneity might arise if distinct regulatory factors and/or cell surface proteins facilitate invasion in different segregants and environments. The possibility of such rewiring of invasive growth is supported by recent work showing that the Σ1278b strain requires the transcription factor Tec1 to express FLO11, while BY does not (Chin et al. 2012), as well as by experiments demonstrating extensive variability in transcription factor binding among progeny from the BYxYJM cross (Zheng et al. 2010). Further supporting such a scenario, some of the genes that we cloned have regulatory functions. For example, GPA1 influences signaling through the MAPK cascade, and the MAPK cascade is known to regulate Ste12, which is a transcriptional activator required for invasion in many pathogenic fungi (Lo and Dranginis 1998; Felden et al. 2014).
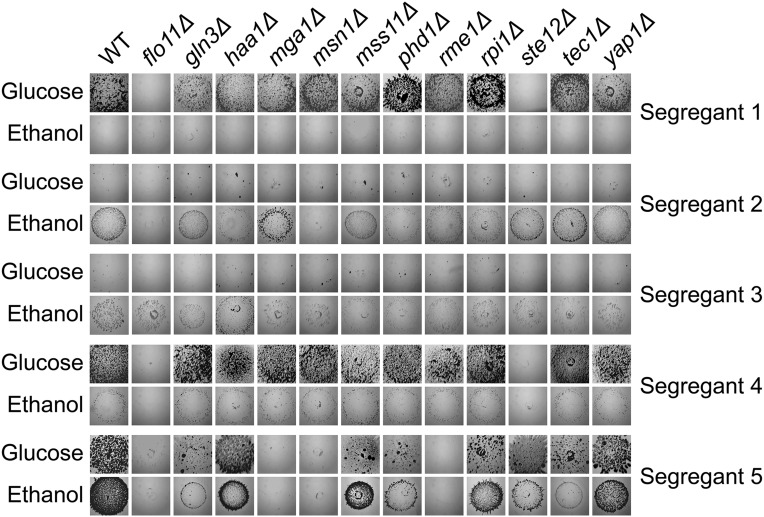
To explore whether regulatory rewiring might contribute to the genetic heterogeneity in our study, we deleted 11 transcription factors that are known to regulate invasion, as well as FLO11, from Segregants 1, 2, and 3 (see Materials and Methods). We also performed these deletions in two additional individuals that showed FLO8-independent invasion on both glucose and ethanol (hereafter referred to as Segregant 4 and Segregant 5). Although some deletions had quantitative effects on invasion (Figure 5), we focused on cases where deletion of one of the examined genes caused inability to invade. Such complete losses of the phenotype indicate genes that are required for a particular segregant to express FLO8-independent invasion.
Figure 5.
Deletion screen of known FLO11 activators. FLO11 and a number of transcription factors that regulate invasive growth were knocked out in Segregants 1–5. These deletion strains then were phenotyped for their ability to invade.
The examined segregants differed in their requirements for FLO11 and four transcription factors—MGA1, MSN1, RME1, and STE12 (Figure 5). None of the deletions caused Segregant 3 to lose its ability to invade, implying that this individual invades in a FLO11-independent manner that may not require the examined transcription factors. In contrast, Segregants 1, 2, 4, and 5 showed FLO11-dependent invasion but differed in the transcription factors that they require. Segregants 1 and 4 lost the ability to invade when STE12 was deleted, suggesting that their ability to invade is MAPK dependent. Segregants 2 and 5 required MSN1, a transcriptional activator that influences many traits in yeast. While MSN1 was the only transcription factor that caused loss of invasion in Segregant 2, Segregant 5 also lost its ability to invade when MGA1 and RME1 were deleted. The finding that individuals differ in the transcription factors and cell surface proteins that they require for invasion supports regulatory rewiring as a cause of genetic heterogeneity in our study.
Conclusion
We have shown that a model phenotype in yeast—haploid invasive growth—exhibits extensive nonallelic genetic heterogeneity. This heterogeneity is caused by genetic variants that change the regulation of invasive growth and enable FLO8-independent invasion in specific cross progeny. Our results from genetic mapping and genetic engineering experiments suggest that multiple distinct regulatory architectures of FLO8-independent invasion segregate in the BYxYJM cross. Although these regulatory architectures require different transcription factors and/or cell surface proteins, they lead to similar abilities to invade.
The present data do not shed light on the specific details of these different regulatory architectures. However, the finding that most BYxYJM segregants that show FLO8-independent invasion require FLO11 suggests that FLO11 expression is an important component of most of the regulatory architectures. This is of note because FLO11 has one of the largest promoters in the yeast genome and is thought to be influenced by at least 8 pathways and 15 transcription factors, as well as linked noncoding RNAs and chromatin remodeling complexes (Bruckner and Mosch 2012). The potential of FLO11 to be regulated by a number of different pathways may facilitate some of the variability in wiring that we have described.
Our finding that different transcription factors and cell surface proteins are required for different genetic backgrounds to invade is similar to the recent discovery of “conditional essential” genes in yeast (Dowell et al. 2010). These conditional essential genes are necessary for viability in some isolates but dispensable in others. Our work suggests that conditional essentiality may arise because genetically distinct individuals express similar phenotypes as a result of different underlying regulatory mechanisms. If this is true, then the essentiality of a gene for a trait will depend on which signaling cascade(s) or pathway(s) an individual employs to express a given phenotype in a particular environment.
Given that we have examined a single phenotype in only one pairwise cross and two conditions, we cannot comment on the broader extent of this heterogeneity across species, traits, and environments. However, we note that our results are comparable to recent studies in humans [as summarized in McClellan and King (2010)] and mice (Shao et al. 2008; Spiezio et al. 2012), which have shown that many genetic perturbations can produce comparable phenotypic outcomes. To some degree, our effort also represents an integration of previous work describing genetic variation in regulatory pathways (Yvert et al. 2003) and transcription factor activity (Zheng et al. 2010; Chin et al. 2012) across yeast isolates. Importantly, we have extended these past studies by connecting changes in signaling and transcription factor activity, as identified via genetic techniques, to phenotypic outcomes.
Supplementary Material
Acknowledgments
We thank Jonathan Lee, Martin Mullis, Matthew Taylor, Lars Steinmetz, and two anonymous reviewers for critically reviewing a draft of this manuscript. We also thank Sammi Ali for technical assistance with this project, Oscar Aparicio for the pGAL-HO plasmid, Charles Nicolet and the USC Epigenome Center staff and Jinliang Li and the staff at Laragen for their help with Illumina sequencing, and Peter Calabrese for comments on this project during its implementation. Our work was supported in part by grants from the National Institutes of Health (R01GM110255 and R21AI108939), the National Science Foundation (MCB1330874), the Army Research Office (W911NF-14-1-0318), the Alfred P. Sloan Foundation, and the Rose Hills Foundation to I.M.E.
Footnotes
Communicating editor: L. M. Steinmetz
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.180661/-/DC1
Literature Cited
- Bruckner S., Mosch H. U., 2012. Choosing the right lifestyle: adhesion and development in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 36: 25–58. [DOI] [PubMed] [Google Scholar]
- Cao F., Lane S., Raniga P., Zhou Z., Ramon K., et al. , 2006. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 17: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. E., Thorner J., 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773: 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin B. L., Ryan O., Lewitter F., Boone C., Fink G. R., 2012. Genetic variation in Saccharomyces cerevisiae: circuit diversification in a signal transduction network. Genetics 192: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Sprague G. F., Jr, 2000. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 97: 13619–13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell R. D., Ryan O., Jansen A., Cheung D., Agarwala S., et al. , 2010. Genotype to phenotype: a complex problem. Science 328: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich I. M., Bloom J., Torabi N., Wang X., Jia Y., et al. , 2012. Genetic architecture of highly complex chemical resistance traits across four yeast strains. PLoS Genet. 8: e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich I. M., Torabi N., Jia Y., Kent J., Martis S., et al. , 2010. Dissection of genetically complex traits with extremely large pools of yeast segregants. Nature 464: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdeniz N., Mortensen U. H., Rothstein R., 1997. Cloning-free PCR-based allele replacement methods. Genome Res. 7: 1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felden J., Weisser S., Bruckner S., Lenz P., Mosch H. U., 2014. The transcription factors Tec1 and Ste12 interact with coregulators Msa1 and Msa2 to activate adhesion and multicellular development. Mol. Biol. Cell 34: 2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo M., Barrales R. R., Ibeas J. I., Jimenez J., 2006. Adaptive evolution by mutations in the FLO11 gene. Proc. Natl. Acad. Sci. USA 103: 11228–11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo M., Barrales R. R., Jimenez J., 2008. Coding repeat instability in the FLO11 gene of Saccharomyces yeasts. Yeast 25: 879–889. [DOI] [PubMed] [Google Scholar]
- Fujimura H. A., 1989. The yeast G-protein homolog is involved in the mating pheromone signal transduction system. Mol. Cell. Biol. 9: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Styles C. A., Feng Q., Fink G. R., 2000. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97: 12158–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A., Bumgarner S., Styles C., Fink G. R., 2004. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell 116: 405–415. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E., 1991. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194: 132–146. [DOI] [PubMed] [Google Scholar]
- Kang C. M., Jiang Y. W., 2005. Genome-wide survey of non-essential genes required for slowed DNA synthesis-induced filamentous growth in yeast. Yeast 22: 79–90. [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Lee S. B., Kang H. S., Oh G. T., Kim T., 2014. Two distinct domains of Flo8 activator mediates its role in transcriptional activation and the physical interaction with Mss11. Biochem. Biophys. Res. Commun. 449: 202–207. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang L., Wu X., Fang O., Wang L., et al. , 2013. Polygenic molecular architecture underlying non-sexual cell aggregation in budding yeast. DNA Res. 20: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Carter D. M., Moses A. M., Warringer J., Parts L., et al. , 2009. Population genomics of domestic and wild yeasts. Nature 458: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Styles C. A., Fink G. R., 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W. S., Dranginis A. M., 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A. D., Macdonald S. J., King E. G., 2014. Dissecting complex traits using the Drosophila Synthetic Population Resource. Trends Genet. 30: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchia M., Cullis J., Turecki G., Rouleau G. A., Uher R., et al. , 2013. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS One 8: e76295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio T. A., Collins F. S., Cox N. J., Goldstein D. B., Hindorff L. A., et al. , 2009. Finding the missing heritability of complex diseases. Nature 461: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J., King M. C., 2010. Genetic heterogeneity in human disease. Cell 141: 210–217. [DOI] [PubMed] [Google Scholar]
- Mosch H. U., Fink G. R., 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogee L. M., Wert S. E., Proffit S. A., Hull W. M., Whitsett J. A., 2000. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am. J. Respir. Crit. Care Med. 161: 973–981. [DOI] [PubMed] [Google Scholar]
- Risch N. J., 2000. Searching for genetic determinants in the new millennium. Nature 405: 847–856. [DOI] [PubMed] [Google Scholar]
- Rupp S., Summers E., Lo H. J., Madhani H., Fink G., 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18: 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D., 2002. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4: 42–50. [DOI] [PubMed] [Google Scholar]
- Shao H., Burrage L. C., Sinasac D. S., Hill A. E., Ernest S. R., et al. , 2008. Genetic architecture of complex traits: large phenotypic effects and pervasive epistasis. Proc. Natl. Acad. Sci. USA 105: 19910–19914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., 1991. Guide to yeast genetics and molecular, pp. 3–21 in Methods in Enzymology, edited by Guthrie C., Fink G. R. Elsevier Academic Press, San Diego. [Google Scholar]
- Sirr A., Cromie G. A., Jeffery E. W., Gilbert T. L., Ludlow C. L., et al. , 2015. Allelic variation, aneuploidy, and nongenetic mechanisms suppress a monogenic trait in yeast. Genetics 199: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. N., Kruglyak L., 2008. Gene-environment interaction in yeast gene expression. PLoS Biol. 6: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Johnson C., Wilson T. E., Kumar A., 2014. Pooled segregant sequencing reveals genetic determinants of yeast pseudohyphal growth. PLoS Genet. 10: e1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiezio S. H., Takada T., Shiroishi T., Nadeau J. H., 2012. Genetic divergence and the genetic architecture of complex traits in chromosome substitution strains of mice. BMC Genet. 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storici F., Lewis L. K., Resnick M. A., 2001. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 19: 773–776. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Delahanty R. J., Prasad H. C., McCauley J. L., Han Q., et al. , 2005. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 77: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. B., Ehrenreich I. M., 2014. Genetic interactions involving five or more genes contribute to a complex trait in yeast. PLoS Genet. 10: e1004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A. H., Evangelista M., Parsons A. B., Xu H., Bader G. D., et al. , 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294: 2364–2368. [DOI] [PubMed] [Google Scholar]
- Wang Y., Shirogane T., Liu D., Harper J. W., Elledge S. J., 2003. Exit from exit: resetting the cell cycle through Amn1 inhibition of G protein signaling. Cell 112: 697–709. [DOI] [PubMed] [Google Scholar]
- Will J. L., Kim H. S., Clarke J., Painter J. C., Fay J. C., et al. , 2010. Incipient balancing selection through adaptive loss of aquaporins in natural Saccharomyces cerevisiae populations. PLoS Genet. 6: e1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray N. R., Maier R., 2014. Genetic basis of complex genetic disease: the contribution of disease heterogeneity to missing heritability. Curr. Epidemiol. Rep. 1: 220–227. [Google Scholar]
- Yvert G., Brem R. B., Whittle J., Akey J. M., Foss E., et al. , 2003. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat. Genet. 35: 57–64. [DOI] [PubMed] [Google Scholar]
- Zheng W., Zhao H., Mancera E., Steinmetz L. M., Snyder M., 2010. Genetic analysis of variation in transcription factor binding in yeast. Nature 464: 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data from our experiments is available from the NCBI Sequence Read Archive under accession numbers SRR2039809–SRR2039935, SRR2039936–SRR2039992, SRR2040045–SRR2040076, SRR2040023–SRR2040044, and SRR2039993–SRR2040022 (Table S1, Table S2, Table S3, Table S4, Table S5, and Table S6). All other data from the paper are provided in the Supplement or are available by request from the authors.