Abstract
Objective
To conduct a systematic review of clinical trials that examined the effectiveness of interventions on balance self-efficacy among individuals with stroke.
Design
Systematic review
Summary of Review
Searches of the following databases were completed in December 2014: MEDLINE (1948-present), CINAHL (1982-present), EMBASE (1980-present) and PsycINFO (1987-present) for controlled clinical trials that measured balance self-efficacy in adults with stroke. Reference lists of selected papers were hand-searched to identify further relevant studies.
Review Methods
Two independent reviewers performed data extraction and assessed the methodological quality of the studies using the Physical Therapy Evidence Database scale. Standardized mean differences (SMD) were calculated.
Results
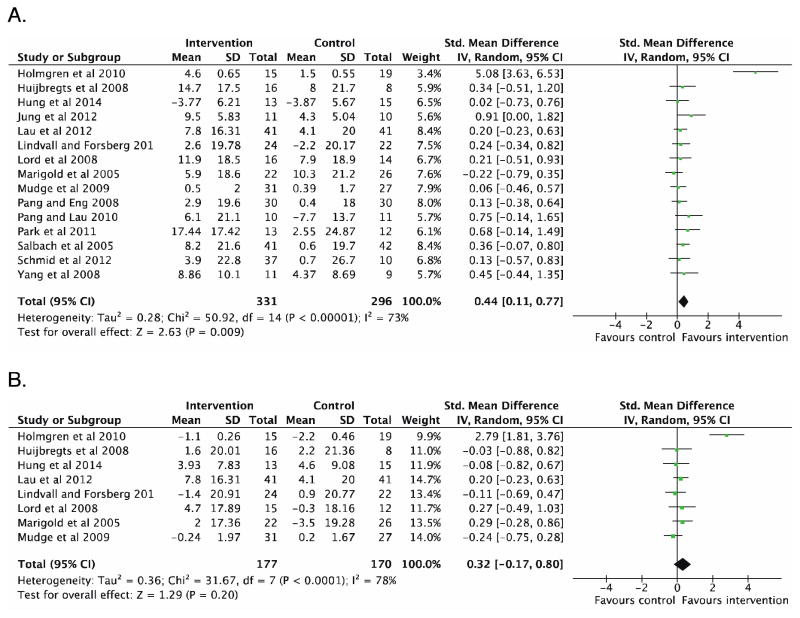
Nineteen trials involving 729 participants used balance self-efficacy as a secondary outcome. Study quality ranged from poor (n=3) to good (n=8). In the meta-analysis of 15 trials that used intensive physical activity interventions, a moderate beneficial effect on balance self-efficacy was observed immediately following the programs (SMD 0.44, 95% CI 0.11–0.77, P=0.009). In the studies that included follow-up assessments, there was no difference between groups across retention periods (8 studies, SMD 0.32, 95% CI −0.17–0.80, P=0.20). In the 4 studies that used motor imagery interventions, there was no between-group difference in change in balance self-efficacy (fixed effects SMD 0.68, 95% CI −0.33–1.69, P=0.18)
Conclusions
Physical activity interventions appear to be effective in improving balance self-efficacy after stroke.
Keywords: Stroke, balance, self-efficacy, systematic review, meta-analysis
Introduction
Impairments in balance and mobility are common, such that the rate of falls after stroke is nearly two times higher relative to age and gender-matched counterparts1. Rehabilitation and recovery interventions typically focus on physical factors such as balance and walking capacity, with gait training being one of most frequently addressed activities2. These interventions are effective in improving balance and mobility outcomes across the continuum of stroke care3–5.
Balance and mobility impairments are also associated with decreased balance confidence6, but the impact of stroke recovery interventions on psychological factors such as balance self-efficacy receives far less attention. Self-efficacy is defined as “an individual’s judgment of his or her ability to organize and execute given types of performances”7. It is a concept that originates from Social Cognitive Theory, which postulates that a person’s perceived level of ability better predicts behavior than their actual physical ability8. Within the context of balance and falls, self-efficacy may be related to either falls self-efficacy, defined as a person’s level of confidence in avoiding falling during daily activities, or balance self-efficacy, a person’s confidence in performing tasks without losing balance or becoming unsteady9. For the purposes of this review, falls self-efficacy and balance self-efficacy will be considered the same construct, and balance self-efficacy is the common term used hereafter.
Balance self-efficacy has been shown to be compromised in community dwelling individuals with stroke10, is a predictor of satisfaction with community reintegration11, a determinant of falls in chronic stroke survivors with low bone mineral density12, and is independently associated with post-stroke activity and participation13. Interventions that improve post-stroke mobility may also contribute to improved self-efficacy by influencing elements of Social Cognitive theory, such as mastery experience (offering opportunities for successful performance), verbal persuasion (positive feedback from instructors or therapists), change in physiological or affective states, or vicarious experience (observing others successes). Importantly, it is anticipated that strategies effective in improving balance self-efficacy are also associated with meaningful clinical endpoints, particularly reduced risk and rate of falling. To prevent a perpetuating cycle of fall incidents, deconditioning and functional decline14, it is important to establish effective interventions to improve balance self-efficacy after stroke.
To our knowledge, there has been no previous review of the effects of post-stroke interventions on balance self-efficacy. The objective of this review was to summarize the results of controlled clinical trials to determine the effectiveness of interventions on improving balance self-efficacy in people with stroke.
Methods
This review was written according to the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses15.
Studies were eligible for inclusion if they compared an intervention to a control group, involved adults with hemorrhagic or ischemic stroke, at any stage or severity along the post-stroke continuum, were conducted in any setting, reported an outcome measure (primary or secondary) related to balance self-efficacy, and were published in English. Case studies, case series, pre-/post-test (non-controlled) studies, dissertations and conference proceedings were excluded, as well as studies that included participants with significant comorbidities affecting balance and mobility.
The following databases were searched up until 4 December 2014: MEDLINE (1946-present), Excerpta Medica database (EMBASE) (1974-present), PsycINFO (1987-present), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982-present). The specific MEDLINE, EMBASE and PsycINFO search strategies are outlined in the Appendix, and equivalent search was applied for the CINAHL database, with appropriate indexing and syntax modifications applied. Reference lists of selected papers were hand searched to identify further relevant studies. Studies were included for further screening even if balance self-efficacy related terms were not mentioned in the title and abstract, provided that all other eligibility criteria were met.
Two independent reviewers initially screened study titles and abstracts for eligibility, then screened and evaluated full text of all relevant studies. If needed, disagreements were resolved through consultation with a third reviewer.
The following data were extracted: study type, details of participant characteristics, interventions, outcome measures, results, and time of follow-up.
For the qualitative assessment, methodological quality of all studies was appraised using the Physical Therapy Evidence Database (PEDro) scale16, a scale that has been used beyond physical therapy interventions, such as pharmacological and non-pharmacological therapies17. Where available, scores were obtained from the PEDro website (www.pedro.org.au); otherwise, scores were determined independently by two reviewers with disagreements resolved by a third reviewer. Study quality was defined using PEDro scores as follows: “good” 6–8 points, “fair” 4–5 points, and “poor” ≤3 points18. Participants, interventions, comparisons, outcomes, and occurrence of adverse events were described.
For the quantitative analysis, the end point outcome measures used were continuous scales of balance self-efficacy or falls self-efficacy. Standardized mean differences (SMD) were used to determine treatment effect sizes, along with 95% confidence intervals. For outcomes with opposite polarity, treatment effects were reversed so that higher scores always indicated better outcome. For all studies, mean change was calculated as the difference between baseline (pre-intervention) and the first post-intervention time points. For studies that included long-term follow-up, mean change between the first and last post-intervention time points was also determined. Effect sizes were defined as small 0.2–0.3, medium 0.5, large >0.819. Fixed effect models were utilized if statistical heterogeneity was low (quantified using the I2 value, which represents the extent of inconsistency among the results that is due to true variation rather than sampling error or chance20). Random effect models were utilized in all other cases. The level of heterogeneity was defined as follows: I2 25% low, 50% moderate, 75% high heterogeneity. Forest plots were generated to illustrate the overall effect of interventions on balance self-efficacy, and funnel plots were used to determine whether publication bias was present. Sensitivity analysis was performed to compare random- and fixed-effect models, and by removing lower quality studies rated as poor or fair quality (PEDro score <6). Statistical analysis was performed using Review Manager software package (RevMan 5.0, Cochrane Collaboration).
Results
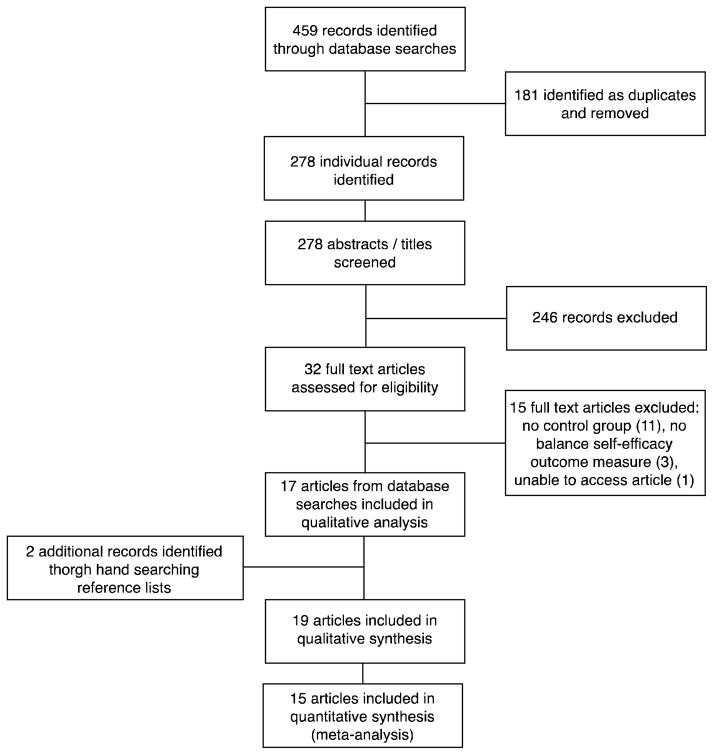
Figure 1 presents the study flow diagram. The initial search identified 459 citations, of which 181 were removed as duplicates. The titles and abstracts of the remaining 278 articles were screened, 246 were excluded, 32 full-text articles were further screened, of which 17 met all eligibility criteria. Two additional articles were identified through searching the reference lists of relevant articles. In total, 19 articles21–39 involving 729 participants, were included in the systematic review (Table 1).
Figure 1.
Study flow diagram
Table 1.
Nineteen trials included in the qualitative synthesis
| Study | Total n, time post-stroke, group n, n (%) men, age, setting | Outcome, time points | PEDro score (/10) | Intervention Group (Duration, type, frequency) | Control Group (Duration, type, frequency) | Between group P |
|---|---|---|---|---|---|---|
| Dickstein et al 201324 | Total n=23, 6 months-2 years post I: n=12, 9 (75%) men, 71.3 years C: n=11, 7 (64%) men, 72.2 years Home |
FES Swedish version (secondary outcome) pre, post, 2 week follow up | 6 | 4 weeks. Integrated imagery practice 3x/week for 15 min | 4 weeks. PT for upper limb function 3x/week for 15 min | 0.67 post-intervention |
| Dickstein et al 201421 | Total n=16, =3 months post, 12 (75%) men, 63 ± 7 years Community |
ABC (secondary outcome) pre, post | 3 | 5 weeks. Motor imagery practice for walking activities 2x/week for 15 min | 5 weeks. Motor imagery practice for upper limb activities 2x/week for 15 min | Not significant |
| Holmgren et al 201027 | Total n=34, 3–6 months post I: n=15, 9 (60%) men, 77.7 ± 7.6 years C: n=19, 12 (63%) men, 79.2 ± 7.5 years Community |
FES-I (secondary outcome) pre, post, 3-and 6-month follow up | 7 | 5 weeks. High intensity functional exercises and activities 3x/week for 90 min; Education 1x/week for 60 min. After 5 weeks, home exercise 3x/week until 3-month follow up. | 5 weeks. Education 1x/week for 60 min | 0.05 post-intervention; 0.03* at 3-months, 0.08 at 6-months |
| Huijbregts et al 200828 | Total n=30, =3 months post I: n=18, 2 (20%) men, 71 ± 7.6 years C: n=12, 3 (33%) men, 63 ± 12.4 years Community |
ABC (secondary outcome) pre, post, 12 weeks follow up | 5 | 8 weeks. Land (stretch, strength, balance) or Pool (endurance) exercise 2x/week for 60 min; Discussion 2x/week for 60 min. One 120-minute booster session provided 6 weeks after program ended. | 6 weeks. Education 1x/week for 90 min | 0.09 |
| Hung et al 201422 | Total n=30, =6 months post I: n=13, 8 (62%) men, 55.5 ± 10.0 years C: n=15, 10 (67%) men, 53.4 ± 10.0 years Outpatient rehabilitation |
FES-I, pre, post, 3 months follow up | 7 | 12 weeks. Maintenance exercises 2x/week plus exergaming weight-shift training 2x/week for 30 min | 12 weeks. Maintenance exercises 2x/week plus conventional weight-shift training 2x/week for 30 min | 0.97 post-intervention; 0.89 at 3-months |
| Hwang et al 201029 | Total n=24, =6 months post I: n=13, 10 (77%) men, 46.4 ± 6.8 years C: n=11, 8 (73%) men, 48.1 ± 5.9 years Community |
ABC (secondary outcome) pre, post | 5 | 4 weeks. Videotape-based locomotor imagery training 5x/week for 25–30 min; Usual care PT 5x/week for 60 min | 4 weeks. Health-related documentary television program 5x/week for 25–30 min; Usual care PT 5x/week for 60 min | <0.001* |
| Jung et al 201226 | Total n=21, >6 months post I: n=11, 7 (64%) men, 60.5 ± 8.6 years C: n=10, 6 (60%) men, 63.6 ± 5.1 years Outpatient services |
ABC pre, post | 6 | 3 weeks. Virtual reality treadmill training with progressive increases in walking speed, 5x/week for 30 min | 3 weeks. Treadmill training without progression, 5x/week for 30 min | <0.05* |
| Lau et al 201225 | Total n=82, >6 months post I: n=41, 26 (63%) men, 57.3 ± 11.3 years C: n=41, 32 (78%) men, 57.4 ± 11.1 years Research laboratory |
ABC – Chinese version, pre, post, 1 month follow up | 8 | 8 weeks. Dynamic exercise combined with whole body vibration, 3x/week for 60 min | 8 weeks. Dynamic exercise on stable platform, 3x/week for 60 min | 0.008*, with large effect sizes pre-vs. post, and pre- vs. follow up. No change post-vs. follow up |
| Lindvall and Forsberg 201423 | Total n=46, >6 months post I: n=24, 12 (50%) men, 62.1 ± 11.4 years C: n=22, 15 (68%) men, 65.5 ± 9.2 years Primary healthcare centre |
ABC pre, post, 14 week follow up | 7 | 8 weeks. Body awareness group sessions 1x/week for 60 min | 8 weeks. No intervention or contact | 0.45 post-intervention, 0.54 at 14 weeks |
| Lord et al 200830 | Total n=30, Post-acute I: n=14, 9 (64%) men, 60.7 ± 17.6 years C: n=16, 9 (56%) men, 64.2 ± 14.8 years Community |
ABC (secondary outcome) pre, post | 6 | 7 weeks. PT assistant-led sessions of functional gait activities 2x/week. Session duration not specified. | 7 weeks. PT 2x/week. Session duration not specified. | 0.93 post-training, 0.47 at 3-month follow up |
| Marigold et al 200531 | Total n=61, =12 months post I: n=22, 17 (77%) men, 68.1 ± 9.0 years C: n=26, 18 (69%) men, 67.5 ± 7.2 years Community |
ABC (secondary outcome) pre, post, 1-month follow up | 6 | 10 weeks. Agility training (dynamic balance and walking) 3x/week for 60 min | 10 weeks. Stretching and weight shifting 3x/week for 60 min | 0.36 |
| Mudge et al 200932 | Total n=58, >6 months post I: n=31, 19 (61%) men, median (min-max) 76.0 (39.0–89.0) years C: n=27, 13 (48%) men, 71.0 years (44.0–86.0) Rehabilitation clinic |
ABC (secondary outcome), pre-, post, 3-months follow up | 7 | 4 weeks. Circuit training (gait, standing balance, lower limb strength) and stretching 3x/week for 50–60 min | 4 weeks. Education or Social sessions 2x/week for 90 min | 0.34 post-training, 0.54 at 3-month follow up |
| Pang Eng 200833 | Total n=60, >12 months post I: n=30, 18 (60%) men, 66.0 ± 8.7 years C: n=30, 13 (43%) men, 65.0 ± 8.5 years Community |
ABC (secondary outcome) pre, post | 6 | 19 weeks. Leg exercise program (aerobic, balance, strength) 3x/week for 60 min | 19 weeks. Arm exercise program 3x/week for 60 min | >0.10 |
| Pang Lau 201034 | Total n=21, >3 months post I: n=10, 7 (70%) men, 64.6 ± 7.2 years C: n=11, 7 (64%) men, 64.5 ± 6.2 years Community |
ABC – Chinese version (secondary outcome) pre, post | 6 | 6 months. Treadmill exercise in community settings 2x/week for 60 min | 6 months. Usual activities (e.g. leisure walking, light household tasks) | 0.018* |
| Park et al 201135 | Total n=25, 6 months-5 years post I: n=13, 7 (54%) men, 59.4 ± 8.5 years C: n=12, 5 (42%) men, 56.9 ± 7.8 years Inpatient rehabilitation |
ABC (secondary outcome) pre, post | 7 | 4 weeks. Ambulation training in community settings 3x/week for 60 min; Usual care PT 5x/week for 60 min | 4 weeks. Usual care PT 5x/week for 60 min | <0.01* |
| Salbach et al 200536 | Total n=91, <12 months post I (n=41, 25 (61%) men) + C (n=42, 26 (62%) men): 71 ± 11 years Community |
ABC (secondary outcome) pre, post | 7 | 6 weeks. Walking intervention 3x/week for 60 min | 6 weeks. Functional upper extremity tasks in sitting 3x/week for 60 min | <0.05* |
| Schmid et al 201237 | Total n=47, <6 months post I: n=37, 20 (54%) men, 63.9 ± 8.7 years C: n=10, 10 (100%) men, 60.2 ± 8.9 Community |
ABC pre, post | 4 | 8 weeks. Yoga (2x/week for 60 min) ± relaxation audio recording (3x/week for 20 min) | 8 weeks. No intervention or contact | >0.016 (NS for multiple comparisons) |
| Schuster et al 201238 | Total n=39, >3 months post Two intervention groups:
|
ABC (secondary outcome) | 5 | 2 weeks:
|
2 weeks. 6 physiotherapy sessions 25–30 min, plus audio recording 17 minutes (total 45–50 min) | >0.05 |
| Yang et al 200839 | Total n=20, >6 months post I: n=11, 5 (45%) men, 55.5 ± 12.2 years C: n=9, 5 (56%) men, 60.9 ± 9.3 years Community |
ABC – Chinese version (secondary outcome) pre, post, 1-month follow up | 6 | 3 weeks. Virtual reality treadmill training 3x/week for 20 min | 3 weeks. Treadmill training 3x/week for 20 min | 0.31 at post-training, 0.78 at 1-month follow up |
P<0.05
Abbreviations: I – Intervention group; C – Control group; FES-I – Falls Efficacy Scale-International; ABC – Activities-specific Balance Confidence Scale; PT – Physical therapy
Qualitative Analysis
Of the studies included in the qualitative analysis, fifteen22–27,30–33,35–39 were RCTs. All but two37,38 were rated as “good” quality (Table 2). Randomization was performed by an independent person or by a computer generated randomization program. The remaining three studies were controlled but not randomized trials, mostly rated “good”34 and “fair”28,29 in quality, with the exception of one that was rated poor quality21 (Table 2). Eleven studies22–25,27,30–32,37–39 included assessments at follow-up time points to evaluate retention of benefits, ranging from 2 weeks to 6 months after the intervention ended. In five studies24,26,31,37,39, loss to follow up was greater than 15%.
Table 2.
Methodological quality (Physical Therapy Evidence Database (PEDro) scores)
| Criterion | Dickstein et al 201324 | Dickstein et al 201421 | Holmgren et al 201027 | Huijbregts et al 200828 | Hung et al 201422 | Hwang et al 201029 | Jung et al 201226 | Lau et al 201225 | Lindvall and Forsberg 201423 | Lord et al 200830 | Marigold et al 200531 | Mudge et al 200932 | Pang and Eng 200833 | Pang and Lau 201034 | Park et al 201135 | Salbach et al 200536 | Schmid et al 201237 | Schuster et al 201238 | Yang et al 200839 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligibility criteria specified* | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Random allocation | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Concealed allocation | N | N | Y | N | Y | N | N | Y | Y | Y | Y | Y | N | N | Y | N | N | Y | Y |
| Groups similar at baseline | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y |
| Subject blinding | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Therapist blinding | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Assessor blinding | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Adequate follow-up | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | N | N |
| Intention-to-treat analysis | N | N | Y | Y | N | Y | N | Y | Y | N | N | Y | N | Y | N | Y | N | Y | N |
| Between-group comparisons | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Point estimates/variability data | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Total score (/10) | 6 | 3 | 7 | 5 | 7 | 5 | 6 | 8 | 7 | 6 | 6 | 7 | 6 | 6 | 7 | 7 | 4 | 5 | 6 |
Criterion does not contribute to total score
Participants
Sample size ranged from 1621 to 9136. Participants’ age ranged from 53 to 80 years, except in one study (<50 years)29. All were ≥3 months post-stroke, with 12 studies involving participants ≥6 months post-stroke22–26,29–33,35,39. Four studies set upper limits on time post-stroke (within 627,37, 1236, or 2424 months). Four studies were conducted in a rehabilitation setting, but participants were 1–5 years post-stroke22,23,32,35. The remaining studies were conducted in the community or laboratory settings. Participants were independent with ambulation with or without assistive devices, except in one study where participants needed only to able to stand with or without a device37. Participants with severe co-morbidities (such as neurological (other than stroke), orthopedic or cardiovascular problems, or any other conditions that precluded study participation) were excluded.
Interventions
Interventions were ≤4 weeks24,26,29,32,35,38,39, one to three months21–23,25,27,28,30,31,36,37, or >5 months33,34 in duration. Intervention frequency ranged from one to five sessions per week.
Fourteen studies involved physical exercise interventions: gait training alone30,34–36 or combined with virtual reality26,39, exergaming22, combination of fitness, mobility and functional exercises27,28,31–33, dynamic exercises combined with whole body vibration25, yoga37, and functional movements combined with body awareness training23. Pool exercises28, home programs27, and education sessions27,28 were also offered. In the four studies that did not use physical activity interventions, motor imagery training was used21,23,24,29,38.
Comparisons
All but 4 studies were randomized controlled trials. In the non-randomized studies, participants self-selected their intervention group based on location and accessibility constraints28, assigned based on order of study enrolment21 or control participants were matched based on age, sex, lesion, time post-stroke or impairment level29,34.
In general, control interventions were comprised of less intensive or lower dose physical activity relative to the Intervention groups. These included upper extremity physical33,36 or mental practice training21, weight shifting and stretching31, dynamic exercises without whole body vibration25, or routine physical therapy30,35. Otherwise, control interventions included stroke educational programming27,28,32,38 health-related documentary programs, which may have been supplemented with routine physical therapy29,38, or treadmill training without an immersive virtual reality environment26,39. In three studies23,34,37, the control group continued with their usual activities but did not receive any intervention. In six studies27,28,34,35,37, groups were not matched for equivalent minutes of attention. Only one study22 was designed such that the control intervention was comparable with respect to time and content of training as the intervention group (weight shift training through exergaming vs. through conventional methods).
Outcomes
None of the trials used measures of balance self-efficacy as the primary outcome. Almost all studies used the Activities-specific confidence scale (ABC) scale (three25,34,39 used the Chinese version40). The Falls Efficacy Scale-International (FES-I)41 and Falls Efficacy Scale-Swedish version were also used22,24,27. The ABC Scale and FES-I have both been shown to have good validity and reliability in community dwelling elderly individuals9,41,42. The ABC Scale has been validated for use in community dwelling individuals both within43 and after one year post-stroke44. The standard error of measurement (SEM) among individuals with stroke is 6.8144. Eight25,26,28–30,35,36,39 of the 13 studies that used the original ABC scale reported improvement in the intervention group that exceeded the SEM.
Adverse Events
Five studies21–23,25,30 reported that no serious adverse events occurred. Two studies reported on occurrence of falls amongst participants: 26 falls involving 5 people in the intervention group and 6 in the control group27, 100 falls involving 16 intervention group participants and 11 in control group31. Adverse events were not reported in the other studies.
Quantitative data analysis
A meta-analysis was performed with the 15 studies that compared more intensive physical exercise-based interventions to less intensive programs22,23,25–28,30–37,39. Immediately following the programs, a medium effect was found favoring interventions over control group to improve balance self-efficacy after stroke (627 participants, SMD 0.44, 95% CI 0.11–0.77, P=0.009) (Figure 2A). When the non-randomized trials were removed from the analysis28,34, the trend towards a beneficial effect of more intensive physical interventions remained (582 participants, SMD 0.43, 95% CI 0.07–0.80, P=0.02). A large effect was found when only the 12 studies that used the ABC scale were included (545 participants, mean difference 3.17, 95% 0.45–5.89, P=0.02). In the eight studies that included follow-up assessments22,23,25,27,30–32,37, there was no difference between groups across retention periods (n=347, SMD 0.32, 95% CI −0.17–0.80, P=0.20) (Figure 2B).
Figure 2.
Meta-analyses of A) 15 studies involving intensive physical activity interventions for training effects immediately after the programs ended, and B) 8 trials that included post-program follow-up assessments
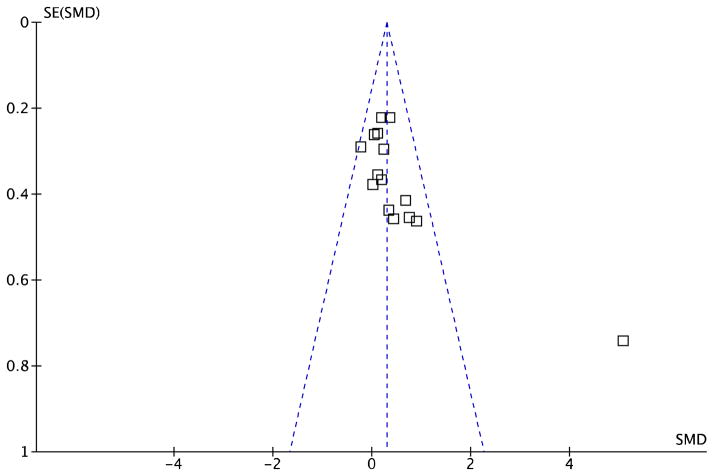
In sensitivity analyses, all randomized trials were of “good” quality (PEDro score ≥6) (Table 2) and as such, no studies were removed based on quality. However, high heterogeneity was noted (I2=82%), and the funnel plot indicated possible publication bias with an outlier study27 (Figure 3). With this study removed, the beneficial effect of intensive physical interventions on balance self-efficacy immediately following the programs remained amongst homogeneous studies (593 participants, fixed effects SMD 0.23, 95% CI 0.07–0.40, P=0.006, I2=0%). In the studies that included follow-up assessments, no difference was observed between groups (313 participants, fixed effects SMD 0.05, 95% CI −0.17–0.28, P=0.65, I2=0%).
Figure 3.
Funnel plot of 15 studies involving intensive physical activity interventions included in meta-analysis
There were 4 studies21,24,29,38 that used motor imagery interventions. There was no difference in change in balance self-efficacy between groups (102 participants, fixed effects SMD 0.68, 95% CI −0.33–1.69, P=0.18).
Discussion
Results from this systematic review suggest that intensive physical interventions, specifically those that involve strengthening, balance, endurance, and functional exercises are more effective than less intensive interventions for improving balance self-efficacy after stroke. There were no differences between groups in follow-up studies.
Post-stroke balance impairment is common and can contribute to mobility restriction and increased risk of falls, but balance self-efficacy is also an important predictor of fall risk11, activity, and participation13. It is important to establish interventions that not only address the physical factors that contribute to improved balance and walking after stroke, but also benefit psychological factors, such as balance self-efficacy. The interventions in these trials were not specifically targeted towards improving balance self-efficacy, as the measures selected were included as a secondary (not primary) outcome. Thus, the studies may not have been adequately powered to detect change in this outcome. Indeed, when individually considered, many of the studies reported non-significant effects of training on balance self-efficacy, but when study results were combined the meta-analysis, we found that intensive physical interventions were effective in improving balance self-efficacy after stroke.
These programs may have offered the necessary elements of Social Cognitive Theory to influence balance self-efficacy8, which may account for the positive benefit observed. Indeed, Huijbregts and colleagues28 designed the intervention arm of their study with enhancing self-efficacy in mind. In all other trials27,30–37,39, physical activity interventions may have influenced self-efficacy through mastery experience by offering opportunities for successful performance of tasks and activities that challenge and improve balance. Further, verbal persuasion may have been incorporated through positive feedback from class instructors, and participants would also experience change in physiological or affective states during the interventions. In trials that offered group classes28,31–33,37, vicarious experience may be gained from observing others successfully perform a task. Arguably, increasing self-efficacy after stroke is relevant only if it also leads to reduced occurrence of falls. Future research may focus on establishing the effectiveness of interventions on improving both balance self-efficacy after stroke and clinical endpoints of risk and rate of falls.
In an earlier meta-analysis of the effectiveness of exercise interventions on balance self-efficacy among older adults without neurological conditions, Tai Chi was more effective than strengthening, functional or task-specific activities45. The authors postulated that the sensory-motor balance elements of Tai Chi, combined with and cognitive and emotional stimuli of relaxation and awareness, contributed improved greater improvements in balance self-efficacy compared to physical activity interventions alone45. For individuals with stroke, similar interventions that concurrently address physical and cognitive factors may yield greater benefit to balance self-efficacy than either form of intervention alone. To date, no studies have examined the effects of Tai Chi on balance self-efficacy after stroke, but one pilot study reported improvements in ABC score with post-stroke yoga37. The authors attribute these positive effects to the active mind-body connection and complex coordination of movement and breathing that is offered through yoga37. Future studies may also examine the effects of interventions that explicitly incorporate relevant components of Social Cognitive Theory foundations8 to improve balance self-efficacy.
The heterogeneity of the included studies was quite high, such that one study27 demonstrated the largest effect on balance self-efficacy and was identified as an outlier. When this study was removed from the meta-analysis, the trend towards improved balance self-efficacy was retained, although there was a reduction in the overall effect. Of the five trials where groups were not matched for attention27,28,34,35,37, this study had the largest disparity (60 vs. 450 minutes/week for control and intervention groups, respectively27). This difference in contact time may account for the greater between-group interaction effect.
There were no differences between groups in studies that included follow-up assessment time points. It is possible that intervention-related improvements in balance self-efficacy wane over time, or programs of longer duration are required for durability of benefits. This may also be a product of the smaller number of trials included in the analysis and thus, there was less sensitivity for detecting change.
The three studies that used motor imagery interventions had disparate findings. Hwang et al29 found a large treatment effect, but also enrolled younger participants (4729 vs. 6338 and 7224 years) and provided the greatest total training time (5 30-minute sessions per week for 4 weeks (total 600 minutes)29 vs. 3 15-minute sessions per week for 4 weeks (180 minutes)24 and 3 50-minute sessions per week for 2 weeks (300 minutes)38). Given the discrepancy in study results and differences in program design, further research focusing on imagery-related interventions is needed to establish its effectiveness on balance self-efficacy.
The major limitation to this systematic review is that none of the trials had the primary aim of examining the effectiveness of post-stroke interventions on balance self-efficacy as the primary outcome. RCTs designed and adequately powered to improve balance self-efficacy among individuals with stroke are warranted. There was also a range in methodological quality across the studies, and differences between control and intervention group with respect to treatment type, delivery, and attention time, which may have influenced the results. Moreover, due to the small number of studies and participants, secondary analyses to compare participant subgroups or intervention types were not performed. As the body of evidence continues to develop, more in depth analyses will be permitted that may examine the differential effects across stages of stroke recovery (early to late), across interventions (physical, cognitive, psychological, combination), or across levels of functional mobility (low to high). Further, more studies that include follow up assessments to determine the long-term effects of post-stroke interventions on balance self-efficacy are warranted.
Clinical Messages.
Physical activity interventions involving strengthening, balance, endurance, and functional exercises appear to be effective in improving balance self-efficacy after stroke
Addressing psychological factors related to balance ability after stroke can be an important strategy for breaking the cycle of fall occurrence, activity restrictions and functional decline
Acknowledgments
We would like to thank Charlotte Beck in assisting with our search strategy.
Finance Support
AT (Tang) is supported by personnel awards from the Heart and Stroke Foundation, Ontario Provincial Office (CS I 7468), the Canadian Institutes of Health Research (MFE-98550) and the Michael Smith Foundation for Health Research (MSFHR) (ST-PDF-03003(11-1)CLIN), and JJE was supported by the CIHR (MSH-63617).
Appendix. Search strategy
exp Stroke/
(stroke* or CVA* or cerebrovascular stroke* or apoplexy or cerebrovascular accident* or cerebral stroke* or hemipar* or hemipleg*).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
1 or 2
(fear adj3 fall*).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
(balance adj3 (confidence or “self efficacy” or self-efficacy)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
(fall* adj3 (“self efficacy” or self-efficacy)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
4 or 5 or 6
Accidental Falls/
Fear/
8 and 9
Postural Balance/
self efficacy/
self concept/
self-assessment/
12 or 13 or 14
11 and 15
7 or 10 or 16
3 and 17
Footnotes
Competing interests
The authors declare no conflicts of interest
Contributors
JJE designed the study. AT (Tang), AT (Tao), MS, CT, HT, JT conducted the research and drafted the manuscript. JJE revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Ada Tang, McMaster University School of Rehabilitation Science, Population Health Research Institute, Hamilton; Canadian Partnership for Stroke Recovery, Ottawa. Address: McMaster University, School of Rehabilitation Science, 1400 Main Street West, Hamilton ON CANADA L8S 1C7, Tel: +1 (905) 525-9140 Extension 27818, Fax: +1 (905) 524-0069.
Amy Tao, University of British Columbia, Faculty of Medicine, Department of Physical Therapy, Vancouver. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
Michelle Soh, University of British Columbia, Faculty of Medicine, Department of Physical Therapy, Vancouver. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
Carolyn Tam, University of British Columbia, Faculty of Medicine, Department of Physical Therapy, Vancouver. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
Hannah Tan, University of British Columbia, Faculty of Medicine, Department of Physical Therapy, Vancouver. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
Jessica Thompson, University of British Columbia, Faculty of Medicine, Department of Physical Therapy, Vancouver. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
Janice J. Eng, University of British Columbia, Faculty of Medicine, Department of Physical Therapy; Vancouver Coastal Health; International Collaboration on Repair Discoveries, Vancouver; Canadian Partnership for Stroke Recovery, Ottawa. Address: Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC CANADA V6T 1Z3.
References
- 1.Simpson LA, Miller WC, Eng JJ. Effect of stroke on fall rate, location and predictors: a prospective comparison of older adults with and without stroke. PLoS One. 2011 Apr 29;6(4):e19431. doi: 10.1371/journal.pone.0019431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother. 2007 Oct;7(10):1417–1436. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009 Jan;23(1):5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 4.Stuart M, Benvenuti F, Macko R, Taviani A, Segenni L, Mayer F, et al. Community-based adaptive physical activity program for chronic stroke: feasibility, safety, and efficacy of the Empoli model. Neurorehabil Neural Repair. 2009 Sep;23(7):726–734. doi: 10.1177/1545968309332734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair. 2012 Jan;26(1):85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 6.Yiu J, Miller WC, Eng JJ, Liu Y. Longitudinal analysis of balance confidence in individuals with stroke using a multilevel model for change. Neurorehabil Neural Repair. 2012 Oct;26(8):999–1006. doi: 10.1177/1545968312437941. [DOI] [PubMed] [Google Scholar]
- 7.Bandura A. Self-efficacy: the exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 8.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977 Mar;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995 Jan;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 10.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Arch Phys Med Rehabil. 2006 Mar;87(3):364–370. doi: 10.1016/j.apmr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Pang MY, Eng JJ, Miller WC. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Phys Ther. 2007 Mar;87(3):282–291. doi: 10.2522/ptj.20060142. [DOI] [PubMed] [Google Scholar]
- 12.Pang MY, Eng JJ. Fall-related self-efficacy, not balance and mobility performance, is related to accidental falls in chronic stroke survivors with low bone mineral density. Osteoporos Int. 2008 Jul;19(7):919–927. doi: 10.1007/s00198-007-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid AA, Van Puymbroeck M, Altenburger PA, Dierks TA, Miller KK, Damush TM, et al. Balance and balance self-efficacy are associated with activity and participation after stroke: a cross-sectional study in people with chronic stroke. Arch Phys Med Rehabil. 2012 Jun;93(6):1101–1107. doi: 10.1016/j.apmr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SM, Munoz B, West SK, Rubin GS, Fried LP. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002 Aug;50(8):1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Centre of Evidence-Based Physiotherapy. [Accessed 08/31, 2012];Physiotherapy Evidence Database (PEDro) 2012 Available at: http://www.pedro.org.au/
- 17.Foley NC, Bhogal SK, Teasell RW, Bureau Y, Speechley MR. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther. 2006 Jun;86(6):817–824. [PubMed] [Google Scholar]
- 18.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke Rehabilitation Evidence-Based Review: methodology. Top Stroke Rehabil. 2003 Spring;10(1):1–7. [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickstein R, Levy S, Shefi S, Holtzman S, Peleg S, Vatine JJ. Motor imagery group practice for gait rehabilitation in individuals with post-stroke hemiparesis: a pilot study. NeuroRehabilitation. 2014;34(2):267–276. doi: 10.3233/NRE-131035. [DOI] [PubMed] [Google Scholar]
- 22.Hung JW, Chou CX, Hsieh YW, Wu WC, Yu MY, Chen PC, et al. Randomized comparison trial of balance training by using exergaming and conventional weight-shift therapy in patients with chronic stroke. Arch Phys Med Rehabil. 2014 Sep;95(9):1629–1637. doi: 10.1016/j.apmr.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Lindvall MA, Forsberg A. Body awareness therapy in persons with stroke: a pilot randomized controlled trial. Clin Rehabil. 2014 Dec;28(12):1180–1188. doi: 10.1177/0269215514527994. [DOI] [PubMed] [Google Scholar]
- 24.Dickstein R, Deutsch JE, Yoeli Y, Kafri M, Falash F, Dunsky A, et al. Effects of integrated motor imagery practice on gait of individuals with chronic stroke: a half-crossover randomized study. Arch Phys Med Rehabil. 2013 Nov;94(11):2119–2125. doi: 10.1016/j.apmr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Lau RW, Yip SP, Pang MY. Whole-body vibration has no effect on neuromotor function and falls in chronic stroke. Med Sci Sports Exerc. 2012 Aug;44(8):1409–1418. doi: 10.1249/MSS.0b013e31824e4f8c. [DOI] [PubMed] [Google Scholar]
- 26.Jung J, Yu J, Kang H. Effects of virtual reality treadmill training on balance and balance self-efficacy in stroke patients with a history of falling. J Phys Ther Sci. 2012;24(11):1133–1136. [Google Scholar]
- 27.Holmgren E, Lindstrom B, Gosman-Hedstrom G, Nyberg L, Wester P. What is the benefit of high-intensive exercise program? A randomized controlled trial. Adv Physiother. 2010;12(3):115–124. doi: 10.3109/14038196.2010.488272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huijbregts MP, Myers AM, Streiner D, Teasell R. Implementation, process, and preliminary outcome evaluation of two community programs for persons with stroke and their care partners. Top Stroke Rehabil. 2008 Sep-Oct;15(5):503–520. doi: 10.1310/tsr1505-503. [DOI] [PubMed] [Google Scholar]
- 29.Hwang S, Jeon HS, Yi CH, Kwon OY, Cho SH, You SH. Locomotor imagery training improves gait performance in people with chronic hemiparetic stroke: a controlled clinical trial. Clin Rehabil. 2010 Jun;24(6):514–522. doi: 10.1177/0269215509360640. [DOI] [PubMed] [Google Scholar]
- 30.Lord S, McPherson KM, McNaughton HK, Rochester L, Weatherall M. How feasible is the attainment of community ambulation after stroke? A pilot randomized controlled trial to evaluate community-based physiotherapy in subacute stroke. Clin Rehabil. 2008 Mar;22(3):215–225. doi: 10.1177/0269215507081922. [DOI] [PubMed] [Google Scholar]
- 31.Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. J Am Geriatr Soc. 2005 Mar;53(3):416–423. doi: 10.1111/j.1532-5415.2005.53158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mudge S, Barber PA, Stott NS. Circuit-based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2009 Dec;90(12):1989–1996. doi: 10.1016/j.apmr.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Pang MY, Eng JJ. Determinants of improvement in walking capacity among individuals with chronic stroke following a multi-dimensional exercise program. J Rehabil Med. 2008 Apr;40(4):284–290. doi: 10.2340/16501977-0166. [DOI] [PubMed] [Google Scholar]
- 34.Pang MY, Lau RW. The effects of treadmill exercise training on hip bone density and tibial bone geometry in stroke survivors: a pilot study. Neurorehabil Neural Repair. 2010 May;24(4):368–376. doi: 10.1177/1545968309353326. [DOI] [PubMed] [Google Scholar]
- 35.Park HJ, Oh DW, Kim SY, Choi JD. Effectiveness of community-based ambulation training for walking function of post-stroke hemiparesis: a randomized controlled pilot trial. Clin Rehabil. 2011 May;25(5):451–459. doi: 10.1177/0269215510389200. [DOI] [PubMed] [Google Scholar]
- 36.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. The effect of a task-oriented walking intervention on improving balance self-efficacy poststroke: a randomized, controlled trial. J Am Geriatr Soc. 2005 Apr;53(4):576–582. doi: 10.1111/j.1532-5415.2005.53203.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmid AA, Van Puymbroeck M, Altenburger PA, Schalk NL, Dierks TA, Miller KK, et al. Poststroke balance improves with yoga: a pilot study. Stroke. 2012 Sep;43(9):2402–2407. doi: 10.1161/STROKEAHA.112.658211. [DOI] [PubMed] [Google Scholar]
- 38.Schuster C, Butler J, Andrews B, Kischka U, Ettlin T. Comparison of embedded and added motor imagery training in patients after stroke: results of a randomised controlled pilot trial. Trials. 2012 Jan 23;13:11-6215-13-11. doi: 10.1186/1745-6215-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality-based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait Posture. 2008 Aug;28(2):201–206. doi: 10.1016/j.gaitpost.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Hsu PC, Miller WC. Reliability of the Chinese version of the Activities-specific Balance Confidence Scale. Disabil Rehabil. 2006 Oct 30;28(20):1287–1292. doi: 10.1080/09638280600638414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I) Age Ageing. 2005 Nov;34(6):614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 42.Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing. 2010 Mar;39(2):210–216. doi: 10.1093/ageing/afp225. [DOI] [PubMed] [Google Scholar]
- 43.Salbach NM, Mayo NE, Hanley JA, Richards CL, Wood-Dauphinee S. Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Arch Phys Med Rehabil. 2006 Dec;87(12):1597–1604. doi: 10.1016/j.apmr.2006.08.336. [DOI] [PubMed] [Google Scholar]
- 44.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005 Feb 18;27(4):156–163. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 45.Rand D, Miller WC, Yiu J, Eng JJ. Interventions for addressing low balance confidence in older adults: a systematic review and meta-analysis. Age Ageing. 2011 May;40(3):297–306. doi: 10.1093/ageing/afr037. [DOI] [PMC free article] [PubMed] [Google Scholar]