Abstract
Objectives
To assess whether patients’ willingness to add a blood pressure-lowering drug and the importance they attach to specific treatment characteristics differ among age groups in patients with type 2 diabetes.
Materials and Methods
Patients being prescribed at least an oral glucose-lowering and a blood pressure-lowering drug completed a questionnaire including a discrete choice experiment. This experiment contained choice sets with hypothetical blood pressure-lowering drugs and a no additional drug alternative, which differed in their characteristics (i.e. effects and intake moments). Differences in willingness to add a drug were compared between patients <75 years (non-aged) and ≥75 years (aged) using Pearson χ2-tests. Multinomial logit models were used to assess and compare the importance attached to the characteristics.
Results
Of the 161 patients who completed the questionnaire, 151 (72%) could be included in the analyses (mean age 68 years; 42% female). Aged patients were less willing to add a drug than non-aged patients (67% versus 84% respectively; P = 0.017). In both age groups, the effect on blood pressure was most important for choosing a drug, followed by the risk of adverse drug events and the risk of death. The effect on limitations due to stroke was only significant in the non-aged group. The effect on blood pressure was slightly more important in the non-aged than the aged group (P = 0.043).
Conclusions
Aged patients appear less willing to add a preventive drug than non-aged patients. The importance attached to various treatment characteristics does not seem to differ much among age groups.
Introduction
There is growing interest to tailor drug treatment to the individual patient's clinical and personal needs. In general, the incorporation of patient preferences in treatment decisions is high on the agenda in our society. Particularly in drug treatment it is important to take patient preferences into account since such preferences are related to a patient’s willingness to take a drug. The willingness to take a drug improves adherence and leads to a more effective treatment [1–3].
A patient’s willingness to take a drug is influenced by factors related to the drug, the physician, the disease, and patient’s own characteristics [4–7]. Drug-related factors include expected effects on, for example, life extension or factors related to quality of life, such as good health, ease of taking the drug and burden of adverse drug events (ADEs) [1]. Patients often value life extension as one of the most important drug effects [8–11]. On the other hand, many aged or frail patients are likely to value quality of life over life extension [12–14]. Therefore, one may expect that preferences for specific drugs and the willingness to add a treatment are influenced by a patient’s age or life-expectancy, as has been shown previously [12,15,16].
The role of patient’s age on drug choices may be particularly important for preventive treatment, such as the use of blood pressure-lowering drugs in patients with type 2 diabetes to reduce their risk for cardiovascular morbidity and mortality [17]. With respect to balancing life extension with quality of life, the need for blood pressure-lowering drugs may be less in aged patients since 1) evidence of long-term benefit in aged patients is lacking [18], 2) having a high blood pressure level is usually not perceived as burdensome [19], and 3) adverse events related to these drugs are common in the aged [20,21]. In general, patients with type 2 diabetes appear to have lower necessity beliefs for blood pressure-lowering drugs than, for instance, for glucose-lowering drugs [19,22]. Currently, little is known about preferences for choosing blood pressure-lowering treatment among aged and non-aged patients.
A useful and commonly used method to assess patient preferences for treatment is the discrete choice experiment [23,24]. In such experiments, a hypothetical situation is presented to the patient with treatment alternatives described by their characteristics, or so-called attributes [25]. The relative importance of the attributes can be inferred from the choices made [26]. This method is useful because people have to make trade-offs between positive and negative consequences of a choice, similar to decisions that they have to make in practice.
The aim of this study is to assess whether patients’ willingness to add a blood pressure-lowering drug and the importance they attach to specific treatment characteristics differ among age groups in patients with type 2 diabetes.
Materials and Methods
Study design and participants
This study was conducted in 2014 and has a cross-sectional design in which we compared patients <75 years with ≥75 years of age. Patients were eligible to participate when they were aged ≥18 years and had been prescribed at least an oral glucose-lowering and a blood pressure-lowering drug in the past 6 months. We aimed to include 150 patients in our study, since it has been shown that the precision of discrete choice experiments rapidly decreases at sample sizes less than 150 [26]. Pharmacists in the northern part of the Netherlands sent invitation letters to eligible patients identified from their electronic records. Patients who gave written informed consent received a questionnaire including general questions such as Cantril’s ladder to assess a patient’s quality of life [27], and subsequently the discrete choice experiment to evaluate their treatment preferences. Patients were called when they did not return the questionnaire within two months or in case of missing data in the general questions. In case of missing data in the discrete choice experiment, the questionnaire was returned to the patient for further completion. The Medical Ethics Committee of the University Medical Center Groningen (METc UMCG) in the Netherlands determined that ethical approval was not needed for this study (reference number M14.150721). The study was carried out in accordance with the Code of Ethics of the World Medication Association (Declaration of Helsinki) for experiments involving humans.
Outcome variable
The outcome variable in this study was the choice patients made in a hypothetical situation with respect to adding a blood pressure-lowering drug.
Discrete choice experiment
Attributes in the discrete choice experiment were based on a two-step literature review. In the first step, the literature was assessed for factors which may influence a patient’s willingness to take blood pressure-lowering drugs or drugs for primary cardiovascular disease prevention in general. This review revealed that lowering the blood pressure, achieving risk reduction of complications (myocardial infarction, stroke), reducing ADEs and improving quality of life were relevant factors [4–7]. During the second step, previous studies with discrete choice experiments to assess patient preferences for any drug were screened. This screening revealed two additional attributes, that is, costs of treatment and number of tablets needed per day [28–35]. We decided not to include costs in our experiment since expenditure for preventive drugs, such as blood pressure-lowering drugs, are covered by the health insurance in the Netherlands.
Levels of efficacy were established using clinical trial data of blood pressure-lowering treatment effects and cardiovascular risk reduction between tight and less tight blood pressure control, and using the UKPDS risk engine for assessing differences among different ages [36]. We estimated risks of complications or death within the next 5 years. Levels for ADEs were based on the prevalence of known ADEs for blood pressure-lowering drugs, such as cough and headache, as reported in the national drug compendium for healthcare professionals. Levels for the intake moments were based on possible schemes mentioned in the literature [37,38]. The list of attributes and levels was discussed and finalized by interviews with ten experts (2 nurse practitioners, 3 general practitioners, 2 specialists, and 3 pharmacists) (Table 1).
Table 1. Overview of the attributes and levels used in the discrete choice experiment.
| Attributes | Levels | Coding |
|---|---|---|
| Blood pressure level 1 | Remains 160* | 160 |
| Decrease from 160 to 140 | 140 | |
| Decrease from 160 to 150 ‡ | 150 | |
| Risk of death by a heart attack or stroke in the next 5 years 1 | 13 of the 100 die and 87 don’t* | 0.13 |
| 9 of the 100 die and 91 don’t | 0.09 | |
| 11 of the 100 die and 89 don’t ‡ | 0.11 | |
| Risk of limitations due to a heart attack, such as fatigue and difficulty walking in the next 5 years 1 | 7 of the 100 get limitations and 93 don’t* | 0.07 |
| 5 of the 100 get limitations and 95 don’t | 0.05 | |
| 6 of the 100 get limitations and 94 don’t ‡ | 0.06 | |
| Risk of limitations due to a stroke, such a speech problems and forgetfulness in the next 5 years 1 | 7 of the 100 get limitations and 93 don’t* | 0.07 |
| 5 of the 100 get limitations and 95 don’t | 0.05 | |
| 6 of the 100 get limitations and 94 don’t ‡ | 0.06 | |
| Risk of side effect 3 , such as cough and headache 1 | No side effects* | 0 |
| 5 of the 100 get side effects and 95 don’t | 0.05 | |
| 10 of the 100 get side effects and 90 don’t ‡ | 0.10 | |
| Intake moment 2 | 1 tablet in the morning* | - |
| 1 tablet in the morning and 1 in the evening ‡ | ||
| 1 combination tablet | ||
| 2 tablets in the morning † |
1 Continuous variable;
2 Categorical variable;
3 Side effect used as lay-term for adverse drug events.
* Level used for ‘no additional drug’ option (never used for the additional drug options).
‡ Levels used for the non-preferable drug in the dominant choice set.
† Reference category in the categorical attribute.
A d-efficient design [26] of 30 choice sets, divided in three blocks, was generated using Ngene (version 1.1.1). No restrictions of level combinations were included. Patients were randomly assigned to one of the blocks containing ten choice sets. This blocking was used to reduce the cognitive burden of the patients. Patients had to imagine that they were using one blood pressure-lowering drug and that their blood pressure was uncontrolled (160 mmHg). In the choice sets, patients could indicate how they would want to continue their treatment, choosing from hypothetical treatment options presented as ‘no additional drug’, ‘additional drug A’, or ‘additional drug B’. The profile of ‘no additional drug’ described the situation as presented in the case, whereas the profiles of ‘additional drug A’ and ‘additional drug B’ were generated with the d-efficient design. Patients were asked to complete the ten choice sets plus a choice set in which one drug was preferable on all attributes compared to the other drug. This dominant choice set was added to identify the responders who may not understand the task.
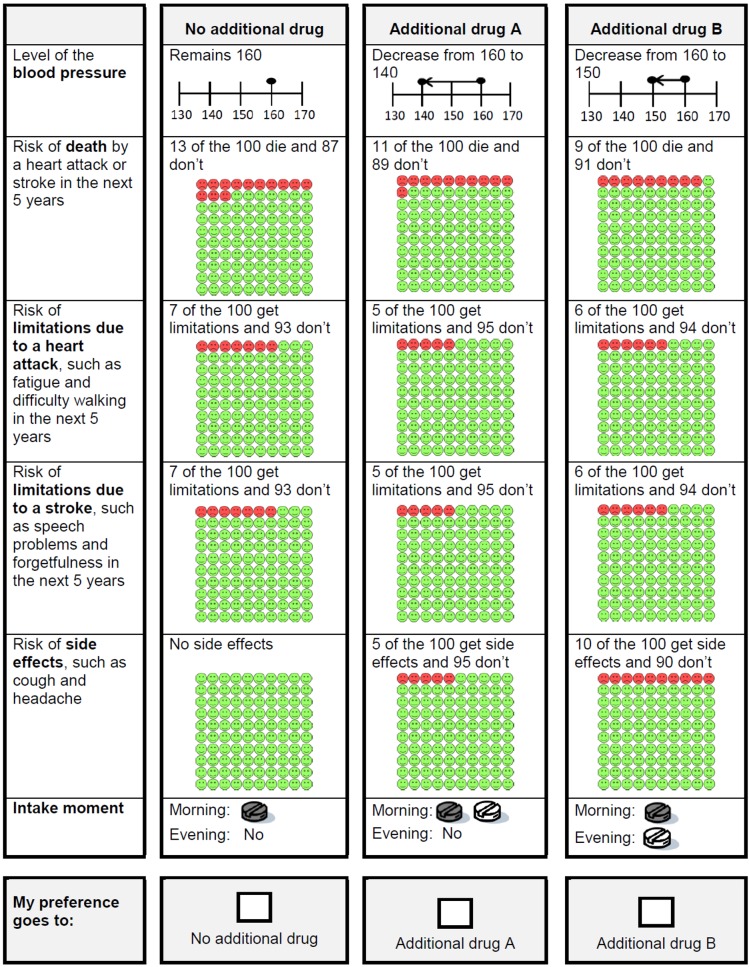
A pilot study was conducted in which ten patients with type 2 diabetes completed the full questionnaire. Based on this pilot study, minor changes in wordings were made and a separate instruction form for the choice sets was included. An example of a final choice set is presented in Fig 1.
Fig 1. Example of a choice set presented in the questionnaire.
Patients’ age and life-expectancy
In the questionnaire, patients were asked their age. We used a cut-off level of ≥75 years to define aged patients. This cut-off level has been used in guidelines [39] and observational studies [40] looking at age differences for preventive treatment. To test its value in relation to perceived life-expectancy, we additionally asked patients an open-ended question: “In 2012, men became on average 75 years old and women on average 80 years old [41]. How old do you think you will become?”. With this we determined that age was a reasonable proxy for self-reported life-expectancy (S1 Table).
Additional data collection
Pharmacists provided the age and gender for all eligible patients, as well as overviews of prescribed drugs in the last six months for those patients who gave written informed consent. The Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization was used to classify the prescribed drugs.
Statistical analyses
Descriptive statistics are presented for patient characteristics. Differences in age and gender between patients who completed the study and those who did not participate or complete the study were assessed using the T-test and Pearson χ2-test respectively. Differences in characteristics between non-aged and aged patients were tested using Pearson χ2-tests for categorical variables and Mann-Whitney U tests for non-normally distributed, continuous variables. The Fisher freeman-halton test was used for categorical variables in which one or more cells contained less than five patients.
Differences in the number of patients who chose at least once an additional drug on the choice sets (willing to add) versus never (unwilling to add) were compared between the age groups using the Pearson χ2-test. The choices were further analysed using multinomial logit models (asclogit function in Stata) to assess 1) the willingness to add a blood pressure-lowering drug when controlling for all attributes, and 2) the relative importance of the attributes. Four models were assessed, that is, one including all patients, one including non-aged patients, one including aged patients, and one with all patients in which the interaction terms between these two age groups and the attributes were included.
We followed the random utility model by assuming that patients choose the alternative in each choice set which maximizes their utility. The estimated model was the following:
with U indicating the utility that a patient assigns to a treatment which is the sum of a systematic, explainable component V and a random, unexplainable component ε. The explainable component is a function of the attributes of the alternatives. The constant β0 indicates the relative weight patients place on choosing an additional blood pressure-lowering drug versus no additional blood pressure-lowering drug when controlling for the attributes. The β1 to β7 coefficients indicate the relative importance of each of the attributes. Coefficients reflect continuous variables of the attributes, except β6 and β7 which reflect the dummy-coding of respectively the level one drug in the morning and one in the evening (coded as 1) versus two drugs in the morning (coded as 0), and the level combination tablet (coded as 1) versus two drugs in the morning (coded as 0). The sign of the beta-coefficients indicates whether the effect on the utility is positive or negative. Attributes with beta-coefficients with a two-sided P-value <0.05 were considered as being important for the treatment choice. Important attributes were ranked according to their relative importance. This was determined by calculating the difference between the smallest part worth utility and the largest part-worth utility of the levels of an attribute, and dividing this difference by the sum of the difference scores for all attributes [42].
Patients who chose the non-preferable drug in the dominant choice set were excluded from the analyses. Sensitivity analyses were conducted in which these patients were not excluded. Additional sensitivity analyses were conducted to assess the multinomial logit model for patients <65 years and patients aged ≥80 years. The analyses were conducted using Stata version 13 (Stata Corp., College Station, TX).
Results
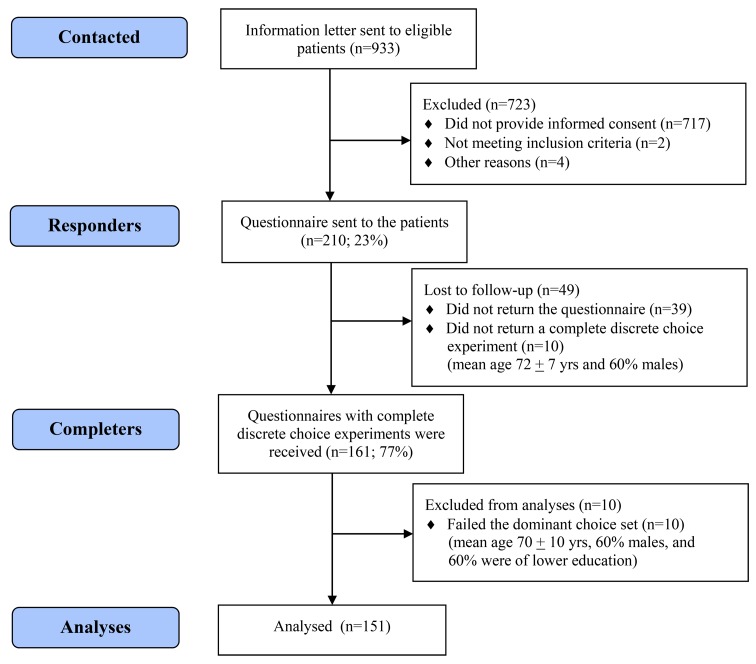
Three pharmacies sent information letters to 933 patients, resulting in 210 eligible consenting patients. Of these, 161 completed the questionnaire (Fig 2). There was no significant age difference between these completers and the non-completers (69 and 69 years, P = 0.310), but less females completed the study (42% versus 52% females, P = 0.025). This difference became insignificant in an additional analysis per age group (data not shown). In the final analyses, 151 were included. The included patients were on average 68 ± 9 yrs and the majority were males (58%). Metformin was the most commonly prescribed glucose-lowering drug in both aged and non-aged patients (Table 2). Agents acting on the renin-angiotensin system (RAS-inhibitors) were the most commonly prescribed blood pressure-lowering drug class in non-aged patients, whereas the β-blockers were the most commonly prescribed class in aged patients. Most of the patients reported that their blood pressure was <160 mmHg, that they used one or two drugs to lower their blood pressure, and that they preferred to leave decisions about their drugs to the general practitioner (Table 2).
Fig 2. Patient inclusion flow-chart.
Table 2. Patient characteristics per age group.
| Characteristic | All | <75 years | ≥75 years | P-value |
|---|---|---|---|---|
| Included patients | 151 | 106 | 45 | |
| Mean age (SD) | 68 (9.2) | 64 (7.1) | 79 (3.6) | |
| Females (%) | 64 (42.4) | 40 (37.7) | 24 (53.3) | 0.076 1 |
| Median body mass index (IQR) | 28 (26–32) | 29 (27–33) | 26 (24–29) | 0.000 2 |
| Education (%) | 0.472 3 | |||
| Lower education a | 86 (57.0) | 59 (55.7) | 27 (60.0) | |
| Middle education b | 44 (29.1) | 34 (32.1) | 10 (22.2) | |
| Higher education c | 17 (11.3) | 11 (10.4) | 6 (13.3) | |
| Other | 4 (2.7) | 2 (1.9) | 2 (4.4) | |
| Smoking | 0.011 1 | |||
| Current smokers | 22 (14.6) | 16 (15.1) | 6 (13.3) | |
| Past smokers | 75 (49.7) | 60 (56.6) | 15 (33.3) | |
| Non smokers | 54 (35.8) | 30 (28.3) | 24 (53.3) | |
| Median quality of life (IQR)◦ | 3 (3–5) | 3 (3–4) | 4 (3–5) | 0.351 2 |
| Classes of prescribed blood pressure-lowering drugs (ATC code)* | ||||
| Centrally acting antihypertensives (C02) | 2 (1.3) | 0 (0.0) | 2 (4.4) | 0.089 3 |
| Diuretics (C03) | 49 (32.7) | 31 (29.5) | 18 (40.0) | 0.210 1 |
| β-Blockers (C07) | 87 (58.0) | 56 (53.3) | 31 (68.9) | 0.077 1 |
| Calcium channel blockers (C08) | 33 (22.0) | 20 (19.1) | 13 (28.9) | 0.182 1 |
| Agents acting on the renin-angiotensin system (C09) | 104 (69.3) | 74 (70.5) | 30 (66.7) | 0.643 1 |
| Combination tablet ‡ | 27 (18.0) | 16 (15.2) | 11 (24.4) | 0.179 1 |
| Classes of prescribed glucose-lowering drugs (ATC code)* | ||||
| Insulin (A10A) | 33 (22.0) | 27 (25.7) | 6 (13.3) | 0.093 1 |
| Biguanides (metformin) (A10BA) | 135 (90.0) | 96 (91.4) | 39 (86.7) | 0.373 1 |
| Sulfonamides (A10BB) | 58 (38.7) | 39 (37.1) | 19 (42.2) | 0.558 1 |
| Combination Metformin and Sulfonamide (A10BD02) | 1 (0.7) | 1 (1.0) | 0 (0.0) | 1.000 3 |
| Thiazolidinediones (A10BG) | 1 (0.7) | 1 (0.95) | 0 (0.0) | 1.000 3 |
| Dipeptidyl peptidase 4 inhibitors (A10BH) | 13 (8.7) | 9 (8.6) | 4 (8.9) | 1.000 3 |
| Liraglutide (A10BX07) | 2 (1.3) | 2 (1.9) | 0 (0.0) | 1.000 3 |
| Use of lipid-lowering drugs (%)* | 0.019 3 | |||
| No lipid-lowering drug | 27 (18.0) | 13 (12.4) | 14 (31.1) | |
| 1 lipid-lowering drug | 117 (78.0) | 88 (83.8) | 29 (64.4) | |
| 2 lipid-lowering drugs | 6 (4.0) | 4 (3.8) | 2 (4.4) | |
| Drug burden expressed as median number of chronic treatments from 8 anatomical chapters (IQR) ø | 3 (3–4) | 3 (3–4) | 4 (3–4) | 0.025 2 |
| High blood pressure | ||||
| How serious do you think that having a high blood pressure is in general? (%) | 0.584 3 | |||
| Very serious | 20 (13.4) | 13 (12.4) | 7 (15.9) | |
| Reasonable serious | 93 (62.4) | 69 (65.7) | 24 (54.6) | |
| A little serious | 28 (18.8) | 18 (17.1) | 10 (22.7) | |
| Not serious | 8 (5.4) | 5 (4.8) | 3 (6.8) | |
| How high was your systolic blood pressure during the last measurement conducted by your general practitioner or nurse practitioner? (%) | 0.162 1 | |||
| <120 mmHg | 10 (6.6) | 7 (6.6) | 3 (6.7) | |
| 120–139 mmHg | 66 (43.7) | 52 (49.1) | 14 (31.1) | |
| 140–159 mmHg | 47 (31.1) | 32 (30.2) | 15 (33.3) | |
| ≥160 mmHg | 16 (10.6) | 9 (8.5) | 7 (15.6) | |
| I do not know | 12 (8.0) | 6 (5.7) | 6 (13.3) | |
| Number of patients who report ever having experienced a symptom of high blood pressure (%) | 36 (23.8) | 25 (23.6) | 11 (24.4) | 0.910 1 |
| Blood pressure-lowering drugs | ||||
| Number of drugs that the patients report to use for high blood pressure | 0.102 1 | |||
| None | 6 (4.0) | 6 (5.7) | 0 (0.0) | |
| One | 79 (52.3) | 55 (51.9) | 24 (53.3) | |
| Two | 38 (25.2) | 29 (27.4) | 9 (20.0) | |
| More than two | 19 (12.6) | 9 (8.5) | 10 (22.2) | |
| I do not know | 9 (6.0) | 7 (6.6) | 2 (4.4) | |
| Have you ever experienced a side effect of a blood pressure-lowering drug | 0.511 1 | |||
| No | 110 (72.9) | 78 (73.6) | 32 (71.1) | |
| Yes | 24 (15.9) | 18 (17.0) | 6 (13.3) | |
| I do not know | 17 (11.3) | 10 (9.4) | 7 (15.6) | |
| I prefer to leave decisions about my drugs to my general practitioner † | 142 (94.0) | 100 (94.3) | 42 (93.3) | 0.811 1 |
a No education; Elementary school; Junior secondary vocational education.
b Junior general secondary education; Senior secondary vocational education.
c Senior general secondary education; Higher professional education; University education.
SD = Standard deviation; IQR = Interquartile range; ATC = Anatomical Therapeutic Chemical.
◦ Measured with Cantril’s ladder [27] with a range of 1 (best)– 10 (worst possible life).
* N = 150 (medication overview of one patient was not extracted at the time of data collection since the patient had not given written informed consent yet).
‡ ATC codes: C03EA01, C07BB02, C09BA03, C09BA04, C09BA06, C09BB04, C09DA01, C09DA03, C09DA04, C09DA06, C09DB02.
ø Drug burden was counted at the anatomical ATC level for the chapters: A, B, C, H, L, M, N, R (maximum of 8).
† Statement adapted from [43]. Scored on a 6-point Likert scale and divided by (partially, totally) agree and (partially, totally) disagree. Number is presented for those who agree.
1 Pearson χ2-test;
2 Mann-Whitney U test;
3 Fisher freeman-halton test.
Influence of age on willingness to add a blood pressure-lowering drug
Of the aged patients, 67% chose an additional drug in at least one of the ten presented choice sets. This percentage was significantly lower than in non-aged patients (84%; P = 0.017). The same is reflected in the drug choice model where the constant is more negative in the aged than in the non-aged (Table 3).
Table 3. Preferences of all patients and divided in patients aged <75 years (non-aged) and ≥75 years (aged).
| Constant and attributes | All patientsa | <75 yearsb | ≥75 yearsc | P-value of the interaction between age groups and preferencesd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P-value | Relative importance (ranking) * | Coefficient (95% CI) | P-value | Relative importance (ranking) * | Coefficient (95% CI) | P-value | Relative importance (ranking) * | ||
| Constant (additional drug) | -1.26 (-1.72 –-0.80) | 0.000 | -1.05 (-1.60 –-0.50) | 0.000 | -1.65 (-2.52 –-0.77) | 0.000 | 0.257 | |||
| Blood pressure | -0.08 (-0.10 –-0.07) | 0.000 | 36.10 (1) | -0.09 (-0.11 –-0.08) | 0.000 | 37.22 (1) | -0.06 (-0.09 –-0.03) | 0.000 | 36.84 (1) | 0.043 |
| Death within the next 5 years | -22.13 (-28.75 –-15.51) | 0.000 | 19.97 (3) | -21.79 (-29.59 –-13.99) | 0.000 | 18.03 (3) | -24.43 (-37.32 –-11.54) | 0.000 | 30.00 (3) | 0.731 |
| Limitations heart attack | -9.16 (-22.29–3.98) | 0.172 | -9.13 (-24.61–6.36) | 0.248 | -11.31 (-36.83–14.22) | 0.385 | 0.886 | |||
| Limitations stroke | -26.65 (-39.89 –-13.41) | 0.000 | 12.03 (4) | -30.22 (-45.83 –-14.61) | 0.000 | 12.50 (4) | -15.71 (-41.42–10.00) | 0.231 | 0.344 | |
| Adverse drug events | -14.14 (-16.89 –-11.39) | 0.000 | 31.90 (2) | -15.59 (-18.86 –-12.31) | 0.000 | 32.24 (2) | -10.80 (-16.02 –-5.58) | 0.000 | 33.16 (2) | 0.128 |
| Additional tablet in the evening | 0.07 (-0.10–0.25) | 0.424 | 0.13 (-0.08–0.34) | 0.216 | -0.10 (-0.44–0.24) | 0.578 | 0.264 | |||
| Combination tablet | 0.13 (-0.05–0.31) | 0.151 | 0.10 (-0.11–0.31) | 0.361 | 0.21 (-0.12–0.54) | 0.206 | 0.566 | |||
a Number of observations 4,530 (151 patients * 10 choice sets * 3 alternatives per choice set).
b Number of observations 3,180 (106 patients * 10 choice sets * 3 alternatives per choice set).
c Number of observations 1,350 (45 patients * 10 choice sets * 3 alternatives per choice set).
d Interaction between preferences and age groups added to the model of all patients.
* Determined by calculating the difference between the smallest part worth utility and the largest part-worth utility of the levels of an attribute, and dividing this difference by the sum of the difference scores for all attributes [42].
CI = Confidence interval.
Influence of age on importance attached to drug attributes
Drug attributes that significantly influenced the choices for an additional blood pressure-lowering drug in the total group were the effect on the blood pressure, the risk of experiencing ADEs, the risk of death within the next 5 years, and the risk on limitations due to stroke within the next 5 years (Table 3). These results were similar in the analyses including only non-aged patients. The risk of limitations due to a stroke within the next 5 years did not significantly contribute to the choice of an additional drug in the aged group. Ranking of the relative importance of the attributes revealed that in both aged and non-aged patients, the effect on the blood pressure was the most influencing attribute, followed by the risk of experiencing ADEs and the risk of death within the next 5 years (Table 3).
A sensitivity analysis in which the patients who failed the dominant choice set were included, revealed similar results (S2 Table). In addition, sensitivity analyses including only younger patients (<65 years) or older patients (≥80 years) showed the same direction for all coefficients but some coefficients became insignificant (S3 Table).
Only the impact of the blood pressure-lowering effect turned out to be significantly different among the age groups (Table 3, interaction, P = 0.043). This finding indicates that the effect of an additional drug on the blood pressure level was seen as more important by non-aged patients than aged patients.
Discussion
This study in patients with type 2 diabetes shows that aged patients were less willing to add a blood pressure-lowering drug than non-aged patients when they had to imagine that their blood pressure was too high. The effect of a drug on the blood pressure was more important for non-aged patients than aged patients. The effects on the risk of death within the next 5 years and experiencing ADEs were important drug characteristics for choosing a drug in both age groups. For non-aged patients, also the risk of limitations due to a stroke was important.
Previously, it was found that patients with a limited life-expectancy have a decreased willingness to add treatment because they may value quality of life over life extension [12,15]. On the other hand, it was also found that a large proportion of such patients remain willing to undergo burdensome treatment for a small risk reduction of death [12]. Our study confirms the finding that aged patients are less willing to add a drug than non-aged patients. The finding that the drug effect on reducing the risk of death within the next 5 years was of similar importance for aged and non-aged patients may be surprising, but fits with the finding that many aged patients remain willing to undergo burdensome treatment for a risk reduction of death [12]. This might be explained by the fact that the aged group still perceived they had sufficient life-expectance.
Previous studies have not directly compared the importance attached to various treatment outcomes between aged and non-aged patients. Our study revealed that most characteristics were similarly valued by aged patients in comparison to non-aged patients. Both groups attached a similar importance, for example, to the risk of ADEs, and in both groups was the effect on the blood pressure ranked as most important attribute. However, the effect on the blood pressure was the only attribute that significantly differed in importance between aged and non-aged patients. This finding could imply that aged patients are less willing to add a blood pressure-lowering drug because they believe that decreasing the blood pressure is of less importance. Whether this is influenced by the current advise of guidelines, and thus practitioners, to take a patient’s age or life-expectancy into account in setting blood pressure targets [44,45], remains to be determined.
In both age groups, the choice for a blood pressure-lowering drug was not significantly influenced by the risk reductions in limitations in daily life due to a heart attack. This finding may be due to a more subjective interpretation of such an attribute compared to an attribute such as death [46]. However, the preferences of the non-aged patients were influenced by a similar attribute, that is, the risk of limitations due to a stroke. Therefore, it seems that reducing potential limitations due to a heart attack from 7% to 5% was not decisive for the patients' treatment choices, whereas a similar reduction for limitations due to stroke was. These findings are comparable with a previous study showing that patients are less willing to risk cognitive disability than physical disability [12]. For the aged patients, however, reducing potential limitations due to a stroke from 7% to 5% was not critical. Possibly, the presented problems, i.e. speech problems and forgetfulness, may have been perceived by aged patients as an expected part of aging [47]. Presenting more severe problems, such as becoming dependent on others, might have increased the relevance of this aspect since maintaining independent is important for aged patients [48,49].
In our study, patients’ preferences for a blood pressure-lowering drug were not significantly influenced by the intake moment of the drug. A previous discrete choice experiment in patients with type 2 diabetes showed that the intake moment influences patient preferences, but that this is more important for patients who are taking less than five drugs a day compared to patients with five or more drugs per day [50]. This may explain the non-significant influence of the intake moment in our study since many patients were prescribed not only glucose-lowering and blood pressure-lowering drugs but also lipid-lowering drugs and drugs for other chronic diseases.
This study was conducted in the north of the Netherlands, which includes a mostly caucasian population. Selection bias may have occurred since only 17% of the contacted patients completed the questionnaire and less females completed the study. Regarding age there were no differences between those who completed the study and those who did not. Although we reached a sample size of 150 patients, it should be noted that the included number of patients per age group was lower which may have reduced the efficiency of the models for the separate groups. This limitation may especially apply to the aged group (≥75 years) since only 45 patients were included. In the aged group, more blood pressure-lowering drugs and especially β-blockers were used than in the non-aged group. However, a post-hoc analysis showed that the reported use of two or more blood pressure-lowering drugs was not associated with less willingness to add a drug (data not shown). In this study, age seemed to be a reasonable proxy for life-expectancy. There are also some strengths and limitations to a discrete choice experiment. A major strength is that it comes close to the trade-offs and choices that have to be made in real life, and can thus provide better estimates of the relative importance of different treatment characteristics than when asking patients to rate the importance of each characteristic separately. The evaluation of several treatment alternatives and attributes at one time reveals a rich source of data [26,51]. Moreover, the ‘no additional drug’ option was included which represents actual choices in practice. However, the choices that have to be made may be complex and may require high cognitive efforts [52]. Aged patients may have more problems with such choices than non-aged patients due to increased cognitive impairment. However, an exploratory study among older patients showed that cognitive impairment had no significant impact on the consistency of responses in a discrete choice experiment [53]. Furthermore, hypothetical situations have to be assessed in discrete choice experiments which do not necessarily represent actual behaviour [54,55]. Some patients may have difficulty in making hypothetical choices. To reduce complexity and effort, patients were presented a sample of ten choice sets and a pilot study in eligible patients was conducted to detect and resolve any difficulties. The pilot study revealed only a need for minor changes in wording and the use of a separate instruction card, and indicated that the task was doable for (aged) patients with type 2 diabetes. In addition, we excluded 10 patients from the analyses who failed the dominant choice set. There is discussion in the literature about the fairness of excluding such responders [56], but the sensitivity analysis in which these patients were included revealed similar results. A final limitation may be that for most of the attributes only a reduction in the frequency of a risk was presented. Other information may be relevant for patients. For instance, patients may want information about the severity and duration of the ADEs.
In conclusion, it is important to acknowledge that aged patients may be less willing to add a blood pressure-lowering drug than non-aged patients. This finding underlines the importance of discussing patients' preferences even when they prefer to leave the final treatment decision to their general practitioner. When choosing a drug, aged patients attach as much importance to reduce their risk of death within the next 5 years and of experiencing ADEs as non-aged patients. Therefore, treatment decisions in clinical practice should focus on quality of life as well as life extension in both age groups in which the individual patient’s preferences and willingness to add a drug should be taken into account.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DTA)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the Research Institute SHARE, University Medical Center Groningen, Groningen, The Netherlands. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aristides M, Weston AR, FitzGerald P, Le Reun C, Maniadakis N. Patient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experiment. Value Health 2004;7(4):442–454. [DOI] [PubMed] [Google Scholar]
- 2. Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther 2001;26(5):331–342. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell AJ, Selmes T. Why don’t patients take their medicine? Reasons and solutions in psychiatry. Adv Psychiatr Treat 2007;13(5):336–346. [Google Scholar]
- 4. Gascon JJ, Sanchez-Ortuno M, Llor B, Skidmore D, Saturno PJ, Treatment Compliance in Hypertension Study Group. Why hypertensive patients do not comply with the treatment: results from a qualitative study. Fam Pract 2004;21(2):125–130. [DOI] [PubMed] [Google Scholar]
- 5. Benson J, Britten N. Patients' decisions about whether or not to take antihypertensive drugs: qualitative study. BMJ 2002;325(7369):873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svensson S, Kjellgren KI, Ahlner J, Saljo R. Reasons for adherence with antihypertensive medication. Int J Cardiol 2000;76(2–3):157–163. [DOI] [PubMed] [Google Scholar]
- 7. Fried TR, Tinetti ME, Towle V, O'Leary JR, Iannone L. Effects of benefits and harms on older persons' willingness to take medication for primary cardiovascular prevention. Arch Intern Med 2011;171(10):923–928. 10.1001/archinternmed.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porzsolt F, Clouth J, Deutschmann M, Hippler HJ. Preferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes 2010;8:125 10.1186/1477-7525-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with Type 2 diabetes using oral glucose-lowering agents. Diabet Med 2009;26(4):416–424. 10.1111/j.1464-5491.2009.02696.x [DOI] [PubMed] [Google Scholar]
- 10. Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP–1 products–-liraglutide and exenatide–-for the treatment of type 2 diabetes. J Med Econ 2010;13(4):655–661. 10.3111/13696998.2010.529377 [DOI] [PubMed] [Google Scholar]
- 11. Hiligsmann M, van Durme C, Geusens P, Dellaert BG, Dirksen CD, van der Weijden T, et al. Nominal group technique to select attributes for discrete choice experiments: an example for drug treatment choice in osteoporosis. Patient Prefer Adherence 2013;7:133–139. 10.2147/PPA.S38408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried TR, Van Ness PH, Byers AL, Towle VR, O'Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med 2007;22(4):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA 1998;279(5):371–375. [DOI] [PubMed] [Google Scholar]
- 14. Sherbourne CD, Keeler E, Unutzer J, Lenert L, Wells KB. Relationship between age and patients' current health state preferences. Gerontologist 1999;39(3):271–278. [DOI] [PubMed] [Google Scholar]
- 15. Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care 2008;46(3):275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dibonaventura MD, Wagner JS, Girman CJ, Brodovicz K, Zhang Q, Qiu Y, et al. Multinational Internet-based survey of patient preference for newer oral or injectable Type 2 diabetes medication. Patient Prefer Adherence 2010;4:397–406. 10.2147/PPA.S14477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zanchetti A, Ruilope LM. Antihypertensive treatment in patients with type–2 diabetes mellitus: what guidance from recent controlled randomized trials? J Hypertens 2002;20(11):2099–2110. [DOI] [PubMed] [Google Scholar]
- 18. van Hateren KJ, Landman GW, Kleefstra N, Houweling ST, van der Meer K, Bilo HJ. Time for considering other blood pressure target values in elderly patients with type 2 diabetes? Int J Clin Pract 2012;66(2):125–127. 10.1111/j.1742-1241.2011.02841.x [DOI] [PubMed] [Google Scholar]
- 19. Stack RJ, Bundy C, Elliott RA, New JP, Gibson JM, Noyce PR. Patient perceptions of treatment and illness when prescribed multiple medicines for co-morbid type 2 diabetes. Diabetes Metab Syndr Obes 2011;4:127–135. 10.2147/DMSO.S17444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan DC, Chen JH, Kuo HK, We CJ, Lu IS, Chiu LS, et al. Drug-related problems (DRPs) identified from geriatric medication safety review clinics. Arch Gerontol Geriatr 2012;54(1):168–174. 10.1016/j.archger.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 21. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM, HARM Study Group. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 2008;168(17):1890–1896. 10.1001/archinternmed.2008.3 [DOI] [PubMed] [Google Scholar]
- 22. de Vries ST, Keers JC, Visser R, de Zeeuw D, Haaijer-Ruskamp FM, Voorham J, et al. Medication beliefs, treatment complexity, and non-adherence to different drug classes in patients with type 2 diabetes. J Psychosom Res 2014;76(2):134–138. 10.1016/j.jpsychores.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 23. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics 2008;26(8):661–677. [DOI] [PubMed] [Google Scholar]
- 24. de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21(2):145–172. 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- 25. Ryan M. Discrete choice experiments in health care. BMJ 2004;328(7436):360–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013;16(1):3–13. 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- 27. Cantril H. The pattern of human concerns. New Brunswick, NJ: Rutgers University Press; 1965. [Google Scholar]
- 28. Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient preferences for topical antibiotic treatment for acne. A randomised controlled trial. Br J Dermatol 2006;154(3):524–532. [DOI] [PubMed] [Google Scholar]
- 29. Herbild L, Bech M, Gyrd-Hansen D. Estimating the Danish populations' preferences for pharmacogenetic testing using a discrete choice experiment. The case of treating depression. Value Health 2009;12(4):560–567. 10.1111/j.1524-4733.2008.00465.x [DOI] [PubMed] [Google Scholar]
- 30. Darba J, Restovic G, Kaskens L, Balbona MA, Carbonell A, Cavero P, et al. Patient preferences for osteoporosis in Spain: a discrete choice experiment. Osteoporos Int 2011;22(6):1947–1954. 10.1007/s00198-010-1382-3 [DOI] [PubMed] [Google Scholar]
- 31. Shafey M, Lupichuk SM, Do T, Owen C, Stewart DA. Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplant 2011;46(7):962–969. 10.1038/bmt.2010.225 [DOI] [PubMed] [Google Scholar]
- 32. Brown TM, Pashos CL, Joshi AV, Lee WC. The perspective of patients with haemophilia with inhibitors and their care givers: preferences for treatment characteristics. Haemophilia 2011;17(3):476–482. 10.1111/j.1365-2516.2010.02401.x [DOI] [PubMed] [Google Scholar]
- 33. Scalone L, Watson V, Ryan M, Kotsopoulos N, Patel R. Evaluation of patients' preferences for genital herpes treatment. Sex Transm Dis 2011;38(9):802–807. [DOI] [PubMed] [Google Scholar]
- 34. Kauf TL, Mohamed AF, Hauber AB, Fetzer D, Ahmad A. Patients' willingness to accept the risks and benefits of new treatments for chronic hepatitis C virus infection. Patient 2012;5(4):265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dwight Johnson M, Apesoa-Varano C, Hay J, Unutzer J, Hinton L. Depression treatment preferences of older white and Mexican origin men. Gen Hosp Psychiatry 2013;35(1):59–65. 10.1016/j.genhosppsych.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 37. Carter BL, Chrischilles EA, Rosenthal G, et al. Efficacy and safety of nighttime dosing of antihypertensives: review of the literature and design of a pragmatic clinical trial. J Clin Hypertens (Greenwich) 2014;16(2):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewanczuk R, Tobe SW. More medications, fewer pills: combination medications for the treatment of hypertension. Can J Cardiol 2007;23(7):573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proeftuin farmacie Groningen. K86, K87, Hypertensie [online]. http://proeftuinfarmaciegroningen.tmade.nl/qmfiles/Hypertensie.pdf. Accessed May 13, 2015.
- 40. van Hateren KJ, Drion I, Kleefstra N, Groenier KH, Houweling ST, van der Meer K, et al. A prospective observational study of quality of diabetes care in a shared care setting: trends and age differences (ZODIAC–19). BMJ Open 2012;2(4):e001387 10.1136/bmjopen-2012-001387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centraal Bureau voor de Statistiek [Central Bureau for Statistics]. Sterfte; kerncijfers naar diverse kenmerken [Mortality; highlights to various characteristics]. Den Haag/Heerlen 19-9-2014.
- 42. Kimman ML, Dellaert BG, Boersma LJ, Lambin P, Dirksen CD. Follow-up after treatment for breast cancer: one strategy fits all? An investigation of patient preferences using a discrete choice experiment. Acta Oncol 2010;49(3):328–337. 10.3109/02841860903536002 [DOI] [PubMed] [Google Scholar]
- 43. Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med 2005;20(6):531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NICE National Institute for Health and Care Excellence. Hypertension. Clinical management of primary hypertension in adults [online]. http://www.nice.org.uk/guidance/cg127/resources/guidance-hypertension-pdf. Accessed May 13, 2015.
- 45.Verenso. Multidisciplinaire Richtlijn Diabetes. Verantwoorde Diabeteszorg bij Kwetsbare Ouderen Thuis en in Verzorgings of Verpleeghuizen. Deel 1. [Multidisciplinary Guideline Diabetes. Responsible Diabetes Care in Vulnerable Elderly at Home and in Residential Care or Nursing Homes. Part 1]. Utrecht, the Netherlands, Verenso, 2011.
- 46. Steel N. Thresholds for taking antihypertensive drugs in different professional and lay groups: questionnaire survey. BMJ 2000;320(7247):1446–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc 2002;50(11):1837–1843. [DOI] [PubMed] [Google Scholar]
- 48. Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns 2011;83(2):278–282. 10.1016/j.pec.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med 2011;171(20):1854–1856. 10.1001/archinternmed.2011.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hauber AB, Han S, Yang JC, Gantz I, Tunceli K, Gonzalez JM, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient Prefer Adherence 2013;7:937–949. 10.2147/PPA.S43465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Viney R, Lancsar E, Louviere J. Discrete choice experiments to measure consumer preferences for health and healthcare. Expert Rev Pharmacoecon Outcomes Res 2002;2(4):319–326. 10.1586/14737167.2.4.319 [DOI] [PubMed] [Google Scholar]
- 52. Hoyos D. The state of the art of environmental valuation with discrete choice experiments. Ecol Econ 2010;69(8):1595–1603. [Google Scholar]
- 53. Milte R, Ratcliffe J, Chen G, Lancsar E, Miller M, Crotty M. Cognitive overload? An exploration of the potential impact of cognitive functioning in discrete choice experiments with older people in health care. Value Health 2014;17(5):655–659. 10.1016/j.jval.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 54. Laba TL. Using Discrete Choice Experiment to elicit patient preferences for osteoporosis drug treatments: where to from here? Arthritis Res Ther 2014;16(2):106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hauber AB, Arellano J, Qian Y, Gonzalez JM, Posner JD, Mohamed AF, et al. Patient preferences for treatments to delay bone metastases. Prostate 2014;74(15):1488–1497. 10.1002/pros.22865 [DOI] [PubMed] [Google Scholar]
- 56. Ryan M, Watson V, Entwistle V. Rationalising the 'irrational': a think aloud study of discrete choice experiment responses. Health Econ 2009;18(3):321–336. 10.1002/hec.1369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DTA)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.