Abstract
Objective
CD4+CD25+FoxP3+ regulatory T cells (TR) are critical regulators of autoimmunity. Yet, TR are paradoxically increased in RA patients and show variable activity in human studies. Our objective is to characterize the expansion and function of TR during the initiation and progression of experimental arthritis.
Methods
To unequivocally identify TR, we crossed FoxP3gfp mice to K/BxN to generate arthritic mice in which TR express green fluorescence protein. We examined the expansion and function of TR and effector T cells (TE) during different stages of arthritis by flow cytometry and cell proliferation.
Results
In K/BxN mice, thymic selection of KRN T cells resulted in an enrichment of FoxP3+ TR. TR numbers increased during arthritis with significant increases in the spleen and draining lymph node, indicating selective tropism to sites of disease. In contrast to the in vitro unresponsiveness when cultured by themselves, substantial fractions of TR proliferated in both non-arthritic and arthritic mice. However, they also underwent greater apoptosis thereby maintaining equilibrium with TE. Similarly, enhanced TR suppressive activity during arthritis was offset by greater resistance by their TE counterparts and antigen presenting cells.
Conclusion
In this well established model of RA, the interplay of TE and TR in K/BxN mice recapitulated many features of human disease. We demonstrated an ordered expansion of TR during arthritis and the dynamic changes in TR and TE functions. By elucidating factors that govern TR and TE development in K/BxNgfp mice, we will gain insight into the pathophysiology and develop novel therapeutics for human RA.
Rheumatoid arthritis (RA) is an autoimmune disease resulting in joint inflammation and destruction. Autoreactive T and B cells are crucial for its pathogenesis. Autoreactive T cells are predominantly deleted in the thymus, but this process is not stringent. Thus autoreactive T cells can and do escape into the periphery; subsequent activation can result in autoimmune pathology. CD4+CD25+FoxP3+ regulatory T cells (TR), comprising 5–10% of CD4+ T cells, are crucial for the maintenance of peripheral tolerance (1, 2).
In adult RA and juvenile idiopathic arthritis (JIA), TR were enriched in the synovial fluid of inflamed joints compared to peripheral blood, suggesting active homing or expansion at inflammatory sites (3–8). These TR expressed FoxP3 and suppressed both proliferation and cytokine production by CD4+CD25− effector T cells (TE). Moreover, increased TR in the synovial fluid directly correlated with limited disease (3), suggesting that TR aid in disease remission. However, it is unknown how TR modulate immunity as they are paradoxically increased during disease and there is also variability in TR function among different studies. For example, Ehrenstein et al demonstrated that TR from RA patients showed compromised function compared to healthy controls (7), while others showed that TR obtained during active disease were equally or more suppressive than healthy controls (8). In this case, the increased suppressive function was offset by the responder TE themselves being more refractory to suppression, and by the presence of inflammatory cytokines (9). Because these studies examined heterogeneous patient populations, disease stages, and therapeutic regimens, these variables likely contributed to differences observed between studies. In addition, investigators differed in their criteria for TR with some studies focusing only on CD25bright while others used all CD25+ T cells (8, 10). Because CD25 is also elevated in activated TE, which are increased during disease, varying degrees of TE contamination can render interpretation difficult. It is also unclear if arthritis resulted from a primary TR dysfunction or a secondary defect due to persistent inflammation.
To eliminate these confounding processes, we examined TR development and function in a well characterized murine model of RA. K/BxN mice were generated by crossing KRN T cell receptor (TCR) transgenic mice with Non Obese Diabetic (NOD) mice (11). The disease is fully penetrant in all progeny and follows a predictable course of progressive symmetrical distal polyarthritis resembling human RA. Arthritis results from autoreactive KRN T cells recognizing peptide 281–293 of the glycolytic enzyme, glucose-6-phosphate isomerase (GPI), bound to I-Ag7 (the NOD specific MHC II allele) (12, 13). Incomplete thymic deletion allows autoreactive KRN CD4+ T cells to persist in the periphery and become activated by endogenously presented GPI. KRN T cells then provide help to GPI-specific B cells, giving rise to arthritogenic antibodies. TR are enriched in arthritic K/BxN mice (14, 15) and loss of TR results in earlier and more extended disease, suggesting that although TR do not prevent arthritis, TR may nevertheless mitigate it (15). Similar findings are found in collagen induced arthritis in which depletion of CD25+ T cells exacerbated arthritis and adoptive transfer of CD4+CD25+ TR or FoxP3 transduced T cells ameliorated disease (16–18). To understand how antigen specific TR develop during the course of arthritis, we crossed K/BxN to FoxP3gfp reporter mice to unequivocally identify TR. Here, we analyzed TR selection in the thymus and followed their expansion and function during the progression of arthritis.
Materials and Methods
Mice
KRN mice have been described (11). FoxP3gfp mice were generously provided by A. Rudensky and were bred to KRN mice to generate KRNgfp mice in which FoxP3+ T cells expressed GFP (19). These were subsequently bred to NOD Lt/J mice (Jackson Laboratories, Bar Harbor, ME) to generate K/BxNgfp mice. Non-arthritic controls were KRNgfp on the C57Bl/6 background. Because FoxP3 is on the X chromosome, only male K/BxNgfp mice were analyzed. K/BxNgfp exhibited arthritis with equivalent severity and time course as K/BxN mice (data not shown). All mice were bred and housed under specific pathogen-free conditions in the animal facility at the Washington University Medical Center in accordance with the standards of the Animal Studies Committee.
Flow cytometry
Thymi, spleens, draining (dLN: popliteal, axillary, and brachial), non-draining (ndLN: inguinal and cervical), and mesenteric (mLN) lymph nodes were harvested from individual mice and analyzed separately. Single cell suspensions (3×106) were treated with αCD16 (2.4G2) prior to surface staining with specific antibodies according to standard protocols. The following antibodies were used: αCD4-APC, αCD4-PE, (GK1.5), αCD8-PE-Cy7 (53-6.7), αCD25-PE-Cy7 (PC61), αBrdU-APC, αCD45RB (C363-16A), Annexin-V-PE, and αBcl-2-PE kit were from BD Biosciences or eBiosciences, San Diego, CA. Streptavidin-PerCP was obtained from BD Biosciences, San Diego, CA. TCR Vβ6 was used to track the KRN transgenic TCR. All samples were analyzed on either a FACSAria or a FACScaliber flow cytometer (BD Biosciences, Mountain View, CA) with FlowJo software. Lymphocytes were gated based on forward and side scatter and 1–2.5×105 gated events were collected per sample.
Lymphocyte isolation from paws
front and rear paws were harvested from each mouse and the skin was removed. Paws from each mouse were minced in 40 ml of RPMI with 5% calf serum, 125 μl Collagenase VIII (10,000U/ml) and 200 μl Dispase (50U/ml). Minced tissues were agitated for two hours at 37°C at 150 rpm with vortexing every 30 minutes. Digested tissues were strained through a 70 μM Nitex membrane. Lymphocytes were harvested over a Ficoll gradient and analyzed by flow cytometry.
In vitro T cell cultures
CD4+ T cells from pooled spleens and dLNs were enriched by positive selection using αCD4 Miltenyi microbeads. Purity was typically >90%. Dendritic cells and B cells were prepared from spleens as described (20). T cells (5×105/well) were stimulated with 5×104 DC in 1 ml of Iscove’s medium containing 10% heat inactivated bovine growth serum (BGS, Hyclone, Logan UT), 2 μM Glutamax (Gibco-BRL, Gaithersburg, MD), 2×10−5 M β-mercaptoethanol, 50 μg/ml gentamicin (referred hence as ISC-10). At various times, 1 mM BrdU was added and cultures analyzed 12 hours later for BrdU incorporation and apoptosis.
T cell suppression assays
CD4+ T cells were enriched by negative selection using αB220 and αCD8, followed by negative selection using goat-anti-rat Ig coupled paramagnetic beads (Chemicell, Berlin, Germany). Cells were stained with αCD4-PE prior to sorting for CD4+FoxP3− and CD4+FoxP3+ T cells using FACSAria. Cell purity was typically >98%. In some experiments, αCD45RB-APC was used to identify naïve and memory T cells. Proliferation assays were performed in triplicate with 1 ×105 CD4+ TE/well with varying ratios of TR and 2×105 irradiated splenocytes and 10 μM GPI peptide (Global Peptide, Fort Collins, CO) in round bottom plates with ISC-10%. For experiments in Figure 6, irradiated purified DCs (1×104/well) or B cells (2×105/well) were used as allogeneic stimulators for polyclonal CD4+ T cells with αCD3 (5 μg/ml). Neutralizing αIL-6 (10μglml, clone MP5-20F3, eBiosciences) or TNFR:Fc(10 μg/ml, etanercept, Immunex) were added as indicated. Cultures were pulsed at 72 hours with 0.2 μCi 3H-thymidine/well and harvested 18 hours later. Proliferation was measured as cpm incorporated.
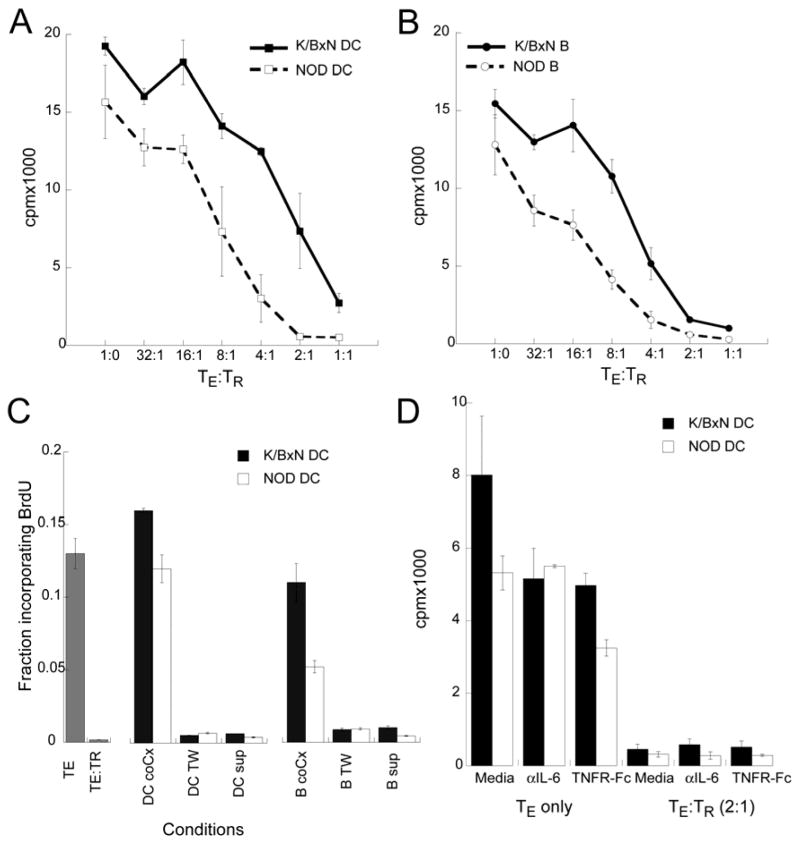
Figure 6. DCs and B cells from arthritic mice reduce TR suppression.
Dendritic cells (A) and B cells (B) were purified from NOD and K/BxN arthritic mice using Miltenyi microbeads. TE and TR were sorted from Foxp3gfp mice. TE (1×105/well) were cultured with either 1×104 DCs (A) or 2×105 B cells (B) and 5 μg/ml αCD3. TR were added to TE cultures at the indicated ratios. Proliferation was quantitated at 72 hours by 3H-thymidine incorporation. Proliferation by TE to APCs were 6874±752 cpm (NOD DC), 17455±788 cpm (K/BxN DC), 4059±298 cpm (NOD B cells), and 8094±1442 cpm(K/BxN B cells). Results represent mean of triplicate wells ± SD. Data is representative of 2 experiments. C) TE and TR were cultured at a 4:1 ratio and stimulated by αCD3 and irradiated splenocytes. DCs and B cells were purified from NOD or arthritic mice and activated by platebound αCD40 for 1 hour and washed. Activated APCs were added to the T cell cultures either directly or separated by a transwell membrane. In some T cell cultures, conditioned supernatant from αCD40 activated DC and B cell 48 hour cultures were added to a 1:1 vol/vol. BrdU incorporation was assayed at 72 hours. Results represent mean of duplicate wells ± SD. D) TE (1×105/well) ± TR (5×104/well) were stimulated by αCD3 and DC (1×104/well) from either NOD or K/BxN arthritic mice in the presence of neutralizing αIL-6 antibodies (10 μg/ml) or TNFR:Fc (10μg/ml). Proliferation was quantitated at 72 hours by 3H-thymidine incorporation. Data represent mean of triplicate wells ± SD. Data is representative of 2 experiments.
Transwell suppression assays
DCs or B cells from NOD or arthritic mice were activated with plated bound αCD40 (5μg/ml, 1C10, R&D) for 1 hour and washed extensively before adding to T cells. TE (5×105), TR (1.25×105) and irradiated splenocytes (5×105) were added in 0.4 μm transwell inserts or co-cultured with αCD40 activated DCs (5×104) or B cells (5×105) in 1 ml ISC-10. T cells were activated by αCD3 (5 μg/ml, 2C11). After 72 hours, 1 mM BrdU was added to each well and BrdU incorporation was assessed by flow cytometry after 12 hours.
Statistical analysis
Wilcoxon Rank Sum test and Student T test were calculated using InStat 2.00.
Results
Thymic Selection of FoxP3+ TR in K/BxNgfp mice
We have used K/BxNgfp mice to examine the development and function of FoxP3+ TR during arthritis. In these mice, TR suppressive activity resided specifically in the FoxP3+ (GFP+) population, regardless of CD25 expression (19). Therefore we defined TR as CD4+FoxP3+ T cells and TE as CD4+FoxP3− T cells.
FoxP3 expression in thymocytes was analyzed in 4 week old KRNgfp and K/BxNgfp mice to minimize effects of chronic inflammation upon thymocyte development. In KRNgfp mice, CD4+ SP thymocytes comprised 1–3% of total thymocytes (Figure 1A). Negative selection by endogenous GPI resulted in a 3 fold decrease in thymic cellularity (21.5±2.37 ×107 in KRNgfp and 8.3±1.75×107 in K/BxNgfp mice), resulting primarily from losses in the DP subset (Figure 1A). In agreement with our prior observation, TR were enriched 2–3 fold among CD4 SP thymocytes in K/BxNgfp mice compared to KRNgfp mice (Figure 1B,C) (14). To examine the effect of KRN TCR expression on FoxP3 selection, we analyzed the expression of the transgene encoded TCR Vβ6 because there is no clonotypic antibody to the KRN TCR. In agreement with results from other transgenic TCR systems, recognition of its cognate peptide appears to be a pre-requisition for KRN TR selection (21, 22). In KRNgfp mice, which lacked GPI-I-Ag7 complexes, Vβ6bright KRN T cells were only seen in TE, suggesting that TR required endogenous TCR expression (resulting in lower Vβ6 levels) for selection (Figure 1B). Conversely, in K/BxNgfp mice, negative selection by endogenous GPI resulted in preferential deletion of Vβ6bright TE. The remaining TE expressed equivalently lower levels of Vβ6 as TR. Statistically higher numbers of CD4+ SP FoxP3+ thymocytes were found in K/BxNgfp mice (Figure 1D). The enrichment of TR in K/BxNgfp mice resulted from both selective deletion of FoxP3− CD4 thymocytes, especially Vβ6bright T cells, and induction of FoxP3+ thymocytes.
Figure 1. Increased FoxP3+ thymocytes selection in K/BxNgfp mice.
A. Thymocytes from 4 week old KRNgfp and K/BxNgfp mice were labeled with CD4-APC and CD8-PE and analyzed by flow cytometry. Live cells were gated by forward and side scatter and analyzed by CD4 and CD8. Numbers indicated the % in each subset. B. Histograms of FoxP3 (top) and Vβ6 (bottom) expression in gated CD4+ SP thymocytes. C and D. The percentage (C) and numbers (D) of FoxP3+ thymocytes seen in CD4+ SP thymocytes in KRNgfp mice (circles, n=8) and K/BxNgfp mice (squares, n=10). Each dot represented an individual mouse and lines indicated means. P values were calculated by Wilcoxon Rank Sum test.
Development of KRN TR during arthritis in K/BxNgfp mice
The frequencies of TR were similarly increased in the spleen and peripheral lymph nodes (LN). While GFP expression correlated largely with CD25 expression in KRNgfp mice, 10–15% of CD25+ were Foxp3− and were activated TE (19). This fraction of CD25+FoxP3− TE was doubled in K/BxNgfp mice with significant overlap with FoxP3+TR, highlighting the power of FoxP3-GFP reporter in this autoimmune model (Figure 2A). To show that FoxP3+ T cells function as TR, sorted CD4+FoxP3+ T cells were tested in a suppression assay using CD4+FoxP3− T cells as responder TE. TR did not proliferate to NOD dendritic cells (DCs) presenting either endogenous GPI or KRN superagonist G7m (13). However, they, nevertheless, suppressed the proliferation of TE when co-cultured in a 1:1 ratio (Figure 2B).
Figure 2. Site specific expansion of FoxP3+TR during arthritis.
A. Splenocytes from 4 week old KRNgfp and K/BxNgfp mice were labeled with CD4-APC and CD25-PE and analyzed by flow cytometry. Live cells were analyzed by CD4 and CD25 (top panels). Lower panels showed the CD25 and FoxP3 expression in gated CD4+ splenocytes. B. TR (CD4+FoxP3+) and TE (CD4+FoxP3−) were sorted and stimulated with irradiated NOD ± 1 μM G7m peptide in separately or in a co-culture at a 1:1 ratio. Results are mean ±SD. C. Percentages of FoxP3+ CD4+ TR in spleens and lymph nodes from non-arthritic (n=8), acutely arthritic (n=10), and chronically arthritic mice (n=6). Results are presented as means ± SD. P values were calculated based on the Wilcoxon Rank sum. For spleen cells, p<.0001 between non-arthritic and acute mice, and p=.0003 between non-arthritic and chronic mice, and NS between acute and chronic mice. For dLN, p=.02 between non-arthritic and acute mice, and p=.0037 between non-arthritic and chronic, and NS between acute and chronic mice. In both acute and chronic mice, P<.005 for comparisons between each of the different organs. There was no statistically significant difference in distribution of TR in the various organs in non-arthritic mice. D. Total numbers of FoxP3+ CD4+ TR cells in spleens and lymph nodes. Results are presented as means ± SD. P <0.05 between non-arthritic spleen and their counterparts in both acute and chronically arthritic mice, and between non-arthritic dLN and chronically arthritic dLN. Within the non-arthritic group, there was no statistically significant difference in the TR numbers in various organs. Within the acutely arthritic mice group, p<0.05 for differences between spleen and dLN, ndLn, and mLN. Within the chronically arthritic group, TR numbers in the spleen and dLN were significantly different from ndLN and mLN. E. Total numbers of CD4+FoxP3− TE in spleen and lymph nodes. Results are presented as means ± SD. F. Lymphocytes were isolated from inflamed paws and analyzed by flow cytometry for CD4-PE and FoxP3-gfp. Live lymphocytes were identified by forward and side scatter, as well as PI exclusion. Numbers indicated % of cells within each quadrant and the number in parenthesis indicated % of CD4+ T cells. Data are representative of 5 non-arthritic, 6 acutely arthritic, and 6 chronically arthritic mice.
To determine TR expansion during arthritis, we quantified the frequency and numbers of TR in the spleen, joint draining LN (dLN), non-joint draining LN (ndLN), and mesenteric LN (mLN) during different phases of arthritis: non arthritic KRNgfp control, acutely arthritic (4–6 weeks) and chronically arthritic (8–10 weeks) K/BxNgfp mice. In all organs surveyed, the frequencies of TR were increased in a hierarchical manner with higher increases in the spleens and joint dLN relative to ndLN and mLN in K/BxNgfp mice compared to KRNgfp mice (Figure 2C). When we quantified the total numbers of TR in arthritic mice, TR numbers were significantly increased in the spleen of acutely arthritic mice and in the spleen and dLN of chronically arthritic mice compared to non-arthritic mice. Therefore, TR first expanded in the spleens and spread to the dLN during disease progression (Figure 2D). Comparison of TE and TR numbers in the spleen and LN showed that TR expansion was met by similar expansion by TE: such that the ratios of TE:TR were maintained during arthritis (Figure 2E).
We also examined TE and TR in the paws. In non-arthritic mice, <1% were CD4+ T cells. Of which, 5.9±3.2% were TR (Figure 2F). During acute arthritis, CD4+ T cells comprised 2.1±0.7% with 58.5±10.9% TR. As the inflammation waned in the paws, TR became less frequent comprising 39.9±10.1% of CD4+T cells. Our data resonated with findings in human RA showing accumulation of TR during active inflammation.
TR experienced greater turnover in vivo than TE in K/BxNgfp mice
Because increased numbers of TR in the spleen and LN during arthritis can arise from increased proliferation or migration, we directly examined TE and TR proliferation by in vivo BrdU incorporation. Non arthritic KRNgfp and acutely arthritic K/BxNgfp mice were injected ip with 1 mg of BrdU 12 hours prior to sacrifice. The fractions of BrdU+ Vβ6+CD4+FoxP3− (KRN TE) and Vβ6+CD4+FoxP3+ (KRN TR) were quantified. In non-arthritic KRNgfp mice, higher fractions of TR (~10%) incorporated BrdU compared to TE (1–3%) (Figure 3A). This tonic proliferation by TR suggests active surveillance at steady state even in non-lymphopenic mice.
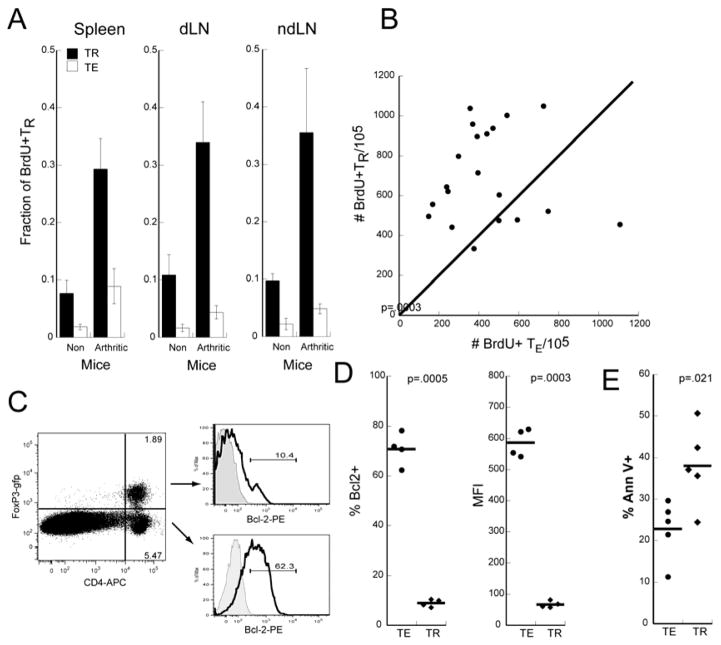
Figure 3. TR proliferate at higher rates than TE in vivo.
A. Fraction of BrdU+ TR and TE in spleens, dLN, and ndLN in KRNgfp and acutely arthritic K/BxNgfp mice. Bars represent mean from 4 mice and error bars show SD. Results are representative of 4 separate experiments. B. The numbers of BrdU+ TR in dLN were plotted against the numbers of BrdU+ TE in each mouse. Each dot represents an individual arthritic mouse. The bisecting diagonal line indicates 1:1 correspondence. N=20. C. Bcl2 expression is increased in TE. Splenic CD4+ TR and TE from arthritic mice were gated and analyzed for intracellular Bcl-2 expression. Black line=Bcl-2 PE and filled gray= isotype control. Numbers in the gate represent % of cells. D. The frequency of Bcl-2+ and MFI of Bcl-2 expression of TE (filled circles) and TR (filled diamonds) in dLN from 4 arthritic mice are depicted. Bars represent mean and p values are calculated by Fisher t-tests. Experiment is representative of two independent experiments. E. The percentage of Annexin V+ TE (filled circles) and TR (filled diamonds) in dLN from 5 arthritic mice are represented with the bar denoting mean. P values are calculated by Fisher t-tests. Experiment is representative of 2 independent experiments.
In arthritic K/BxNgfp mice, recognition of GPI increased the fraction of cycling TE and TR by three fold resulting in 5–10% of TE and 30–35% TR incorporating BrdU. Consistently higher fractions of cycling TR were observed in the LN (Figure 3A) and accounted for the greater increase in T cell numbers seen in the LN as the disease progressed from acute to chronic arthritis (Figure 2 D).
To directly compare the numbers of cycling TE vs TR, we plotted BrdU+TE vs BrdU+TR in dLNs from arthritic mice (Figure 3B). Similar results were seen in the spleens and other LN (data not shown). With few exceptions, more TR incorporated BrdU than TE. In some mice, BrdU+TR were 3 fold higher than BrdU+TE. We, however, did not find any correlation between our gross measurements of paw inflammation with numbers of BrdU+ TR or TE.
Given the higher rate of TR proliferation, the fractions of TR should increase over time. However, this was not the case (Figure 2C). To reconcile this discrepancy, we hypothesized that the increased turnover is balanced by increased death. In support, we found that TR from arthritic mice expressed significantly less anti-apoptotic factor Bcl-2 (Figure 3C,D) and higher levels of apoptosis (Annexin V+) than TE ex vivo (Figure 3E).
FoxP3+TR proliferate and die with different kinetics than FoxP3− TE
To test our hypothesis that TR proliferated and died at a faster rate than TE, we directly examined the kinetics of TR and TE proliferation and attrition in vitro. KRNgfp CD4+ T cells were purified from pooled spleens and LN and cultured with NOD DCs. This approach allowed us to specifically examine intrinsic differences between TR and TE separate from the inflammatory milieu in arthritic mice. We used naïve T cells to synchronize the exposure to antigenic stimuli. At various times, BrdU was added to the cultures and BrdU incorporation was analyzed 12 hours later. Contrary to the in vitro anergy noted when TR were cultured alone, TR showed significant proliferation when cultured in physiologic ratio with TE and stimulated with physiologic amounts of GPI. By day 1, 20% of FoxP3+ TR have entered cell cycle and incorporated BrdU compared to <2% of FoxP3− TE. BrdU incorporation by TR peaked at day 2 and declined over days 3 and 4 (Figure 4A). In contrast, TE did not proliferate to any appreciable degree in the first 24 hours of culture but proliferated vigorously in the subsequent days. Our data showed that TE and TR proliferated with distinct kinetics and magnitude. TR proliferated earlier but the response was short lived. TE proliferation, though delayed, was more sustained.
Figure 4. Kinetics of TR and TE proliferation and cell death upon antigen stimulation.
A. Purified CD4+ T cells from KRNgfp mice were cultured with NOD DCs and 1 mM BrdU was added at varying times. BrdU incorporation was analyzed 12 hours later by flow cytometry. Time indicates time of cell analysis. Results are presented as means of duplicate wells ± SD. Experiment is representative of 3 experiments. B. Purified CD4+ T cells from KRNgfp and arthritic K/BxNgfp mice were cultured with NOD DCs. At various times, apoptotic and dead CD4+ T cells were analyzed by 7-AAD and Annexin V respectively. Results are mean of duplicate wells. SD were <15%. Experiment has been performed 3 times with similar results.
In a parallel experiment, cell death and apoptosis of KRNgfp T cells were analyzed using 7-AAD and Annexin V to identify dead and apoptotic cells respectively. As shown in Figure 4B, the fractions of dead and apoptotic TR were roughly twice that of TE cells observed during this time course. Interestingly, the time course of TR apoptosis correlated with TR proliferation: both peaked at day 2 and declined at days 3 and 4. This finding contrasted with the kinetics of TE proliferation and apoptosis, and provided support that Bcl2 expression in TE conferred protection from apoptosis.
To determine if prior activation conferred protection from apoptosis, TE and TR from arthritic K/BxNgfp mice were also analyzed (Figure 4C). Naive and activated TE displayed similar rates of cell death. In comparison to naïve TR, TR from K/BxNgfp mice displayed less apoptosis and death. However, relative to their TE counterparts, both naïve and activated TR still undergo higher apoptotic rates. Together the data confirmed our hypothesis that increased TR proliferation was offset by increased TR apoptosis, resulting in TE:TR homeostasis.
TR become increasingly suppressive during arthritis
To interrogate TR function during arthritis, we sorted TE and TR from acutely and chronically arthritic mice. Additionally, to determine if there were site specific effects, TE and TR from spleen and dLNs were analyzed separately. We first compared proliferation of TE from the spleen and LN during acute and chronic disease. LN TE proliferated better to GPI compared to splenic TE but there was no statistically significant difference in the proliferation of TE between acute and chronic disease (Figure 5A).
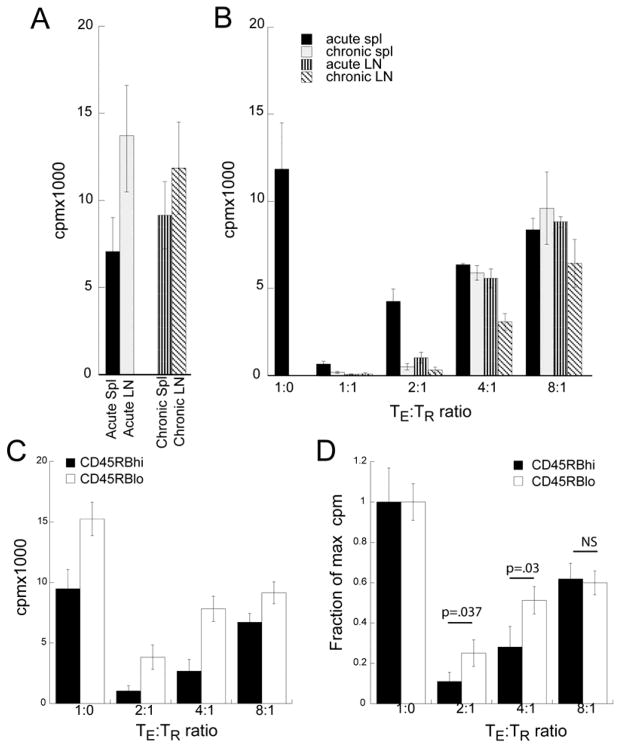
Figure 5. Increased TR activity during arthritis is countered by increased TE resistance to suppression.
A. TE (CD4 +FoxP3−) were sorted from spleens or pooled LN from acutely or chronically arthritic mice. TE (1×105/well) were stimulated with irradiated NOD DCs and cell proliferation was assayed by 3H-thymidine incorporation during the last 16 hour in a 72 hour culture. Data denotes mean of triplicates wells ± SD. B. TR from different sites and disease state varied in their suppressive activity against TE. Data denotes mean of triplicates wells ± SD. C and D. CD4+FoxP3−CD45RBlo(memory) and CD4+FoxP3−CD45RBhi (naïve) TE from arthritic mice were cultured with varying ratios of TR with NOD DCs. Data represents mean cpm of triplicate wells ± SD (C) or normalized as a fraction of max cpm of TE alone (D). P values were <.02 for CD4+FoxP3−CD45RBhi vs CD4+FoxP3−CD45RBlo at each of the TE:TR ratios in (C) and other P values for D are shown. N.S. not significant. Data is representative of three similar experiments.
We next examined the ability of TR to suppress TE proliferation. Because TE from chronically arthritic mice would be targets for TR immunotherapy, we used LN TE from chronic mice as responders. As shown in Figure 5B, TR from different sites and disease states suppressed TE proliferation with differing efficacy. While all TR were effective at a 1:1 ratio, splenic TR from acutely arthritic mice were the least suppressive. Conversely, LN TR from chronically arthritic mice were the most effective. Thus, TR activity increased during arthritis and at sites of active inflammation.
To determine if the TE activation state influences its susceptibility to suppression, we sorted FoxP3−CD45RBhi (naïve) TE, FoxP3−CD45RBlo (memory) TE, and FoxP3+ TR from pooled splenic and dLN T cells from arthritic mice. Naïve CD45RBhi TE cells were less proliferative and more easily suppressed than experienced CD45RBlo TE (Figure 5C). To determine if this was due to differences in the magnitude in T cell response, we normalized the response to the proliferation in the absence of TR. At TE:TR ratios of 2:1 and 4:1, TR suppression was two fold less effective with CD45RBlo TE (Figure 5D). Thus, during arthritis, both TE and TR enhanced their response to GPI. Such that increased TR suppression is met by increased TE resistance, resulting in an immunologic détente.
Inflammatory APCs contribute to Resistance to TR Suppression
Pro-inflammatory cytokines produced by monocytes and DC can abrogate TR function by increasing TR apoptosis or by increasing TE resistance (9, 23, 24). In K/BxN mice, DCs and B cells comprised two major APC for the activation of KRN T cells (20). DCs were the most efficacious at stimulating KRN T cells while B cells were the most abundant. We therefore compared the efficacy of TR suppression in the presence of DC and B cells from chronically arthritic K/BxN mice or control NOD mice. We have previously shown that APCs from arthritic K/BxN mice showed greater presentation of GPI peptide compared to non-arthritic APCs (20). To eliminate the role of GPI dose, we used polyclonal T cells from FoxP3gfp mice on the H-2k background in which the APCs would provide a strong allostimulation in addition to αCD3. As expected, DCs from arthritic mice attenuated the suppressive effect of TR (Figure 6A). With NOD APCs, TR suppressed TE proliferation by 50% at a TE:TR 8:1. Four fold higher numbers of TR were necessary in the presence of arthritic DCs. Arthritic B cells similarly inhibited the suppressive effect of TR (Figure 6B).
To determine the mechanism by which DC and B cell inhibit TR suppression, we added αCD40-activated DC and B cells to TE:TR suppression assays either in the same wells or separated by transwell membranes. Proliferation of TE was assayed by BrdU incorporation by flow cytometry. As shown in Figure 6C, αCD40-activated DCs and B cells abrogated TR suppression only when they are co-cultured with the TE and TR. Restoration of TE proliferation was absent when DCs and B cells were separated by transwell membranes or when cultured supernatants from DC and B cell cultures were added. The finding that inflammatory DCs can reverse TR suppression has been described (9, 24). However, these reports attributed abrogation of TR activity to soluble factors such as TNFα and IL-6. Here, we found that neutralization IL-6 or TNFα had no effect on TR suppressive activity (Figure 6D). Similar results were seen with B cells as APCs (data not shown). Together, our data demonstrated that both arthritic DCs and B cells decrease TR activity in a cell-cell contact or close contact dependent mechanism. The finding that arthritic B cells can also inhibit TR suppression provides a potential mechanism for the efficacy of B cell depletion in controlling RA.
Discussion
There is considerable interest in harnessing CD4+CD25+ TR to control autoimmune diseases (25, 26). In RA patients, CD4+CD25+ TR are increased in inflamed joints and showed variable activity against their TE counterparts. Results differed among studies in part due to differences in the criteria for TR, disease states, and patient population. It is also not clear what factors control their tropism, function, growth, and death during disease. We have used K/BxNgfp mice to model the development and function of arthritogenic TE and TR in a well characterized murine model of RA. K/BxN mice displayed many advantages relative to human RA patients. The progression of arthritis has been extensively characterized and follows a predictable course: K/BxN mice develop overt joint inflammation at 4–5 weeks, peaking at 7–8 weeks, followed by a chronic phase in which joint destruction and deformity predominates (27). Additionally, the arthritis shows regional involvement with the distal joints more affected than proximal and axial. This feature allowed us to determine if there is regional expansion of TR at sites of disease. Moreover, by examining GPI-reactivity, we focus specifically on the arthritogenic KRN TE and TR in this system. Lastly, we have used FoxP3gfp to unequivocally identify TR.
These experiments describe for the first time the expansion and function of antigen specific TR during the initiation and progression of spontaneous arthritis. Here, we confirmed our prior findings that incomplete deletion of KRN thymocytes by endogenous GPI resulted in increased frequency of GPI-specific TR in K/BxNgfp mice through selective deletion of Vβ6bright FoxP3− TE and induction of FoxP3+TR in the thymus (14). In contrast to other models of autoimmune disease in which TR deficits resulted in pathology, K/BxN mice displayed enhanced populations of GPI-specific TR (14, 15, 28–30). These TR proliferated quite well both in vitro and in vivo in response to endogenously presented GPI, were functional, and suppressed TE proliferation in vitro. TR numbers expanded in parallel with TE during arthritis in the spleens and lymph nodes. In K/BxN mice with an ubiquitous self-antigen, both TE and TR responses initiated in the spleen with subsequent extension to the lymph nodes with preference for the dLN, indicating selective tropism to inflamed joints from increased inflammation and/or GPI presentation during disease. However, this enhanced activity was offset by increased resistance by GPI-specific TE and APCs to suppression. A recent study described similar dynamic changes in the spatio-temporal expansion of TE and TR in the draining LNs during the induction and progression of adjuvant arthritis that presumably reflected changes in the concentration of the inciting antigen (31). Their findings complemented our studies here with the exception that their waxing/waning TR course likely reflected the monophasic course in adjuvant arthritis compared to the progressive expansion of TE and TR in the K/BxN model.
Despite the enrichment in TR activity, K/BxN mice nevertheless developed arthritis. Our in vitro studies demonstrate effective TE suppression at ratios that approximate the physiologic ratio found in vivo. Why then are TR ineffective in vivo? There are several possible explanations. First, TR and TE are differentially regulated during ontogeny. FoxP3− CD4 SP thymocytes reached mature levels during the first week of life. In contrast, FoxP3+ CD4+ SP thymocytes development was substantially delayed and did not achieve mature levels until 21 days of age (32). The delayed appearance of TR in K/BxN mice would result in unopposed TE reactivity at the initiation of arthritis. Second, the precursor frequency of GPI specific T cells is supra-physiologic in K/BxN mice. The self antigen is also ubiquitously expressed and can be presented by all APCs, including B cells. Under such conditions, autoreactive B cells can be driven into memory B cells despite abundant TR (33). Thus for a humorally driven arthritis, TR may be less effective than for a T cell driven disease like colitis. We propose that the developmental regulation and numerical superiority of KRN TE overcome TR suppression at the initiation of disease. TR might simply arrive too late and in too few numbers.
During the ensuing arthritis, TR are highly activated with >30% of TR proliferating in a 12 hour period. However, this vigorous TR activity is attenuated by three factors: TR undergo shorter proliferative bursts and higher apoptosis rates than TE upon antigen stimulation, TE become more refractory to TR suppression as they differentiate into memory T cells, and APCs from arthritic mice abrogate TR suppression. This inhibition of TR suppression required close contact but it is unknown whether this is due to increased resistance of APCs to TR or enhanced antigen presentation to TE. It is also unclear whether these are due to changes in the co-stimulatory molecules or secretion of paracrine cytokines in activated APCs from arthritic mice. Current studies are on-going to dissect these mechanisms. Our findings parallel observations in human RA and provide an explanation for the paradoxical occurrence of disease in the presence of abundant TR. By elucidating factors and mechanisms that augment TR activity, it may be possible to achieve disease control. K/BxNgfp mice thereby provide a valuable model to examine TE:TR homeostasis during disease and therapy.
Acknowledgments
This work is supported by grants (K12-HD01487 1 and K08 AR051980-01) from the NIH and a pilot and feasibility grant (IRG-58-010-49) from the American Cancer Society.
We are grateful to Drs. D. Mathis and C. Benoist and INSERM/IGBMC for the use of KRN mice. We thank Dr. A. Rudensky for FoxP3gfp mice. We are grateful to Drs. P. Tarr, C. Pham, and J. P. Atkinson for critical review of the manuscript.
Footnotes
The authors do not have any financial disclosures.
Author Contributions: Dr. Shih had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Design: Shih
Acquisition of data: Monte, Wilson, Shih
Analysis and interpretation of data: Shih
Manuscript preparation: Shih
Statistical analysis: Shih
References
- 1.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nature Reviews Immunology. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172(10):6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 4.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201(11):1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33(1):215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 6.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6(4):R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200(3):277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50(9):2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 9.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, et al. Proinflammatory mediator-induced reversal of CD4+, CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56(3):732–42. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 10.Cao D, Borjesson O, Larsson P, Rudin A, Gunnarsson I, Klareskog L, et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63(6):444–52. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 11.Korganow A-S, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 13.Basu D, Horvath S, Matsumoto I, Fremont DH, Allen PM. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. Journal of Immunology. 2000;164:5788–5796. doi: 10.4049/jimmunol.164.11.5788. [DOI] [PubMed] [Google Scholar]
- 14.Shih FF, Mandik-Nayak L, Wipke BT, Allen PM. Massive thymic deletion results in systemic autoimmunity through elimination of CD4+ CD25+ T regulatory cells. Journal of Experimental Medicine. 2004;199:323–335. doi: 10.1084/jem.20031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56(2):509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 16.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48(5):1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 17.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52(7):2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 18.Ohata J, Miura T, Johnson TA, Hori S, Ziegler SF, Kohsaka H. Enhanced efficacy of regulatory T cell transfer against increasing resistance, by elevated Foxp3 expression induced in arthritic murine hosts. Arthritis Rheum. 2007;56(9):2947–56. doi: 10.1002/art.22846. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Shih FF, Racz J, Allen PM. Differential MHC class II presentation of a pathogenic autoantigen during health and disease. J Immunol. 2006;176(6):3438–48. doi: 10.4049/jimmunol.176.6.3438. [DOI] [PubMed] [Google Scholar]
- 21.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 22.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200(10):1221–30. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–9. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 27.Mandik-Nayak L, Wipke BT, Shih FF, Unanue ER, Allen PM. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14368–14373. doi: 10.1073/pnas.182549099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 29.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99(19):12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–8. discussion 98–105, 106–14. [PubMed] [Google Scholar]
- 31.Nolte-’t Hoen EN, Boot EP, Wagenaar-Hilbers JP, van Bilsen JH, Arkesteijn GJ, Storm G, et al. Identification and monitoring of effector and regulatory T cells during experimental arthritis based on differential expression of CD25 and CD134. J Leukoc Biol. 2008;83(1):112–21. doi: 10.1189/jlb.0607436. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202(7):901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guay HM, Larkin J, 3rd, Picca CC, Panarey L, Caton AJ. Spontaneous autoreactive memory B cell formation driven by a high frequency of autoreactive CD4+ T cells. J Immunol. 2007;178(8):4793–802. doi: 10.4049/jimmunol.178.8.4793. [DOI] [PubMed] [Google Scholar]