Abstract
Daytime and nighttime symptoms of posttraumatic stress disorder (PTSD) are common among combat veterans and military service members. However, there is a great deal of heterogeneity in how symptoms are expressed. Clarifying the heterogeneity of daytime and nighttime PTSD symptoms through exploratory clustering may generate hypotheses regarding ways to optimally match evidence-based treatments to PTSD symptom profiles. We used mixture modeling to reveal clusters based on six daytime and nighttime symptoms of 154 combat veterans with insomnia and varying levels of PTSD symptoms. Three clusters with increasing symptom severity were identified (N1=50, N2=70, N3=34). These results suggest that, among veterans with insomnia, PTSD symptoms tend to exist on a continuum of severity, rather than as a categorical PTSD diagnosis. Hypotheses regarding possible targeted treatment strategies for veterans within each identified cluster, as well as ways to generalize these methods to other groups within the military, are discussed.
Keywords: Veterans, chronic insomnia, sleep, PTSD, personalized medicine, clustering
Posttraumatic Stress Disorder (PTSD) is among the most widespread mental health diagnoses in combat veterans and military service members (Weiss et al., 1992; Hoge et al., 2004, 2008; Thomas, 2010). It refers to stress reactions that persist for more than one month following exposure to threatening events, and includes both daytime and nighttime symptoms of intrusions, avoidance, hyperarousal, and dysphoria (American Psychiatric Association [APA], 2013). PTSD is highly disabling and distressing, as veterans with the diagnosis often struggle in social, work, school, and family settings. Soldiers with functional impairment as a result of PTSD and who redeploy may not be able to perform their duties as effectively or efficiently as expected. Those who return home with PTSD often struggle with readjustment to civilian life, finding gainful employment, and success in returning to school.
There is substantial heterogeneity in the expression of PTSD symptoms. A DSM-5 PTSD diagnosis requires at least 1 of 5 intrusion, 1 of 2 avoidance, 2 of 7 dysphoria, and 2 of 6 hyperarousal symptoms. These criteria result in various presentations depending on which symptoms are most commonly endorsed and/or symptom severity (e.g., see Runyon et al., 2014; Galatzer-Levy & Bryant, 2013). It is also important to account for the high prevalence, and significant heterogeneity, of veterans with sub-clinical PTSD—veterans who struggle to manage symptoms and experience distress and functional impairment but do not meet full DSM-5 diagnostic criteria. For example, sleep disturbances, including insomnia and nightmares, are symptoms within the hyperarousal and intrusion criteria, respectively. However, they are not always accompanied by a sufficient number of daytime symptoms to meet PTSD diagnostic criteria and put the veteran at risk of not receiving appropriate treatment for all symptoms (Germain et al. 2012; Seelig et al., 2010; Ulmer et al., 2011; Raskind et al., 2003, 2013).
Given the heterogeneity of PSTD symptom presentations, it is not surprising that PTSD is difficult to treat and that no single evidence-based treatment adequately addresses all symptom profiles (Green, 2013; IOM 2014). Current pharmacological and psychological therapies to treat overall PTSD symptoms include prolonged exposure therapy (PE) (Foa et al., 2007), cognitive processing therapy (CPT) (Resick et al., 1993, 2002), trauma-focused cognitive behavioral therapies (CBT) (e.g., Ehlers et al., 2005; Belleville et al., 2011), and selective serotonin reuptake inhibitors (SSRIs). However, these approaches can have variable efficacy depending on the specific daytime and nighttime symptoms experienced (Brady et al., 2000; Zayfert et al., 2004; Davidson et al., 2006; Belleville et al., 2011; Gutner et al., 2013). Alternatively, there are treatments designed specifically for nighttime symptoms, including prazosin (Raskind et al., 2003, 2013), imagery rehearsal therapy (IRT) (Krakow et al., 2000), and sleep-focused cognitive and/or behavioral interventions (e.g., Germain et al., 2012; Krakow et al, 2001; Talbot et al., 2014). Although these treatments effectively reduce nightmares and insomnia, they do not target daytime symptoms and, thus, do not always yield full remission of PTSD.
In light of these challenges, the Institute of Medicine (IOM) recently emphasized the importance of developing new evidence-based PTSD treatment strategies for veterans (2014). However, because existing treatments are not equally effective for all, we propose that clinicians should have an array of evidence-based treatments they can optimally match to specific symptom profiles of veterans. To accomplish this, however, it is first necessary to capture the heterogeneity of PTSD symptoms to identify common symptom profiles. This goal is consistent with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) project that seeks to reveal new classes of psychiatric disease that are agnostic to current DSM diagnoses and to optimally match treatments to these classes (Insel et al., 2010). It also aligns with the IOM's call for precision medicine and clarification of heterogeneity in PTSD (2011, 2014).
The aims of this manuscript are to 1) demonstrate how clustering can be used to clarify the heterogeneity of daytime and nighttime PTSD symptoms within a sample of combat veterans, 2) characterize each cluster based on clinical and demographic characteristics to suggest symptom profiles, and 3) generate hypotheses surrounding how treatments might be targeted towards veterans matching specific symptom profiles. As an initial approach, we focus on a clearly defined research sample of 154 veterans with chronic insomnia and varying levels of PTSD symptoms. Insomnia is one of the most common reasons for referral to mental health services in active duty service members, is the most frequently reported symptom of post-9/11 veterans with PTSD, and is a significant risk factor for developing PTSD (Cozza et al., 2004; Hoge et al., 2008; McLay et al. 2010; Wright et al., 2011; Bramoweth & Germain, 2013; Pigeon et al., 2013). Thus, veterans with insomnia are an ideal, high-risk population that could readily benefit from the IOM's request for improved PTSD treatment. After demonstrating our approach in this sample, we discuss subsequent steps required to develop an array of empirically-based targeted treatment strategies and ways to extend these methods to other military contexts.
Methods
Participants and Procedures
Our aim was to develop a sample of combat veterans with insomnia, drawn from a larger pool of combat veterans enrolled in one of several research studies focused on sleep. Participants for all studies were recruited from the general population through television advertisement, recruitment fliers, and word of mouth. Across all studies, combat veterans were eligible if they: 1) were between 18 and 65 years old; 2) did not have a diagnosis of psychotic or bipolar disorder; 3) did not experience current concussive symptoms; 4) did not meet criteria for current (< 3 months) alcohol or substance use disorders; and 5) did not present with symptoms of sleep-disordered breathing, periodic leg movement disorder, narcolepsy, REM sleep behavior disorder, or restless leg syndrome. As part of the screening process, the Structured Clinical Interview for DSM-IV (SCID), Research Version, was conducted by trained assessors and used to diagnose sleep disorders (including insomnia) and other psychiatric disorders (First et al., 2002). Upon study entry, trained assessors diagnosed PTSD based on the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995; Weathers et al., 2001) using the 1-2 score rule: a frequency score of 1 (scale 0 = “none of the time” to 4 = “most or all of the time”) and an intensity score of 2 (scale 0 = “none” to 4 = “extreme”) is required for a particular symptom to meet criterion (Weathers et al., 1999). All studies were approved by the University's Institutional Review Board and the Department of Defense Human Research Protection Office.
To draw our sample of veterans with insomnia from the larger pool of veterans involved in the studies described above, we first considered all participants (n = 271) who completed the clinician-administered interviews to assess sleep disorders. Of these 271, 89% (n = 242) had an insomnia diagnosis. Sixty four percent (n=154) of those with insomnia had complete baseline data on all measures used for clustering. Table 1 shows clinical and demographic characteristics of the final sample of 154.
Table 1.
Demographic and clinical characteristics of the sample. Ranges are provided for clustering variables. Cells show the mean (SD) unless otherwise noted.
| Full Sample (N=154) | Cluster 1 (N=50) | Cluster 2 (N=70) | Cluster 3 (N=34) | Statistic | Pairwise Differences | |
|---|---|---|---|---|---|---|
| Daytime And Nighttime Symptoms Used for Clustering | ||||||
| Sleep Quality (PSQI; Range = 0 - 21) | 9.8 (3.5) | 8.8 (4) | 9.9 (2.8) | 11 (3.5) | F=4.6*** | 1 < 3 |
| DNB (PSQI –A; Range = 0 – 21) | 3.4 (3) | 1 (1.1) | 3.7 (2.5) | 6.3 (3) | F=56.3*** | 1 < 2 < 3 |
| Daytime Intrusions (PCL; Range = 4 –20) | 7.6 (3.3) | 4.9 (0.9) | 7.3 (1.6) | 12.4 (3) | F=168.4*** | 1 < 2 < 3 |
| Daytime Avoidance (PCL; Range = 2-10) | 4.3 (2.2) | 2.5 (0.6) | 4.2 (1.6) | 7.2 (1.5) | F=126.6*** | 1 < 2 < 3 |
| Daytime Dysphoria (PCL; Range = 7-35) | 13.7 (5.6) | 9.5 (2) | 13 (3.2) | 21.1 (6) | F=101.0*** | 1 < 2 < 3 |
| Daytime Hyperarousal (PCL; Range = 2-10) | 4.4 (2.3) | 2.7 (0.8) | 4.5 (1.9) | 6.9 (2) | F=67.4*** | 1 < 2 < 3 |
| Trauma and Combat History | ||||||
| Trauma (“Happened To”) (LEC) | 5.3 (2.5) | 4.5 (2.4) | 5.2 (2.5) | 6.7 (2.1) | F=8.7*** | (1, 2) < 3 |
| Trauma (“Witnessed”) (LEC) | 3.2 (2.7) | 3 (3.1) | 3 (2.2) | 4 (3) | F=2 | |
| Combat Exposure (CES), N=146 | 14.6 (10.9) | 10.4 (9.6) | 15.6 (10.1) | 19.5 (12.5) | F=7.3*** | 1 < (2, 3) |
| Total Number of Deployments, N=142 | 1.3 (0.7) | 1.3 (0.9) | 1.3 (0.7) | 1.1 (0.3) | F=1.1 | |
| Years Since Last Deployment, N=95 | 7 (10.7) | 8 (12.6) | 7.4 (10.1) | 4.7 (8.6) | F=6 | |
| Demographic Characteristics | ||||||
| Army, %(n), N=123 | 65.4 (80) | 59.5 (25) | 66.0 (35) | 71.4 (20) | X2=1.1 | |
| Navy, %(n), N=123 | 18.7 (23) | 23.8 (1) | 17.0 (9) | 14.3 (4) | FE | |
| Air Force, %(n), N=123 | 5.7 (7) | 4.0 (2) | 4.5 (3) | 5.9 (2) | FE | |
| Marines, %(n), N=123 | 10.6 (13) | 11.9 (5) | 11.3 (6) | 7.1 (2) | FE | |
| Military Officer, %(n), N=118 | 22.9 (27) | 30.8 (12) | 21.6 (11) | 14.3 (4) | X2=2.2 | |
| Age | 36.8 (12) | 39 (13.2) | 35.4 (10.7) | 36.6 (12.8) | F=1.3 | |
| Female, %(n) | 14.9 (23) | 14 (7) | 14.3 (10) | 17.6 (6) | X2=.25 | |
| Caucasian, %(n) | 83.1 (128) | 80.0 (40) | 81.4 (57) | 91.2 (31) | X2=2.1 | |
| Clinical Characteristics | ||||||
| Total PCL | 35.3 (12.6) | 23.7 (3.4) | 34.1 (4.9) | 54.6 (9.2) | F=294.7*** | 1 < 2 < 3 |
| Total CAPS | 40.1 (21.2) | 26.5 (15.5) | 40.3 (18.8) | 59.5 (17.9) | F=35.6*** | 1 < 2 < 3 |
| CAPS B (Re-experiencing) | 9.5 (7.4) | 5.2 (5.3) | 9.2 (7.1) | 16.4 (5.7) | F=32.1*** | 1 < 2 < 3 |
| CAPS C (Avoidance, Numbing) | 12.6 (10.3) | 6.6 (7.1) | 12.9 (9.2) | 20.6 (10.9) | F=24.6*** | 1 < 2 < 3 |
| CAPS D (Hyperarousal) | 18 (7) | 14.7 (6.3) | 18.2 (7) | 22.6 (5) | F=15.6*** | 1 < 2 < 3 |
| Anxiety (BAI), N=151 | 6.4 (6.3) | 2.2 (2.3) | 6.2 (5) | 12.8 (7.3) | F=45.2*** | 1 < 2 < 3 |
| Depression (BDI), N=151 | 9.2 (6.8) | 5.2 (4.1) | 8.8 (5.7) | 15.5 (7.5) | F=32.6*** | 1 < 2 < 3 |
| PTSD Diagnosis, % (n) | 46.8 (72) | 20 (10) | 48.6 (34) | 82.4 (28) | X2=31.78*** | 1 < 2 < 3 |
| Taking Any Psychotropic Medications, % (n) | 50 (77) | 32 (16) | 51.4 (36) | 73.5 (25) | X2=14.07*** | 1 < 3 |
Note. ANOVA, Chi-square, and Fisher's exact (FE) tests were used to test for overall differences among clusters. Cells in the “Statistic” column with an “FE” indicate that Fisher's exact test was used. All pair-wise differences were adjusted for multiple comparisons using either Tukey's method (continuous measures) or Bonferroni's correction (dichotomous measures). PSQI = Pittsburgh Sleep Quality Index; PSQI-A = Pittsburgh Sleep Quality Index Addendum; PCL = Posttraumtic Stress Disorder (PSTD) Checklist; LEC = Life Events Checklist; CES = Combat Exposure Scale; CAPS = Clinician-Administered PSTD Scale; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; DNB = disruptive nocturnal behaviors.
p<.05.
p<.01.
p<.001.
Among the group of 242 veterans with insomnia, the 154 with complete data were younger than the 88 with missing data (mean (SD) age complete data = 36.8 (12.0), mean (SD) age missing data = 40.8 (13.3), t = 2.35, df = 240, p = .019). Those with and without complete data did not differ significantly on other demographic characteristics, specifically: gender (X2 = .28, df = 1, p=.600), percentage Caucasian versus other races (X2 = .27, df = 1, p=.604), military branch (X2 = 7.51, df = 3, p = .057), and percentage who were officers (X2 = .41, df = 1, p=.521). Overall, considering the studies' entry criteria and differences between veterans with and without complete data, veterans in our sample may be relatively young with few additional psychiatric comorbidities or chronic physical illnesses relative to the larger universe of combat veterans.
Measures
We selected two nighttime symptoms (sleep quality, disruptive nocturnal behaviors [DNB]) and four daytime symptoms (intrusions, avoidance, hyperarousal, and dysphoria) for clustering. Sleep quality and DNB were chosen because they cover the full array of nighttime symptoms indicative of veterans with PTSD symptoms (Germain et al., 2008). The four daytime symptoms were chosen to reflect the DSM-5 PTSD symptom clusters.
The Pittsburgh Sleep Quality Index (PSQI) measured sleep quality. It is a widely used measure that captures general sleep problems such as difficulty initiating sleep and nighttime awakenings. It has high test-retest reliability and validity among adults with insomnia (Backhaus et al., 2002). Scores > 5 indicate poor sleep quality (Buysse et al., 1989; range = 0-21). The PSQI Addendum (PSQI-A) measured DNB. DNB include parasomnia-like episodes that are often reported by adults with PTSD, in addition to nightmares related to traumatic events. These episodes include panic attacks, night sweats, disturbing dreams unrelated to traumatic events, acting out dreams, and/or other complex vocal or motor behaviors (e.g., see Mysliviec et al., 2014; Husain et al., 2001; and Ohayon and Shapiro, 2000). The PSQI-A assesses the self-reported frequency of seven DNB and has been shown to have high validity. Scores > 4 may be associated with a PTSD diagnosis (Germain et al., 2005; Insana et al., 2013; range = 0-21).
Daytime intrusions, avoidance, hyperarousal, and dysphoria were measured using factors from the PTSD Checklist (PCL), a self-report screening tool for PTSD with high validity and high test-retest reliability (Weathers et al., 1993; Simms et al., 2002; Pratt et al., 2006). PCL symptoms are rated on a scale from 1 to 5 with respect to how bothersome they have been in the last month: 1 “not at all,” 2 “a little,” 3 “moderately,” 4 “quite a bit,” and 5 “extremely.” Items are summed to calculate the total score and factor scores. When calculating the factor scores, we omitted two nighttime-specific symptoms (“sleep quality” and “disturbing dreams”) in order to retain focus on daytime PTSD symptoms and to reduce overlap with the PSQI and PSQI-A. Hyperarousal consisted of “hypervigilance” and “exaggerated startle response” (range = 2–10); intrusions consisted of “intrusive thoughts,” “flashbacks,” “emotional reactivity,” and “physiological reactivity” (range = 4–20); avoidance consisted of “avoiding thoughts of trauma” and “avoiding reminders of trauma” (range = 2–10); and dysphoria consisted of “inability to recall traumatic events,” “loss of interest,” “detachment,” “restricted effort,” “sense of foreshortened future,” “irritability,” and “difficulty concentrating” (range = 7–35).
The Combat Exposure Scale (CES; Keane et al., 1989) and the life events checklist (LEC; Blake et al., 2000) assessed combat and non-combat-related trauma. Other scales included the CAPS and Beck Depression and Anxiety Inventories (Beck et al., 1961, 1988). Additional characteristics included age, gender, race, deployment history, military branch, and rank.
Data Analysis
We used normal mixture modeling (Fraley & Raftery, 1998) to identify clusters based on the six daytime and nighttime symptoms. This method uses a likelihood-based approach to optimally divide the data into a pre-selected number of clusters. We considered models with 1- 6 clusters and used the Bayesian Information Criterion (BIC) to select the best-fitting solution. After selecting the best model, we summarized the uncertainties (i.e., probabilities of individuals actually not being members of their assigned cluster) of all the individuals in the sample.
Clusters were characterized based on the four daytime and two nighttime symptoms as well as other clinical and demographic measures. ANOVA, Chi-square, and Fisher's Exact tests were used to reveal differences across the clusters. If the overall test was significant, we conducted pair-wise tests adjusted for multiple comparisons using Tukey's method and Bonferroni's correction for continuous and dichotomous variables, respectively. Cohen's d effect sizes further compared differences in symptom levels across clusters. The ANOVA F-statistics were ranked to compare each clustering variable's overall influence in the model. We used the statistical software package R (R Core Team, 2013) for all analyses. Mixture modeling was performed using the Mclus function from the mclus package (Fraley & Raftery, 2012).
Results
The best-fitting model based on the BIC had three clusters (N1 = 50, N2 = 70, N3 = 34) that were generally characterized by increasing levels of severity on the four daytime and two nighttime symptoms (Table 1 and Figure 1). On average, the veterans in cluster 1 reported daytime symptoms on the PCL as “not at all” bothersome and reported almost no DNB on the PSQI-A. The veterans in cluster 2 rated individual daytime symptoms as “a little” bothersome and reported mild DNB. The veterans in cluster 3 rated daytime symptoms as “moderately” to “quite a bit” bothersome and reported significant DNB. Not surprisingly, individuals in all three clusters had poor sleep quality. Based on these characteristics, we refer to cluster 1 as “Poor Sleep Quality”, cluster 2 as “Poor Sleep Quality with Mild Daytime Symptoms and DNB”, and cluster 3 as “Poor Sleep Quality with Significant Daytime Symptoms and DNB.”
Figure 1.
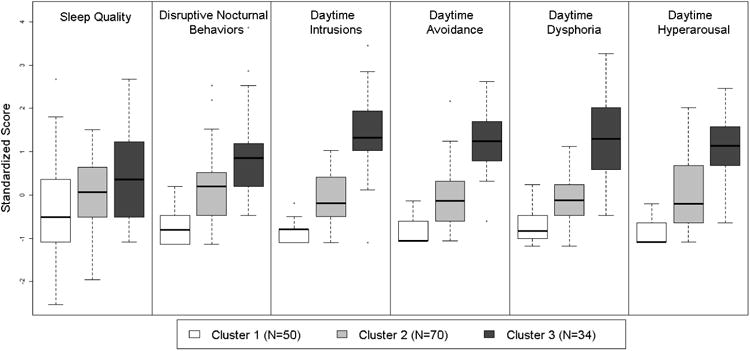
Box-and-whisker plots of the standardized distributions of the six daytime and nighttime symptoms within each cluster. The box length indicates the interquartile range and the middle line indicates the median. “Whiskers” extending vertically from the box indicate the most extreme data point that is no more than 1.5 times the interquartile range from the box. Sleep quality and disruptive nocturnal behaviors (DNB) are measured with the Pittsburgh Sleep Quality Index (PSQI) and the PSQI-Addendum, respectively. Daytime symptoms are measured with the PTSD Checklist (PCL).
Magnitudes of the Cohen's d effect sizes comparing daytime symptoms and DNB among clusters were all very large in size (range = 1.33, 4.37). Magnitudes of the Cohen's d effect sizes comparing clusters based on sleep quality were medium in size (range = .31, .59). F-statistics indicated that daytime intrusions had the most influence in the cluster model, followed by avoidance, dysphoria, hyperarousal, DNB, and sleep quality. The median (25th percentile, 75th percentile) uncertainty was 0.005 (0.0007, 0.05). These small uncertainty values suggest that the vast majority of observations were clustered correctly.
Veterans in the “Poor Sleep Quality” and “Poor Sleep Quality with Mild Daytime Symptoms and DNB” clusters reported experiencing significantly less lifetime trauma than veterans in the “Poor Sleep Quality with Significant Daytime Symptoms and DNB” cluster. Veterans in the “Poor Sleep Quality” cluster reported significantly less combat exposure than veterans in the other two clusters. Only ten veterans (20.0%) in the “Poor Sleep Quality” cluster had a PTSD diagnosis based on the CAPS. About half (48.6%, N=34) had a CAPS PTSD diagnosis in the “Poor Sleep Quality with Mild Daytime Symptoms and DNB” cluster. The majority (82.5%, N=28) had a CAPS PTSD diagnosis in the “Poor Sleep Quality with Significant Daytime Symptoms and DNB” cluster. There were no significant differences in demographic characteristics across clusters.
Discussion
Using a sample of combat veterans with insomnia and varying PTSD expressions, we demonstrated how exploratory cluster analysis can be used to clarify heterogeneity in a sample, thereby revealing new symptom profiles that might not be detected through clinical observation alone. Our analyses suggested three symptom profiles largely differentiated by overall severity of PTSD symptoms: 1) “Poor Sleep Quality”, 2) “Poor Sleep Quality with Mild Daytime Symptoms and DNB”, and 3) “Poor Sleep Quality with Significant Daytime Symptoms and DNB”. These profiles may be used to generate hypotheses surrounding new, personalized treatment approaches that can be tested through further research, with the ultimate goal of providing clinicians with an array of evidence-based treatments they can optimally match to individual veterans based on their presenting characteristics.
Limitations
The findings of this exploratory study need to be considered in the context of some limitations. Our use of mixture modeling to identify symptom profiles and generate treatment hypotheses highlights an important new strategy for developing an array of empirically-based treatments. However, mixture modeling is only one of many different clustering methods, some of which could produce different results and thus suggest alternative treatments. Another limitation is that our sample size of 154 is relatively small for the kind of analysis performed. Although one can certainly generate hypotheses based on our exploratory research, validation using an independent sample is required to move beyond the hypothesis-generation stage.
The results revealed by the novel strategy demonstrated herein are highly dependent on the sample. As an initial demonstration, we chose to use a well-defined research sample of veterans with insomnia so that our work could be easily validated and continued in other treatment studies with similar inclusion and exclusion criteria. However, because veterans in our sample are relatively young and had few additional psychiatric comorbidities or chronic physical illnesses, our results may not be generalizable to groups of veterans with chronic physical and psychiatric disabilities. Thus, in addition to validating our findings in similar samples to the one used herein, it will also be important to perform similar exploratory cluster analyses using samples of service members and combat veterans seen in different settings.
Clinical Implications
The symptom profiles revealed in this exploratory study can help to inform new approaches to personalized treatment for veterans with insomnia. Veterans in the “Poor Sleep Quality” cluster may benefit from a treatment aimed at improving overall sleep quality, but would not be likely to require additional PTSD-specific treatments. Veterans in the “Poor Sleep Quality with Mild Daytime Symptoms and DNB” cluster may benefit from sequenced treatment first aimed at reducing PTSD-related sleep difficulties like fear of sleep and nightmares (e.g., see Krakow et al., 2001; Germain et al., 2012; Talbot et al., 2014) prior to reassessment of daytime symptoms to evaluate the need for PTSD-focused treatment. Finally, veterans in the “Poor Sleep Quality with Significant Daytime Symptoms and DNB” cluster may benefit from an intervention that integrates sleep-specific PTSD treatments (e.g., cognitive and/or behavioral interventions for insomnia and nightmares and/or prazosin) with existing PTSD treatment approaches (e.g., PE, CPT, or SSRIs). A sequenced treatment—sleep-focused followed by trauma-focused—may also benefit veterans in this cluster, especially if there are barriers to engaging in trauma-focused therapies, such as stigma regarding mental health services, time commitment of therapy, or a wait-list to receive services. Receipt of sleep-focused treatment first may be considered less “psychiatric” and more “medical” in nature and can help veterans develop strong therapeutic rapport with a mental health professional. Furthermore, improved sleep may improve overall stability, thereby making it easier for veterans to tolerate the more challenging trauma-focused therapies.
Our findings highlight the need for clinicians to consider PTSD symptoms on a continuum in veterans with chronic insomnia rather than as a dichotomous disorder (i.e., absent vs. present). This is an important shift in conceptualization because most current PTSD interventions are only provided to those who meet full criteria, leaving those with sub-threshold presentations to receive no or suboptimal treatment. Emphasis on a symptom continuum may reduce the perceived stigma of psychiatric diagnoses and help increase access to care for veterans with sub-threshold PTSD and those who under-report symptoms. A better match between symptom presentation and intervention also has significant operational implications for the military. For instance, a soldier with symptoms matching the “Poor Sleep Quality” or “Poor Sleep Quality with Mild Daytime Symptoms and DNB” clusters may be effectively managed in theater without comprising the unit capabilities. They may also be able to be treated by clinicians without specialty training in trauma-related therapies (e.g., a primary care psychologist vs. a PTSD clinic psychologist). Severity-based treatment is also consistent with mental health care in the Veterans Health Administration (VHA), where many combat-exposed veterans receive care. Primary Care Mental Health Integration in VHA aims to manage mild symptoms of psychiatric disorders (i.e., “Poor Sleep Quality” or “Poor Sleep Quality with Mild Daytime Symptoms and DNB”) in the Primary Care setting through co-located collaborative care with the primary care and mental health providers in Patient Aligned Care Teams. Veterans with more severe symptoms (i.e., “Poor Sleep Quality with Significant Daytime Symptoms and DNB”) can then receive more intensive care through specialty mental health clinics with providers who are able to dedicate more time and services to veterans who need more specialized care.
Future Directions
Consistent with the hypotheses laid out in the NIMH RDoC project (Insel et al., 2010), the clusters we identified suggest that symptoms exist on a continuum rather than the presence or absence of a categorical PTSD diagnosis. However, to move our research forward within the RDoC framework, incorporating objective measures that tap into underlying physiological and neural processes will be critical. This type of application would allow researchers to develop personalized treatments that are targeted not only to a profile of self-reported symptoms, but also to specific combinations of biomarkers including genes, immune function, structural or functional changes in the brain, polysomnographic characteristics, and/or cardiometabolic parameters (e.g., see Yehuda et al., 2013, 2014; Eraly et al., 2014; Tylee et al., 2015; Nievergelt et al., 2015). This would provide clinicians with an array of empirically-based treatments that are targeted to specific underlying disease mechanisms rather than self-reported symptoms.
In future research, it will be important to validate these exploratory findings using a similar but independent sample of combat veterans to determine whether analogous symptom profiles are revealed. After validation, the next step would be to determine which existing treatments are most appropriate for specific symptom profiles and to develop new treatments, or combinations of treatments, if necessary. Next, it will be critical to perform treatment studies to determine whether individuals have a better outcome when given treatments that were personalized for their specific symptom profile. Finally, it is also important to take preference into consideration when testing treatments personalized to symptom profiles. Regardless of “match to symptom profile,” if a veteran's preferences, or lack of attention thereto, results in less effective treatment, the benefits of personalization based on symptoms and biomarkers alone will be limited. Future research utilizing symptom, biomarker, and preference personalization will allow mental health practitioners to use all available information to treat veterans with insomnia comorbid with PTSD symptoms, and the full spectrum of psychiatric disorders, more effectively and efficiently.
Acknowledgments
This research was supported by grants K01 MH096944 (PI: Wallace) and PR054093, PT073961, MH080696, MH083035, and Log11293006 (PI: Germain). Dr. Bramoweth is supported by funds from the VISN 4 Mental Illness Research, Education and Clinical Center (MIRECC, Director: D. Oslin; Pittsburgh Site Director: G. Haas), VA Pittsburgh Healthcare System.
Footnotes
Disclosures: Drs. Wallace, Iyengar, Bramoweth, and Germain have no conflicts of interest. Dr. Frank has the following conflicts of interest: 1) American Psychiatric Press, Editorial Consultant; 2) Psychiatric Assessments Inc, Stockholder; 3) American Psychological Association Press and Guilford Press, Royalties; 4) Servier International, Advisory Board. The contents of this article do not represent the views of the Department of the Veterans Affairs of the United States Government.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Backhaus J, Junghans K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. http://dx.doi.org/10.1016/S0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. http://dx.doi.org/10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. http://dx.doi.org/10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70(4):318–327. doi: 10.1016/j.jpsychores.2010.09.022. http://dx.doi.org/10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, Keane T, Buckley TC. Clinician-Administered PTSD Scale: Life Events Checklist. National Center for Posttraumatic Stress Disorder Behavioral Science Division, Boston Neurosciences Division; West Haven: 2000. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. http://dx.doi.org/10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837–1844. doi: 10.1001/jama.283.14.1837. http://dx.doi.org/10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15(10):401–404. doi: 10.1007/s11920-013-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–232. doi: 10.1016/0165-1781(89)90047-4. http://dx.doi.org/10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cozza SJ, Benedek DM, Bradley JC, Grieger TA, Nam TS, Waldrep DA. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ, editors. The Iraq War Clinician Guide. 2nd. National Center for PTSD and Department of Defense; 2004. [Google Scholar]
- Davidson J, Rothbaum BO, Tucker P, Asnis G, Benattia I, Musgnung JJ. Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study. J Clin Psychopharmacol. 2006;26(3):259–267. doi: 10.1097/01.jcp.0000222514.71390.c1. http://dx.doi.org/10.1097/01.jcp.0000222514.71390.c1. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM, Hackmann A, McManus F, Fennell M. Cognitive therapy for post-traumtic stress disorder: development and evaluation. Behav Res Ther. 2005;43(4):413–431. doi: 10.1016/j.brat.2004.03.006. http://dx.doi.org/10.1016/j.brat.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkasuskas DA, Biswas N, Agorastos A, O'Connoer DT, Baker DG Marine Resiliency Study Team. Assessment of plasmas C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(14):423–431. doi: 10.1001/jamapsychiatry.2013.4374. http://dx.doi.org/10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; Nov, 2002. [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford, England: Oxford University Press; 2007. [Google Scholar]
- Fraley C, Raftery AE. How many clusters? Which clustering method? Answers via model-based cluster analysis. The Computer Journal. 1998;41(8):578–588. http://dx.doi.org/10.1093/comjnl/41.8.578. [Google Scholar]
- Fraley C, Raftery AE, Murphy TB, Scrucca L. mclust Version 4 for R: normal mixture modeling for model-based clustering, classification, and density estimation. Department of Statistics, University of Washington: 2012. [Google Scholar]
- Galatzer-Levy IR, Bryant RA. 636,120 Ways to have post-traumatic stress disorder. Perspectives on Psychological Science. 2013;8(6):651–662. doi: 10.1177/1745691613504115. http://dx.doi.org/10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. http://dx.doi.org/10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Hall M, Krakow B, Shear KM, Buysse DJ. A brief sleep scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19(2):233–244. doi: 10.1016/j.janxdis.2004.02.001. http://dx.doi.org/10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Germain A, Richardson R, Moul DE, Mammen O, Haas G, Forman SD, Rode N, Begley A, Nofzinger EA. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72(2):89–96. doi: 10.1016/j.jpsychores.2011.11.010. http://dx.doi.org/10.1016/j.jpsychores.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. Post-traumatic stress disorder: New directions in pharmacotherapy. Advances in Psychiatric Treatment. 2013;19:181–190. http://dx.doi.org/10.1192/apt.bp.111.010041. [Google Scholar]
- Gutner CA, Casement MD, Stavitsky GK, Resick PA. Change in sleep symptoms across Cognitive Processing Therapy and Prolonged Exposure: A longitudinal perspective. Behav Res Ther. 2013;51(12):817–822. doi: 10.1016/j.brat.2013.09.008. http://dx.doi.org/10.1016/j.brat.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. http://dx.doi.org/10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. http://dx.doi.org/10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148–157. doi: 10.1097/00004691-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF, editors. The AASM manual for the scoring of sleep and associated events: rules, terminology, and specifications. 1st. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Insana SP, Hall M, Buysse DJ, Germain A. Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26(2):192–200. doi: 10.1002/jts.21793. http://dx.doi.org/10.1002/jts.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Stanislow C, Wang P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. http://dx.doi.org/10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Toward Precision Medicine: Building a Knowledge Network or Biomedical Research and a New Taxonomy of Disease. Washington, DC: National Acadamies Press; 2011. [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Treatment for posttraumatic stress disorder in military and veteran populations: Final Assessment. Washington, DC: National Academies Press; 2014. [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1(1):53–55. http://dx.doi.org/10.1037/1040-3590.1.1.53. [Google Scholar]
- Krakow B, Hollifield M, Schrader R, Koss M, Tandberg D, Lauriello J, McBride L, Warner TD, Cheng D, Edmond T, Kellner R. A controlled study of imagery rehearsal for chronic nightmares in sexual assault survivors with PTSD: a preliminary report. J Trauma Stress. 2000;13(4):589–609. doi: 10.1023/A:1007854015481. http://dx.doi.org/10.1023/a:1007854015481. [DOI] [PubMed] [Google Scholar]
- Krakow B, Johnston L, Melendrez D, Hollifield M, Warner TD, Chavez-Kennedy D, Herlan MJ. An open-label trial of evidence-based cognitive behavior therapy for nightmares and insomnia in crime victims with PTSD. Am J Psychiatry. 2001;158(12):2043–2047. doi: 10.1176/appi.ajp.158.12.2043. http://dx.doi.org/10.1176/appi.ajp.158.12.2043. [DOI] [PubMed] [Google Scholar]
- McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Military Medicine. 2010;175(10):759–762. doi: 10.7205/milmed-d-10-00193. http://dx.doi.org/10.7205/MILMED-D-10-00193. [DOI] [PubMed] [Google Scholar]
- Mysliwiec V, O'Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and ERM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143–1148. doi: 10.5664/jcsm.4120. http://dx.doi.org/10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, Logue MW, Geyer MA, Risbrough VB, O'Connor DT, Baker DG. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. http://dx.doi.org/10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Comp Psychiatry. 2000;41(6):469–478. doi: 10.1053/comp.2000.16568. http://dx.doi.org/10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Campbell CE, Possemato K, Ouimette P. Longitudinal relationships of insomnia, nightmares, and PTSD severity in recent combat veterans. J Psychosoma Res. 2103;75(6):546–550. doi: 10.1016/j.jpsychores.2013.09.004. http://dx.doi.org/10.1016/j.jpsychores.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Pratt EM, Brief DJ, Keane TM. Recent advances in psychological assessment of adults with posttraumatic stress disorder. In: Follette VM, Ruzek JI, editors. Cognitive-behavioral therapies for trauma. 2nd. New York, NY: Guilford; 2006. pp. 34–61. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Joff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. http://dx.doi.org/10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP, Rohde K, O'Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003–1010. doi: 10.1176/appi.ajp.2013.12081133. http://dx.doi.org/10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Resick PA, Schnicke MK. Cognitive processing therapy for rape victims: A treatment manual. Newbury Park, CA: Sage Publications, Inc; 1993. [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. http://dx.doi.org/10.1037/0022-006X.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon MK, Deblinger E, Steer RA. PTSD symptom cluster profiles of youth who have experienced sexual or physical abuse. Child Abuse Negl. 2014;38(1):84–90. doi: 10.1016/j.chiabu.2013.08.015. http://dx.doi.org/10.1016/j.chiabu.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Seelig AD, Jacobson IG, Smith B, Hooper TI, Boyko EJ, Gackstetter GD, Gehrman P, Macera CA, Smith TC. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33(12):1615–1622. doi: 10.1093/sleep/33.12.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms LJ, Watson D, Doebbeling BN. Confirmatory factor analyses of posttraumatic stress symptoms in deployed and nondeployed veterans of the Gulf War. J Abnorm Psychol. 2002;111(4):637–647. doi: 10.1037//0021-843x.111.4.637. http://dx.doi.org/10.1037//0021-843X.111.4.637. [DOI] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Perlis ML, Posner DA, Weiss B, Ruoff L, Varbel J, Neylan TC. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. http://dx.doi.org/10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614–623. doi: 10.1001/archgenpsychiatry.2010.54. http://dx.doi.org/10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Tylee DS, Chandler SD, Nievergelt CM, Liu X, Pazol J, Woelk CH, Lohr JB, Kremen WS, Baker DG, Glatt SJ, Tsuang MT Marine Resiliency Study Investigators. Psychoneuroendocrinology. 2015;51:472–494. doi: 10.1016/j.psyneuen.2014.09.024. http://dx.doi.org/10.1016/j.psyneuen.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer CS, Edinger JD, Calhoun PS. A multi-component cognitive-behavioral intervention for sleep disturbance in veterans with PTSD: a pilot study. J Clin Sleep Med. 2011;7(1):57–68. [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. http://dx.doi.org/10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD checklist: reliability, validity, & diagnostic utility; Paper presented at the Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. Oct, [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment. 1999;11:124–133. http://dx.doi.org/10.1037/1040-3590.11.2.124. [Google Scholar]
- Weiss DS, Marmar CR, Schlenger WE, Fairbank JA, Jordan BK, Hough RL, Kulka RA. The prevalence of lifetime and partial post-traumatic stress disorder in vietnam theater veterans. J Trauma Stress. 1992;5(3):365–376. http://dx.doi.org/10.1002/jts.2490050304. [Google Scholar]
- Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as a predictor versus outcome of PTSD and depression among Iraq combat vetearns. J Clin Psychol. 2011;67(12):1240–1258. doi: 10.1002/jclp.20845. http://dx.doi.org/10.1002/jclp.20845. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Neylan TC, Flory JD, McFarlane AC. The use of biomarkers in the military: from theory to practice. Psychoneruoendoctrinology. 2013;38(9):1912–1922. doi: 10.1016/j.psyneuen.2013.06.009. http://dx.doi.org/10.1016/j.psyneuen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Pratchett LC, Elmes MW, Lehrner A, Daskalakis NP, Koch E, Makotkine I, Flory JD, Bierer LM. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus. 2014;4(5) doi: 10.1098/rsfs.2014.0048. http://dx.doi.org/10.1098/rsfs.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17(1):69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. http://dx.doi.org/10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]


