Abstract
Bilinguals’ ability to control which language they speak and to switch between languages may rely on neurocognitive mechanisms shared with non-linguistic task switching. However, recent studies also reveal some limitations on the extent control mechanisms are shared across domains, introducing the possibility that some control mechanisms are unique to language. We investigated this hypothesis by directly comparing the neural correlates of task switching and language switching. Nineteen Spanish-English bilingual university students underwent a functional magnetic resonance imaging (fMRI) study employing a hybrid (event-related and blocked) design involving both color-shape switching and language switching paradigms. We compared the two switching tasks using within-subject voxel-wise t-tests for each of three trial types (single trials in single blocks, and stay and switch trials in mixed blocks). Comparing trial types to baseline in each task revealed widespread activation for single, stay, and switch trials in both color-shape and language switching. Direct comparisons of each task for each trial type revealed few differences between tasks on single and switch trials, but large task differences during stay trials, with more widespread activation for the non-linguistic than for the language task. Our results confirm previous suggestions of shared mechanisms of switching across domains, but also reveal bilinguals have greater efficiency for sustaining the inhibition of the non-target language than the non-target task when two responses are available. This efficiency of language control might arise from bilinguals’ need to control interference from the non-target language specifically when not switching languages, when speaking in single- or mixed-language contexts.
Keywords: bilingualism, fMRI, linguistic control, executive control, task switching
1. Introduction
During natural conversation, bilinguals appear to effortlessly manage activation of two languages, fluently switching between them at will without any obvious difficulty. A current debate in the literature on bilingualism concerns how bilinguals manage to accomplish this skill so seamlessly. Some general consensus has emerged that bilinguals recruit at least some non-linguistic mechanisms of executive control to manage dual-language activation and language switching (Abutalebi & Green, 2007; Gollan & Ferreira, 2009; for review, Bialystok, Craik, Green, & Gollan, 2009; Hernandez, 2009), but the extent to which non-linguistic mechanisms of executive control are used remains uncertain. While some results suggest completely overlapping control mechanisms across domains, others suggest at least partially unique and specialized mechanisms for language control (e.g., Abutalebi et al., 2008; Prior & Gollan, 2013; Weissberger, Wierenga, Bondi, & Gollan, 2012). The goal of the present study was to investigate this question by comparing the neural underpinnings of linguistic and non-linguistic control using functional magnetic resonance imaging (fMRI) techniques with an eye towards identifying differences between domains that might signal the existence of specialized language control mechanisms.
Evidence that non-linguistic executive control mechanisms are recruited to achieve bilingual language control comes from a growing literature documenting bilingual advantages on non-linguistic tasks of executive function (for review see Bialystok et al., 2009; though recent literature reviews have called this into question, see Paap, 2014). For example, bilinguals exhibited reduced Stroop interference effects (e.g., Bialystok, Craik, & Luk, 2008) and were faster to resolve response conflict in the Simon Task (Bialystok et al., 2004) and the Attentional Network Task (Costa, Hernández, Costa-Faidella, & Sebastián-Gallés, 2009; Costa, Hernández, & Sebastián-Gallés, 2008) when compared to matched monolinguals.
A topic of particular interest in investigating bilingual language control, and the possible role of executive control, is switching. Non-linguistic task switching is more transparently linked to bilingual language switching than other tasks that have shown bilingual advantages (e.g., Flanker Task). Supporting the notion of a shared “switch mechanism”, studies of bilingual switching ability reveal explicit relationships between task switching and language switching (e.g., Prior & Gollan, 2011; Weissberger et al., 2012). In switching paradigms, there are single task blocks (in which participants perform just one task), and mixed task blocks (in which participants are cued to switch between tasks from trial to trial). Within mixed blocks, there are trials in which the individual is cued to switch tasks (switch trial) and trials in which the individual is cued to perform the same task as the preceding trial (stay trial). Studies implementing these paradigms report robust switching costs (comparing switch to stay trials) and mixing costs (comparing stay trials to single-task trials) for both linguistic and non-linguistic tasks (Chrisoffels, Firk, & Schiller, 2007; Meuter & Allport, 1999; Rubin & Meiran, 2005), and studies comparing bilinguals to monolinguals on non-linguistic task switching have found a significant reduction in switching, but not mixing costs, for bilinguals (e.g., Prior & Gollan, 2011; Prior & MacWhinney, 2010).
Follow-up studies directly comparing task to language switching suggest some overlap but also some differences in control mechanisms across linguistic and non-linguistic domains (Calabria, Hernández, Branzi, & Costa, 2012; Prior & Gollan, 2011; Weissberger et al., 2012). For example, Weissberger et al. (2012) found both similarities and differences between tasks by examining aging effects across the two tasks. Suggestive of shared control mechanisms, a subset of older bilinguals was unable to complete the task switching paradigm at better than chance levels of performance, and these same bilinguals also exhibited greater switching costs during the language switching paradigm than matched controls. However, in support of unique control mechanisms, there was a crossover interaction between age and task such that aging effects appeared to be far greater for non-linguistic than for linguistic switching; whereas young bilinguals responded more slowly on language than on task switching, the reverse was true for older bilinguals.
Another source of evidence comes from fMRI studies, which suggest shared mechanisms between non-linguistic and linguistic control. Such studies reveal apparent overlapping neural circuits responsible for both types of control, though direct comparisons are limited. For example, imaging studies of monolinguals performing task switching, Stroop, and Simon tasks show activation in the dorsal executive system including the prefrontal cortex (PFC), dorsolateral PFC, anterior PFC, parietal cortex, the anterior cingulate cortex (ACC), and the left caudate nucleus (e.g., Braver, et al., 2003; Corbetta & Shulman, 2002; Digirolamo et al., 2001; Dosenbach et al., 2006; Fan, Flombaum, McCandliss, Thomas, & Posner, 2003; Hyafil, Summerfield, & Koechlin, 2009; Jimura & Braver, 2009; Liu et al., 2004; Lungu et al., 2007; MacDonald et al., 2000; Peterson et al., 2002; Sohn et al., 2000). Imaging studies of bilingual language switching reveal activation in similar brain regions (Abutalebi & Green, 2007; Abutalebi et al., 2012; Crinion et al., 2006; Hernandez, 2009; Hernandez, Dapretto, Mazziotta, & Bookheimer, 2001; Hernandez, Martinez, & Kohnert, 2000; Hosoda et al., 2012; Rodriguez-Fornells et al., 2005; Wang, Wang, Jiang, Wang, & Wu, 2009; Wang, Xue, Chen, Xue, & Dong, 2007; for review, see Hervais-Adelman, Moser-Mercer, & Golestani, 2011) and support the notion that linguistic control is accomplished through general mechanisms of executive control.
To date, few imaging studies have examined language control and non-linguistic executive control within the same study and the few studies that do this have evidence for both shared and unique mechanisms of control (Abutalebi et al., 2008; Abutalebi et al., 2012; Hosoda et al., 2012). For example, Abutalebi and colleagues (2012) reported evidence for shared mechanisms of control across domains using a flanker task to measure non-linguistic control and a picture-naming task to measure linguistic control. A conjunction analysis that extracted regions of overlap between the two tasks revealed the anterior cingulate cortex to be active in both tasks. However, Abutalebi et al. (2012) also found greater activation during the flanker task in certain regions that were not recruited during the language task, suggesting the presence of some unique control mechanisms for switching across domains. Another study that compared bilingual performance on a within-language task (name the picture or a related verb in the same language) and a between-language task (name pictures in either language) revealed the left caudate and anterior cingulate cortex were active during the between-language paradigm, but not during the within-language paradigm, suggesting these regions were recruited to manage between-language competition (Abutalebi et al., 2008). However, follow-up studies reported anterior cingulate and left caudate involvement during non-linguistic executive control tasks (e.g., Abutalebi et al., 2012; Wang, Wang, Jiang, Wang, & Wu, 2012) and raise questions regarding the degree to which these regions are domain-specific.
Thus, to date, there is both behavioral and neuroimaging evidence supporting the proposal of shared mechanisms for linguistic and non-linguistic control as well as evidence supporting at least partially non-overlapping control mechanisms across domains. However, methodological differences across studies, and sometimes tasks used within the same study, leave a critical gap in this comparison and may account for the contrasting findings in the literature. The present study aimed to close this gap by comparing linguistic and non-linguistic task switching in the same group of bilinguals while minimizing methodological differences between tasks, with the goal of identifying both similarities and differences in control mechanisms across domains.
Following Prior and Gollan (2011; 2013) we tested bilinguals on color-shape switching and language switching paradigms behaviorally and in the scanner to investigate neural mechanisms underlying single, stay, and switch trial-types. Imaging studies that have investigated language switching in bilinguals have not decomposed single, stay, and switch trial-types in the way that is commonly done in the behavioral literature. This is due to the limitation of using either a blocked design that only allows for between block comparisons (non-mixed block vs. mixed block) or an event-related design that eliminates single blocks and allows for only between-trial comparisons in a mixed block (stay trials vs. switch trials). Thus, to decompose the regions of activation associated with switch and stay trials of mixed blocks, and single trials within single blocks, we implemented a hybrid event-related and blocked fMRI design (see Braver et al., 2003 for use of this design with task switching) that more closely mirrors the design in behavioral studies. We opted to focus on between-task comparisons separately for each trial type, rather than comparing tasks on switch and mix costs as is typically done for response time data, since it is not clear what the neural instantiation of mix and switch costs really reflect. Decomposing trial-types provides greater transparency of the neural substrates underlying components of language and task switching. Given previously reported bilingual advantages in switching and other connections between language and task switching (Prior & MacWhinney, 2010; Prior & Gollan, 2011; Weissberger et al., 2012), we predicted that differences between tasks would be smallest on switch trials and relatively greater on stay trials within the mixed task block. Alternatively, similarities and differences between tasks might be found on both switch and stay trials, given reported associations between tasks in the size of mixing costs (Prior & Gollan, 2013), and limitations on the extent to which bilinguals are advantaged in switching per se (e.g., Hernandez, Martin, Barceló, & Costa, 2013; Paap, 2014; Paap & Greenberg, 2013; Prior & Gollan, 2011). For single task blocks, we expected to observe considerable similarities given the absence of switching and requirement of covert naming in both tasks, and given the relatively high level of proficiency for producing the relatively easy names needed to complete the language task even when responding in their non-dominant language (Klein, Milner, Zatorre, Meyer, & Evans, 1995). Any differences observed between tasks in single task blocks might be due to differences in the stimuli (colored circles and triangles vs. Arabic numerals) and specific names required to complete colors/shape vs. number naming tasks.
2. Methods
2.1 Participants
Twenty-one college-aged English-Spanish bilinguals (15 women; M age = 21; SD = 1.7) participated in the study for monetary compensation (with the exception of one student who received course credit for participation). Participants were recruited through a study on voluntary language switching at the University of California, San Diego (UCSD) and were classified as bilinguals based on their ability to name pictures in both languages (see Table 1). Most participants (n=14) were born in the United States; five were born in Mexico, one in Spain, and one in Peru. All participants were strongly right handed (Oldfield, 1971). Participants were excluded if they had metal in their body other than dental fillings, and female participants were excluded if they were pregnant or trying to become pregnant. At the end of the imaging procedures, one participant was excluded due to a structural brain abnormality, and a second participant only completed the language switching task due to discomfort in the scanner1. Thus, 20 participants were included in the language switching analysis, and 19 participants were included in the task switching analysis. Participant characteristics for the final group of participants included in fMRI analyses are presented in Table 1.
Table 1.
Participant characteristics for the 20 participants included in the fMRI analyses. Language history information and picture naming data were obtained from the language switching study from which participants were recruited.
| M | SD | |
|---|---|---|
| Age | 20.45 | (1.9) |
| Education | 14.3 | (1.7) |
| % Female | 75 | - |
| % Right Handed | 100 | - |
| % English Dominant/%Balanced/%Spanish Dominant based on self-report | 70/15/15 | - |
| Age 1st Exposure to English | 5.1 | (3.5) |
| Age 1st Exposure to Spanish | 0.3 | (0.5) |
| % of Current Spanish Use | 28 | (18.0) |
| % of Childhood Spanish Use | 49.6 | (18.4) |
| How often speak to bilinguals currentlya | 4.9 | (1.7) |
| How often speak to bilinguals growing upa | 6.4 | (1.3) |
| English Speakb | 6.5 | (0.8) |
| English Listenb | 6.3 | (1.6) |
| English Readb | 6.4 | (0.8) |
| English Writeb | 6.2 | (0.9) |
| Spanish Speakb | 6.0 | (1.2) |
| Spanish Listenb | 6.4 | (0.9) |
| Spanish Readb | 5.7 | (0.8) |
| Spanish Writeb | 5.5 | (1.1) |
| % English Dominant based on picture naming reaction time performancec | 90% | - |
| % English Dominant based on picture naming error ratesc | 90% | - |
| Tendency to switch languages when speaking to bilingualsd | 3.47 | (1.3) |
The following 7-point scale was used: 1 “rarely or never”, 2 “less than 1 hr/day”, 3 “about 1 hr/day”, 4 “about 2 hr/day”, 5 “about 3–4 hr/day”, 6 “about 5 hr/day”, and 7 “6 or more hr/day”.
Self-ratings were based on a 7-point scale: 1 “almost none”, 2 “very poor”, 3 “fair”, 4 “functional”, 5 “good”, 6 “very good”, and 7 “like native speaker”.
Participants named pictures in English only and in Spanish only in the language switching study they were recruited from. Those with faster reaction times (RTs) in English and less error rates were considered to be English dominant. The same two subjects who were Spanish dominant based on RTs were also Spanish dominant based on error rates.
Participants filled out a questionnaire asking them to report their tendency to switch between English and Spanish in conversations with other bilinguals. The following 6-point scale was used: 1 “never”, 2 “very infrequently”, 3 “occasionally”, 4 “frequently”, 5 “almost constantly”, and 6 “constantly”.
2.2 Experimental Design and Behavioral Task
Participants completed consent procedures, were screened for MRI safety, and were then tested on the behavioral version of the color-shape and language switching paradigms on a laptop computer. Practice with the behavioral versions of both tasks was administered prior to scanning to familiarize participants with the switching procedures and to minimize superficial differences between tasks related to prior experience with language switching (which would benefit little from practice relative to task switching; Prior & Gollan, 2013). In brief, the language switching task required participants to name numbers 1–9 in either English or Spanish based on a cue; the color-shape task required participants to name the shape or color of a figure based on a cue. Participants were given the option of completing the color-shape task in either English or Spanish. All participants chose to complete both tasks in English. Prior to beginning the behavioral task, participants completed practice trials with feedback (12 single and 16 mixed responses in each task). All participants successfully completed the practice trials on the first attempt with over 80% accuracy.
Specific parameters for the behavioral version of the paradigm were almost identical to those described in Weissberger et al. (2012). Specifically, in the language task, participants named numbers in English or Spanish based on a cue (American flag for English, and Mexican flag for Spanish). Each trial was preceded by a fixation point that lasted for 500 ms. The fixation point was replaced by a cue that appeared on the screen throughout the remainder of the trial, after which the stimuli appeared alongside the cue. After a delay of 750 ms, another fixation point appeared for 1750 ms, after which it was replaced by the stimulus. The cue and stimuli remained on the screen until the subject responded or 2000 ms passed. At the end of each 20-trial block, a fixation point appeared on the screen for a prolonged period (5 s). Preceding each run, the participant was presented with brief instructions prompting them to “Press any button to begin”. Stimuli were presented using a 17-inch MacBook Pro laptop through PsyScope X (Cohen, MacWhinney, Flatt, & Provost, 1993) software. Naming times were recorded using headset microphones connected to a PsyScope response box. Responses were also tape-recorded with permission of the participants and responses were later coded for accuracy off-line.
We made one modification to the task implemented by Weissberger et al. (2012). Specifically, participants responded verbally, stating the color (red or green) or shape (circle or triangle) of the figure in the color-shape task instead of using button presses. This modification was made to equate methodological implementations across domains by minimizing differences related to mode of response (i.e., to avoid the confound of language production response in language switching and motor response in task switching) that might obscure similarities in switching mechanisms across domains. Although this modification reduced the extent to which the color-shape task is strictly “non-linguistic”, the relevant distinction between tasks was still maintained given that the color-shape task is clearly not a bilingual switching task. Thus, if there are specific mechanisms related to switching between languages (our main question of interest), they will still be captured in our comparison of the two tasks. In both tasks, responses were univalent, involving unique (non-overlapping) sets of responses for each task (“red” or “green” for color; “circle” or “triangle” for shape; or numbers 1–9 in English or Spanish). We also ensured that timing parameters of the two tasks were kept constant, and both tasks employed cues that were conceptually transparent and compatible in shape and size. In addition, the likelihood of each trial-type (single, stay, switch) was equated across tasks and each stimulus was equally likely to appear with each cue.
2.3 fMRI Functional Specifications
We used a hybrid design including both event-related and blocked manipulations in order to enable decomposition of single trials in single blocks, stay trials in mixed blocks, and switch trials in mixed blocks (Braver et al., 2003). A limitation of the hybrid design is that overt naming is challenging due to associated task-related motion artifact. Thus, unlike the pre-scan behavioral version of the task (in which bilinguals overtly spoke their responses), bilinguals were instructed to covertly name each stimulus while also pressing a button to indicate that they had responded using a fiber-optic button box designed for use in the magnet. Other modifications made for fMRI compatibility included displaying the stimulus on the screen for 2 seconds, rather than removing it with the bilingual’s response, and varying the intertrial intervals pseudorandomly between 500 ms and 5500 ms to mitigate the effects of periodic or quasi-periodic physiological noise and to allow the hemodynamic response to return to baseline before the subsequent trial was presented.
As in the behavioral version and based on Rubin and Meiran (2005), we used a sandwich design such that participants completed two single-task blocks and four mixed-task blocks, followed by two more single-task blocks in each task (e.g., color-shape and language). Each scan run was composed of two blocks (single-single, or mixed-mixed) and spanned a total of 6 minutes and 42.5 seconds, for a total of 4 runs per task. 161 functional images were acquired during each run. A 37.5 second fixation block preceded and followed each block to allow the hemodynamic response to return to baseline for block comparisons. Each trial was preceded by a fixation point that lasted for 500 ms to 5500 ms based on the intertrial interval. The fixation point was replaced by a cue that appeared on the screen throughout the remainder of the trial. After 750 ms with just a cue on the screen, a fixation point appeared in the place of the upcoming stimulus and remained for a 1750 ms delay. After the delay, the stimulus appeared on the screen alongside the cue for 2000 ms, after which both were replaced by the inter-trial fixation point that preceded the next stimulus presentation. For a diagram of the order of events, see Figure 1.
Figure 1.
Order of events and computer screen display for the color-shape task (top panel) and language switching task (bottom panel). Images appeared in color during actual task.
Within each task, the order of English versus Spanish and color versus shape was counterbalanced within subjects for the single-task blocks (the counterbalancing order used in the scanner task was the same order as that used in the pre-scan behavioral version); and between subjects with respect to which response type was first or second. Furthermore, order of stimuli presentation (e.g., numbers 1–9, or red/green circle or triangle) was pseudo-randomized; after randomization, we ensured that no numbers were repeated more than twice in succession.
Stimuli were presented with the same equipment (i.e., lap top, software) used in the behavioral design and presented via an LCD projector onto a screen situated at the end of the scanner bed. The participants viewed the stimuli through a mirror mounted on the head cage. An example of the display in the two tasks is shown in Figure 1.
2.4 Image Acquisition
Participants were scanned on a 3.0 Tesla General Electric Medical Systems EXCITE whole body imager with an 8-channel receive-only head coil at UCSD. Head movement was constrained with padding and tape to secure head position. A quick localizer scan was acquired initially to allow selection of the block of slices to be acquired during functional scanning and to assure good head placement in the scanner. Functional BOLD was obtained with a 1-shot gradient echo EPI scan (24 cm FOV, 64 × 64 matrix, 3.75 mm × 3.75 mm in-plane resolution, TR= 2500 msec, TE= 30 msec, flip angle=70 degrees). Forty-one 4 mm thick sagittal slices covering the whole brain were acquired. Two field maps were collected to correct for distortions in EPI images due to susceptibility artifact. A structural MRI sequence included a high resolution T1-weighted Fast Spoiled Gradient Recall (3D FSPGR) scan to provide anatomic reference (172 1 mm contiguous sagittal slices, FOV = 25 cm, TR = 8 ms, TE = 3.1 ms, flip angle = 12, T1 = 600, 256 × 192 matrix, Bandwidth = 31.25 kHZ, frequency direction = S-I, NEX =1, scan time = 8 min 13 sec).
2.5 Data Analysis
2.5.1 Behavioral data
Mean reaction time (RT) for single, stay, and switch trials were calculated to investigate differences between color-shape and language switching paradigms. We also calculated mix costs (RTs of stay trials in mixed block minus RTs of single trials in single block), and switch costs (RTs of switch trials in mixed block minus RTs of stay trials in mixed block) as previously reported for these tasks (e.g., Prior & Gollan, 2011). Error rates were also calculated to ensure that each bilingual successfully completed the tasks. Paired sample t-tests were also used to compare language and color-shape single, stay, and switch RTs, mixing and switching costs, and error rates. RTs for incorrect responses were excluded from analyses and outlier RTs were trimmed for individual participants by calculating mean RT across all trials and excluding any response deviating by more than 3 standard deviations of their mean.
2.5.2 Imaging Analyses
fMRI data were analyzed and overlaid onto structural images with the Analysis of Functional Neuroimaging (AFNI) program from the National Institutes of Health (Cox, 1996). To minimize the effects of head motion, each participant’s functional time series was corrected for motion using a three-dimensional iterated, linearized, weighted least-squares method with Fourier interpolation, and time-points with uncorrected motion outliers were excluded from statistical analysis. Images were visually inspected for gross artifacts and quality control procedures were applied to the data to detect residual motion or susceptibility artifact. Slice timing correction was applied and the eight imaging runs were detrended of low frequency signal drifts (Birn et al., 2011).
Statistical analyses were performed using a general linear model (GLM), with individual events (single, stay, switch trials) modeled using AFNI’s 3dDeconvolve TENT function on each participant’s time-series for each task (language and color-shape). The following predictors were used in each model: a constant, a linear trend, three parameters indicating the degree of motion correction performed in three rotational angles, and 3 stimulus vectors indicating the onset of event-related cues to 1) switch trials in mixed blocks, 2) stay trials in mixed blocks, and 3) single trials in single blocks, to model the hemodynamic response for each trial type (item effects). Functional data were scaled to percent signal change (PSC) and spatially smoothed with a Gaussian kernel of 4mm full-width at half-maximum. The T1-weighted anatomic images and the functional activation maps were warped to the coordinates of the co-planar stereotactic atlas of Talairach & Tournoux (Talairach, 1988) and resampled at a 4mm3 resolution.
Within group comparisons of each trial-type (single, stay, switch) to a baseline were conducted for each task (color-shape, language) using voxel-wise student’s t-tests with percent signal change as the dependent variable. To guard against false-positives, Monte-Carlo simulations using 3dClustSim indicated that clusters larger than 4 contiguous voxels (256 mm3) at a threshold of p<0.05 (with a peak voxel of p<0.001) were considered significant. To directly investigate the relationship between language and color-shape switching, we conducted three additional within-group t-tests comparing each task on each of the three trial-types (single, stay, and switch), using the same cluster threshold and single-voxel p-value (.001) as with the baseline comparisons.
3. Results
3.1. Behavioral Results
We report behavioral results for all participants included in the fMRI analyses, with the exception of one participant whose reaction time data were unavailable due to equipment malfunction. This participant was able to complete both tasks with fewer than 3% errors.
Mean RTs and error rates for single, stay, and switch trials, as well as mix and switch costs for each task are reported in Table 2. Both tasks elicited mix and switch costs (all ps ≤ .006). RTs for single and stay trials did not differ between tasks (both ps≥.37), but RTs for switch trials were marginally longer for language than color-shape (t(18) = −1.28; MSE = 18.32; p=.08). Color-shape and language switching did not differ in the magnitude of mix costs (p=.15) and were marginally different in magnitude of switch costs, in the direction of larger switch costs for the language task (t(18) = −1.72; MSE = 13.13; p=.10).2
Table 2.
Means and standard deviations of Reaction Times (RTs) and Number of Errors for the color-shape and language behavioral tasks (N=19).
| Color-shape | Language | |||||||
|---|---|---|---|---|---|---|---|---|
| RTs | Errorsa | RTs | Errorsa | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Single | 642 | (142) | 0.1 | (0.3) | 629 | (105) | 0.2 | (0.4) |
| Stay | 697 | (161) | 0.5 | (0.8) | 708 | (161) | 0.5 | (0.7) |
| Switch | 729 | (185) | 1.2 | (1.3) | 763 | (182) | 1.2 | (1.8) |
| Mix Costs | 55 | (63) | - | - | 79 | (74) | - | - |
| Switch Costs | 32 | (46) | - | - | 55 | (44) | - | - |
Error rates can be calculated by dividing number of errors reported in table by number of single, stay, and switch trials; for color-shape: total single trials = 80; total stay trials = 39; total switch trials = 41; for Language: total single trials = 80; total stay trials = 40; total switch trials = 40.
All participants produced ≤6.3% errors across both tasks; thus, no one was excluded from fMRI analyses on the basis of behavioral performance. Breaking down error rates by trial type, switch trials had the highest number of errors on average (3.2%) in both language and color-shape tasks (M = 1.2, SD = 1.8 and M = 1.2, SD = 1.3, respectively) compared to single trials (0.2%; M = .2, SD = 0.4 and M = 0.1, SD = 0.3, respectively) and stay trials (1.2%: M = 0.5, SD = 0.7 and M = 0.5, SD = 0.8, respectively). Errors made on color-shape and language switching did not differ across all three trial-types (all ps ≥ .43; see Table 2).
3.2 fMRI Results
Performance during the scan was monitored by button presses. All participants indicated their response using the button box and none had to be reminded to do so during their scan session.
3.2.1. Single, Stay, and Switch Relative to Baseline
Comparison of each trial type (single, stay, and switch) to baseline for both language and color-shape tasks revealed widespread activation in multiple brain regions including bilateral frontal, sensory/motor, parietal, temporal, occipital, cingulate, insular, and subcortical regions, including regions of the basal ganglia (all corrected ps ≤ .001; volume threshold = 256 mm3; see Supplementary Tables 1–3; see Figures 2, 3, and 4 for display of single, stay, and switch images). Visual examination of these images revealed many regions of overlap between language and color-shape on single and switch trials. In contrast, whereas color-shape stay trials elicited one large cluster, language stay trials revealed 26 smaller and less significant clusters (see Figure 3).
Figure 2.

Within group comparisons of single trials to baseline for color-shape and language switching (R = right, L = left, P = posterior, S = superior; C = cluster ≥ 256 mm3). Color bars refer to p-values for individual voxels.
Figure 3.

Within group comparisons of stay trials to baseline for color-shape and language switching (R = right, L = left, P = posterior, S = superior; C = cluster ≥ 256 mm3). Color bars refer to p-values for individual voxels.
Figure 4.

Within group comparisons of switch trials to baseline for color-shape and language switching (R = right, L = left, A = anterior, P = posterior, S = superior, I = inferior; C = cluster ≥ 256 mm3). Color bars refer to p-values for individual voxels.
3.2.2. Task Comparisons
To identify significant differences between the tasks across single, stay, and switch trials we directly compared language and color-shape task using three voxel-wise paired sample t-tests, one for each trial-type. We initially used a corrected individual p-value of .001. However, doing so revealed no significant clusters of activation for single and switch trials, because at this statistical threshold level, only the comparison of language stay to color-shape stay revealed differences (13 significant clusters of activation showed greater response for color-shape versus language stay trials). Therefore, to enable consideration of possible differences between tasks on all three trial-types, we raised the individual p-value threshold to .025. The predetermined cluster size in this case was 17 contiguous voxels (i.e., 1088 mm3), which also protected for a whole-brain p-value of .05.
The comparison of language to color-shape single and switch trials revealed few differences between the two tasks (see Figure 5 and Table 3). Specifically, brain response was greater for language than color-shape for single trials in the right and left thalamus, parahippocampal gyrus, and posterior cingulate gyrus. For switch trials, brain response was greater for language than color-shape in the thalamus, right caudate, and cingulate gyrus. There were no regions in which brain response was greater for color-shape than language during single and switch trials. By contrast, the comparison of tasks on stay trials revealed greater brain response for color-shape vs. language in multiple widespread brain regions including frontal, parietal, temporal, occipital, cingulate, insular, and subcortical areas (all ps ≤ .025; volume threshold ≤1088 mm3; see Figure 5 and Table 3). There were no regions in which brain response was greater for language than color-shape during stay trials3. This is the opposite pattern reported for single and switch trials). Thus, in relative terms, and interpreting greater activation as an indication of greater effort required (e.g., recruitment of additional neural resources in order to perform the task at a comparable level; Petersen et al., 1998), the language task was more demanding on single and switch trials, whereas the color-shape task was more demanding on stay trials.
Figure 5.
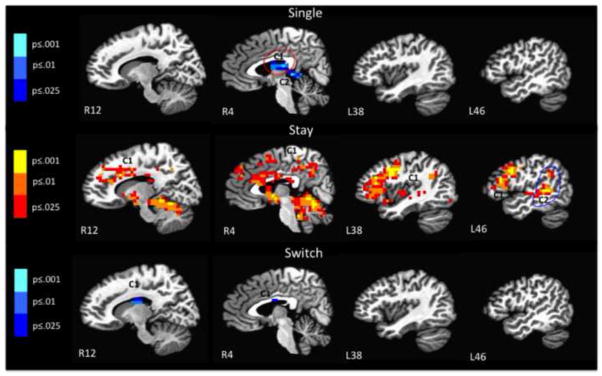
Within group comparisons of color-shape single to language single, color-shape stay to language stay, and color-shape switch to language switch (R = right, L= left; C = cluster ≥ 1088 mm3); Blue = greater activation for language than color-shape; Red = greater activation for color-shape than language. Color bars refer to p-values for individual voxels.
Table 3.
Brain areas showing significant differences in activation between language and color-shape during single, stay, and switch trials (BA = Brodmann’s Area; R = right, L = left, C = cluster ≥ 256 mm3, PSC = Percent Signal Change).
| Single: language > color-shape | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical Location | BA/subregion | Volume (mm3) | Center (x,y,z) | T-value at Peak Intensity | PSC Lang | PSC Task | ||||
| C1 | Focus Point | Right Thalamus | - | 1600 | R2 | P21 | S16 | −4.10 | 1.72 | 1.08 |
| Right Medial Dorsal Nucleus | - | |||||||||
| Left Thalamus and Medial Dorsal Nucleus | - | |||||||||
|
| ||||||||||
| C2 | Focus Point | Right Thalamus | - | 1536 | R2 | P33 | S4 | −4.34 | 2.31 | 1.46 |
| Left Thalamus | - | |||||||||
| Parahippocampal Gyrus | - | |||||||||
| Posterior Cingulate Gyrus | - | |||||||||
| Stay: color-shape > language | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical Location | BA/subregion | Volume (mm3) | Center (x,y,z) | T-value at Peak Intensity | PSC Lang | PSC Task | ||||
| C1 | Focus Point | Left Precentral Gyrus | 155520 | L38 | A7 | S36 | 5.71 | 0.22 | 0.81 | |
| Focus Point | Left Precentral Gyrus | L BA 6, 9, 44 | ||||||||
| Fronal Regions | Inferior, Middle, Medial, Superior Frontal Gyrus | R & L BA 6, 8, 9, 10, 13, 32, 45, 46, 47; L BA 13, 44 | ||||||||
| Paracentral Lobule | R & L BA 5, 31 | |||||||||
| Parietal | Precuneus | R & L BA 7, 31 | ||||||||
| Temporal | Superior and Right Middle Temporal Gyrus | R & L BA 13, 22; R BA 21, 38 | ||||||||
| Occipital | Middle Occipital Gyrus | |||||||||
| Left Cuneus Gyrus | ||||||||||
| Left Lingual Gyrus | L BA 17, 18, 19 | |||||||||
| Fusiform Gyrus | R & L BA 37 | |||||||||
| Cingulate | Anterior, Posterior Cingulate Gyrus | R & L BA 23, 24, 25, 31, 32; R BA 33; L BA 30 | ||||||||
| Insular Cortex | Insula | R & L BA 13, 22; R BA 21; L BA 47 | ||||||||
| Subcortical | Parahippocampal Gyrus | R & L BA 19, 30, 35, 36, 37; L BA 28 | ||||||||
| Hippocampus | ||||||||||
| Amygdala | ||||||||||
| Thalamus and Hypothalamus | ||||||||||
| Claustrum | ||||||||||
| Subcallosal Gyrus | R & L BA 34 | |||||||||
| Basal Ganglia | Caudate, Lentiform Nucleus, Putamen, Globus Pallidus | |||||||||
|
| ||||||||||
| C2 | Focus Point | Left Superior Temporal Gyrus | L BA 13, 21, 22, 39 | 4544 | L46 | P37 | S4 | 4.84 | 0.18 | 0.51 |
| Left Middle Temporal Gyrus | L BA 22 | |||||||||
| Temporal | Left Supramarginal Gyrus | L BA 40 | ||||||||
| Left Angular Gyrus | ||||||||||
| Left Inferior Parietal Lobule | L BA 40 | |||||||||
| Subcortical | Left Paraphippocampal Gyrus | |||||||||
| Left Caudate | ||||||||||
| Switch: language > color-shape | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical Location | BA/subregion | Volume (mm3) | Center (x,y,z) | Peak Intensity | PSC Lang | PSC Task | ||||
| C1 | Focus Point | Right Thalamus | - | 1728 | R10 | P13 | S20 | −3.47 | 1.68 | 1.03 |
| Right Caudate | - | |||||||||
| Cingulate Gyrus | - | |||||||||
| Left Thalamus, Left Midline Nucleus, Left Lateral Dorsal Nucleus | - | |||||||||
Clusters shown survived our cluster threshold alpha-protection procedure (clusters: individual voxel p < 0.025, volume > 1088 mm3, whole brain p < 0.05; see text for details).
4. Discussion
Our study aimed to identify regions of overlap as well as differences in the neural mechanisms underlying bilingual linguistic and non-linguistic control. Based on previous behavioral and imaging studies, we predicted that there should be considerable overlap in brain regions underlying language and color-shape task, particularly on switch trials. In some ways, our data confirmed this prediction (e.g., widespread activation for each task and trial type compared to baseline), corroborating recent behavioral studies that reported significant relationships between language and color-shape switching (Prior & MacWhinney, 2010; Prior & Gollan, 2011), and in line with previous neuroimaging research showing that aspects of bilingual language control recruit similar neural substrates as non-linguistic tasks of executive control (e.g., Abutalebi et al., 2012; for review see Hervais-Adelman et al., 2011). However, in other ways, our data also revealed differences across domains, and implied that the precise manifestation of similarities versus differences can vary with trial-type, supporting studies that suggested only partial overlap in mechanisms of control in language and task switching (e.g., Weissberger et al., 2012; Prior & Gollan, 2011).
Imaging data comparing single, stay, and switch trials to baseline revealed widespread activation for both language and color-shape switching tasks across many overlapping regions including cortical (e.g., frontal, temporal, parietal, cingulate) and subcortical (e.g., caudate, thalamus) regions. This widespread activation is consistent with previous task switching studies that report regions of activation that are not only specific to “executive” brain areas, but also include regions that are relevant to the specific tasks at hand (e.g., color/shape naming, number naming; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000). To supplement these findings, we also conducted a post-hoc conjunction analysis to statistically identify the regions of overlap between color-shape and language for each of the three trial-types. This confirmed extensive overlap for single and switch trials but not stay trials (corrected for an individual p-value of .00001). Overall, behavioral data patterned similarly to the baseline comparisons and post-hoc analyses in that there were no significant differences between tasks either in RTs or in error rates on single, stay, and switch trials, and on mixing and switching costs across tasks.
Direct task comparisons for each trial-type reveal more nuanced results than demonstrated by behavioral data, the baseline comparisons, and the post-hoc conjunction analyses alone. Comparing tasks on single and switch trials revealed relatively few differences, with significantly greater brain response for language than for color-shape on single and switch trials in the bilateral thalamus, posterior cingulate, and parahippocampal gyrus (single trials) and in the bilateral thalamus, right caudate, and cingulate gyrus during (switch trials). In contrast, comparing tasks on stay trials revealed greater activation in frontal, parietal, temporal, occipital, cingulate, insular, and subcortical areas for color-shape than language4.
Although there were few differences in activation for switch trials between the tasks, the bilateral thalamus, right caudate, and cingulate gyrus were significantly more active during language switch trials compared to color-shape switch trials (though these regions were involved in both tasks). Neuroimaging studies of language processing have reported the importance of the thalamus for linguistic processing (e.g., Crosson, 1999; Crosson, Zawacki, Brinson, Lu, & Sadek, 1997; Fabbro, Peru, & Skrap, 1997). Many imaging studies have also reported the importance of the caudate and cingulate cortex during tasks of language control (e.g., Abutalebi et al., 2008; Crinion et al., 2006; Hosoda et al., 2012; for meta-analysis, see Luk, Green, Abutalebi, & Grady, 2012). However, most of the language switching studies report activation of the left caudate (e.g., case study by Abutalebi, Miozzo, & Cappa, 2000; Crinion et al., 2006; Hosoda et al., 2012; case study by Wang et al., 2012), whereas we found greater activation for language switch trials in the right caudate (though it should be pointed out that the left caudate was also active during language switch trials as indicated by our baseline comparison). One question that arises is why the right caudate was activated to a greater extent than the left caudate during language switching. Studies of bilingual language lateralization report greater right hemisphere involvement in early (i.e., both languages acquired before the age of 6) bilinguals compared to late bilinguals (see meta-analysis by Hull & Vaid, 2007), and 15 of the 20 bilinguals in our sample were early bilinguals. In addition, one previous study of language switching in second-language learners also reported right caudate activation during switching specifically (Wang et al., 2007). Thus, based on our comparison of language switching to color-shape switching, it is clear that the left caudate facilitates both linguistic and non-linguistic switching, while the right caudate may be a region that is recruited more specifically to achieve language control and may represent a unique region necessary for bilingual language control, especially in early bilinguals. Future studies may consider focusing on the role of the caudate in linguistic and non-linguistic control to further explore this notion.
Of particular interest is why the difference in activation between color-shape and language tasks was greatest during stay trials. More experience with a task, corresponding to greater task efficiency, can lead to reduced activation (Petersen et al., 1998), whereas inefficient performance can lead to increased activation (Suskauer et al., 2008). Thus, if greater activation is interpreted as greater effort needed to accomplish the task at hand, the results of the baseline and cross-task comparisons suggest that sustaining more than one task-set (i.e., stay trials) is more challenging for non-linguistic than for linguistic control in bilinguals. Broadly, these findings suggest that regions shared during stay trials require less neural activation during linguistic than non-linguistic processing. This finding is consistent with recent characterization of language as an “expert task” when compared with the color-shape task (Weissberger et al., 2012), and further suggests efficiency in sustaining a task set during mixed task blocks as a more specific locus for the relevant expertise.
Consideration of how bilinguals communicate on a daily basis may shed light on this finding. A wealth of behavioral data suggest that, even when bilinguals speak in just a single language, both languages remain active (e.g., Colomé, 2001; for review, see Kroll, Bobb, Misra, & Guo, 2008). Kroll et al. (2008) suggested that rather than learning to avoid competition between languages, bilinguals become skilled in dealing with the constant activation of two languages. The bilinguals in the present study reported speaking in mixed language contexts frequently, especially throughout childhood (see Table 1). Although switching between languages is common practice in some bilingual communities, even in conversations with frequent switches, non-switches (i.e., staying within the same language) would nevertheless occur with much greater frequency. Consequently, though bilinguals who switch often have practice switching, they likely have even more practice staying within the same language in situations where both languages are active response alternatives. In contrast, color and shape decisions are not naturally competing tasks, thus there is no prior expertise for maintaining these response alternatives simultaneously.
Bilinguals might also become “staying experts” from conversations in monolingual contexts. When bilinguals speak with monolinguals, they may need to maintain consistent inhibition of the non-target language (de Bruin, Roelofs, Dijkstra, & FitzPatrick, 2014; Guo et al., 2011), and if so they would have constant practice “staying” in one language. In line with this, a recent imaging study by Guo et al. (2011) found evidence for two types of inhibition involved in bilingual language processing; global inhibition in situations which require sustained processing in one language (e.g., monolingual contexts) and local inhibition situations requiring rapid and frequent language switches. While the dorsal anterior cingulate cortex and supplementary motor area played important roles for local inhibition, the dorsal left frontal gyrus and parietal cortex were important during global inhibition. Frequent interactions with monolinguals essentially place bilinguals in an everlasting “stay” trial in which they recruit neural resources for sustained language control during conversation on a daily basis. Although single blocks are more analogous to monolingual contexts than mixed blocks, it is nevertheless possible that practice in single-language contexts could transfer to trials without a switch in mixed-language blocks (i.e., the stay trials). Though we did not find greater efficiency in response (i.e., decreased neural activation) in single language blocks than in single color-shape blocks (possibly evidence against the idea that practice in monolingual contexts transfers to “staying” in bilingual contexts), this could reflect idiosyncrasies of the tasks chosen for study. That is, the color-shape task only had two response alternatives in single task blocks, whereas the language task had 9 response alternatives. This might explain why the “staying” advantage was observed in our data only in mixed blocks which entail elevated control demands, possibly overriding any demands associated with the greater number of response alternatives.
Finally, if there is greater task efficiency for language stay relative to task stay, one question is why we did not find differences between language stay and color-shape stay reaction times in the behavioral tasks. A previous behavioral study using similar paradigms by Weissberger et al. (2012) reports faster overall reaction times across all three trial-types for color-shape switching than language switching (main effect of task), but not differentially for each trial-type (i.e., no interaction effect). This faster responding could be due to use of button-presses in place of vocal responses in the color-shape task. In support of this, a study by Prior & Gollan (2013) that used vocal responses in place of button presses found overall slower color-shape reaction times across all three trial types. This suggests that behavioral differences between tasks may be due to differences in methodological implementations. Notably, lack of significant behavioral differences between the language and color-shape tasks in our study suggests that the differences in brain activation are not due to methodological differences related to task difficulty, error monitoring, or time spent on each task. Rather, our imaging data reveal a more nuanced relationship between linguistic and non-linguistic control depending on the trial-type under investigation that is not dependent on methodological differences between the tasks.
Although this study is the first to our knowledge to directly compare non-linguistic task switching to bilingual language switching, there were some methodological limitations that deserve to be addressed. First, participants were required to covertly provide responses to both task paradigms while in the scanner and thus accuracy was not measured. To ensure that participants were focused on completing the task, button presses were monitored by a flashing light and the scan operator checked in with the participant between each scan run. Additionally, an examination of error rates during the behavioral version of the tasks completed prior to the scan session for each participant verified their ability to complete the tasks with minimal errors. Another related limitation is the fact that covert naming during both tasks may have introduced a potential confound related to speech inhibition. However, because we were interested in differences between the tasks, our comparison analyses will have eliminated any shared regions related to speech inhibition as a result of covert naming. A third potential limitation is the difference in response options between the two tasks. Whereas the color-shape task had four response options (“red”, “green”, “circle”, “triangle”), the language switching task had 18 (numbers 1–9 in English or Spanish). It could be argued that this difference might require greater effort to initiate a response during the language switching task due to less frequent repetition of stimuli. If this were indeed the case, we would expect greater activation for language switching than color-shape switching across all three trial types; however, behavioral and imaging data do not confirm this suggestion. A final potential limitation relates to our decision to introduce the behavioral version of the task prior to imaging to reduce the novelty of the color-shape task, minimize error rates, and to minimize superficial differences between tasks in familiarity. A recent study reported greater practice effects for the color-shape than for the language task (Prior & Gollan, 2013). Thus, the results reported here likely did not reflect such practice effects given our finding of a double dissociation with respect to apparent efficiency such that language appeared to be more efficient than color-shape on stay trials whereas the reverse was true for single and switch trials.
5. Conclusion
The present study examined the relationship between linguistic and non-linguistic control. Results revealed more similarities than differences between the tasks in relation to switching task-sets (switch trials) and more differences than similarities related to maintaining a task-set (stay trials), potentially as a result of the cognitive demands of successfully communicating as a bilingual. Consistent with previous studies, our findings support the notion that some aspects of executive control are necessary for successful language switching, especially when in a bilingual context in which mixing languages is appropriate and frequently done. However, our findings are also unique in that they suggest greater efficiency in bilinguals for sustaining inhibition of a non-target language than a non-target task. This efficiency may arise due to frequent interactions in both single and mixed language contexts in which bilinguals must control dual language activation to avoid mixing languages. On this view, bilingual language use, in both bilingual and monolingual contexts, does not lead to switching expertise, but rather results in speakers who are experts at “staying” in a single language when faced with linguistic alternatives.
Supplementary Material
Highlights.
Single/stay/switch trials were compared across tasks
Greatest differences in activation were found between language stay and color-shape stay trials.
Findings suggest greater efficiency for maintaining 2 languages, than 2 non-linguistic tasks.
Bilinguals are experts at “staying” in a single language when faced with linguistic alternatives.
Acknowledgments
We would like to thank Sheena Dev and Sarah Jurick for their help with data collection. This work was supported by an F31 from NIA awarded to Gali Weissberger (AG039177), by R01s from NICHD (HD050287) and NIDCD (R01 DC011492) awarded to Tamar H. Gollan, by NIA grants (K24 AG026431 and R01AG012674) awarded to Mark W. Bondi, and a VA CSR&D Career Development Award to Christina E. Wierenga (CDA-2-022-08S).
Footnotes
This participant pressed the panic button during the second half of the fMRI experiment, which was the color-shape component, due to anxiety in the scanner. Notably, the participant did not complain of discomfort during the entirety of the language switching task and thus the data were still included in analyses. However, to ensure that this participant’s data did not impact our findings, we conducted the language switching baseline analyses without this participant. Findings did not differ and widespread activation was still found across regions reported in the supplementary tables; thus, we included the participant in the baseline analyses of language switching.
Below (see footnote 4), we report some significant language dominance effects in the fMRI results. Note that behavioral data exhibited no significant differences (see also Prior & Gollan, 2011) between English and Spanish RTs on single and switch trials (both ps ≥ .34), and only a non-significant trend towards reversed dominance effects on stay trials with English responses (M = 725, SD = 168 ms) being slower than Spanish responses (M = 701; SD = 154; p = .06, even though English was the dominant language for these bilinguals).
To further assess the robustness of this effect, we examined whether the pattern observed for stay trials appeared to be present in each individual bilingual. Of note, all but three bilinguals showed a pattern in which PSC for color-shape was greater than for language during stay trials. The three bilinguals that did not show this pattern did not differ systematically from the other bilinguals in self-reported language switching behavior or proficiency ratings (i.e., all three rated their language proficiency in both languages as high and self-reported as English dominant).
Though we tested early and relatively proficient bilinguals in the present study and we did not observe significant dominance effects in the RTs (see footnote 2), fMRI data might be more sensitive to dominance effects; if so, it might seem possible that some of the above-reported between-task differences might reflect production of Spanish, the non-dominant language, during some trials of the language task (the color-shape task was done exclusively in English). To consider this possibility, we examined whether activation differed within the language task for responses produced in English versus Spanish. We conducted these analyses separately for single, stay, and switch trials with three t-tests comparing regions of activation for English and Spanish (all corrected ps ≤ .001; volume threshold = 256 mm3). Of greatest interest, we found no significant differences between English and Spanish responses on stay trials within the mixed blocks. Thus, our main conclusion, which is that language stay trials are executed more efficiently within the brain than color-shape stay trials is not an artifact of language dominance effects. We did observe some significant differences between English and Spanish responses on single and switch trials. In both cases Spanish elicited greater activation than English (with a larger number of regions exhibiting language dominance effects on switch than on single trials). During switch trials, activation was greater for Spanish than English across multiple brain regions including the paracentral lobule, postcentral gyrus, superior and dorsal parietal regions, left insula, caudate, and thalamus. However, a follow-up analysis comparing English switch trials to color-shape switch trials demonstrated that these language dominance effects cannot explain the between-task differences we reported for switch trials. Specifically, even when including only responses that were produced in English in the language task (excluding Spanish responses), language switch trials still showed significantly greater activation than color-shape switch trials across several brain regions (p ≤ .001; volume threshold = 256 mm3). Thus, this result too appears to not be driven exclusively by language dominance effects (i.e., more effort to produce Spanish responses). Finally, comparing Spanish to English single trials revealed greater activation in the bilateral cuneus for Spanish trials relative to English trials. Although the cuneus is found in language switching studies (e.g., Guo et al., 2011), its role remains unclear, and future studies may focus on its role in language control.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Kahteb A. Language control and lexical competition in bilinguals: An event-related fMRI study. Cerebral Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Costa A. Bilingualism tunes the Anterior Cingulate Cortex for conflict monitoring. Cerebral Cortex. 2012;22:2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green D. Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics. 2007;20:242–275. [Google Scholar]
- Abutalebi J, Miozzo A, Cappa SF. Do subcortical structures control ‘Language Selection’ in polygots? Evidence from pathological language mixing. Neurocase. 2000;6:51–56. [Google Scholar]
- Bialystok E, Craik FIM, Green DW, Gollan TH. Bilingual minds. Psychological Science in the Public Interest. 2009;10:89–129. doi: 10.1177/1529100610387084. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Klein R, Viswanathan M. Bilingualism, aging, and cognitive control: Evidence from the Simon task. Psychology and Aging. 2004;19:290–303. doi: 10.1037/0882-7974.19.2.290. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Luk G. Cognitive control and lexical access in younger and older bilinguals. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:859–873. doi: 10.1037/0278-7393.34.4.859. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Ryan J. Executive control in a modified antisaccade task: Effects of aging and bilingualism. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:1341–1354. doi: 10.1037/0278-7393.32.6.1341. [DOI] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. Neuroimage. 2001;14(4):817–826. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Calabria M, Hernández M, Branzi FM, Costa A. Qualitative differences between bilingual language control and executive control: evidence from task-switching. Frontiers in Psychology. 2012;2:399. doi: 10.3389/fpsyg.2011.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphical system for designing and controlling experiments in the Psychology laboratory using Macintosh computers. Behavioral Research Methods, Instrumentation and Computation. 1993;25:257–271. [Google Scholar]
- Colomé A. Lexical activation in bilingual’s speech production: Language-specific or language-independent? Journal of Memory and Language. 2001;45:721–736. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers in Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: Lexical-semantic mechanisms and the thalamus. Brain and Cognition. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Crosson B, Zawacki T, Brinson G, Lu L, Sadek JR. Models of subcortical functions in language: Current status. Journal of Neurolinguistics. 1997;10:277–300. [Google Scholar]
- de Bruin A, Roelofs A, Dijkstra T, FitzPatrick I. Domain-general inhibition areas of the brain are involved in language switching: FMRI evidence from trilingual speakers. Neuroimage. 2014;90:348–359. doi: 10.1016/j.neuroimage.2013.12.049. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. NeuroReport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex in task switching: an event-related fMRI study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Fabbro F, Peru A, Skrap M. Language disorders in bilingual patients after thalamic lesions. Journal of Neurolinguistics. 1997;10:347–367. [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez-Pujadas A, Belloch V, Ávila C. Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. Neuroimage. 2010;53:1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kim, Chobok K, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. The Journal of Neuroscience. 2013;33:387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Ferreira VS. Should I stay or should I switch? A cost-benefit analysis of voluntary language switching in young and aging bilinguals. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:640–665. doi: 10.1037/a0014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Weissberger GH, Runqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A multilingual naming test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition. 2011;15:594–615. doi: 10.1017/S1366728911000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Liu H, Misra M, Kroll JF. Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. Neuroimage. 2011;56:2300–2309. doi: 10.1016/j.neuroimage.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: What’s next? Brain and Language. 2009;109:133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: An fMRI study. NeuroImage. 2001;14:510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, Kohnert K. In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain and language. 2000;73:421–431. doi: 10.1006/brln.1999.2278. [DOI] [PubMed] [Google Scholar]
- Hernández M, Martin CD, Barceló F, Costa A. Where is the bilingual advantage in task-switching? Journal of Memory and Language. 2013;69:257–276. [Google Scholar]
- Hervais-Adelman AG, Moser-Mercer B, Golestani N. Executive control of language in the bilingual brain: integrating the evidence from neuroimaging to neuropsychology. Frontiers in Psychology. 2011;2:234. doi: 10.3389/fpsyg.2011.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda C, Hanakawa T, Nariai T, Ohno K, Honda M. Neural mechanisms of language switch. Journal of Neurolinguistics. 2012;25:44–61. [Google Scholar]
- Hull R, Vaid J. Bilingual language lateralization: A meta-analytic tale of two hemispheres. Neuropsychologia. 2007;45:1987–2008. doi: 10.1016/j.neuropsychologia.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hyafil A, Summerfield C, Koechlin E. Two mechanisms for task switching in the prefrontal cortex. The Journal of Neuroscience. 2009;29:5135–5142. doi: 10.1523/JNEUROSCI.2828-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Braver TS. Age-related shifts in brain activity dynamics during task-switching. Cerebral Cortex. 2010;20:1420–1431. doi: 10.1093/cercor/bhp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D’Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Cognitive Brain Research. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substratates underlying word generation: A bilingual functional-imaging study. Proceedings of the National Academy of Sciences. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács ÁM. Early bilingualism enhances mechanisms of false-belief reasoning. Developmental Science. 2009;12:48–54. doi: 10.1111/j.1467-7687.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- Kovács ÁM, Mehler J. Cognitive gains in 7-month old bilingual infants. Proceedings of the National Academy of Sciences. 2009;106:6556–6560. doi: 10.1073/pnas.0811323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Bobb SC, Misra M, Guo T. Language selection in bilingual speech: Evidence for inhibitory processes. Acta Psychologica. 2008;2008:416–430. doi: 10.1016/j.actpsy.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Green DW, Abutalebi J, Grady C. Cognitive control for language switching in bilinguals: A quantitative meta-analysis of functional neuroimaging studies. Language and Cognitive Processes. 2012;27:1479–1788. doi: 10.1080/01690965.2011.613209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu OV, Binenstock MM, Pline MA, Yeaton JR, Carey JR. Neural changes in control implementation of a continuous task. The Journal of Neuroscience. 2007;27:3010–3016. doi: 10.1523/JNEUROSCI.5051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mariën P, Abutalebi J, Engelborghs S, De Deyn PP. Pathophysiology of language switching and mixing in an early bilingual child with subcortical aphasia. Neurocase. 2005;11:385–398. doi: 10.1080/13554790500212880. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paap K. The role of componential analysis, categorical hypothesizing, replicability and confirmation bias in testing for bilingual advantages in executive functioning. Journal of Cognitive Psychology 2014 [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy of Sciences of the USA. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A, Gollan TH. Good language-switchers are good task-switchers: Evidence from Spanish-English and Mandarin-English bilinguals. Journal of the International Neuropsychological Society. 2011;17:682–691. doi: 10.1017/S1355617711000580. [DOI] [PubMed] [Google Scholar]
- Prior A, Gollan TH. The elusive link between language control and executive control: A case of limited transfer. Journal of Cognitive Psychology. 2013 doi: 10.1080/20445911.2013.821993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A, MacWhinney B. A bilingual advantage in task switching. Bilingualism: Language and Cognition. 2010;13:253–262. doi: 10.1017/S1366728909990526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, van der Lugt A, Rotte M, Britti B, Heinze H, Münte TF. Second language interferes with word production in fluent bilinguals: Brain potential and functional imaging evidence. Journal of Cognitive Neuroscience. 2005;17:422–433. doi: 10.1162/0898929053279559. [DOI] [PubMed] [Google Scholar]
- Rubin O, Meiran N. On the origins of the task mixing cost in the cuing task-switching paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y-Y, Jiang T, Wang Y-Z, Wu C-X. Direct evidence of the left caudate’s role in bilingual control: An intra-operative electrical stimulation study. Neurocase. 2012;i:1–8. doi: 10.1080/13554794.2012.701635. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. Neuroimage. 2007;35:862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Weissberger GH, Wierenga CE, Bondi MW, Gollan TH. Partially overlapping mechanisms of language and task control in young and older bilinguals. Psychology and Aging. 2012;27:959–974. doi: 10.1037/a0028281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



