Abstract
The goal of the present study was to quantify and correlate the contribution of the cytosolic p67phox subunit of NADPH oxidase 2 to mitochondrial oxidative stress in the kidneys of the Dahl salt-sensitive (SS) hypertensive rat. Whole kidney redox states were uniquely assessed using a custom-designed optical fluorescence three-dimensional cryoimager to acquire multichannel signals of the intrinsic fluorophores NADH and FAD. SS rats were compared with SS rats in which the cytosolic subunit p67phox was rendered functionally inactive by zinc finger nuclease mutation of the gene (SSp67phox-null rats). Kidneys of SS rats fed a 0.4% NaCl diet exhibited significantly (P = 0.023) lower tissue redox ratio (NADH/FAD; 1.42 ± 0.06, n = 5) than SSp67phox-null rats (1.64 ± 0.07, n = 5), indicating reduced levels of mitochondrial electron transport chain metabolic activity and enhanced oxidative stress in SS rats. When fed a 4.0% salt diet for 21 days, both strains exhibited significantly lower tissue redox ratios (P < 0.001; SS rats: 1.03 ± 0.05, n = 9, vs. SSp67phox-null rats: 1.46 ± 0.04, n = 7) than when fed a 0.4% salt, but the ratio was still significantly higher in SSp67phox rats at the same salt level as SS rats. These results are consistent with results from previous studies that found elevated medullary interstitial fluid concentrations of superoxide and H2O2 in the medulla of SS rats. We conclude that the p67phox subunit of NADPH oxidase 2 plays an important role in the excess production of ROS from mitochondria in the renal medulla of the SS rat.
Keywords: optical fluorescence cryoimaging, hicotinamide adenine dinucleotide, flavin adenine dinucleotide, NADPH oxidase, p67phox, mitochondrial redox state, oxidative stress, hypertension, Dahl salt-sensitive rat, three-dimensional
essential hypertension affects 972 million (26.4%) of the world adult population and 67 million (31%) of United States population over the age of 18 yr (10, 24). Nearly half of adults with hypertension in the United States exhibit enhanced blood pressure sensitivity to dietary salt intake, reaching as high as 75% in African-Americans (2, 47, 52). The Dahl salt-sensitive (SS) rat mimics many of the traits found in African-American hypertensive patients (6, 17, 26) and has been studied extensively as an animal model of a complex multifactorial genetic disease (13, 14, 21, 34, 46). A number of mechanisms have been recognized to contribute physiologically to the development of hypertension in SS rats, with the most prominent of these being the reduced ability of the kidneys to excrete Na+ as characterized by a reduction in the pressure-natriuresis relationship (38, 40). It is recognized that an imbalance in the redox state of the kidney contributes importantly to the pressure-natriuresis relationship and renal dysfunction in the SS rat, which exhibit elevated levels of ROS in the kidney (7, 32, 48–50).
The sources and localization of ROS production within the kidney have been of great interest given the need to develop therapeutic strategies that can reduce ROS in the kidney, where excess levels are associated with aging, diabetes, and hypertension. Elevated ROS production within the renal outer medulla of SS rats has been largely attributed to NADPH oxidase (Nox) (49). Analyses of tissue homogenates have found medullary superoxide (O2·−) production is greater in SS rats and accounted for by greater Nox activity compared with a salt-insensitive control strain (49). ROS production was further reduced by more than half in both rat strains when pretreated with the mitochondrial uncoupler dinitrophenol, indicating the importance of the mitochondria in basal ROS production in the outer medullary region of the kidney (49). Inhibition of xanthine oxidase, nitric oxide synthase, and cycloxygenase had no effect on ROS production, and levels of l-arginine, nitric oxide synthase, superoxide dismutase, catalase, and glutathione peroxidase activities did not differ between the SS strain and a salt-resistant control strain (49).
These and other studies have indicated that Noxs are major sources of O2·− and H2O2 in the kidney and that mitochondria are importantly involved in this process (3, 23, 39, 43). There is also evidence of mitochondrial cross-talk with membrane Noxs in ROS production (35), although the details of these mechanisms are not well understood (27, 28, 33, 37). Of the seven identified Nox isoforms, Nox2 and Nox4 appear to be the two most robustly expressed in the kidney (22). Unlike Nox4, the activity of Nox2 is critically dependent on the activation and docking of the cytosolic subunit p67phox protein (29). Therefore, in the present study, the contribution of Nox2 to the redox states of the kidney of SS rats was assessed and correlated with oxidative stress. This assessment was done by determining the effects of high- and low-salt diets in SS rats in which the p67phox subunit was rendered nonfunctional using zinc finger nuclease (ZFN) genetic engineering technologies (12, 15, 16).
The redox states of the whole kidney were uniquely assessed using a novel, custom-designed optical fluorescence three-dimensional (3-D) cryoimager to acquire fluorescence signals of the intrinsic fluorophores NADH and FAD. This technique provided a number of advantages over previous methods, such as microdialysis of interstitial fluids and measurement of tissue enzyme activities in homogenized tissue (48–50). First, it enabled a 3-D visualization, quantification, and correlation of the contribution of the mitochondrial redox states toward the production of ROS in the kidney. Second, it circumvented the various potential distortions associated with measurements made in homogenized tissues. Third, since the technique is based on the detection of the well-recognized autofluorescence signals of NADH and FAD (the oxidized form of FADH2), it did not require the utilization of exogenous labels (4). Using the cryoimager, the tissue redox ratio (NADH/FAD) was imaged in 3-D, which enabled us to assess and correlate mitochondrial electron transport chain (ETC) metabolic activity and oxidative stress in the kidneys obtained from SS rats and SS rats with a null mutation of the Nox2 subunit p67phox (SSp67phox-null rats) fed either 0.4% or 4.0% NaCl diets for 21 days. The results showed that SSp67phox-null rats exhibited a significantly greater redox ratio than SS rats, indicating an enhancement of mitochondrial ETC metabolic activity and a reduction of oxidative stress in this region of the kidney of SSp67phox-null rats.
MATERIALS AND METHODS
Experimental animals.
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Male Dahl SS rats (SS/JrHsdMcwi) and SSp67phox-null rats were obtained as weanlings from colonies maintained at the Medical College of Wisconsin. SSp67phox-null rats were produced using ZFN techniques (15, 16). Sequencing of p67phox cDNA of this novel rat strain verified a 5-bp deletion (GAGAA), and functional studies demonstrated a complete loss of functional respiratory burst activity in macrophages obtained from these rats as well as protection from the severe hypertension observed in the SS rat when fed a high-salt diet (11, 12).
Protocol.
Rats were fed a custom AIN-76 diet (Dyets, Bethlehem, PA) containing 0.4% NaCl since weaning. At 6 wk of age, a subgroup of these rats was switched to a diet with 10-fold higher salt content (4.0% NaCl diet) for 21 days before the kidneys were collected. On the day of tissue harvest, rats were deeply anesthetized with pentobarbital sodium (60 mg/kg), and a catheter placed in the aorta for a rapid infusion of 20 ml of cold isotonic saline to flush the kidneys of blood. The left kidney was then quickly removed, hemisected, and dropped into a container of isopentane cooled by liquid nitrogen. After 2 min in the cooled isopentane, the kidney was moved to liquid nitrogen and then stored at −80°C until cryoimaging was performed.
Optical fluorescence 3-D cryoimaging.
The cryoimager is a custom-designed and custom-fabricated optical fluorescence imaging system (Fig. 1) from the Biophotonics laboratory at the University of Wisconsin (Milwaukee, WI) and has been previously described (42, 45). A mercury arc lamp (200 W, Oriel) is used as a light source, and desired wavelengths are selected by filtering the broad band light to excite the selected fluorophores from the exposed surface of the frozen tissue block. The emission light is also filtered to eliminate reflected and unwanted lights. The excitation filter for NADH is centered at 350 nm (80-nm bandwidth, UV Pass Blacklite, HD Dichroic, Los Angeles, CA), and the corresponding FAD filter is centered at 437 nm (20-nm bandwidth, 440QV21, Omega Optical, Brattleboro, VT). Moreover, the emission filter for NADH is centered at 460 nm (50-nm bandwidth, D460/50M, Chroma, Bellows Falls, VT), and the emission filter for FAD is centered at 537 nm (50-nm bandwidth, QMAX EM 510-560, Omega Optical). Filters for excitation and emission wavelengths are controlled using two motorized filter wheels (Oriental Motor Vexta Step Motor PK268-01B), and images are captured using a charge-coupled device camera (QImaging, Rolera EM-C2, 14 bit) with 1,004 × 1,002 pixel arrays. Separate images for NADH and FAD autofluorescence are obtained from each tissue slice, and from these images, 3-D images of the kidney are compiled. A motorized microtome blade (Delaware Diamond Knives, profile D, WC125cryo) sequentially slices the tissue; for the present study, 30-μm slices were made in the z-direction, which resulted in 400 z-slices/kidney. During the slicing, tissue is maintained in the frozen state at −40°C.
Fig. 1.
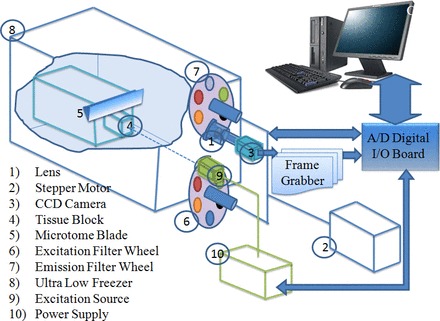
Schematic of the custom-designed cryoimaging system (18, 43, 45). CCD, charge-coupled device.
Image processing.
NADH and FAD autofluorescence images from each group of kidneys were analyzed using MATLAB (The Mathworks). 3-D representations of the kidneys were rendered using z-stacks of all image slices for both NADH and FAD signals. The intensity at each pixel in the NADH and FAD images is an indicator of the concentration of the fluorophore at that pixel. The 3-D volume-averaged NADH-to-FAD redox ratio of each kidney was calculated voxel by voxel according to the following equation:
| (1) |
The corresponding histograms for each group of kidneys, which represent the distributions of redox ratio intensities in 3-D volumes, were plotted. The histogram is a scaled probability density function of the redox ratio in a kidney for quantitative analogy between the groups. Arithmetic mean values of the histograms were calculated according to the following equation (42):
| (2) |
where Nx, Ny, and Nz are the numbers of pixels in the x-, y-, and z-directions; the pixel size in x- and y-directions was 10 μm, but the pixel size in the z-direction was 30 μm.
Statistical evaluation of data.
Values are reported as means ± SE; n is the number of rats used. Two-way ANOVA followed by a Holm-Sidak post hoc test for multiple comparisons was used, and significance was determined at the P < 0.05 level for strain and dietary salt.
RESULTS
Representative examples of 3-D images of the NADH and FAD fluorescence signals and their redox ratios (NADH/FAD) obtained from a representative SS rat and SSp67phox-null rat on the 0.4% NaCl diet and a rat of each strain on the 4.0% NaCl diet are shown in Fig. 2. As shown in Fig. 2, higher NADH and lower FAD fluorescence signals were observed within the renal medulla region of SSp67phox-null rats compared with SS rats fed a 0.4% NaCl diet. When fed a 4.0% NaCl diet for 21 days, both strains of rats exhibited relatively lower levels of redox ratios throughout the kidney and especially in the mitochondria-rich renal medulla, as apparent by the pseudored color intensity. Notably, the SSp67phox-null rat remained relatively well protected from the high salt-induced oxidative stress, as reflected by the high redox ratio values compared with the SS rat. As NADH and FAD signals originate exclusively from the mitochondria (4), these results indicate greater mitochondrial ETC metabolic activity and decreased oxidative stress in the mitochondria-rich renal medulla of SSp67phox-null mutant rats.
Fig. 2.
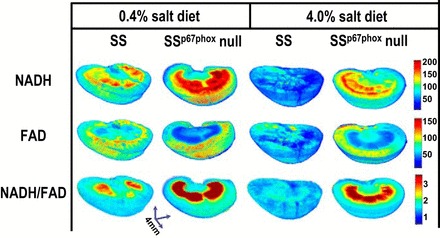
Representative three-dimensional rendered images of kidneys from each strain [Dahl salt-sensitive (SS) rats and SS rats with a null mutation of the NADPH oxidase subunit p67phox (SSp67phox-null rats)] on the two salt diets (0.4% NaCl and 4.0% NaCl) are shown. The fluorescence patterns for NADH, FAD, and the tissue redox ratio (NADH/FAD) are shown. The intensity scale bar indicates that those regions most red are the highest in the redox ratio, thus protected from the oxidative stress as observed in the SSp67phox-null rat.
Figure 3 shows the corresponding histogram plots of redox ratios of the same four kidneys shown in Fig. 2 to display the 3-D intensity distribution throughout the kidney tissue. The computed mean values of the histograms (see materials and methods) clearly showed that the redox ratio was reduced in both SSp67phox-null and SS rats in response to a high-salt diet. However, SSp67phox-null rat kidneys exhibited lower overall levels of state of oxidative stress (higher redox ratio) throughout all regions.
Fig. 3.
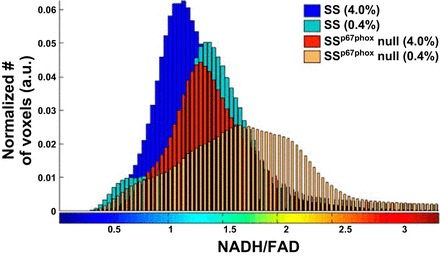
Intensity histogram distribution of the tissue redox ratio (NADH/FAD) for the four kidneys shown in Fig. 2. The mean value of each histogram was determined for each imaged kidney. a.u., Arbitrary units.
Figure 4 shows the images of redox ratios of the individual kidneys for the two diet treatment groups used in the analysis shown in Fig. 5. The consistency in the redox ratio between different rats (biological replicates) in a particular group signified our research findings correlating reduced redox ratio to oxidative stress in the kidneys of SS rats.
Fig. 4.
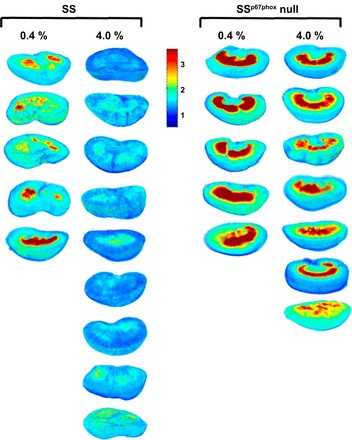
Images of the tissue redox ratio for each kidney in the four groups.
Fig. 5.
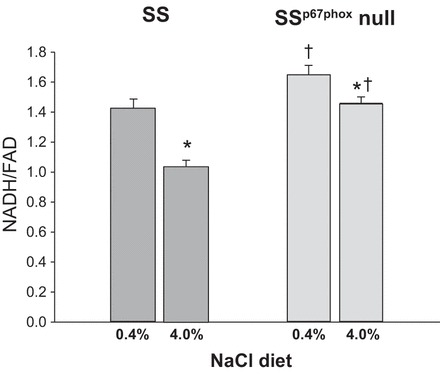
Tissue redox ratio (NADH/FAD) mean values of the histograms obtained from each kidney for the two strains and two salt levels. The redox ratio of the SS strain on a 0.4% NaCl diet was significantly lower than that of the SSp67phox-null rat on the same diet (P = 0.023). The redox ratio was significantly reduced in both strains after 21 days of 4.0% NaCl diet. *Significant difference in redox ratios within strain between 0.4% and 4.0% NaCl diets (P < 0.05); †significant difference of redox ratios between strains on the same salt diet (P < 0.05).
Figure 5 shows group data for the redox ratio for the two rat strains on the two salt diets. Kidneys from SS rats fed a 4.0% NaCl diet (n = 9, 1.03 ± 0.05) had a significantly lower tissue redox ratio compared with kidneys from SS rats maintained on a 0.4% NaCl diet (n = 5, 1.42 ± 0.06), a consequence of a substantial reduction of NADH relative to FAD fluorescence. A similar significant reduction of the mean redox ratio of kidneys from SSp67phox-null rats fed a 4.0% NaCl diet (n = 7, 1.46 ± 0.04) was measured compared with kidneys from 0.4% NaCl diet-fed rats of the same strain (n = 5, 1.64 ± 0.07). Importantly, the tissue redox ratio was significantly less in SS rats compared with SSp67phox-null rats at both salt levels. Based on two-way ANOVA, there was a statistically significant difference due to strain (P < 0.001) and diet (P < 0.001) but not a significant interaction of the two (P = 0.0748).
DISCUSSION
A recent study (12) conducted by our group has shown ZFN knockdown of the Nox2 cytosolic subunit p67phox in SS rats (SSp67phox-null rats) resulted in a 40% reduction of salt-induced hypertension and a substantial reduction of renal injury. These experiments were the first to demonstrate a relevant role of p67phox in chronic blood pressure regulation and salt sensitivity. The present study applied a novel fluorescence cryoimaging technique to determine whether these protective effects of the null mutation of p67phox were associated with alterations of regional metabolism and oxidative stress within the kidney. The higher NADH signal and lower FAD signal observed in the mitochondria-rich renal medulla of SSp67phox-null rats indicate that in the mitochondria, there was more NAD present in its reduced form (NADH) and less FADH2 present in its oxidized form (FAD) compared with SS rats. This would result in enhanced pairing of electrons with hydrogen and less leakage of electrons in the mitochondrial ETC to available oxygen and less production of ROS in the kidneys of SSp67phox-null rats.
Although it is known that the cytosolic protein p67phox is required to activate membrane Nox2 (29), the present study is the first to globally assess the contribution of this essential Nox subunit to renal oxidative stress in the SS rat. The results show that the kidney of SS rats was particularly vulnerable to oxidative stress and that a reduction of Nox2 in SSp67phox-null rats resulted in a significant reduction of oxidative stress in this region. The renal medullary region of the kidney is rich in mitochondria contained in the medullary thick ascending limbs of Henle, which require high levels of ATP to handle the reabsorption of nearly 25% of all NaCl filtered by the glomeruli (31, 36). The 3-D fluorescence images showed that a significant difference existed in the redox ratio between SS and SSp67phox-null rats even when fed a low-salt diet, which was significantly changed in both strains as a consequence of high-salt (4% NaCl) feeding.
Ultrastructural abnormalities of mitochondria within the medullary thick ascending limbs of Henle have been found in SS rats, as represented by a lower proportion of long mitochondria in SS rats (19), and SS rats exhibit lower levels of O2 consumption compared with salt-insensitive consomic SS.13BN rats (53). This, in turn, was associated with a significant attenuation of renal injury in SSp67phox-null rats (12). We now show that these abnormalities are associated with an increased redox ratio in the kidneys of SSp67phox-null rats. This is consistent with an earlier report (12) from our group showing that H2O2 and O2·− production were significantly reduced in the renal medulla of SSp67phox-null rats compared with their wild-type SS littermates, as determined in renal medullary tissue homogenates and microdialysis experiments. Other studies have shown that increased levels of ROS within the renal medulla alone result in a reduction of medullary blood flow, Na+ retention, and hypertension (30, 48, 49, 54). Recently, we found that SSp67phox-null rats are protected from reduced medullary blood flow compared with their wild-type SS littermates (11). Therefore, mitochondrial metabolic dysfunction associated with the excess production of ROS was captured with the present 3-D imaging experiments of NADH and FAD.
Biological meaning of the tissue redox ratio (NADH/FAD).
Although we have presented technical details of fluorescent cryoimaging in bioengineering journals (18, 42, 45), it is useful to emphasize several elements of this technique that are important to understand. It was first demonstrated by Chance and colleagues (4, 5) that the autofluorescence signals from tissues originate almost exclusively from NADH and FAD in the mitochondria. The contribution of NADH present in the cytosol is small, since as soon as NADH is produced by glycolysis in the cytosol, the electron from NADH is transported to the mitochondria through the malate-aspartate shuttle and is oxidized to NAD (20). The NADH autofluoresence signals detected in our experiments therefore represent mitochondrial signals. FAD is produced and strictly localized within the mitochondria (20, 41), so the signal detected is also derived only from the mitochondria (1, 25).
Changes in the tissue redox ratio therefore provide a quantitative marker of the bioenergetic status of the mitochondria (42). Neither NAD nor FADH2 are autofluorescent, and, in the presence of oxidative stress, NADH and FADH2 accumulate in their oxidized forms, NAD and FAD. The autofluoresence signal of NADH fluorescence is therefore reduced and that of FAD is elevated, resulting in a reduction of the redox ratio. Of importance, rapid freezing and imaging at low temperatures (−40°C) are important parts of this methodology for several reasons. First, the metabolic state of the mitochondria is preserved at a particular instance in time. Second, the low temperature elevates the quantum yield of NADH and FAD fluorophores compared with imaging techniques used at room temperature. The higher quantum yield results in a stronger signal and a more accurate measurement. Finally, by evaluating the ratio of these two fluorophores, some of the confounding factors in determining the oxidative state are eliminated, such as blood, mitochondrial protein concentration, and the number of mitochondria or mitochondrial density (5).
Summary.
The results from the present study show that the kidneys of the SS rat exhibit a significantly lower redox ratio than those of the SSp67phox-null rat. These observations indicate reduced levels of ETC metabolic activity and enhanced oxidative stress in this model of SS hypertension, particularly in the renal medulla. We conclude that the p67phox subunit of Nox2 plays an important role in the excess production of ROS from mitochondria in the kidneys of the SS rat, which contributes to salt-sensitive hypertension.
GRANTS
This work was supported by a University of Wisconsin-Milwaukee Research Growth Inititative grant (to M. Ranji) and National Heart, Lung, and Blood Institute Grants HL-116264 (to A. W. Cowley, Jr.) and HL-82798 (to A. W. Cowley, Jr.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.S., C.Y., N.N.Z., and T.K. performed experiments; F.S., Z.G., A.W.C.J., and M.R. analyzed data; F.S., R.K.D., A.W.C.J., and M.R. interpreted results of experiments; F.S., A.W.C.J., and M.R. prepared figures; F.S. drafted manuscript; F.S., C.Y., R.K.D., A.W.C.J., and M.R. edited and revised manuscript; F.S., Z.G., C.Y., N.N.Z., T.K., R.K.D., A.W.C.J., and M.R. approved final version of manuscript; A.W.C.J. and M.R. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Meredith Skelton for the careful review of the manuscript.
REFERENCES
- 1.Aldakkak M, Stowe DF, Lesnefsky EJ, Heisner JS, Chen Q, Camara AK. Modulation of mitochondrial bioenergetics in the isolated Guinea pig beating heart by potassium and lidocaine cardioplegia: implications for cardioprotection. J Cardiovasc Pharmacol 54: 298–309, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 43: 707–713, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cabral PD, Hong HJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185–F1192, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem 233: 736–739, 1958. [PubMed] [Google Scholar]
- 5.Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem 254: 4764–4771, 1979. [PubMed] [Google Scholar]
- 6.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet 7: 829–840, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley AW Jr, Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 308: F179–F197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of helath examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 377: 568–577, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Evans LC, Ryan RP, Broadway E, Skelton MM, Kurth T, Cowley AW Jr. Null mutation of the NADPH oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and GFR. Hypertension 65: 561–568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng D, Yang C, Geurts A, Kurth T, Liang M, Lazar J, Mattson D, O'Connor P, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flister MJ, Hoffman MJ, Reddy P, Jacob HJ, Moreno C. Congenic mapping and sequence analysis of the renin locus. Hypertension 61: 850–860, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flister MJ, Tsaih SW, O'Meara CC, Endres B, Hoffman MJ, Geurts AM, Dwinell MR, Lazar J, Jacob HJ, Moreno C. Identifying multiple causative genes at a single GWAS locus. Genome Res 23: 1996–2002, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 597: 211–225, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW Jr. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 65: 447–455, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanian Z, Ranji M. Organ specific optical imaging of mitochondrial redox state in a rodent model of hereditary hemorrhagic telangidctasis-1. J Biophotonics 7: 799–809, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Liu Y, Usa K, Tian Z, Cowley AW Jr, Liang M. Ultrastructure of mitochondria and the endoplasmic reticulum in renal tubules of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 306: F1190–F1197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikal AA. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomarkers Med 4: 241–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman MK, Flister MK, Nunez L, Ziao B, Greene AS, Jacob HJ, Moreno C. Female-specific hypertension loci on rat chromosome 13. Hypertension 62: 557–563, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Manikoshi T, Thallas-Bonke VM, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provide renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25: 1237–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Koke JR, Wylie W, Wills M. Sensitivity of flavoprotein fluorescence to oxidative state in single isolated heart cells. Cytobios 32: 139–45, 1981. [PubMed] [Google Scholar]
- 26.Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease-a delicate balance. N Engl J Med 368: 1229–1237, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Li WG, Miller FJ Jr, Zhang HJ, Spitz Dr Oberley LW, Weintraub NL. H2O2-induced O2. production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem 276: 29251–29256, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maehara Y, Miyano K, Sumimoto H. Role for the first SH3 domain of p67phox in activation of superoxide-producing NADPH oxidases. Biochem Biophys Res Commun 379: 589–593, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Mandel LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int 29: 3–9, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25: 311–317, 2005. [DOI] [PubMed] [Google Scholar]
- 33.McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol 25: 1623–1628, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW Jr. Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics 31: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Ohsaki Y, O'Connor P, Mori T, Ryan RP, Dickinson BC, Chang CJ, Lu Y, Ito S, Cowley AW Jr. Increase of sodium delivery stimulates the mitochondrial respiratory chain H2O2 production in rat renal medullary thick ascending limb. Am J Physiol Renal Physiol 302: F95–F102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J am Soc Nephrol 10: 676–687, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33: 1451–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Roman RJ. Abnormal renal hemodynamics and pressure-natiruresis relationship in Dahl salt-sensitive rats. Am J Physiol Renal Fluid Electrolyte Physiol 251: F57–F65, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997. [PubMed] [Google Scholar]
- 40.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Scholz R, Thurman RG, Williamson JR, Chance B, Bucher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem 244: 2317–24, 1969. [PubMed] [Google Scholar]
- 42.Sepehr R, Staniszewski K, Maleki S, Jacobs ER, Audi S, Ranji M. Optical imagining of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress. J Biomed Opt 17: 046010, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 202. [DOI] [PubMed] [Google Scholar]
- 44.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol 48: 9–31, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Staniszewski K, Audi SH, Sepehr R, Jacobs ER, Ranji M. Surface fluorescence studies of tissue mitochondrial redox state in isolated perfused rat lungs. Ann Biomed Eng 41: 827–836, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoll M, Cowley AW Jr, Tonellato PJ, Greene AS, Kaldunski ML, Roman RJ, Dumas P, Schork NJ, Wang Z, Jacob HJ. A genomic-systems biology map for cardiovascular function. Science 294: 1723–1726, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan J, Prewitt RL, Ratts TE. Sodium sensitivity in normotensive and borderline hypertensive humans. Am J Med Sci 295: 370–377, 1988. [DOI] [PubMed] [Google Scholar]
- 48.Taylor NE, Cowley AW Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Taylor NE, Maier KG, Roman RJ, Cowley AW Jr. NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Tian Z, Greene AS, Usa K, Matus IR, Bauwens J, Pietrusz JL, Cowley AW Jr, Liang M. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension 51: 899–904, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–90, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Zheleznova NN, Yang C, Ryan RP, Halligan BD, Liang M, Greene AS, Cowley AW Jr. Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics 44: 829–842, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]


