Abstract
Chemoprevention of breast cancer is feasible with the use of non-toxic phytochemicals from edible and medicinal plants. Benzyl isothiocyanate (BITC) is one such plant compound that prevents mammary cancer development in a transgenic mouse model in association with tumor cell apoptosis. Prior studies from our laboratory have demonstrated a role for reactive oxygen species (ROS)-dependent Bax activation through the intermediary of c-Jun N-terminal kinases in BITC-induced apoptosis in human breast cancer cells. The present study demonstrates that truncated Recepteur d’Origine Nantais (sfRON) is a novel regulator of BITC-induced apoptosis in breast cancer cells. Overexpression of sfRON in MCF-7 and MDA-MB-361 cells resulted in augmentation of BITC-induced apoptosis when the apoptotic fraction was normalized against vehicle control for each cell type (untransfected and sfRON overexpressing cells). ROS generation and G2/M phase cell cycle arrest resulting from BITC treatment were significantly attenuated in sfRON overexpressing cells after normalization with vehicle control for each cell type. Increased BITC-induced apoptosis by sfRON overexpression was independent of c-Jun N-terminal kinase or p38 mitogen-activated protein kinase hyperphosphorylation. On the other hand, activation of Bax and Bak following BITC exposure was markedly more pronounced in sfRON overexpressing cells than in controls. sfRON overexpression also augmented apoptosis induction by structurally diverse cancer chemopreventive phytochemicals including withaferin A, phenethyl isothiocyanate, and D,L-sulforaphane. In conclusion, the present study provides novel mechanistic insights into the role of sfRON in apoptosis regulation by BITC and other electrophilic phytochemicals.
Keywords: benzyl isothiocyanate, sfRON, apoptosis, chemoprevention
INTRODUCTION
Chemical constituents of edible and medicinal plants remain appealing for prevention of breast cancer, which continues to be a serious public health concern for women worldwide [1–3]. More than 40,000 deaths are linked to breast cancer each year in the United States alone. Phytochemicals with in vivo preclinical evidence of mammary cancer prevention have been identified from common edible plants (e.g., watercress and garden cress) and certain medicinal plants (e.g., herbal plant Withania somnifera) [4–6]. Benzyl isothiocyanate (BITC), which occurs as a thioglucoside conjugate in certain cruciferous vegetables (e.g., garden cress) appears promising for prevention of breast cancer based on the following observations: (a) BITC administration prior to the carcinogen challenge inhibited 7,12-dimethylbenz[a]anthracene-induced mammary tumor formation in female Sprague-Dawley rats [7]; (b) dietary administration of 1 or 3 mmol BITC/kg diet for 25–29 weeks to female mouse mammary tumor virus-neu (MMTV-neu) transgenic mice produced a statistically significant decrease in cumulative incidence of hyperplasia and carcinoma as well as self-renewal of breast cancer stem cells [4,8]; (c) BITC treatment retarded in vivo growth of MDA-MB-231 human breast cancer cells implanted in female athymic mice [9]; (d) solid tumor growth as well as pulmonary metastasis of 4T1 murine mammary carcinoma cells orthotopically injected in syngeneic female BALB/c mice was inhibited after daily gavage with 5 and 10 mg BITC/kg body weight/day [10]; and (e) dietary BITC administration inhibited high fat diet-stimulated growth of 4T1 cells in obesity-resistant BALB/c mice [11]. Moreover, epidemiological studies have suggested an inverse association between intake of broccoli, a well-known dietary source of isothiocyanates, and breast cancer risk in premenopausal women [12].
Apoptosis induction is a well-established mechanism in cancer protective effect of BITC [4,10,13,14]. For example, in vivo inhibition of 4T1 tumor growth by BITC treatment was accompanied by increased Bax expression and cleavage of procaspase-3 and poly-(ADP-ribose)-polymerase [10]. Cell death induction by BITC in human breast cancer cells was closely linked to inhibition of complex III of the electron transport chain leading to production of reactive oxygen species (ROS) and eventually c-Jun N-terminal kinase (JNK) and p38 mitogen activated protein kinase (MAPK)-dependent activation of multidomain proapoptotic protein Bax [14]. Resistance of mitochondrial DNA deficient Rho-0 variant of MDA-MB-231 cells to Bax activation as well as apoptosis induction provided additional evidence for a role of this molecular pathway in BITC-induced cell death [14]. Studies have also revealed BITC-mediated induction of p53 upregulated modulator of apoptosis and suppression of X-linked inhibitor of apoptosis protein in cultured and xenografted MDA-MB-231 cells [15,16]. A role for FoxO1-mediated autophagy in the overall cell death by BITC has also been suggested previously [17]. The above molecular effects of BITC are apparent at pharmacologically relevant concentrations [13–18]. BITC is known to inhibit epithelial-mesenchymal transition in vitro and in vivo [19,20].
The breast cancer stem cell inhibition by BITC was accompanied by downregulation of full-length Recepteur d’Origine Nantais (RON) as well as its truncated form (sfRON) [8]. The sfRON, which retains the transmembrane and the intracellular domains, is constitutively phosphorylated and exhibits strong intrinsic receptor tyrosine kinase activity [21]. Furthermore, sfRON is sufficient to promote spontaneous metastasis [22]. The present study was undertaken to determine whether breast cancer cell growth inhibition by BITC was altered by sfRON status.
MATERIALS AND METHODS
Reagents and Cell Lines
Cell culture reagents (medium, fetal bovine serum, and antibiotics) were purchased from Invitrogen-Life Technologies (Carlsbad, CA, USA). Antibodies were purchased from the following vendors: anti-phospho-(T182)-p38 MAPK antibody was from Santa Cruz Biotechnology (Dallas, TX, USA); anti-phospho-(S70)-Bcl-2 and anti-phospho-(T183/Y185)-JNK antibodies were from Cell Signaling Technology (Beverly, MA, USA); monoclonal 6A7 antibody specific for detection of active Bax (for immunofluorescence microscopy) was from BD Biosciences (San Diego, CA); and anti-actin antibody was from Sigma-Aldrich (St. Louis, MO). An antibody specific for detection of active Bak (clone-TC-100) for immunofluorescence microscopy was from Calbiochem (Billerica, MA). 4′,6-diamidino-2-phenylindole (DAPI) was from Sigma-Aldrich (St. Louis, MO, USA). MitoSOX Red was from Invitrogen-Life Technologies. Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit was purchased from BD Biosciences. Sources of the test agents including BITC, phenethyl isothiocyanate (PEITC), withaferin A (WA), and D,L-sulforaphane (SFN) have been described by us previously [4–6,23]. Wild-type (untransfected) MCF-7 and MDA-MB-361 cells and those with stable overexpression of sfRON were generously provided by Dr. Alana L. Welm (University of Utah, Salt Lake City, UT [22]. Each cell line was maintained at 37°C in an atmosphere of 95% air and 5% CO2 according to the recommendations of the provider.
Cell Viability and Apoptosis Assays
Cell viability was determined by cell proliferation assay using a kit from Promega (Madison, WI). Apoptosis was assessed by quantitation of histone-associated DNA fragment release into the cytosol using a kit from Roche Applied Sciences (Indianapolis, IN). Annexin V/propidium iodide Apoptosis Detection kit was also used to quantify apoptotic (early + late) fraction by flow cytometry as suggested by the supplier. The results were normalized against corresponding dimethyl sulfoxide (DMSO)-treated control for each cell type (untransfected wild-type and sfRON overexpressing cells).
Detection of ROS
ROS production was measured by flow cytometry following staining with MitoSOX Red as described by us previously [24]. Cells were treated with DMSO (control) or desired concentrations of BITC for the indicated times and then incubated with 5 µM MitoSOX Red for 30 min. Cells were collected, washed with phosphate-buffered saline (PBS) and MitoSOX Red fluorescence was measured using BD Accuri flow cytometer. The results were normalized against corresponding DMSO-treated control for each cell type (untransfected wild-type cells and sfRON overexpressing cells).
Analysis of Cell Cycle Distribution
To determine cell cycle distribution, cells were treated with DMSO as a control or BITC for 3 or 6 h. After treatment, both floating and adherent cells were collected, washed with PBS, and fixed with 70% ethanol overnight at 4°C. The cells were then treated with 80 µg/mL RNase A and 50 µg/mL PI for 30 min and analyzed using BD Accuri flow cytometer. The results were normalized against corresponding DMSO-treated control for each cell type (untransfected wild-type and sfRON overexpressing cells).
Western Blotting
Control and BITC-treated cells were processed for immunoblotting essentially as described by us previously [25]. Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and wet transferred onto membrane. Membrane was incubated overnight with the primary antibody and then with an appropriate secondary antibody. Signals were detected using chemiluminescence reagent. Densitometric quantitation was done using UN-SCAN-IT software version 5.1 (Silk Scientific Corporation, Orem, Utah).
Immunocytochemical Analysis for Active Bax and Active Bak
Desired cells (2.5×104 cells/mL) were cultured on coverslips, allowed to attach, and then exposed to DMSO (control) or 2.5 or 5 µM BITC for 6 h. Cells were then fixed with paraformaldehyde and permeabilized with Triton X-100. Next, the cells were treated with PBS containing 0.5% bovine serum albumin and 0.15% glycine for 1 h and incubated with anti-active Bak or anti-active Bax antibody overnight at 4°C followed by incubation with Alexa Fluor 488-conjugated secondary antibody for 1 h at room temperature. Cell nuclei were stained with DAPI (10 ng/mL) for 5 min at room temperature. The cells were examined under a Leica DC 300F fluorescence microscope at 100× objective magnification.
RESULTS
sfRON overexpression augmented BITC-induced apoptosis in breast cancer cells
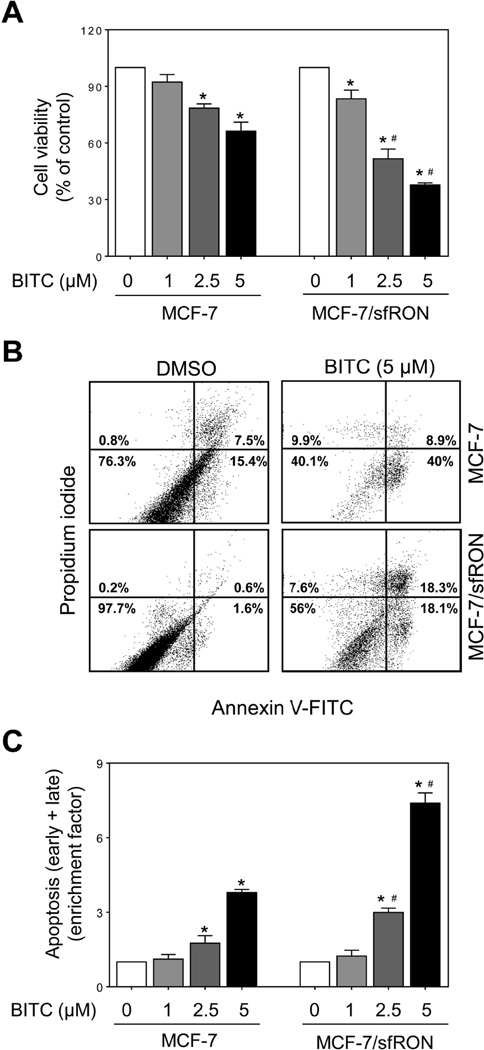
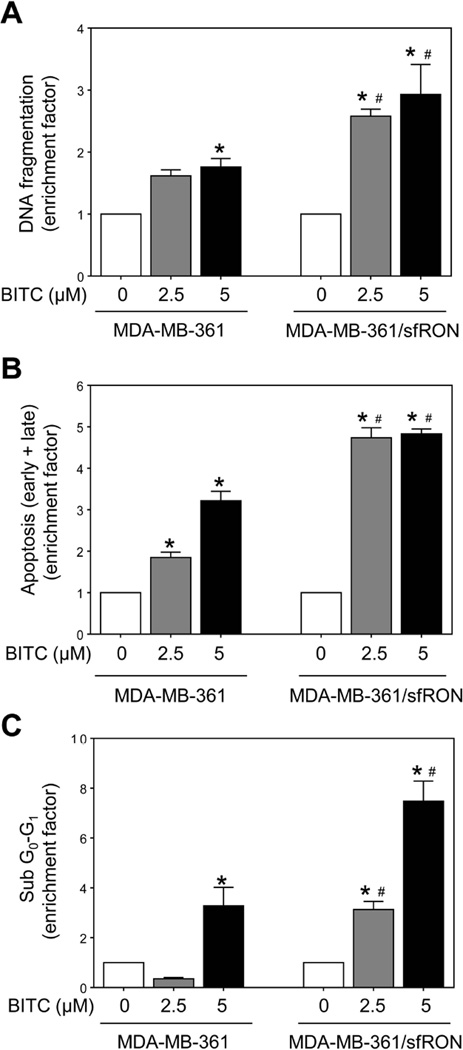
Overexpression of sfRON in stably transfected MCF-7 and MDA-MB-361 cells was confirmed by western blotting prior to functional assays (results not shown). Dose-dependent decrease in cell viability was observed in both MCF-7/sfRON and MCF-7 cells after 12 h treatment with BITC (Figure 1A). Overexpression of sfRON resulted in a modest but statistically significant increase in cell killing by BITC (Figure 1A). Figure 1B shows representative flow histograms for early (Annexin V+ and PI−) and late apoptotic cells (Annexin V+ and PI+) in control and sfRON overexpressing MCF-7 cultures following 24 h treatment with DMSO or BITC. In three independent experiments, basal apoptosis was relatively less pronounced in sfRON overexpressing MCF-7 cells than in untransfected (wild-type) control cells. Treatment with BITC resulted in a dose-dependent increase in apoptotic fraction in both MCF-7 and MCF-7/sfRON cells, but the apoptosis was significantly more pronounced in the MCF-7/sfRON cells than in control cells when the results were normalized against corresponding DMSO-treated control for each cell type (Figure 1C). Overexpression of sfRON augmented BITC-induced apoptosis in MDA-MB-361 cells as judged by quantitation of cytoplasmic histone-associated DNA fragment release into the cytosol (Figure 2A), Annexin V/PI positive cells (Figure 2B), and sub-diploid fraction (Figure 2C) when the values were normalized against corresponding DMSO control for each cell type. Similar to MCF-7 cells, basal cytoplasmic histone-associated DNA fragment release into the cytosol (in two independent experiments) as well as early + late apoptotic fraction (in two independent experiments) was reduced by sfRON overexpression in MDA-MB-361 cells compared with wild-type cells. These results indicated that sfRON overexpression sensitized breast cancer cells to BITC-induced apoptosis, and this association was not a cell line-specific phenomenon.
Fig 1.
Effect of sfRON overexpression on BITC-induced apoptosis in MCF-7 cells. (A) Cell viability in MCF-7 and MCF-7/sfRON cultures after 12 h treatment with DMSO or increasing concentrations of BITC. Results are normalized against respective DMSO control for each cell type. (B) Flow histograms showing apoptotic fraction in control and BITC-treated cells (24 h treatment). (C) Quantitation of apoptotic cells (enrichment factor) relative to corresponding DMSO-treated control for each cell type is shown (mean ± S.D.; n = 3). Significantly different (P < 0.05) compared with *respective DMSO-treated control and #between MCF-7 and MCF-7/sfRON cells by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Similar results were obtained from replicate experiments.
Fig 2.
Effect of sfRON overexpression on BITC-induced apoptosis in MDA-MB-361 cells. Quantitation of (A) histone-associated DNA fragment release into the cytosol, (B) apoptotic fraction (Annexin V/PI method), and (C) sub-diploid apoptotic fraction in MDA-MB-361 and MDA-MB-361/sfRON cells after 24 h treatment with DMSO or BITC. Quantitation of apoptotic fraction (enrichment factor) relative to corresponding DMSO-treated control for each cell type is shown (mean ± S.D.; n = 3). Significantly different (P < 0.05) compared with *respective DMSO-treated control and #between MDA-MB-361 and MDA-MB-361/sfRON cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were observed in repeated experiments.
Effect of sfRON overexpression on ROS generation by BITC
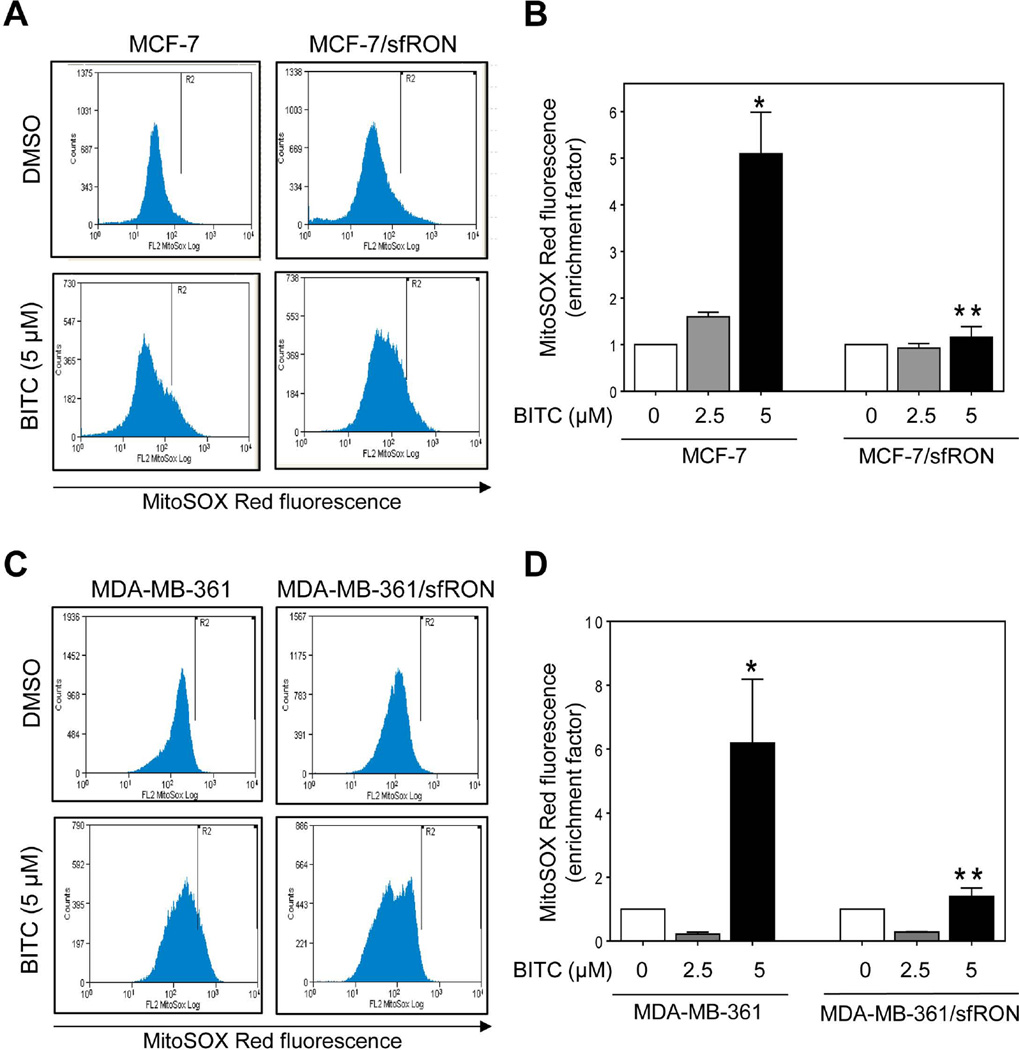
ROS is consistently implicated in BITC-induced apoptosis in different types of cancer cells [13,14,26,27]. Figure 3A shows flow histograms for MitoSOX Red fluorescence in wild-type MCF-7 cells or those overexpressing sfRON after 6 h treatment with DMSO or BITC. Treatment of MCF-7 cells with BITC resulted in enrichment of MitoSOX Red fluorescence that was statistically significant at the 5 µM dose (Figure 3B). However, ROS production upon treatment with BITC was not observed in MCF-7/sfRON cells (Figure 3B). Moreover, sfRON overexpression resulted in increased basal ROS in sfRON overexpressing MCF-7 cells (in two separate experiments) when compared with wild-type cells. Protection against BITC-induced ROS generation under similar treatment conditions was also observed in sfRON overexpressing MDA-MB-361 cells (Figure 3C,D). Unlike MCF-7 cells, however, sfRON overexpression failed to increase basal ROS in MDA-MB-361 cells at least in two independent experiments. These results indicated that BITC was able to trigger apoptosis in sfRON overexpressing cells without increasing ROS production.
Fig 3.
Effect of sfRON overexpression on BITC-mediated ROS generation. Flow histograms showing MitoSOX Red fluorescence in (A) wild-type MCF-7 and MCF-7/sfRON cells and (C) wild-type MDA-MB-361 and MDA-MB-361/sfRON cells after 6 h treatment with DMSO or BITC. MitoSOX Red fluorescence enrichment relative to corresponding DMSO-treated control in (B) wild-type MCF-7 cells and MCF-7/sfRON cells and (D) wild-type MDA-MB-361 cells and MDA-MB-361/sfRON cells (mean ± S.D., n = 3). Significantly different (P < 0.05) compared with *respective DMSO-treated control and **between wild-type and sfRON overexpressing cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Results were consistent in replicate experiments.
Effect of sfRON overexpression on BITC-mediated G2/M phase cell cycle arrest
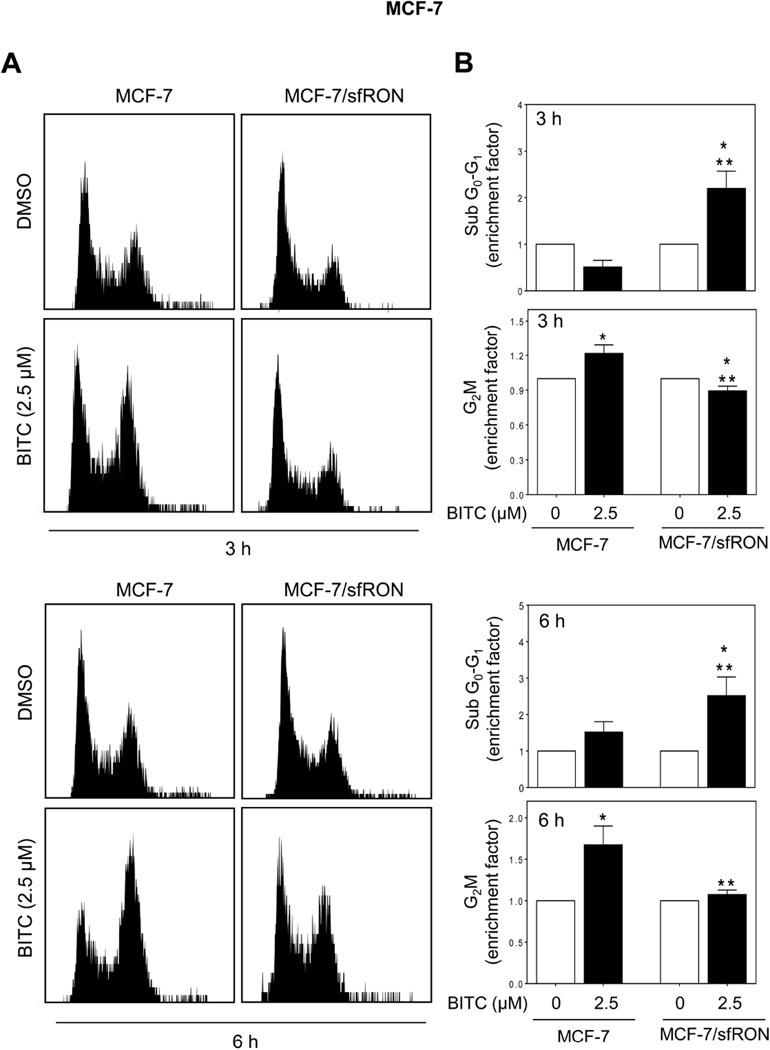
We have shown previously that BITC-treated MCF-7 and MDA-MB-231 cells are arrested in G2/M phase of the cell cycle [13]. Flow cytometry (Figure 4A) revealed statistically significant enrichment of G2/M fraction, but not sub-diploid apoptotic population, after 3 h or 6 h treatment of wild-type MCF-7 cells with 2.5 µM BITC when compared with DMSO control (Figure 4B). Basal G2/M fraction was comparable in wild-type and sfRON overexpressing MCF-7 cells. The G2/M phase arrest resulting from BITC treatment was abolished upon sfRON overexpression (Figure 4B). Instead, the sfRON overexpressing MCF-7 cells exhibited a significant increase in sub-diploid fraction after BITC treatment at both 3 h and 6 h time points. These results suggested that following BITC treatment, sfRON overexpression might override cell cycle arrest to trigger early onset of apoptosis.
Fig 4.
Effect of sfRON overexpression on BITC-induced G2/M phase cell cycle arrest. (A) Flow histograms demonstrate cell cycle distribution after 3 or 6 h treatment with DMSO or BITC (2.5 µM). (B) Sub G0-G1 and G2/M fractions in MCF-7 cells and MCF-7/sfRON cells after 3 h or 6 h treatment with DMSO or BITC (2.5 µM). Results are shown as enrichment factor relative to respective DMSO-treated control for each cell type (mean ± S.D., n = 3). Significantly different (P < 0.05) compared with *respective DMSO control and **between MCF-7 and MCF-7/sfRON cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were obtained from replicate experiments.
Effect of sfRON overexpression on BITC-induced activation of JNK and p38 MAPK
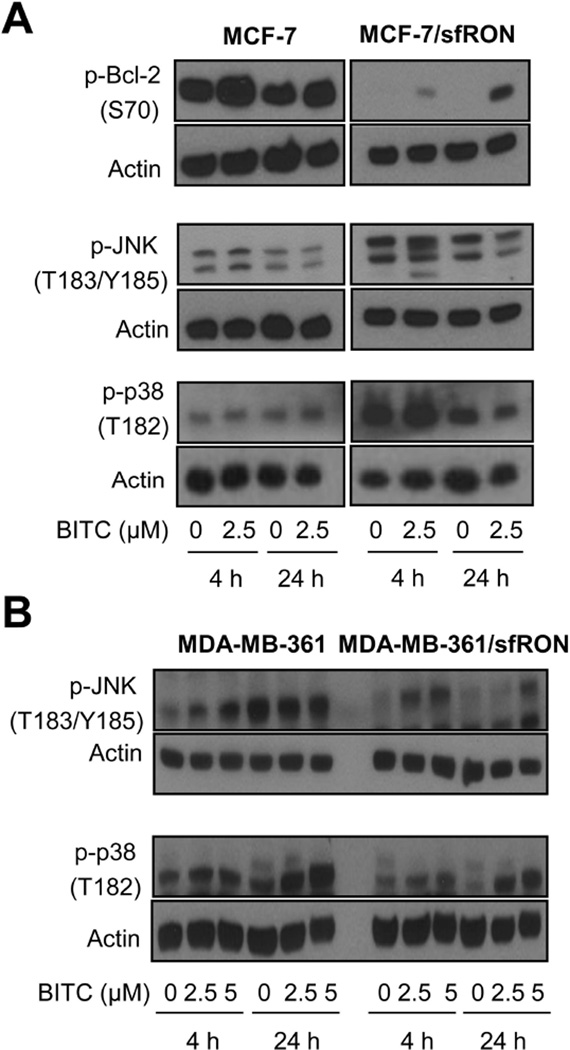
The key mechanism upstream of caspase activation in BITC-induced apoptosis in breast cancer cells involves ROS-dependent activation of JNK and p38 MAPK leading to activation of Bax [14]. BITC-induced apoptosis in some models is also accompanied by p38 MAPK-mediated phosphorylation of Bcl-2 [28]. Even though BITC treatment failed to produce ROS in sfRON overexpressing cells (Figure 3), it was of interest to determine the status of JNK and p38 activation as sfRON retains intrinsic kinase activity. We therefore determined the effect of BITC treatment on Bcl-2 (S70) phosphorylation, and activation of JNK and p38 MAPK in sfRON overexpressing MCF-7 and MDA-MB-361 cells and corresponding wild-type cells. In MCF-7 cells, BITC treatment caused an increase in S70 phosphorylation of Bcl-2 at both 4 h and 24 h time points (Figure 5A). Phosphorylation of JNK1/2 was increased 4 h after exposure to 2.5 µM BITC, followed by a decrease at the 24 h time point in MCF-7 cells (Figure 5A). Phosphorylation of p38 was more pronounced at 24 h time point in response to BITC treatment in MCF-7 cells in comparison with DMSO control. In contrast, MCF-7/sfRON cells showed complete loss of constitutive Bcl-2 phosphorylation that was partly restored as a function of time after treatment with BITC (Figure 5A). Interestingly, overexpression of sfRON resulted in increased constitutive activation of both JNK and p38 even in the absence of BITC treatment in MCF-7 cells, but BITC treatment failed to further activate them in MCF-7/sfRON cells (Figure 5A). In MDA-MB-361 cells, BITC treatment resulted in dose-dependent activation of JNK and p38 MAPK and these effects were sustained in sfRON overexpressing cells (Figure 5B). Cell line-specific differences in BITC-mediated activation of JNK and p38 MAPK were also discernible. In particular, unlike MCF-7 cells, constitutive activation of JNK or p38 by sfRON overexpression was not evident in MDA-MB-361 cells (Figure 5B). These results suggested that potentiation of BITC-induced apoptosis by sfRON overexpression was likely not related to Bcl-2 phosphorylation or JNK and p38 activation.
Fig 5.
Effect of sfRON overexpression on BITC-induced JNK and p38 MAPK activation. Western blotting for p-Bcl-2, p-JNK and/or p-p38 using lysates from (A) MCF-7 and MCF-7/sfRON cells, and (B) MDA-MB-361 and MDA-MB-361/sfRON cells after 4 or 24 h treatment with DMSO or BITC (2.5 or 5 µM). Each experiment was done at least twice and representative data from one such experiment are shown. BITC treatment was performed in medium supplemented with serum.
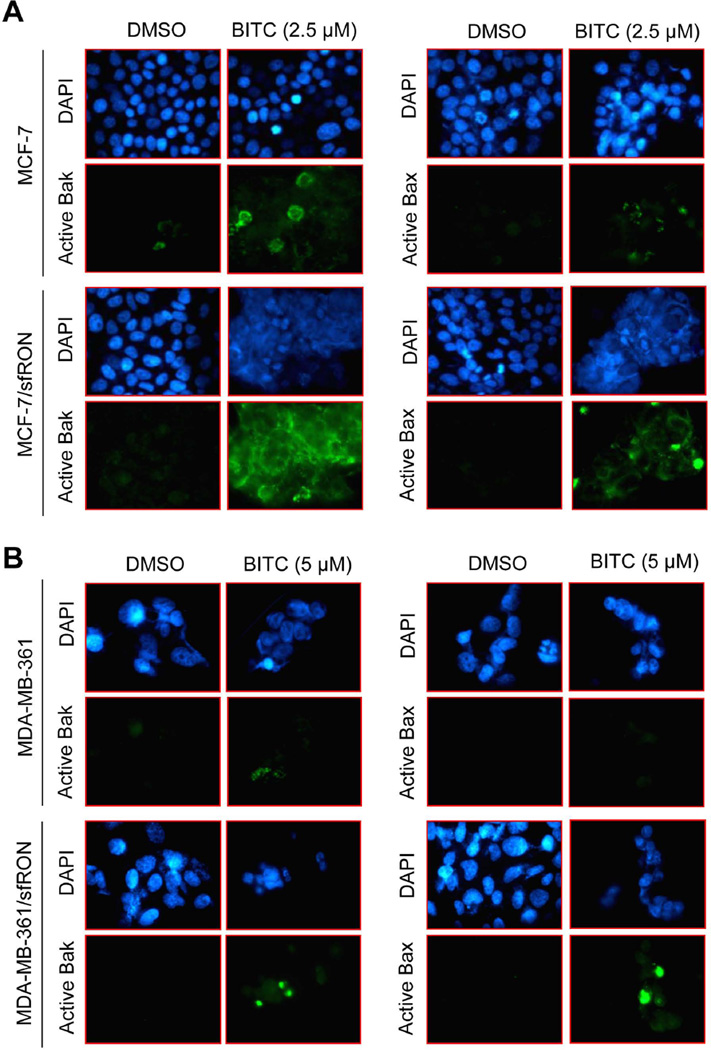
sfRON overexpression increased BITC-mediated activation of Bak and Bax
Activation of Bax is a critical event in BITC-induced apoptosis in breast cancer cells [14]. Therefore we determined the role of sfRON in BITC-mediated activation of Bax and Bak by immunofluorescence microscopy using antibodies specific for detection of respective activated proteins. BITC treatment for 6 h resulted in a modest increase in fluorescence associated with the active Bak and active Bax in both MCF-7 (Figure 6A) and MDA-MB-361 cells (Figure 6B) in comparison with DMSO-treated control. However, BITC exposure showed robust increase in Bak- or Bax-associated green fluorescence in sfRON overexpressing MCF-7 and MDA-MB-361 cells. These results indicated that overexpression of sfRON augmented BITC-mediated activation of Bak and Bax despite a lack of ROS production at least in MCF-7 and MDA-MB-361 cells.
Fig 6.
BITC-induced activation of Bax/Bak is augmented by sfRON overexpression. Fluorescence microscopy for analysis of active Bak and active Bax in (A) MCF-7 and MCF-7/sfRON cells, and (B) MDA-MB-361 and MDA-MB-361/sfRON cells following 6 h treatment with DMSO or BITC (2.5 or 5 µM). The results were consistent in replicate independent experiments.
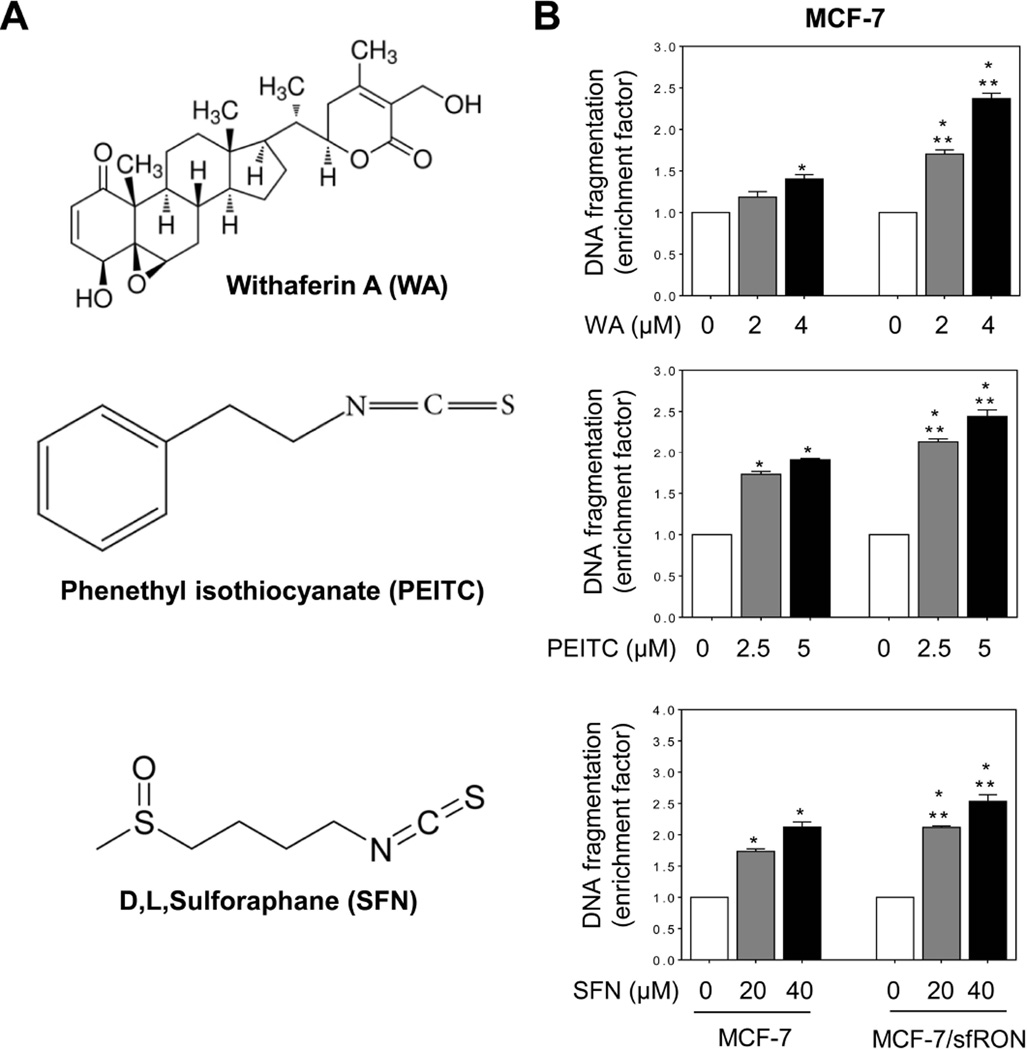
The role of sfRON in apoptosis induction by other cancer chemopreventive phytochemicals
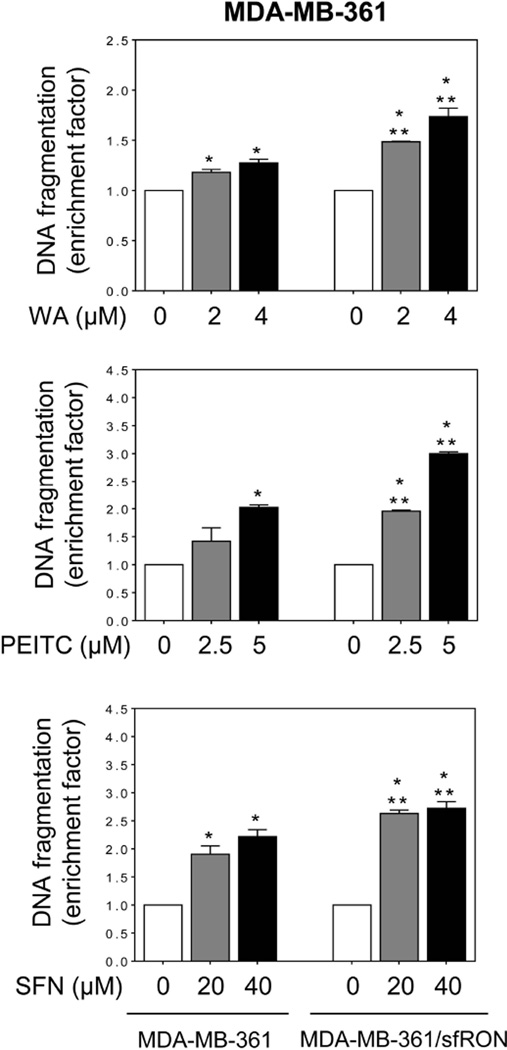
We used several cancer chemopreventive phytochemicals including WA, PEITC, and SFN [5,6,29] to determine if augmentation of apoptosis by sfRON overexpression was unique for BITC. Some of these agents are already under clinical investigation (www.ClinicalTrials.gov). WA (Figure 7A) is derived from a medicinal plant (Withania somnifera) used extensively in the Ayurvedic medicine practice [29]. WA administration inhibits mammary cancer development in MMTV-neu mice and MDA-MB-231 xenograft growth in athymic mice [6,30]. Overexpression of sfRON resulted in a significant increase in WA-induced apoptosis in both MCF-7 (Figure 7B) and MDA-MB-361 cells (Figure 8). PEITC is a close structural analogue of BITC, but noticeable differences in their preventive efficacy against chemically-induced cancers in rodents have been reported [31]. Similar to BITC, PEITC-induced apoptosis was significantly increased by overexpression of sfRON in both MCF-7 (Figure 7B) and MDA-MB-361 cells (Figure 8). Similar results were observed with SFN, which is a synthetic racemic analogue of broccoli constituent L-sulforaphane (Figure7B and Figure 8). Cancer chemopreventive effects of SFN have also been noted [23,32,33].
Fig 7.
Effect of sfRON overexpression on apoptosis induction by other electrophilic phytochemicals in MCF-7 cells. (A) Structures of WA, PEITC, and SFN. (B) Quantitation of histone-associated DNA fragment release into the cytosol in MCF-7 cells and MCF-7/sfRON cells after 24 h treatment with DMSO or the indicated compound. Data are shown as enrichment factor relative to DMSO-treated control for each cell type (mean ± S.D., n = 3). Significantly different (P < 0.05) compared with *respective DMSO-treated control and **between MCF-7 and MCF-7/sfRON cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were obtained from replicate experiments.
Fig 8.
Effect of overexpression of sfRON on apoptosis induction by WA, PEITC, and SFN in MDA-MB-361 cells. Quantitation of histone-associated DNA fragment release into the cytosol in MDA-MB-361 cells and MDA-MB-361/sfRON cells after 24 h treatment with DMSO or the indicated compound. Results are shown as enrichment factor relative to corresponding DMSO-treated control for each cell type (mean ± S.D., n = 3). Significantly different (P < 0.05) compared with *respective DMSO-treated control and **between MDA-MB-361 and MDA-MB-361/sfRON cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were observed in replicate experiments.
DISCUSSION
The RON receptor tyrosine kinase belonging to the c-Met proto-oncogene sub-family is expressed as a full-length (5 kb) and truncated forms (2 kb, sfRON) in breast cancer [34]. The sfRON, which is transcribed from an alternate transcriptional start due to a second promoter in exon 10 of RON, lacks the N-terminal including most of the extracellular domain while retaining the transmembrane and cytoplasmic domains as well as intrinsic kinase activity [22]. The role of sfRON in breast cancer is still poorly understood, but mammary-specific overexpression of RON induces highly metastatic tumors in mice [35]. Moreover, invasive potential of sfRON overexpression is shown to be dependent on phosphoinositide 3-kinase signaling [22]. Recent work from our own laboratory has revealed that expression of sfRON is markedly suppressed after treatment with BITC in cultured and xenografted breast cancer cells [8]. The present study shows that overexpression of sfRON sensitizes cells to BITC-induced apoptosis in both MCF-7 and MDA-MB-361 cell lines. The translational implication of this finding is that breast cancer cells with higher levels of sfRON may be more sensitive to killing by BITC than those with low expression.
The molecular circuitry of BITC-induced apoptosis is fairly well-mapped out at least in breast cancer cells [13,14]. These studies point towards a critical role for ROS, which are generated due to compromised complex III of the electron transport chain, in BITC-induced apoptosis [14]. A role for ROS in cell death induction by BITC has also been observed in other types of cancer cells [26,27]. It is interesting to note that ROS generation by BITC treatment is nearly fully abolished upon overexpression of sfRON in both MCF-7 and MDA-MB-361 cells (present study). It is still unclear if sfRON overexpression influences expression or assembly of complex III subunits. BITC is an electrophilic compound capable of reacting with protein sulfhydryl [36]. Thus it is possible that BITC post-translationally modifies one or more subunit(s) of complex III and this effect is hindered by sfRON overexpression. Clarification of this possibility awaits further investigation.
The known molecular events downstream of ROS generation in BITC-induced apoptosis include MAPK-mediated activation of Bax [14]. The present study shows that sfRON overexpression bypasses MAPK in BITC-mediated Bak/Bax activation in execution of BITC-induced apoptosis. Because basal activation of Bax or Bak is not affected by sfRON overexpression alone in either MCF-7 cells or MDA-MB-361 cells, it is reasonable to conclude that BITC treatment in sfRON overexpressing cells triggers alternate signaling to activate Bax and Bak without involving MAPK.
The G2/M phase cell cycle arrest is a common response to BITC treatment in breast and other types of cancer cells [13,26,37,38]. The G2/M phase arrested cells show increased sensitivity to BITC-induced apoptosis than non-arrested cells [28]. The mechanism underlying growth arrest by BITC seems complex involving downregulation of key proteins involved in G2/M progression (cyclin B1, cyclin-dependent kinase1, and cell division cycle 25C), covalent modification of tubulins, and activation of p38 [13,28,39]. BITC was shown to arrest Jurkat cells in G2/M phase of the cell cycle through activation of p38 MAPK because treatment with its inhibitor, but not a JNK-specific inhibitor, resulted in attenuation of the G2/M arrest [28]. However, lack of G2/M arrest in BITC-treated sfRON overexpressing cells is likely independent of p38 MAPK or JNK as marked differences are apparent in their activation after BITC treatment in MCF-7 versus MDA-MB-361 cells (present study). sfRON overexpression causes constitutive activation of both JNK and p38 MAPK in MCF-7 cells but not in the MDA-MB-361 cell line. sfRON overexpressing MDA-MB-361 cells, but not MCF-7/sfRON cells, are sensitive to JNK and p38 MAPK activation by BITC. Nevertheless, abrogation of cell cycle arrest likely contributes to increased apoptosis by BITC in sfRON overexpressing cells.
In conclusion, the present study reveals that sfRON overexpression augments BITC-induced apoptosis in association with ROS-independent activation of Bax and Bak, whose basal activation is not affected by sfRON alone. We demonstrate further that the positive association of sfRON overexpression with apoptosis is not unique to BITC but shared by other electrophilic cancer chemopreventive phytochemicals.
ACKNOWLEDGMENTS
The authors thank Diane Lowe for technical assistance. This work was supported by the grant RO1 CA129347-08 (to SVS), awarded by the National Cancer Institute, National Institutes of Health. This research project used the Flow Cytometry Facility supported in part by the Cancer Center Support Grant P30CA047904.
Abbreviations
- ANOVA
analysis of variance
- BITC
benzyl isothiocyanate
- DAPI
4’,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- JNK
c-Jun N-terminal kinases
- MAPK
mitogen-activated protein kinase
- MMTV-neu
mouse mammary tumor virus-neu
- PBS
phosphate-buffered saline
- PEITC
phenethyl isothiocyanate
- PI
propidium iodide
- RON
Recepteur d’origine nantais
- ROS
reactive oxygen species
- SFN
D,L-sulforaphane
- sfRON
short-form of RON
- WA
withaferin A
Footnotes
Conflict of interest: None of the authors has any conflict of interest.
REFERENCES
- 1.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;16:1–10. doi: 10.1208/s12248-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 4.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SV, Kim SH, Sehrawat A, et al. Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J Natl Cancer Inst. 2012;104:1228–1239. doi: 10.1093/jnci/djs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahm ER, Lee J, Kim SH, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically-relevant mouse model. J Natl Cancer Inst. 2013;105:1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Sehrawat A, Singh SV. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev Res (Phila) 2013;6:782–790. doi: 10.1158/1940-6207.CAPR-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol Carcinog. 2010;49:500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EJ, Hong JE, Eom SJ, Lee JY, Park JH. Oral administration of benzyl-isothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Res Treat. 2011;130:61–71. doi: 10.1007/s10549-010-1299-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Cho HJ, Kwon GT, et al. Benzyl isothiocyanate suppresses high-fat diet-stimulated mammary tumor progression via the alteration of tumor microenvironments in obesity-resistant BALB/c mice. Mol Carcinog. 2014 doi: 10.1002/mc.22159. In press. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 13.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 14.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antony ML, Kim SH, Singh SV. Critical role of p53 upregulated modulator of apoptosis in benzyl isothiocyanate-induced apoptotic cell death. PLoS ONE. 2012;7:e32267. doi: 10.1371/journal.pone.0032267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Singh SV. p53-Independent apoptosis by benzyl isothiocyanate in human breast cancer cells is mediated by suppression of XIAP expression. Cancer Prev Res (Phila) 2010;3:718–726. doi: 10.1158/1940-6207.CAPR-10-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao D, Bommareddy A, Kim SH, Sehrawat A, Hahm ER, Singh SV. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS ONE. 2012;7:e32597. doi: 10.1371/journal.pone.0032597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res (Phila) 2011;4:1107–1117. doi: 10.1158/1940-6207.CAPR-10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–873. doi: 10.1093/carcin/bgs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardella C, Costa B, Maggiora P, Patane' S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64:5154–5161. doi: 10.1158/0008-5472.CAN-04-0600. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zhao L, Derose YS, et al. Short-form Ron promotes spontaneous breast cancer metastasis through interaction with phosphoinositide 3-kinase. Genes Cancer. 2011;2:753–762. doi: 10.1177/1947601911421924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vyas AR, Hahm ER, Arlotti JA, et al. Chemoprevention of prostate cancer by D,L-sulforaphane is augmented by pharmacological inhibition of autophagy. Cancer Res. 2013;73:5985–5995. doi: 10.1158/0008-5472.CAN-13-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahm ER, Sakao K, Singh SV. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74:1209–1221. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 26.Huang SH, Wu LW, Huang AC, et al. Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways. J Agric Food Chem. 2012;60:665–675. doi: 10.1021/jf204193v. [DOI] [PubMed] [Google Scholar]
- 27.Liu BN, Yan HQ, Wu X, et al. Apoptosis induced by benzyl isothiocyanate in gefitinib-resistant lung cancer cells is associated with Akt/MAPK pathways and generation of reactive oxygen species. Cell Biochem Biophys. 2013;66:81–92. doi: 10.1007/s12013-012-9456-9. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi N, Uchida K, Osawa T, Nakamura Y. A link between benzyl isothiocyanate-induced cell cycle arrest and apoptosis: involvement of mitogen-activated protein kinases in the Bcl-2 phosphorylation. Cancer Res. 2004;64:2134–2142. doi: 10.1158/0008-5472.can-03-2296. [DOI] [PubMed] [Google Scholar]
- 29.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 30.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 32.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 33.Singh SV, Warin R, Xiao D, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angeloni D, Danilkovitch-Miagkova A, Ivanova T, Braga E, Zabarovsky E, Lerman MI. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene. 2007;26:4499–4512. doi: 10.1038/sj.onc.1210238. [DOI] [PubMed] [Google Scholar]
- 35.Zinser GM, Leonis MA, Toney K, et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with β-catenin activation. Cancer Res. 2006;66:11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
- 36.Mi L, Xiao Z, Veenstra TD, Chung FL. Proteomic identification of binding targets of isothiocyanates: a perspective on techniques. J Proteomics. 2011;74:1036–1044. doi: 10.1016/j.jprot.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakubikova J, Bao Y, Sedlak J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005;25:3375–3386. [PubMed] [Google Scholar]
- 38.Wu CL, Huang AC, Yang JS, et al. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 39.Mi L, Xiao Z, Hood BL, et al. Covalent binding to tubulin by isothiocyanates: a mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]