Abstract
Preclinical, epidemiological and prior clinical trial data suggest that green tea catechins (GTCs) may reduce prostate cancer (PCa) risk. We conducted a placebo-controlled, randomized clinical trial of Polyphenon E® (PolyE), a proprietary mixture of GTCs, containing 400 mg (–)-epigallocatechin-3-gallate (EGCG) per day, in 97 men with high-grade prostatic intraepithelial neoplasia (HGPIN) and/or atypical small acinar proliferation (ASAP). The primary study endpoint was a comparison of the cumulative one-year PCa rates on the two study arms. No differences in the number of PCa cases were observed: 5/49 (PolyE) versus 9/48 (placebo), P=0.25. A secondary endpoint comparing the cumulative rate of PCa plus ASAP among men with HGPIN without ASAP at baseline, revealed a decrease in this composite endpoint: 3/26 (PolyE) versus 10/25 (placebo), P<0.024. This finding was driven by a decrease in ASAP diagnoses on the Poly E (0/26) compared to the placebo arm (5/25). A decrease in serum prostate specific antigen (PSA) was observed on the PolyE arm [−0.87 ng/ml (95%CI: −1.66, −0.09)]. Adverse events related to the study agent did not significantly differ between the two study groups. Daily intake of a standardized, decaffeinated catechin mixture containing 400 mg EGCG per day for 1 year accumulated in plasma and was well tolerated but did not reduce the likelihood of PCa in men with baseline HGPIN or ASAP.
Introduction
Prostate cancer (PCa) remains the most common non-cutaneous malignancy among men in the United States, despite advances in the treatment of localized and metastatic disease over the past decade (1). The development of PCa is a long-term process driven by genetic and epigenetic changes (2) and characterized by abnormal cell and tissue differentiation (3). Although the natural history of high-grade prostatic intraepithelial neoplasia (HGPIN) is not completely understood, it is considered by many to be a premalignant lesion (4). The frequency of HGPIN (5, 6) and the autopsy prevalence of prostate cancer (7) are similar in populations with widely differing prostate cancer incidence and mortality rates, suggesting an environmental influence on the expression of this disease and the possibility of preventing it through pharmacological means (8–10). The 5-alpha-reductase inhibitors, finasteride and dutasteride, which block the conversion of testosterone to dihydrotestosterone, have been evaluated for PCa chemoprevention in large, phase III chemoprevention trials (11–13). Although these agents significantly reduced the risk of PCa, their use was also associated with increased detection of high-grade disease, severely limiting their clinical adoption and underscoring the need to identify novel prostate cancer prevention agents (12).
Green tea catechins (GTCs) influence proliferation, apoptosis and other hallmarks of carcinogenesis with an acceptable safety profile, making them attractive candidates for chemoprevention (14). Twenty percent of green tea is consumed in Asian countries where PCa mortality rates are among the lowest in the world (15) and the risk of PCa appears to be increased among Asian men who abandon their original dietary habits upon migrating to the U.S. (15). However, case-control and cohort studies addressing the relationship between GTC consumption and prostate cancer risk have been mixed (16, 17).
(GTCs) include ( )-epigallocatechin-3-gallate (EGCG), ( )-epicatechin (EC), ( )-epigallocatechin (EGC), and ( )-epicatechin-3-gallate (ECG). Among these compounds, laboratory studies have identified EGCG as the most potent modulator of molecular pathways thought to be relevant to prostate carcinogenesis (14, 16–19). Preclinical studies of GTCs (20–23) have shown significant reductions in tumor size and multiplicity in the PCa TRAMP mouse model, as well as potent and selective pro-apoptotic activity in PCa cell lines (16, 18–23). Phase I/II studies (24–30) have demonstrated good bioavailability and tolerability at doses ranging from 200–1200 mg EGCG per day. Bettuzzi et al. reported a significant reduction in prostate cancer in men with HGPIN randomized to receive one-year of EGCG (24, 25). However, nearly all of the prostate cancer risk-reduction in that study occurred at the 6-month biopsy, suggesting that the results may have been biased by a non-random distribution of occult prostate cancer at baseline. To further explore the potential role of GTCs for prostate cancer chemoprevention, we conducted a randomized, double-blind, placebo controlled trial of PolyE, a standardized formulation of GTCs containing 400 mg EGCG per day, in men with HGPIN and/or ASAP.
Materials and Methods
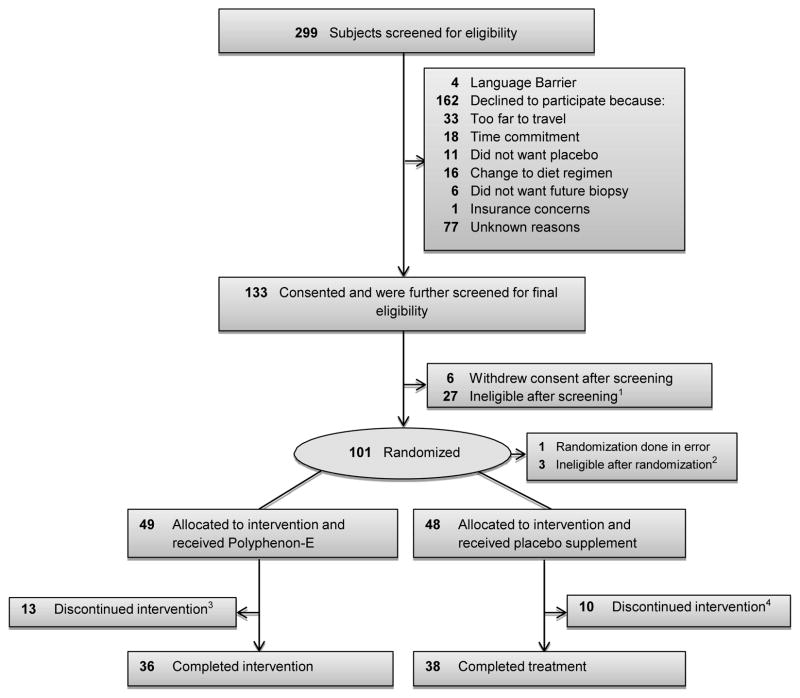
The study and the consent procedures were approved by the institutional review boards of all participating institutions. A consort diagram depicting the number of subjects screened, enrolled, randomized and completed intervention is shown in Figure 1.
Figure 1.
Consort Diagram
1Previously undetected exclusionary medical factors included 13 patients with elevated liver function results, 5 with other lab abnormality, 3 with history of GI disorder, 5 with PSA>10, and 1 taking exclusionary medication.
2Further review of pathology revealed PCa in 2 subjects (ASAP), 1 repeat diagnostic biopsy positive for PCa.(HGPIN)
32 subjects withdrew consent and 11 off study due to AE (4 unrelated, 6 probably related, and 1 possibly related to study)
43 subjects withdrew consent; 7 off study due to AE (5 unrelated and 2 probably related to study)
Eligibility and recruitment
Men between ages 30–80 with a biopsy-proven diagnosis of HGPIN and/or ASAP less than 3 months before randomization, with no history of cancer, hepatic or renal disease, restricted from taking steroid or other supplements, or more than 6–12 cups of green tea a day, were eligible. The original plan was to include only men with HGPIN. However, to expedite accrual, we expanded the inclusion criteria to also include men with ASAP, as this diagnosis has also been associated with prostate cancer risk. All prostate biopsies were reviewed by a central pathology laboratory and all pathologists were unaware of the treatment-group assignment. Discordant interpretations were arbitrated by a referee pathologist (senior pathologist at Moffitt Cancer Center), and concordance was achieved in all cases. Participants were enrolled at the Moffitt Cancer Center, James A. Haley VA Hospital, Tampa and University of Florida, Jacksonville, Florida from September 2008– March 2013. Less than 5% of subjects were recruited from other sites. Potential participants were identified by the primary surgeon and invited for eligibility screening. Screened subjects were recruited to a run-in period where a 10 day supply of multivitamin/mineral supplements, food intake and symptom logs were provided, designed to assure compliance with supplement intake and maintenance of the required study logs. Confirmation of diagnosis by central pathology review, ≥85% compliance to instructions during the run-in period, review and confirmation of inclusion and exclusion criteria and normal lab results were required for randomization.
Randomization and Blinding
After eligibility was confirmed and consent obtained, participants were assigned to the intervention or placebo arm (1:1) using the SRAR system, a web-delivered subject registration application, stratified by diagnosis (HGPIN or ASAP). All study staff and participants, with the exception of the clinical pharmacist and biostatistician, were blinded to the assignments until the completion of the trial. At randomization, baseline assessments of lower urinary tract symptoms (LUTS) using the LUTS Symptoms Scale, (31), quality of life (QOL), using the Rand Short-form (SF)-36 (32), PSA and plasma catechin levels were obtained. Blood samples, urine and tissue from diagnostic biopsy were collected for baseline measurements and banked for future studies.
Intervention
Polyphenon E™ (PolyE), an investigational agent manufactured by Mitsui Norin Co., Ltd., Shizuoka, Japan, was used in this clinical trial. The active pharmaceutical ingredient of PolyE is a purified tea fraction containing 80% to 98% total catechins by weight; the main component of which is EGCG, comprising 50% to 75% of the material. PolyE contains minimal amounts of caffeine, (<1.0%) theobromine (<1.0%) and gallic acid (<0.5%). The investigational product used in this study was a hard gelatin formulation containing 200 mg EGCG/capsule. PolyE and matching placebo capsules were manufactured under contract to NCI, DCP in compliance with current good manufacturing practice regulations. An investigator-initiated IND (77626 Kumar NB PI) was obtained for this agent at this dose and for this indication. Periodical testing was conducted to ensure drug stability with full potency of agent documented until March 2014. To minimize the use of other supplements, a standard vitamin and mineral formulation containing 100% U.S. recommended daily allowance was provided to all participants for the duration of the study.
Participant Follow-up
LUTS (31), QOL(32), plasma catechin levels, PSA and nutritional intake data were evaluated at baseline, 3 and 6 months, and at end-of-study (EOS). Monthly assessments of toxicity (CTCAE 4.0), concomitant medications and organ function, including hepatic panel, PT/PTT and LDH, were performed. Repeat biopsies were performed at six months for (a) PSA velocity >0.75ng/ml or (b) documentation of a prostate nodule on digital rectal examination. All participants who did not have PCa detected on an interim biopsy underwent EOS biopsy at 1 year. Any toxicities (adverse events) occurring during the study were reviewed by the treating physician and managed according to standard medical practice. The intervention was terminated if a participant developed PCa or a serious adverse event. All subjects were contacted 7±3 days following the 1-year intervention to assess toxicity and concomitant medications.
Adherence
Compliance with study agent intake was measured during monthly visits via pill counts and self-reported daily study-agent intake logs. Adherence was assessed by measuring plasma catechin levels at baseline, 6 months and EOS. A validated liquid chromatography triple quadrupole mass spectrometry (LC/MS/MS) method (Thermo Scientific, San Jose, CA) was used to determine plasma catechin levels. We were able to successfully quantitate the four catechins (EGCG, EGC, ECG and EC) using methods previously described (26, 33, 34).
Study End Points
The primary endpoint was a comparison of the cumulative number of PCa diagnoses at 1-year on the two study arms. As a pre-specified secondary endpoint we also compared the cumulative rate of PCa plus ASAP at 1-year among the men with HGPIN without ASAP at baseline. (Clinical Trials.gov NCT00596011). Additional secondary endpoints included comparisons of treatment-related adverse events (AEs) and the effect of PolyE on serum PSA values from baseline to 6 and 12 months.
Data Management and Study Monitoring
All collected data were entered from source documents or case report forms (CRF’s) directly into the web-based ONCORE system at each site by authorized, trained staff. Toxicities were monitored continuously through the trial by the PI and study physician at each site. The study was monitored in accordance with the Protocol Review and Monitoring System at the Moffitt Cancer Center and an External Data and Safety Monitoring Board (EDSMB).
Statistical Analysis
The original assumptions for the statistical power calculations for this study were derived from the trial by Bettuzzi et al. (24, 25) in which only 1 PCa was diagnosed among 30 men on the GTC arm (1/30;3%) at 1 year compared to nine in the placebo arm (9/30;30%), suggesting a 90% chemoprevention efficacy for this intervention in men with HGPIN. Based on prior reports of PCa rates among men with HGPIN (20%) (8, 35, 36) and ASAP (40%) (8, 37, 38), we anticipated that the overall one-year rate of PCa on the placebo arm would be 30%. This study had 79% power (2-sided) to detect a change from 30% to 9% with 50 patients per arm (derived from PASS 2008); with a power of 98% if the true rate of progression were .03 in the better group and .30 in the inferior group. The overall rate of PCa diagnoses among men with HGPIN or ASAP on baseline biopsy in the two treatment groups was compared using the log-rank test, with event times at either 6 or 12 months. A pre-specified secondary endpoint comparing the cumulative 1-year rate of PCa plus ASAP among men with HGPIN without ASAP at baseline was performed using the Barnard unconditional test, as no cases of PCa were detected before the 12 month EOS biopsy in this group. An intention-to-treat analysis was used for the primary efficacy endpoint. Baseline participant characteristics were compared between the two groups using Fisher exact tests for categorical variables and Wilcoxon rank-sum test for continuous variables. Trend for adverse events by group, grade and causality were compared using the Jonckheere-Terpstra test and toxicity symptoms using the Barnard unconditional test. Plasma EGCG levels, nutritional intake, LUTS and QOL were compared by study arm from baseline to end of intervention using 2-sided Wilcoxon rank-sum test. We estimated the overall treatment effect on serum PSA between the two arms using the GEE model which accounts for all 74 patients with PSA values at 6 and 12 months. To assess the effect of treatment on PCa grade in subjects who developed PCa while on study, we compared Gleason categories using a Fisher exact test at α = .05 for the 2×4 contingency table.
Results
Of a total of 299 men meeting all eligibility requirements, 97 were randomized on study (Figure 1). Forty-nine participants were randomized to the PolyE arm and 48 to the placebo arm, with 70 completing the 12-month intervention and 74 reaching the primary endpoint, with at least a 6 month biopsy. Table 1 displays the baseline characteristics of all study participants. The 2 study arms were well matched for potential predictive markers, including age, race, PSA, number of positive cores and body mass index (BMI).
Table 1.
Demographic characteristics of all study participants randomized to the clinical trial (N= 97)
| Variables | Levels | Poly E (N=49) | Placebo (N=48) | P value* |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (years) | Mean (SD) | 62.0 (7.9) | 64.1 (7.9) | 0.24 |
| Race | Black Or African American | 8 (16.3) | 12 (25.0) | |
| White | 41 (83.7) | 36 (75.0) | 0.32 | |
| Ethnicity | Hispanic | 6 (12.2) | 3 (6.3) | |
| Non-Hispanic | 42 (85.7) | 45 (93.8) | ||
| Unknown | 1 (2.0) | 0 (0.0) | 0.40 | |
| Family History of Prostate Cancer | N | 42 (85.7) | 45 (93.8) | |
| Y | 7 (14.3) | 3 (6.3) | 0.32 | |
| Body Mass Index (Weight in Kgs/height in m2) | Mean (SD) | 29.6 (4.9) | 29.8 (4.9) | 0.91 |
| PSA (ng/ml) | Mean (SD) | 4.5 (1.8) | 4.6 (2.1) | 0.67 |
| Subjects with baseline HGPIN | 32(65.3) | 34(70.8) | 0.66 | |
| No. of cores with baseline HGPIN | Mean (SD) | 1.8 (1.4) | 2 (1.1) | 0.13 |
| Subjects with baseline ASAP | 17 (34.7) | 14(29.2) | 0.66 | |
| No. of cores with baseline ASAP | Mean (SD) | 1.3 (0.6) | 1.5 (0.8) | 0.44 |
Abbreviations: ASAP, atypical small acinar proliferation; HGPIN, high-grade prostatic intraepithelial neoplasia; PSA, prostate-specific antigen; SD, standard deviation.
P values were computed by Fisher’s exact test for categorical variables, Wilcoxon rank-sum test for continuous variables
The primary endpoint of this study was not met as significant differences in PCa rates were not observed between the two study arms: 5/49 (10.2%) Poly E versus 9/48 (18.8 %%) placebo, P=0.25 (Table 2a). However, in a pre-specified secondary analysis performed in men with HGPIN without ASAP at baseline, a decrease in the composite endpoint of PCa plus ASAP was observed for the PolyE arm (3/26 PolyE versus 10/25 placebo, P<0.024). In addition, fewer men with HGPIN without ASAP at baseline were subsequently diagnosed with ASAP on the PolyE (0/26) than on the placebo arm (5/25) (Table2b). In this subgroup of men with HGPIN-only at baseline, no subjects met the criteria for biopsy at 6 months in either the PolyE or placebo arm. Among the men with ASAP at baseline, 2/17 in the PolyE arm versus 4/14 in the placebo arm were subsequently diagnosed with PCa over the 12 month study. Among the 10 cancers diagnosed on the 12-month biopsy, no significant effect of the intervention on tumor grade could be detected. No significant differences between the treatment and placebo arms were observed in LUTS and QOL scores from baseline to end of study(data not shown).
Table 2a.
Diagnosis of Prostate Cancer by treatment arm of men with baseline diagnosis of HGPIN and ASAP (N=97)
| Treatment | Total subjects | Censored** due to AE before 6 months (N) | PCa at 6 months (N) | Censored** due to AE between 6–12 months (N) | PCa at 12 months (N) | Censored** at 12 months (N) | Total PCa events | Log-rank P value* |
|---|---|---|---|---|---|---|---|---|
| Placebo | 48 | 12 | 3 | 1 | 6 | 26 | 9 | 0.25 |
| Polyphenon E | 49 | 14 | 1 | 1 | 4 | 29 | 5 | |
| Total | 97 | 26 | 4 | 2 | 10 | 55 | 14 |
The log-rank test is an overall comparison on the diagnosis of PCa, as a time-to-event endpoint.
Censored patients due to AEs.
Table 2b.
Combined rate of Prostate cancer + ASAP at 12 months in a subgroup of men with baseline HGPIN without ASAP (N=51)
| Treatment | Total subjects (N) | ASAP or PCa at 6 months (N) | ASAP at 12 months (N) | PCa at 12 months (N) | Combined rate of ASAP+PCa at 12 months (N) | P value* |
|---|---|---|---|---|---|---|
| Placebo | 25 | 0 | 5 | 5 | 10 | 0.024 |
| Polyphenon E | 26 | 0 | 0 | 3 | 3 | |
| Total | 51 | 0 | 5 | 8 | 13 |
Barnard’s unconditional test
A summary of all toxicities by final attribution appears in Table 3. There were more possible and probable events in the treatment arm compared to the placebo, all but one of which were Grade I or II. One participant on the PolyE arm had Grade III nausea possibly related to study agent. Based on the directive from FDA, eleven (11) subjects met off-study criteria due to AEs in the treatment arm compared to seven (7) in the placebo arm (data not shown). The number of subjects who met FDA-imposed off-study criteria due to grade I-II toxicity related to LFTs was not significantly different between the two groups. Both PolyE and the matching placebo used in the trial were hard gelatin capsules with no difference in appearance, taste or smell and subject to periodical testing to ensure drug stability, potency and other attributes of the study agent. Since Poly E was caffeine-free, there were no differences in patient reported caffeine-related or other symptoms. Although grade I-II toxicities were more frequently observed in the PolyE arm, these were determined from monthly CMP panels and not subject reported symptoms. Subjects and study staff were thus unable to guess group assignment based on reported symptoms, ensuring successful double blinding. Adherence to agent/placebo was greater than 90% as indicated by pill count, self-reported agent logs and plasma catechin levels.
Table 3.
Summary of Toxicities by Final Attribution and Treatment Arm - All Patients (N=97)
| Final Attribution | Treatment Arms | ||
|---|---|---|---|
| N (%) | Placebo | Polyphenon E | Total |
| Definite | 0 (0) | 0 (0) | 0 (0) |
| Possible | 3(1.78) | 7(3.30) | 10(2.62) |
| Probable | 1(0.59) | 5(2.36) | 6(1.57) |
| Unlikely | 9(5.33) | 7(3.30) | 16(4.20) |
| Unrelated | 156(92.31) | 193(91.04) | 349(91.60) |
| Total | 169(44.36) | 212(55.64) | 381(100.0) |
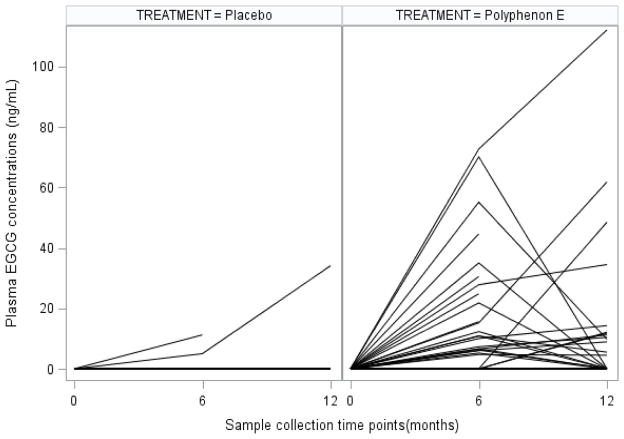
A significantly greater number of subjects in the treatment arm had an increase in plasma catechin EGCG concentrations at 6 and 12 months (p<.0001 and p=.0002, respectively) (Table 4). Greater individual change in plasma concentrations of EGCG was observed in the treatment arm at 6 and 12 months (Figure 2) in the PolyE arm compared to placebo. Although compliance was verified by pill counts and diaries, individual EGCG concentrations decreased in the second half of the year in the PolyE arm. Other catechins were non-detectable or below quantifiable levels in the plasma of all subjects. No significant change in intake of specific nutrients from baseline to the end of study was observed, indicating that compliance was maintained on both study arms (data not shown).
Table 4.
Plasma concentrations of EGCG from baseline to post intervention by study arm (N=74)
| Time (months) | Placebo | Polyphenon E | P value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N(EGCG>0) | 25% Quartile ng/mL | Median ng/mL | 75% Quartile ng/mL | N(EGCG>0) | 25% Quartile ng/mL | Median ng/mL | 75% Quartile ng/mL | ||
| 0 | 0/38 | 0 | 0 | 0 | 0/36 | 0 | 0 | 0 | 1 |
| 6 | 2/37 | 0 | 0 | 0 | 22/35 | 0 | 6.6 | 21.8 | <.0001 |
| 12 | 1/31 | 0 | 0 | 0 | 13/28 | 0 | 0 | 11.5 | 0.0002 |
P value comparing all individual patients’ plasma EGCG concentrations at each time point between placebo vs. poly E was calculated from Wilcoxon rank-sum test, 2-sided
Figure 2.
Change in individual plasma EGCG concentrations at baseline, 6 and 12 months by treatment arm.
Most subjects in the placebo arm had non-detectable plasma
EGCG concentrations at each time point.
A comparison of the estimated overall treatment effect showed a greater reduction of serum PSA in men on the PolyE arm (−0.87 ng/ml;95%CI: −1.66, −0.09) (Table 5). No effect on PSA was observed among the 14 men in whom PCa was diagnosed during the study.
Table 5.
GEE model of change in Serum PSA (ng/mL) from baseline to post intervention by study arm (N=74)
| Treatment | Time (Months) | N | 25% Quartile ng/mL | Median ng/mL | 75% Quartile ng/mL | Treatment effect on overall PSA change* | P value* |
|---|---|---|---|---|---|---|---|
| Placebo | 0 | 37 | 3.70 | 4.80 | 6.10 | −0.87 ng/ml (95%CI: −1.66, −0.09) | .029 |
| 6 | 36 | 3.55 | 5.45 | 6.30 | |||
| 12 | 28 | 3.65 | 4.90 | 6.15 | |||
| Polyphenon E | 0 | 36 | 3.50 | 4.45 | 5.60 | ||
| 6 | 35 | 2.80 | 3.70 | 5.20 | |||
| 12 | 29 | 2.80 | 3.50 | 5.10 |
The p-value and overall serum PSA change from GEE model accounts for all values at 6 and 12 months, and estimates an overall treatment effect during the study.
Discussion
In this phase II, randomized, placebo-controlled trial of PolyE in men with HGPIN and/or ASAP, no significant differences in PCa rates between the two study arms were observed at one year. Although PolyE was associated with a decrease in the composite endpoint (PCa plus ASAP), that finding was largely driven by the absence of ASAP on EOS biopsies on the PolyE arm. ASAP is an entity that reflects a broad group of lesions of varying clinical significance with insufficient cytological or architectural atypia to establish a definitive diagnosis of PCa (8, 38). To date, there is no clear evidence that HGPIN and ASAP represent steps on a linear path to PCa. Consequently, these findings should be interpreted with caution.
Notably, only 6 of 31 men with baseline ASAP were subsequently diagnosed with prostate cancer during this 1-year study. Previous reports of cancer detection rates within one year of a diagnosis of ASAP have ranged from 40% (8) to 59.1% (37–39) depending on the number of cores sampled on the diagnostic and follow-up biopsies, suggesting that this diagnosis may reflect a poorly sampled PCa (40–43). Our requirement of at least 8 cores on the diagnostic biopsy may explain the relatively low rates of cancer detected on subsequent biopsies in patients with ASAP in this study.
As our power calculation assumptions were based on a higher incidence of prostate cancer on the placebo arm than was ultimately observed (30% versus 18.8%), and as only 65% of our participants had HGPIN at baseline (53% with HGPIN-only and 12% with HGPIN + ASAP), this study did not have sufficient power to detect small differences in prostate cancer rates in the HGPIN cohort. However, these data are in sharp contrast to the large effect size suggested by Bettuzzi, et al., who reported a 90% reduction in prostate cancer among men with HGPIN randomized to receive GTCs for one year. As noted above, the fact that nearly all of the benefit in that study occurred by 6-months suggests that sampling error may have contributed to their findings (24, 25).
The PolyE dose and administration guidelines, e.g., required to be taken with food, were selected to minimize toxicity. While the tolerability of EGCG at doses of up to 1200 mg/day (24–26, 33, 34, 44, 45) has been well documented, legitimate concerns persisted regarding the safety of prolonged administration. Increased oral bioavailability occurs when GTCs are consumed in a fasting state (34) and increased toxicity, including hepatotoxicity had been reported in animal studies (46, 47) and in anecdotal reports in humans (48–50). Therefore, the FDA restricted the PolyE dosage to 200 mg BID EGCG and required that it be taken with food. Due to reports of liver toxicity in clinical and preclinical trials (46–50), the FDA also required that a liver panel be obtained at baseline and every four weeks during treatment. Following any elevation in alanine transferase, the study drug was withheld (grade 1) or discontinued (grade 2), and serum enzymes monitored until recovery to normal.
A significant increase in plasma EGCG concentration was achieved in the treatment arm at 6 and 12 months, although mean plasma EGCG concentrations [6 months: 14.7 ng/ml SD:19.9 and 12 months: 12.3 ng/ml SD:24.8] were lower than reported in previous phase I trials (34, 45). Nguyen et al. (45) reported plasma EGCG concentrations of 68.8 ng/ml after 3–6 weeks of PolyE (dosed at 800 mg EGCG per day) during the pre-prostatectomy period. Notably, catechin levels in prostate tissue were low to undetectable following the short-term administration of PolyE in that study, raising questions regarding the plausibility that this agent could have an effect at the tissue level. Studies on single doses in fasting and fed conditions using 400, 800 and 1200 mg EGCG per day have reported higher plasma EGCG concentrations in fasting conditions relative to fed conditions. Studies using varying doses (400 mg, 800 mg EGCG) of GTCs and PolyE administered in single and repeated dosing schedules for 4 weeks have reported maximum concentrations of EGCG levels 390.3 6 ng/ml and 287.6 ng/ml (800 mg EGCG) and 161.4 and 155.4 ng/ml (400 mg EGCG) respectively (33). On the other hand, Lee et al reported much lower peak plasma concentrations of EGCG [34.72 ng/ml (SD 22.87)] compared to others with a single administration of 2 mg EGCG/kg body weight.(51). Additionally, similar to previous observations (33, 34, 45) not all subjects in the treatment arm had detectable levels of EGCG. Although instructed to take the dose of PolyE within 4 hours of the blood draw, with travel time and scheduling challenges, these subjects may have not complied with these instructions. In addition, individual variation in absorption cannot be discounted.
The value of PSA changes in a chemoprevention setting is debatable. Despite this drawback, serum PSA as a continuous variable has been widely used in PCa chemoprevention trials (52–54) as well as in clinical practice, where PSA levels are used to define risk categories (37, 55–57). In contrast to prior reports (25, 45), PolyE was associated with a decrease in serum PSA in the current trial. However, among the 14 men who were diagnosed with PCa during the study, a significant decrease in PSA was not observed. Although the mechanism(s) that could explain the PSA reduction are unclear, there is emerging evidence from epidemiological, histopathological and molecular pathological studies that inflammation plays role in the etiology of PCa (58–60). It is, therefore, tempting to speculate that the reduction in serum PSA with GTCs could be due to reduced inflammation. However, several challenges remain regarding the inflammation hypothesis, including the determination of the cause(s) of chronic inflammation in the prostate and whether inflammation plays a causative role in prostate carcinogenesis (58–60).
LUTS represents a common conglomeration of storage, voiding, and post-micturition symptoms with potentially debilitating effects on quality of life (31, 61, 62). Studies have demonstrated an increased prevalence of LUTS in men over age 60 and in those with benign prostatic hyperplasia (BPH) (31, 61, 62). We were not able to evaluate the effect of PolyE on LUTS as the study participants were generally asymptomatic at baseline.
Randomized chemoprevention trials using agents similar to readily available over-the-counter supplements present unique challenges to recruitment and retention (63). Although several infrastructure, protocol-related and personal factors were taken into consideration while designing the clinical trial, safety monitoring imposed by the FDA continued to challenge recruitment and retention. Only thirty-three percent of an eligible pool of 299 men were ultimately randomized on-study: 162 were unwilling to comply with protocol requirements and 77 refused to participate for unknown reasons. Although those men were unwilling to document their reason for not participating, stringent protocol requirements requiring monthly blood draws was a contributing factor. Similarly, the completion rate of this one-year intervention was only 76% (74/98), much lower than other chemoprevention trials (11–13, 24, 25). The low completion rate was related to FDA-imposed early stopping rules for grade I–II toxicity. Although attention to safety is critical in developing interventions targeting healthy populations, imposing safety requirements typical of cancer treatment trials can undermine chemoprevention agent development efforts, especially when the concerns are derived from animal studies and case reports of adverse events associated with significantly higher doses than those being proposed.
The strengths of our study include the randomized, placebo-controlled, double-blinded design, the use of a standardized agent, prior clinical, as well as preclinical and epidemiological evidence of potential efficacy, the relatively long, one-year study duration and the use of a clinically relevant endpoint, prostate cancer, as the primary study objective. The study was guided by an FDA IND, with stringent eligibility criteria, frequent and extensive toxicity monitoring, and early stopping rules for all grades of toxicity and was conducted with the same rigor by which most therapeutic agents are evaluated. While these factors contributed to the rigor of the study design and conduct, they adversely affected accrual and study completion rates. In addition, as the rate of PCa in our placebo group was lower than expected, and given that only about half of the study participants had HGPIN at baseline, our study was ultimately underpowered to detect small reductions in prostate cancer rates with GTCs in men with HGPIN.
Conclusion
Daily intake of a standardized catechin mixture containing EGCG, 200 mg BID, for one year, accumulated in plasma and was well tolerated but did not reduce the likelihood of a subsequent PCa diagnosis in men with baseline HGPIN or ASAP. Our study confirmed the observations of Epstein (8) and others (53, 54) that the risk of PCa on biopsy within one year following a diagnosis of HGPIN is only about 20% if good sampling is initially performed. In addition, the very low one-year rate of PCa observed in men with ASAP in this trial suggests that earlier reports may have over-estimated the true risk of cancer in that cohort, possibly due to poor initial sampling. Apart from meticulously selecting promising agents, validated biomarkers and study endpoints, future PCa chemoprevention trials should ideally enroll larger cohorts of men at higher risk for this disease, perhaps with durations of interventions that continue beyond one year.
Acknowledgments
Financial Support:
The research funding was awarded to Nagi B Kumar by the National Institute of Health - National Cancer Institute R01 CA12060-01A1.
We thank the following study participants for their dedicated contributions and acknowledge the contribution of Anthony M. Neuger (Moffitt Cancer Center) for performing the plasma catechin assays; Domenico Coppola(Moffitt Cancer Center) for providing central verification of pathological findings; Kyle Anderson (Minneapolis VA Medical Center, Minneapolis, MN); Eduard Trabulsi (Jefferson Medical College, Philadelphia, PA); Tajammul Fazili (Overton Brooks VA, Shreveport, LA); Gregory Zagaja (University of Chicago, Chicago, IL.) for their efforts in recruitment of subjects in this trial; Edward Giovanucci, MD,(Harvard University) for his assistance with identifying epidemiological questionnaire; Omer Kucuk, MD (Emory University), Phyllis Bowen, PhD (University of Illinois at Chicago) and Steven Clinton, MD, PhD (Ohio State University) for their contribution as External Data Monitoring Board members.
Footnotes
Trial Registration: Clinical Trials.gov Identifier: NCT00596011
Publications/Presentations:
- Proffered abstract presentation at the AACR Thirteenth Annual International Conference on Frontiers in Cancer Prevention Research, New Orleans, LA on September 28 - October 1, 2014.
- Poster presentations at the AACR Seventh Annual Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved Conference, November 9–12, 2014 in San Antonio, TX.
Disclaimers: No authors listed in this manuscript have direct or indirect commercial financial incentive associated with publishing the article.
References
- 1.American Cancer Society. http://www.cancer.org/Cancer/ProstateCancer/DetailedGuide/prostate-cancer-key-staistics.
- 2.Lippman SM, Hong WK. Cancer prevention science and practice. Cancer research. 2002;62:5119–25. [PubMed] [Google Scholar]
- 3.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–30. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 4.Montironi R, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M. Mechanisms of disease: high-grade prostatic intraepithelial neoplasia and other proposed preneoplastic lesions in the prostate. Nature clinical practice. Urology. 2007;4:321–32. doi: 10.1038/ncpuro0815. [DOI] [PubMed] [Google Scholar]
- 5.Han KS, Jeong IG, Joung JY, Yang SO, Chung J, Seo HK, et al. Prevalence of high-grade prostatic intraepithelial neoplasia in prostate gland of Korean men: comparisons between radical prostatectomy and cystoprostatectomy. Urology. 2007;70:1100–3. doi: 10.1016/j.urology.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Tan PH, Tan HW, Tan Y, Lim CN, Cheng C, Epstein JI. Is high-grade prostatic intraepithelial neoplasia on needle biopsy different in an Asian population: a clinicopathologic study performed in Singapore. Urology. 2006;68:800–3. doi: 10.1016/j.urology.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Zlotta AR, Egawa S, Pushkar D, Govorov A, Kimura T, Kido M, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. Journal of the National Cancer Institute. 2013;105:1050–8. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820–34. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 9.Kelloff GJ, Lieberman R, Steele VE, Boone CW, Lubet RA, Kopelovitch L, et al. Chemoprevention of prostate cancer: concepts and strategies. European urology. 1999;35:342–50. doi: 10.1159/000019906. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman R. Prostate cancer chemoprevention: Strategies for designing efficient clinical trials. Urology. 2001;57:224–9. doi: 10.1016/s0090-4295(00)00981-x. [DOI] [PubMed] [Google Scholar]
- 11.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton RJ, Kahwati LC, Kinsinger LS. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2164–71. doi: 10.1158/1055-9965.EPI-10-0082. [DOI] [PubMed] [Google Scholar]
- 13.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 14.Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutrition and cancer. 2012;64:4–22. doi: 10.1080/01635581.2012.630158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K. Prostate cancer in Asian men. Nature reviews Urology. 2014;11:197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 16.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. International journal of cancer Journal international du cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. The American journal of clinical nutrition. 2013;98:1676S–81S. doi: 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochemical pharmacology. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer control : journal of the Moffitt Cancer Center. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 20.Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1947–53. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, et al. Clinical Development Plan: Tea extracts, green tea polyphenp;s, epigallocatechin gallate. J Cell Biochem. 1996;26:236–57. [PubMed] [Google Scholar]
- 22.Khan N, Mukhtar H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochemical pharmacology. 2013;85:667–72. doi: 10.1016/j.bcp.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. The Journal of biological chemistry. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 24.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer research. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 25.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. European urology. 2008;54:472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 26.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:53–8. [PubMed] [Google Scholar]
- 27.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. The Prostate. 2007;67:1576–89. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 29.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutrition and cancer. 2009;61:836–41. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SJ, Amankwah E, Connors S, Park HY, Rincon M, Cornnell H, et al. Safety and chemopreventive effect of Polyphenon E in preventing early and metastatic progression of prostate cancer in TRAMP mice. Cancer prevention research. 2014;7:435–44. doi: 10.1158/1940-6207.CAPR-13-0427-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marberger M. Medical management of lower urinary tract symptoms in men with benign prostatic enlargement. Advances in therapy. 2013;30:309–19. doi: 10.1007/s12325-013-0022-7. [DOI] [PubMed] [Google Scholar]
- 32.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:3312–9. [PubMed] [Google Scholar]
- 34.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 35.Hoedemaeker RF, Kranse R, Rietbergen JB, Kruger AE, Schroder FH, van der Kwast TH. Evaluation of prostate needle biopsies in a population-based screening study: the impact of borderline lesions. Cancer. 1999;85:145–52. [PubMed] [Google Scholar]
- 36.O'Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553–9. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 37.Amin MM, Jeyaganth S, Fahmy N, Begin L, Aronson S, Jacobson S, et al. Subsequent prostate cancer detection in patients with prostatic intraepithelial neoplasia or atypical small acinar proliferation. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2007;1:245–9. doi: 10.5489/cuaj.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iczkowski KA. Current prostate biopsy interpretation: criteria for cancer, atypical small acinar proliferation, high-grade prostatic intraepithelial neoplasia, and use of immunostains. Archives of pathology & laboratory medicine. 2006;130:835–43. doi: 10.5858/2006-130-835-CPBICF. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang RC, Kenwright DN, Nacey JN, Delahunt B. The presence of atypical small acinar proliferation in prostate needle biopsy is predictive of carcinoma on subsequent biopsy. BJU international. 2001;87:70–4. doi: 10.1046/j.1464-410x.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 40.Adamczyk P, Wolski Z, Butkiewicz R, Nussbeutel J, Drewa T. Significance of atypical small acinar proliferation and extensive high-grade prostatic intraepithelial neoplasm in clinical practice. Central European journal of urology. 2014;67:136–41. doi: 10.5173/ceju.2014.02.art4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199–202. discussion -3. [PubMed] [Google Scholar]
- 42.Presti JC, Jr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–6. discussion 6–7. [PubMed] [Google Scholar]
- 43.Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166:86–91. discussion -2. [PubMed] [Google Scholar]
- 44.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer prevention research. 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, et al. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer prevention research. 2012;5:290–8. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapetanovic IM, Crowell JA, Krishnaraj R, Zakharov A, Lindeblad M, Lyubimov A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260:28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu KM, Yao J, Boring D. Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. International journal of toxicology. 2011;30:19–20. doi: 10.1177/1091581810387445. [DOI] [PubMed] [Google Scholar]
- 48.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. European journal of clinical pharmacology. 2009;65:331–41. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 49.Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, et al. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12:1892–5. doi: 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- 50.Rohde J, Jacobsen C, Kromann-Andersen H. Toxic hepatitis triggered by green tea. Ugeskrift for laeger. 2011;173:205–6. [PubMed] [Google Scholar]
- 51.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1025–32. [PubMed] [Google Scholar]
- 52.Gee J, Bailey H, Kim K, Kolesar J, Havighurst T, Tutsch KD, et al. Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. The Prostate. 2013;73:970–8. doi: 10.1002/pros.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herawi M, Kahane H, Cavallo C, Epstein JI. Risk of prostate cancer on first re-biopsy within 1 year following a diagnosis of high grade prostatic intraepithelial neoplasia is related to the number of cores sampled. J Urol. 2006;175:121–4. doi: 10.1016/S0022-5347(05)00064-9. [DOI] [PubMed] [Google Scholar]
- 54.Taneja SS, Morton R, Barnette G, Sieber P, Hancock ML, Steiner M. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:523–9. doi: 10.1200/JCO.2012.41.7634. [DOI] [PubMed] [Google Scholar]
- 55.Bryant RJ, Lilja H. Emerging PSA-based tests to improve screening. The Urologic clinics of North America. 2014;41:267–76. doi: 10.1016/j.ucl.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 57.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nature reviews Cancer. 2008;8:268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 58.Kryvenko ON, Jankowski M, Chitale DA, Tang D, Rundle A, Trudeau S, et al. Inflammation and preneoplastic lesions in benign prostate as risk factors for prostate cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:1023–32. doi: 10.1038/modpathol.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schatteman PH, Hoekx L, Wyndaele JJ, Jeuris W, Van Marck E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. European urology. 2000;37:404–12. doi: 10.1159/000020161. [DOI] [PubMed] [Google Scholar]
- 60.Yli-Hemminki TH, Laurila M, Auvinen A, Maattanen L, Huhtala H, Tammela TL, et al. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU international. 2013;112:735–41. doi: 10.1111/bju.12153. [DOI] [PubMed] [Google Scholar]
- 61.Hung SF, Chung SD, Kuo HC. Increased serum C-reactive protein level is associated with increased storage lower urinary tract symptoms in men with benign prostatic hyperplasia. PloS one. 2014;9:e85588. doi: 10.1371/journal.pone.0085588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao CH, Chung SD, Kuo HC. Serum C-reactive protein levels are associated with residual urgency symptoms in patients with benign prostatic hyperplasia after medical treatment. Urology. 2011;78:1373–8. doi: 10.1016/j.urology.2011.04.076. [DOI] [PubMed] [Google Scholar]
- 63.Kumar N, Crocker T, Smith T, Pow-Sang J, Spiess PE, Egan K, et al. Challenges and potential solutions to meeting accrual goals in a Phase II chemoprevention trial for prostate cancer. Contemp Clin Trials. 2012;33:279–85. doi: 10.1016/j.cct.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]