Abstract
Objectives
To perform a systematic review of the growing body of literature evaluating the prognostic value of epicardial fat volume (EFV) quantified by cross-sectional imaging for adverse clinical outcomes.
Methods
Two independent reviewers performed systematic searches on both PubMed and Scopus using search terms developed with a medical librarian. Peer-reviewed articles were selected based on the inclusion of outcome data, utilization of epicardial fat volume and sufficient reporting for analysis.
Results
A total of 411 studies were evaluated with 9 studies meeting the inclusion criteria. In all, the studies evaluated 10,252 patients. All 9 studies were based on CT measurements. Seven studies evaluated the prognostic value of EFV unadjusted for calcium score, and 6 of these studies found a significant association between EFV and clinical outcomes. Seven studies evaluated the incremental value of EFV beyond calcium scoring, and 6 of these studies found a significant association.
Conclusions
The majority of studies suggest that EFV quantification is significantly associated with clinical outcomes and provides incremental prognostic value over coronary artery calcium scoring. Future research should use a binary cut-off of 125mL for evaluation of EFV to provide consistency with other research.
Keywords: Epicardial Fat, Coronary Artery Calcium, Cardiac Computed Tomography, Major Adverse Cardiac Events, Prognostic Value
Introduction
Epicardial fat volume (EFV) has been correlated with numerous disease processes including coronary artery disease, atrial fibrillation and diabetes [1-5]. It has been demonstrated that epicardial fat is metabolically different from other visceral fat both biochemically and in terms of its correlation to cardiac risk factors [6-9]. Emerging techniques for the measurement of EFV are reducing the complexity of its measurement and opening the potential for its inclusion into clinical workflows [10; 11].
EFV measurements by computed tomography (CT) have been shown to be predictive of myocardial ischemia by SPECT [12] and PET [13]. Several studies have investigated the prognostic value of EFV using non-contrast CT and cardiac CT angiography [7; 14-19]. These studies postulate a prognostic role of EFV for clinical outcomes although there is variation in classification and methodology of fat measurement [7; 14-18; 20]. In particular, it is currently not clear whether EFV quantification provides incremental diagnostic value over coronary artery calcium (CAC) scoring on CT. This is of particular clinical interest, since it would provide the rationale for adding routine quantification of EFV to the evaluation of CAC scoring studies.
The present review, therefore, sought to summarize the available evidence on the prognostic value of EFV measurements on cross-sectional imaging for clinical outcomes through a systematic review of the literature.
Methods
Search Strategy
The present study sought to investigate the prognostic value of EFV for major adverse cardiac events (MACE) or all-cause mortality. In coordination with a medical librarian (T.L.H.) experienced in systemic literature reviews, these elements were used to develop a comprehensive search strategy for PubMed and Scopus. The PubMed search included the medical subject headings (MeSH) terms “diagnostic imaging”, “adipose tissue” and “pericardium”, as well as the keywords (“epicardial” OR “pericardial”) and (“fat” OR “adipose”). The results were also filtered for human subjects and English language which yielded 218 relevant publications. The Scopus search included the terms and filters mentioned above and excluded the Medline results and review publications yielding 193 relevant studies. Searches were carried out in June of 2014. We additionally hand-searched the references list of all eligible studies and relevant review articles and consulted a cardiac imaging expert in order to ensure that no relevant studies had been missed.
Search Strategy
Two investigators independently reviewed the search results and determined study eligibility. Studies were evaluated for inclusion into the systematic review if they (a) were performed with cross-sectional imaging - CT (both contrast-enhanced and non-contrast examinations) or magnetic resonance imaging - and (b) included prognostic analysis using MACE or all-cause mortality as the endpoint. Because thickness of cardiac fat has been shown to be widely anatomically variable by region of measurement [21], sonographic studies of fat thickness were not included. Since we were specifically interested in the prognostic value of EFV, we only included longitudinal studies reporting the association between EFV and adverse events occurring after the imaging examination. We did not consider cross-sectional studies on the association between EFV and the prevalence of cardiovacular disease at the time of the imaging examination. The Framingham Heart Study Offspring cohort has previously demonstrated a significant cross-sectional association between EFV and prevalent cardiovascular disease [22].
Data extraction
Two independent investigators (J.V.S. and M.R.) extracted information on the following variables: Number of patients included into the study; inclusion and exclusion criteria; endpoint definition; gender; age; presence or absence of diabetes or hypertension; measurements of body mass index, Framingham Risk Score, CAC score; EFV; years of follow up; number of events; statistical model used; multivariate adjustments; and methods of EFV aggregation. Discrepancies between the two investigators were resolved by discussion and re-examination of the corresponding studies together with a senior investigator (F.G.M.). The number of events was derived from the original studies for each type of endpoint. If the absolute number of events was not directly provided in the manuscript, they were derived from the provided information whenever possible. We extracted the hazard ratios (HR) or odds ratios (OR) and the corresponding 95% confidence intervals of the individual studies as well as the corresponding increments of EFV. In order to minimize confounding, we used the most extensively adjusted HR / OR derived from multivariate regression analysis, if available.
Study quality assessment
Study quality assessment was performed to allow readers to judge the overall quality of the studies included in this systematic review. We did not exclude any studies on the grounds of insufficient study quality. Study quality indicators were chosen as described in a previous meta-analysis [23] and included clear description of the target population, clear description of and justification for exclusion of patients after enrolment, presence of an endpoint committee, quantification of EFV blinded to outcome, outcome assessment blinded to EFV, adjustment for age, gender and cardiovascular risk factors, clear description of EFV quantification method, clear description of endpoints. Each item was rated by two independent reviewers (J.V.S. and A.W.K.) as either 1 for completely fulfilled, 0.5 for partially fulfilled or 0 for not described/not fulfilled. Thus a study’s quality score could theoretically range from 0 to 8.
Results
Study Selection and Characteristics
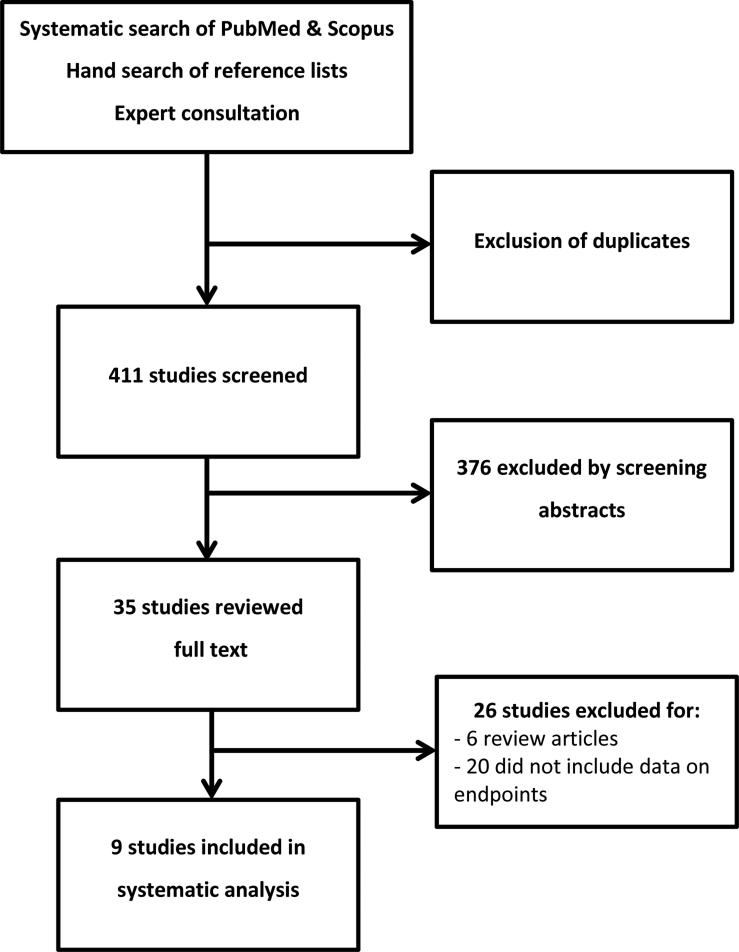
After exclusion of duplicates, a total of 411 studies were identified using our search criteria in Scopus and PubMed and hand-searching reference lists (Figure 1). Of these 376 were discarded based on the abstract. The 35 remaining studies were analysed in full text. Nine of them met the criteria for inclusion in this systematic review. The characteristics of the included studies are summarized in (Table 1). The majority of included studies had a single-centre design (5 of 9) and were conducted in Asia, Europe or the United States (2, 1 and 6, respectively). Of the 9 studies there were one prospective study [15], three case control studies (two of which matched from the same cohort) [16; 18; 24] and five retrospective re-analyses of previously published prospective studies on CAC scoring [7; 14; 17; 19; 20].
Figure 1.
Diagram of study selection process as recommended for systematic reviews and meta-analyses in the QUOROM statement [31].
Table 1.
Summary of Included Studies
| First Author (Ref #) | Year | Inclusion criteria | Exclusion criteria | Primary Endpoints |
|---|---|---|---|---|
| Britton [20] | 2013 | Third generation of Framingham heart study who underwent multi-detector CT | None | MACE as myocardial infarction, angina pectoris, coronary insufficiency, cerebrovascular accident, transient ischemic attack, intermittent claudication, congestive heart failure, cardiovascular death, cancer (excluding non-melanoma skin cancer) or all-cause mortality |
| Cheng [16] | 2010 | Men ≥ 55 years or women ≥ 65 years; men ≥ 45 years with at least 1 traditional CAD risk factor | History of myocardial infarction, coronary revascularization, cardiomyopathy, peripheral artery disease, angina or stroke; prior CAC score; invasive angiogram; active pregnancy; significant comorbidity | MACE as cardiac death, myocardial infarction, stroke, late percutaneous or surgical revascularization |
| Ding [24] | 2009 | randomly selected from Multi-Ethnic Study of Atherosclerosis (MESA) participants and MESA participants who developed incident coronary heart disease | Individuals with physician-diagnosed cardiovascular disease or any related procedures at baseline were not eligible | Coronary heart disease events as myocardial infarction, resuscitated cardiac arrest, angina, or fatal coronary heart disease |
| D'Marco [17] | 2013 | Renagel in New Dialysis – CKD stage 5 with no history of dialysis at outset, transplant, coronary bypass graft or intra coronary stenting, atrial fibrillation or atrial flutter | Error in the image file, or inadvertent exclusion of parts of the pericardium at the time of image acquisition | All-cause mortality |
| Forouzandeh [14] | 2013 | Age > 18 years; chest pain within 24 hours suggestive of ischemia, admission under observational status | prior CAD, ischemic electrocardiogram findings diagnostic of an acute coronary syndrome, troponin >0.1 ng/mL, hemodynamic instability | MACE as cardiac death, nonfatal myocardial infarction, and unstable angina pectoris |
| Greif [15] | 2012 | coronary artery stenosis (1 >50% blockage in coronary angiography) or prior myocardial infarction | acute coronary syndrome, advanced ischemic cardiomyopathy, or coronary artery by-pass grafting | severe cardiac events as cardiac death, myocardial infarction or coronary revascularization |
| Kunita[19] | 2014 | no proven CAD, no history of myocardial infarction, prior coronary revascularization, status of acute coronary syndrome or typical effort angina | proven cardiomyopathy, severe valvular heart disease, renal impairments (creatinine > 1.5 mg/dL), serious life-threatening illness or early coronary revascularizations | cardiac death, non-fatal myocardial infarction, or unstable angina requiring hospitalization, and late coronary revascularizations |
| Mahabadi [7] | 2013 | Heinz Nixdorf Recall -population-based cohort study with subjects randomly selected from mandatory lists of residence | Known coronary artery disease, history of myocardial infarction, or open heart surgery (including bypass and valve surgery) | Nonfatal myocardial infarction or cardiac death |
| Shmilovich [18] | 2011 | Asymptomatic status with no known cardiovascular disease, diabetes mellitus, or smoking; CAC = 0, LDL < 160, triglyceride < 500, FRS < 6% | Pericardial effusion or thickening, lower-than-excellent image quality on non contrast-enhanced cardiac CT | MACE as cardiovascular death, myocardial infarction, stroke, percutaneous or surgical coronary artery revascularization |
CAC – coronary artery calcium score; CAD – coronary artery disease; CKD – chronic kidney disease; FRS – Framingham Risk Score; LDL – low density lipoprotein; MACE – major adverse cardiac event;
In all, the studies evaluated 10,252 patients. The patient population was dominated by two large population-based cohort studies with 4,093 and 3,086 patients [7; 20], respectively, with a total of 3,073 patients in the remaining seven studies (Table 2). One study specifically included patients with acute chest pain suggestive of ischemia [15]. Two of the studies used the same population from a registry of 232 asymptomatic patients with no known cardiac disease [16; 18]. One study was a random selection from the MESA cohort study [24]. One study was a subset of patients with CAC scores in a low to intermediate risk category [14]. One study included only patients on haemodialysis [17]. One study included patients undergoing calcium scoring without proven CAD [19]. A summary of the inclusion and exclusion criteria of all studies is shown in Table 1. Table 3 summarizes the length of follow-up and number of events for each study.
Table 2.
Summary of Patient Characteristics of Included Studies
| First Author (Ref #) | Total Number | Male | Age* (years) | BMI* (kg/m2) | Diabetes | Hypertension | FRS* | CAC* | EFV* (mL) |
|---|---|---|---|---|---|---|---|---|---|
| Britton [20] | 3,086 | 51% | 50.2±10 | 27.7±5.2 | 5.4% | 27% | NR | NR | 111±43 |
| Cheng [16] | 232 | 79% | 61±9 | 28.5±4.9 | 18% | 65% | 13±7 | 50.4±7.8 | 89±41 |
| Ding [24] | 1,119 | 47% | 60±10 | 27.9 | 10.7% | 37% | NR | NR | 82 |
| D'Marco [17] | 95 | 61% | 58±15 | 25.4±15.3 | 59% | 97% | NR | 95.7 | 113 |
| Forouzandeh [14] | 760 | 41% | 54±14 | 30.6±7.3 | 15% | 57% | 8±8 | 125±429 | 127±61 |
| Greif [15] | 145 | 65% | 60±10 | NR | 17% | 74% | NR | 847±1,555 | 240±110 |
| Kunita[19] | 722 | 61% | 65±11 | 23.7±3.5 | 33% | 58% | NR | 24 | 107 |
| Mahabadi [7] | 4,093 | 47% | 59±8 | NR | 12% | 32% | NR | NR | 86 |
| Shmilovich [18] | 226 | 51% | 52±9 | 26.8±4.9 | 0% | 34% | 2 | NR | 65 |
BMI – body mass index; CAC – coronary artery calcium score; EFV – epicardial fat volume; FRS – Framingham Risk Score;
* values are represented as averages±standard deviation, otherwise values are medians
Table 3.
Adverse Events by Study
| First Author (Ref #) | Follow-Up, yrs | Total, n | Deaths from any cause, n | Deaths from cardiac cause, n | MACEs, n |
|---|---|---|---|---|---|
| Britton [20] | 5 | 3,086 | 71 | NR | 90 |
| Cheng [16] | 4 | 232 | NR | 4 | 58 |
| Ding [24] | 2 | 1,119 | NR | NR | 147 |
| D'Marco [17] | 4.1 | 95 | 27 | NR | NR |
| Forouzandeh [14] | 3.3 | 760 | NR | 6 | 45 |
| Greif [15] | 5.4 | 145 | 6 | 4 | 32 |
| Kunita [19] | 3.7 | 722 | NR | 5 | 37 |
| Mahabadi [7] | 8 | 4,093 | NR | 39 | 130 |
| Shmilovich [18] | 4 | 290 | NR | 4 | 58 |
MACE – major adverse cardiac event; NR – not reported
All nine studies were based on CT measurements; no eligible studies on magnetic resonance imaging were identified. Seven of the 9 studies measured only the adipose tissue contained within the pericardium (regardless of whether this was referred to as “epicardial” or “pericardial” fat). Two studies measured fat around the heart both within (“epicardial”) and adjacent to the pericardium (“paracardial”) and referred to the sum of both as “pericardial” fat [15; 24]. Endpoints were MACE in 8 studies, and all-cause mortality in 2 studies (1 studies used both endpoints). Mortality from cardiac causes was also reported in 7 studies but not used for the prognostic analysis.
There was great variability in the statistical methods used for evaluation, primarily due to differences in study design. The six cohort studies performed Cox regression analysis and calculated hazard ratios. The three case-control studies calculated Odds ratios based on multivariate logistic regression. The increments of EFV used varied widely. Some studies evaluated it using incremental gradations [17]. Some studies used tertiles [20] or doublings [7]. And yet others used a binary threshold from the literature of 125 mL [14]. One study, reevaluating the same population as Cheng et al., used the binary threshold normalized to the surface area of the patient [18] which they referred to as indexed EFV. Greif and colleagues evaluated the hazard ratio at 200 mL increments [15]. Kunita et al based EFV hazard ratios on individual values being above or below the median (107.2 mL) of the included patients [19]. Ding et al measured EFV only in a limited stack of images at the level of the left main coronary artery (this method was validated against total EFV in a small subset of patients) and used a binary cutoff for their analysis of prognostic value [24].
Study quality
The quality assessment results are shown in Figure 2. The overall mean of the quality scores was high (mean 6.4, range 4.5 – 7.5) with two studies scoring below six on our 0 to 8 point scale. Only four studies used endpoint committees and only four clearly described blinding assessment of outcomes. Similarly, blinding of patient information for EFV measurement was only sufficiently described in four studies. Descriptions were very clear, however, for study populations, exclusion criteria, EFV measurement techniques and endpoints. All studies included adjustments for risk factors, though risk factor selection varied (Table 4).
Figure 2.
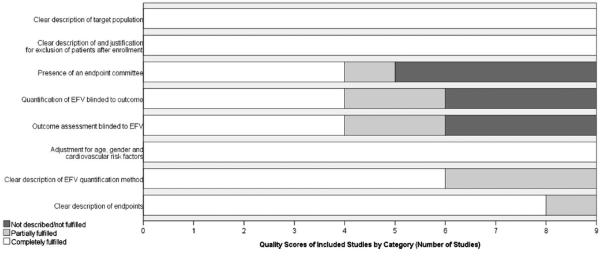
Summary of study quality of articles included in the systematic review. EFV – Epicardial fat volume
Table 4.
Prognostic Value of EFV
| First Author (Ref #) | Total, n | Outcome | Events | EFV Aggregation | OR | HR | Multivariate Adjustments |
|---|---|---|---|---|---|---|---|
| Britton [20] | 3,086 | All-cause mortality | 71 | One standard deviation increments | 1.31 (1.10-1.55) | Age, gender | |
| MACE | 90 | 1.24 (1.05-1.46) | |||||
| All-cause mortality | 71 | 1.17 (0.95-1.44) | Age, gender, systolic blood pressure, diabetes, total cholesterol, HDL cholesterol, smoking, hypertension treatment, and BMI | ||||
| MACE | 90 | 1.11 (0.91-1.35) | |||||
| Ding [24] | 1,119 | MACE | 147 | One standard deviation | 1.26 (1.01-1.59) | age, sex, ethnicity, BMI, smoking, alcohol, physical activity, education, systolic blood pressure, antihypertensive medication, cholesterol, fasting glucose, diabetes medication, and C-reactive protein. | |
| Forouzandeh [14] | 760 | MACE | 45 | > 125 mL | 2.31 (1.21-4.42) | FRS and BMI | |
| Greif [15] | 143 (2 excluded) | MACE | 32 | 200 mL Increments | >200 ml: 2.1(1.4-3.2) >400 ml: 2.2(1.5-3.4) >600 ml: 2.5 (1.6-3.9) |
Age, gender and coronary risk factors | |
| Kunita [19] | 722 | MACE | 37 | > 107.2 mL | 2.65 (1.23-5.70) | Adjusted for age, sex, BMI, hypertension, diabetes mellitus, and smoking | |
| Mahabadi [7] | 4,093 | MACE | 130 | Doubling of Volume | 1.54 (1.09-2.19) | Age, gender and traditional risk factors | |
| Shmilovich [18] | 226 | MACE | 58 | > 68 mL/m2 | 3.1 (1.4-6.9) | Unadjusted (EFV indexed to BSA – roughly equivalent to 125 mL) |
BMI – body mass index; BSA – body surface area; EFV – epicardial fat volume; FRS – Framingham Risk Score; HDL – high density lipoproteins; HR – hazard ratio; MACE – major adverse cardiac event; OR – odds ratio;
Prognostic value of EFV
Seven studies with a total of 10,149 patients evaluated the prognostic value of EFV unadjusted for CAC score (Table 4). Six of these studies evaluating a total of 7,063 patients found a significant prognostic value of EFV for future adverse events. Of these studies, five were adjusted for age, gender and cardiovascular risk factors and one reported an unadjusted OR. The seventh study analysing 3,086 patients found a significant association of EFV with outcome when adjusting only for age and gender, but the prognostic value was no longer significant when cardiovascular risk factors were added as covariates.
Incremental prognostic value of EFV beyond CAC scoring
Seven studies with a total of 6,271 patients evaluated the prognostic value of EFV adjusted for CAC score to determine if there is an incremental prognostic value of EFV beyond CAC scoring (Table 5). In all of these studies, a multivariate analysis was performed with CAC score, age, gender and cardiovascular risk factors as covariates. Six of these studies with a total of 5,511 patients reported that EFV is an independent predictor of adverse events after adjusting for CAC score. One of these analysed a population of 760 patients and found a trend towards a prognostic value (HR=1.59 (0.81-3.09) for EFV > 125 mL), which did not reach statistical significance. One study found that indexing EFV to body surface area improved its incremental prognostic value over CAC scoring and cardiovascular risk factors [18].
Table 5.
Incremental Prognostic Value of EFV beyond Coronary Artery Calcium Scoring
| First Author (Ref #) | Total, n | Outcome | Events | EFV Aggregation | OR | HR | Multivariate Adjustments |
|---|---|---|---|---|---|---|---|
| Cheng [16] | 232 | MACE | 58 | Log of Volume in Continuous Increments | 1.74 (1.03-2.95) | Age, traditional risk factors, CAC and FRS | |
| D'Marco [17] | 95 | All-cause mortality | 27 | 10 mL Increments | 1.06 (1.01-1.05) | Age, gender, race, BMI, HDL cholesterol, total CAC | |
| Forouzandeh [14] | 760 | MACE | 45 | > 125 mL | 1.59 (0.81-3.09) | FRS, BMI and CAC | |
| Greif [15] | 143 (2 excluded) | MACE | 32 | 200 mL Increments | CAC>400, EFV>200: 2.9 (1.9-4.5) CAC>400, EFV>400: 3.0 (1.9-4.5) CAC>400, EFV>600: 3.0 (1.9-4.9) CAC>800, EFV>200: 4.0(2.1-5.0) CAC>1600, EFV>200: 7.1(4.1-10.2) |
Age, gender, coronary risk factors and CAC | |
| Kunita [19] | 722 | MACE | 37 | > 107.2 mL | 2.48 (1.16-5.31) | age, sex, BMI, hypertension, hypercholesterolemia, diabetes mellitus, current smoking and CAC > 100 | |
| Mahabadi [7] | 4,093 | MACE | 130 | Doubling of Volume | 1.50 (1.07-2.11) | Age, gender, traditional risk factors and CAC | |
| Shmilovich [18] | 226 | MACE | 58 | > 68 mL/m2 * | 2.8 (1.3-6.4) | CAC and FRS (EFV indexed to BSA – roughly equivalent to 125 mL) |
BMI – body mass index; BSA – body surface area; CAC – coronary artery calcium score; EFV – epicardial fat volume; FRS – Framingham Risk Score; HDL – high density lipoproteins; HR – hazard ratio; MACE – major adverse cardiac event; OR – odds ratio;
* this is roughly equivalent to 125mL/1.7m2 (close to the average adult BSA)
Discussion
Our study performed a systematic review of the available literature on the prognostic value of EFV quantification on cross-sectional imaging for clinical outcomes. Although the findings are not consistent across all of the 9 studies, there is a clear trend that EFV has value as a prognostic metric for future adverse events and improves risk prediction beyond CAC scoring.
A number of studies have established a unique pathophysiological role of epicardial fat that distinguishes it from thoracic fat [16], aortic fat [20] and other adipose tissue in the body [9; 25]. While the findings are somewhat mixed depending on the study design, it is clear that fat tissue surrounding the heart plays a unique role in cardiac disease. It is thought that the fat directly surrounding the coronary arteries fosters development of atherosclerosis [21], arterial stiffness [26] and calcification, although the exact process is not fully understood. It has been shown that this fat tissue has metabolic activity and produces cytokines implicated in the pathophysiology of coronary atherosclerosis [27]. One challenge to determining the specific pathology is an unclear relationship between epicardial fat and pericardial fat [28]. There is even some disagreement as to the meaning of each of these terms. Choi et al. provided a clear delineation by measuring both values and defining epicardial fat as all fat within the border of the pericardium and pericardial fat as being exterior and adjacent to the pericardium [28]. Based on the inconsistency of terminology found in the literature, we recommend that these definitions be consistently applied in future research to avoid misinterpretation.
Our systematic review demonstrated that the majority of studies reported an incremental prognostic value of EFV quantification beyond CAC scoring. Thusly, the potential clinical role of EFV could be projected as an “add-on” analysis of CAC scoring CT studies. As such, EFV quantification does not require additional radiation exposure or acquisition time. Recent studies on coronary CT angiography datasets suggested that EFV quantification can be performed semi-automatically with good accuracy thus reducing the time required for the analysis to less than two minutes [10]. It is reasonable to assume that a similar time would be necessary for semi-automated EFV quantification on non-contrast CAC scoring CT data. Similar to CAC scoring, EFV quantification can be easily performed by a non-radiologist (such as a technologist) after a moderate amount of training [10].
We had initially intended to also synthesize the available evidence on the prognostic value of EFV in the form of a meta-analysis. Unfortunately, due to the wide variability in the study designs and in the statistical methods associated with EFV evaluation – in particular the various EFV increments used, calculation of pooled ORs or HRs was not possible. The results of our analysis indicate a need to standardize the quantitative evaluation of EFV. In CAC scoring, standardized quantitative categories (0, 1-100, 101-400, 401-1000, >1000) have been established and are used in all pertinent studies with minor variations. Similarly, it would be beneficial to establish standardized quantitative categories for EFV, which would allow direct comparison of prognostic metrics between studies, synthesis of their results and provide guidance in the interpretation of quantitative EFV measurements in clinical routine. The study by Greif and colleagues [15] measured fat both inside and outside the pericardium (epicardial plus pericardial fat according to the definitions by Choi [28]) and demonstrated that increasing the binary cutoff for EFV beyond 200mL did not significantly improve prognostic value. Thus, based on the available evidence, cutoffs of 125mL for epicardial fat and 200mL for epicardial plus pericardial fat appear most appropriate for prognostic risk stratification, if binary cutoffs are used. Thus far, only one relatively small study [18] has demonstrated that indexing EFV to body surface area (68mL/m2) improves its prognostic value; this should be confirmed in larger cohorts.
In most studies included in our analysis, there was a substantial overlap in EFV values between patients who did and did not develop events during follow-up. Accordingly, EFV alone should never be used to determine the appropriate management of an individual patient. However, in combination with established clinical risk factors and CAC score, EFV quantification can provide patients and their health care providers with a more accurate risk estimate than would otherwise be possible. A substantial number of studies did not describe who determined outcomes (presence of an endpoint committee) or whether outcome assessment was performed blinded to EFV measurements and vice versa. This is remarkable considering that the derivation of endpoint is a crucial step in the data evaluation.
The results of our study should be viewed in light of the study design and its limitations. One limitation is that we did not assess whether EFV quantification provides incremental value over findings on contrast-enhanced coronary CT angiography. Considering the high prognostic value of CT angiography incremental to CAC scoring [29], the quantification of EFV may not offer any additional benefit, if CT angiography is performed. CT angiography is an appropriate diagnostic test in selected stable but symptomatic patients [30]. EFV quantification as an “addon” to CAC scoring may be of particular benefit in the asymptomatic patients for whom coronary CT Angiography is not recommended. As a general limitation of systematic reviews, the validity of our findings depends on the quality of the included studies. As discussed above, the available data did not lend itself to a meta-analysis due to variability in the study designs and statistical methods used. Individual patient outcome data could not be derived from the published data, which could have provided more insights and allowed for subgroup analyses for specific subgroups of patients.
Despite these limitations, the available evidence suggests that EFV quantification is a significant predictor of clinical outcomes and provides incremental prognostic value over traditional cardiovascular risk factors and CAC scoring.
Key points.
Epicardial fat volume (EFV) has prognostic value for adverse cardiac events.
Establishment of standardized quantitative categories for EFV is needed.
Quantification of EFV could improve risk assessment with calcium scoring.
Acknowledgments
Disclosure of sources of funding
UJS is a consultant for and receives research support from Bayer, Bracco, GE and Siemens. Dr. Nietert was funded by the National Center for Advancing Translational Sciences (Award Number UL1TR000062); the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Abbreviations
- CAC
coronary artery calcium scoring
- CT
computed tomography
- EFV
epicardial fat volume
- HR
hazard ratio
- MACE
major adverse cardiac event
- OR
odds ratio
Footnotes
Potential conflicts of interest:
The other authors have no potential conflicts of interest to disclose.
References
- 1.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 2.Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.06.013. 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi R, Rajani R, Cheng VY, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis. 2011;218:363–368. doi: 10.1016/j.atherosclerosis.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 4.Raggi P, Alakija P. Epicardial adipose tissue: A long-overlooked marker of risk of cardiovascular disease. Atherosclerosis. 2013 doi: 10.1016/j.atherosclerosis.2013.02.030. 10.1016/j.atherosclerosis.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Stojanovska J, Kazerooni EA, Sinno M, et al. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol. 2015 doi: 10.1007/s00330-015-3643-1. 10.1007/s00330-015-3643-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 7.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 8.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 9.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 10.Spearman JV, Meinel FG, Schoepf UJ, et al. Automated Quantification of Epicardial Adipose Tissue Using CT Angiography: Evaluation of a Prototype Software. Eur Radiol. 2013 doi: 10.1007/s00330-013-3052-2. 10.1007/s00330-013-3052-2. [DOI] [PubMed] [Google Scholar]
- 11.Dey D, Suzuki Y, Suzuki S, et al. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–153. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 12.Tamarappoo B, Dey D, Shmilovich H, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janik M, Hartlage G, Alexopoulos N, et al. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J Nucl Cardiol. 2010;17:841–847. doi: 10.1007/s12350-010-9235-1. [DOI] [PubMed] [Google Scholar]
- 14.Forouzandeh F, Chang SM, Muhyieddeen K, et al. Does quantifying epicardial and intrathoracic fat with noncontrast computed tomography improve risk stratification beyond calcium scoring alone? Circ Cardiovasc Imaging. 2013;6:58–66. doi: 10.1161/CIRCIMAGING.112.976316. [DOI] [PubMed] [Google Scholar]
- 15.Greif M, Leber AW, Saam T, et al. Determination of Pericardial Adipose Tissue Increases the Prognostic Accuracy of Coronary Artery Calcification for Future Cardiovascular Events. Cardiology. 2012;121:220–227. doi: 10.1159/000337083. [DOI] [PubMed] [Google Scholar]
- 16.Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Marco LG, Bellasi A, Kim S, Chen Z, Block GA, Raggi P. Epicardial adipose tissue predicts mortality in incident hemodialysis patients: a substudy of the Renagel in New Dialysis trial. Nephrol Dial Transplant. 2013;28:2586–2595. doi: 10.1093/ndt/gft264. [DOI] [PubMed] [Google Scholar]
- 18.Shmilovich H, Dey D, Cheng VY, et al. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. Am J Cardiol. 2011;108:1680–1685. doi: 10.1016/j.amjcard.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunita E, Yamamoto H, Kitagawa T, et al. Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis. 2014;233:447–453. doi: 10.1016/j.atherosclerosis.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastarrika G, Broncano J, Schoepf UJ, et al. Relationship between coronary artery disease and epicardial adipose tissue quantification at cardiac CT: comparison between automatic volumetric measurement and manual bidimensional estimation. Acad Radiol. 2010;17:727–734. doi: 10.1016/j.acra.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apfaltrer P, Schindler A, Schoepf UJ, et al. Comparison of epicardial fat volume by computed tomography in black versus white patients with acute chest pain. Am J Cardiol. 2014;113:422–428. doi: 10.1016/j.amjcard.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Brinkley TE, Hsu FC, Carr JJ, et al. Pericardial fat is associated with carotid stiffness in the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2011;21:332–338. doi: 10.1016/j.numecd.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi TY, Ahmadi N, Sourayanezhad S, Zeb I, Budoff MJ. Relation of vascular stiffness with epicardial and pericardial adipose tissues, and coronary atherosclerosis. Atherosclerosis. 2013 doi: 10.1016/j.atherosclerosis.2013.03.003. 10.1016/j.atherosclerosis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Labounty TM, Gomez MJ, et al. Incremental prognostic value of coronary computed tomographic angiography over coronary artery calcium score for risk prediction of major adverse cardiac events in asymptomatic diabetic individuals. Atherosclerosis. 2014;232:298–304. doi: 10.1016/j.atherosclerosis.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]