Highlights
-
•
MJ smokers exhibited poorer performance and altered activation on the Stroop.
-
•
MJ smokers reported significantly higher impulsivity than control subjects.
-
•
Poorer performance and neural alterations were primarily related to early MJ onset.
-
•
Early onset smokers reported higher levels of MJ use than late onset smokers.
-
•
Earlier MJ onset and increased MJ use predicted poorer Stroop performance.
Keywords: Marijuana, Stroop, Age of onset, fMRI, Executive function, Cognition
Abstract
Marijuana (MJ) use is on the rise, particularly among teens and emerging adults. This poses serious public health concern, given the potential deleterious effects of MJ on the developing brain. We examined 50 chronic MJ smokers divided into early onset (regular MJ use prior to age 16; n = 24) and late onset (age 16 or later; n = 26), and 34 healthy control participants (HCs). All completed a modified Stroop Color Word Test during fMRI. Results demonstrated that MJ smokers exhibited significantly poorer performance on the Interference subtest of the Stroop, as well as altered patterns of activation in the cingulate cortex relative to HCs. Further, early onset MJ smokers exhibited significantly poorer performance relative to both HCs and late onset smokers. Additionally, earlier age of MJ onset as well as increased frequency and magnitude (grams/week) of MJ use were predictive of poorer Stroop performance. fMRI results revealed that while late onset smokers demonstrated a more similar pattern of activation to the control group, a different pattern was evident in the early onset group. These findings underscore the importance of assessing age of onset and patterns of MJ use and support the need for widespread education and intervention efforts among youth.
1. Introduction
Marijuana (MJ) is predicted to become a multi-billion dollar industry within the next five years. Currently twenty-three states and the District of Columbia have legalized medical MJ, while four (Colorado, Washington, Oregon, and Alaska) have also approved recreational use. As voters say “yes” to MJ, benefits of use are often underscored, while negative effects may be overshadowed. This shift in national attitudes is occurring despite mounting evidence of the deleterious effects of MJ (Lisdahl et al., 2014, Lubman et al., 2014), particularly on the developing brain. Although once considered to be complete by early adolescence, longitudinal studies demonstrate that the brain continues to develop well into adulthood (Casey et al., 2005, Giedd et al., 1999, Gogtay et al., 2004), leaving adolescents and emerging adults particularly vulnerable to the potentially adverse effects of MJ on cognitive processes.
MJ continues to be the most widely used illicit substance and over the past several years, despite decreasing rates of use for other substances, rates of MJ use are climbing among youth (Johnston et al., 2014, Kann et al., 2014). According to the 2013 Monitoring the Future survey, more than 36% of 12th graders used MJ in the past year, and 6.5% used daily (Johnston et al., 2014). Moreover, survey data indicate that the perceived risk of MJ use is approaching historically low levels; less than half of high school students view MJ as harmful. Adolescents who perceive greater risk from MJ are less likely to try MJ, and as a result, the decrease in perception of harm is directly related to the recent increase in use. This trend also impacts public safety: the number of teens driving under the influence of MJ has surpassed the number of teens who drive drunk (Johnston et al., 2014). Youth are also initiating MJ use at alarmingly early ages; among those who began smoking during adolescence, the average age of onset was 16.3-years old (Substance Abuse and Mental Health Services Administration, 2013). This poses serious public health concerns given research findings demonstrating that MJ use is related to cognitive impairments particularly in those who initiate use during adolescence (Crane et al., 2013, Crean et al., 2011, Gruber et al., 2012a, Lisdahl et al., 2014, Solowij and Pesa, 2010), and neuroimaging studies, which highlight the relationship between cognitive decrements and alterations in the brain (Batalla et al., 2013, Gruber et al., 2012b, Gruber et al., 2013, Wrege et al., 2014).
Lisdahl et al. (2014) reported that MJ-smoking youth experience deficits in a variety of cognitive domains, including processing speed, attention, memory, and executive function. These findings support previous work, which revealed that MJ smokers exhibit processing deficits during frontally-mediated cognitive tasks, resulting in altered decision-making and behavioral inhibition (Gruber and Yurgelun-Todd, 2005), and that the deficits observed in MJ smokers were primarily attributable to those with early MJ onset (Gruber et al., 2012a). Additionally, Fontes et al. (2011) reported that early onset MJ smokers demonstrated significantly worse performance than both controls and late onset smokers on a neurocognitive battery designed to measure sustained attention, impulse control, and executive functioning. Neuroimaging studies have also revealed a relationship between age of MJ onset and functional alterations (Tapert et al., 2007), including work demonstrating a differential pattern of brain activation between early and late onset MJ smokers on the Multi-Source Interference Task (MSIT), a measure of inhibitory function (Gruber et al., 2012b). Several investigations have also reported a relationship between functional alterations and increased MJ use (Bolla et al., 2005, Hester et al., 2009, Nestor et al., 2010).
Impulsive personality traits have been identified as a risk factor and predictor of substance use (Brady et al., 1998, Guy et al., 1994, Heil et al., 2006, Vitaro et al., 1998). Impulsivity is characterized by weak executive control that compromises higher-order cognitive processes including inhibition, set-shifting, and utilizing feedback (Wrege et al., 2014). Higher levels of self-reported impulsivity, as measured by the Barratt Impulsiveness Scale (BIS), and risk taking have been reported in substance abusers (Lejuez et al., 2002, Lejuez et al., 2003, Gruber and Yurgelun-Todd, 2005). One study suggests that MJ smokers with higher levels of impulsivity held fewer negative expectancies related to MJ, and used MJ more often than those reporting lower levels of impulsivity (Vangsness et al., 2005). Further, Squeglia and colleagues (2014) found that poorer performance on tasks of cognitive inhibition/interference prior to initiation of substance use during early adolescence predicted increased frequency of MJ use in late adolescence. In combination with the neuroimaging findings that report alterations in response inhibition and decision-making (Gruber and Yurgelun-Todd, 2005, Gruber et al., 2012b, Hester et al., 2009, Jacobus et al., 2009, Schweinsburg et al., 2008, Tapert et al., 2007), impulsivity may reflect a stable trait which precedes substance use in MJ smokers and which may predict increased MJ use. In addition, recent work has demonstrated not only that MJ smokers reported higher levels of impulsivity than non-MJ smoking individuals, but that within early onset MJ smokers (regular use prior to age 16), higher levels of impulsivity were correlated with lower levels of white matter organization and coherence (Gruber et al., 2013). This suggests that MJ is related to both impulsivity and, particularly in early onset MJ smokers, alterations of white matter, critical for efficient communication between brain regions.
Overall, research has demonstrated that MJ smokers exhibit altered frontal function and suggests that cognitive deficits may compromise function, impairing the ability to make good decisions and inhibit inappropriate actions. Given these findings, we hypothesized that MJ smokers would perform more poorly on the Interference condition of the Stroop Color Word Test and would exhibit significantly different patterns of brain activation relative to healthy controls (HC). We also predicted that performance deficits in the MJ smokers would be specifically related to early onset of MJ use, and that these performance differences would correspond with a markedly different pattern of activation than the late onset smokers. Further, we expected that these results would be influenced by heavier patterns of MJ use in terms of frequency (smokes/week) and magnitude (grams/week).
2. Materials and methods
2.1. Participants
Participants were recruited from the Greater Boston Area community and included 50 chronic, heavy MJ smokers (age range: 17–46), divided into those with early onset (initiation of regular MJ use prior to age 16; n = 24) and late onset (regular MJ use at age 16 or later; n = 26). Thirty-four non-MJ-smoking, HCs (age range: 18–48) were also included. Participants were excluded if they met criteria for any Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Axis I pathology (with the exception of MJ abuse/dependence in the smoking group), as assessed by the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P; First et al., 1994). Individuals were also excluded if they reported any significant head injury with loss of consciousness, history of a neurological disorder or serious medical problem, previous and/or current use of psychotropic medications, or were non-native English speakers. Further, no participant was enrolled if they reported more than 15 lifetime uses of any illicit drugs (except MJ for the smoking group) or recreational use of prescription or over-the-counter (OTC) medications. While a number of participants had completed other studies which have previously been published (Gruber et al., 2012a, Gruber et al., 2012b), approximately 30% of the participant pool was newly recruited and had not participated in lab-based clinical research prior to the current study. Further, cognitive and fMRI data collected for the current study has not previously been reported.
All MJ smokers were well-characterized as chronic MJ smokers. In order to qualify for study enrollment, MJ smokers had to have reported smoking a minimum of 2500 times in their lives, used MJ at least five out of the last seven days, tested positive for urinary cannabinoids, and met DSM-IV criteria for MJ abuse or dependence. In addition, in order to ensure that performance on measures of cognitive tasks was not impacted by acute intoxication, MJ smokers were required to abstain from smoking at least 12 h prior to their study visit. All participants were required to provide a urine sample upon arrival at the laboratory to assess for the presence of illicit substances, and in order to ensure adherence to the required 12-h abstinence, MJ participants were led to believe that our researchers could use this sample to detect MJ use within this time frame, a method we have successfully utilized in the past (Gruber et al., 2011, Gruber et al., 2012a, Gruber et al., 2012b, Gruber et al., 2013). Study participants who reported MJ use that violated the abstinence schedule or who appeared intoxicated were assessed for recent use and rescheduled for a later date. Individuals who tested positive for any illicit substance other than MJ were disqualified.
MJ use was quantified using a modified timeline follow-back procedure (Sobell et al., 1998). Participants provided information regarding history of MJ use, including age of onset, and duration of use (years), as well as current frequency (smokes/week), magnitude (grams/week), and mode of use using guided interview questions (i.e., When did you first try MJ? Who were you with? How did you use it?), which significantly facilitated recall. Lifetime use was also determined via the SCID-P. As previously mentioned, MJ smokers were further divided into two groups based on age of onset of regular MJ use in order to determine the potential differential impact of age of onset on cognitive function. For this study, age of ‘regular’ use of MJ was defined as the age at which subjects began using MJ on a routine, expected and consistent basis, and not the age at which they tried MJ for the first time. Although no uniform definition of early and late onset exists, these parameters have been utilized in several studies (Ehrenreich et al., 1999, Gruber et al., 2011, Gruber et al., 2012a, Gruber et al., 2012b, Gruber et al., 2013, Kempel et al., 2003, Pope et al., 2003).
Prior to participation, study procedures were thoroughly explained, and all participants were required to read and sign an informed consent form approved by the McLean Hospital Institutional Review Board. This document describes the procedures, risks, benefits, and voluntary nature of the study.
2.2. Study design
As part of a larger neuroimaging study, participants completed a modified version of the Stroop Color Word Test previously used with healthy control participants (Gruber et al., 2002) while undergoing functional magnetic resonance imaging (fMRI) scanning. Prior to scanning, individuals completed a brief practice version of the task in which they became familiar with the type of task stimuli as well as the instructions. The Stroop Test contains three subtests: Color Naming, Word Reading, and Interference. During the Color Naming subtest, individuals are instructed to name the color of rectangles out loud (vocalized response). The Word Reading subtest requires participants to read words printed in black ink, while the Interference trials consists of words printed in an incongruous ink color (i.e. the word “green” printed in red ink). Participants are required to inhibit the automatic tendency to read the words, and instead must name the color of the ink for each word. Each subtest begins with a period of 30 s of rest in which a fixation point is viewed on the screen, followed by 30 s of stimuli (6 stimuli per slide), 30 s fixation, a second 30 s period of stimuli, and ends with a final 30 s fixation, for a total run time of 2 min 30 s per subtest. Performance is measured by percent accuracy and error types: commissions (incorrect responses) and omissions (missed responses) for each subtest are recorded separately (MacLeod, 1991).
Study participants also completed a battery of assessments to assess current clinical state and to ensure that groups were well matched for different aspects of mood and levels of anxiety. Briefly, these measures included the Profile of Mood States (POMS; Pollock et al., 1979), a measure that reflects current mood state and provides scores for the individual domains of vigor, anger, confusion, tension, and depression, as well as a total mood disturbance score; the Beck Depression Inventory (BDI; Beck et al., 1961); the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983); the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988); the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979); the Hamilton Anxiety Scale (HAM-A; Hamilton, 1959); and the Young Mania Rating Scale (YMRS; Young et al., 1978). The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was also completed by all participants in order to assess current level of nicotine use, and the Addiction Severity Index (ASI; McLellan et al., 1980) was administered to calculate days of alcohol use within the past month. Finally, the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) was used to assess reported levels of impulsivity. This BIS-11 yields scores for three subscales of impulsivity, attention, motor, and non-planning, in addition to a total impulsivity score. All participants were administered either the Wechsler Abbreviated Scale of Intelligence (WASI) or an abbreviated version of the Wechsler Adults Intelligence Scale – Revised (WAIS-R), both of which yield an estimate of IQ (Wechsler, 1999, Wechsler, 1981).
2.3. Statistical analyses
One-way analyses of variance (ANOVAs) with Scheffé post hoc comparisons were used to assess between-group differences for two different comparisons: two-group (HCs vs all MJ smokers) and three-group (HCs vs early MJ onset smokers vs late MJ onset smokers) where age of MJ onset was used as a categorical variable (early vs late). Univariate logarithmic regression analyses were conducted to further assess the relationship between MJ use (age of MJ onset, frequency of MJ use, and magnitude of MJ use), and to determine the extent to which each of these variables predicted performance on the Stroop task. Logarithmic equations were selected, as they provided a best fit for the data, and in these analyses, age of onset was used as a continuous variable.
2.4. Imaging methods
All imaging was performed on a Siemens Trio whole body 3T MRI scanner (Siemens Corporation, Erlangen, Germany) using a 12-channel phased array head coil. Forty contiguous coronal slices were acquired from each participant, ensuring whole brain coverage (5 mm thick), and images were collected with TR = 3000, using a single shot, gradient pulse echo sequence (TE = 30 ms, flip angle = 90, with a 20 cm field of view and a 64 × 64 acquisition matrix; in plane resolution 3.125 mm × 3.125 mm× 3.125 mm). A total of 50 images per slice were collected in order to ensure comparability of tasks with previous studies (Gruber et al., 2002, Gruber and Yurgelun-Todd, 2005).
2.5. Image processing and analysis
fMRI images were analyzed using SPM8 (version 4290, Wellcome Department of Imaging Neuroscience, University College, London, UK) software package running in MATLAB (version R2010b, MathWorks, Natick, MA, USA). First, blood–oxygen-level dependent fMRI data were corrected for motion in SPM8 using a 2-step intra-run realignment algorithm that uses the mean image created after the first realignment as a reference. A criterion of 3 mm of head motion in any direction was used as an exclusionary criterion. The realigned images were then normalized to an EPI template in Montreal Neurological Institute (MNI) stereotactic space. Normalized images were re-sampled into 2 mm cubic voxels and then spatially smoothed using an isotropic Gaussian kernel with 8 mm full width at half maximum (FWHM). Global scaling was not used, high-pass temporal filtering with a cut-off of 128 s was applied, and serial autocorrelations were modeled with an AR(1) model in SPM8.
Statistical parametric images were calculated individually for each subject for the Interference task using a general linear model. These images were subsequently entered into second level model, subjected to a voxel-wise contrast and t-test to assess statistical significance. Direct comparisons between the MJ smokers and HCs were made, as well as early vs late smokers. Movement parameters were included as regressors in the design. The region of interest (ROI) mask was created using the Wake Forest University Pickatlas utility, and included the cingulate cortex. Contrast analysis was conducted for the (Interference-rest/fixation)–(Color Naming-rest/fixation) condition. The statistical threshold was set at 0.0001 uncorrected and a minimum cluster extent (k) of 10 contiguous voxels.
3. Results
3.1. Demographics, clinical state, and MJ use
Across the study groups, subjects were well-matched for age and IQ. Chi square analyses of the frequencies of race and gender distributions indicated no significant differences across the HC, early MJ onset, and late MJ onset groups. Analyses also revealed no significant differences between HCs and MJ smoking groups for days of alcohol used, as assessed by the ASI, or nicotine use (FTND). See Table 1, Table 2.
Table 1.
Healthy controls vs MJ smokers t-test comparisons.
|
Variable Mean (SD) |
Healthy controls | MJ smokers |
Sig 2-tailed p (η2) |
|---|---|---|---|
| N | 34 (21 M, 13 F) | 50 (42 M, 8 F) | – |
| Handedness | 32R, 2L | 46R, 4L | – |
| % Caucasian | 67.65% | 76.00% | – |
| Age | 24.53 (6.57) | 23.98 (6.95) | NS |
| Full Scale IQ (FSIQ) | 123.15 (10.94) | 117.56 (15.03) | NS |
| Past month days of alcohol use | 5.19 (5.16) | 7.25 (5.76) | NS |
| FTNDa | 0.00 (0.00) | 0.29 (0.86) | NS |
| BIS-11b |
Sig 1-tailed p (η2) |
||
| Attention | 14.61 (3.75) | 16.65 (2.61) | .003(.096) |
| Motor | 19.58 (4.65) | 22.61 (4.55) | .003(.097) |
| Non-planning | 23.19 (4.96) | 26.61 (4.61) | .002(.113) |
| Total | 57.39 (10.28) | 65.87 (9.40) | <.001(.157) |
| Stroop Color Word Test |
Sig 1-tailed p (η2) |
||
| Color naming | |||
| Percent accuracy | 94.28 (5.68) | 92.32 (9.25) | NS |
| Omissions | 4.53 (5.79) | 6.70 (9.98) | NS |
| Commissions | 2.09 (2.31) | 2.52 (2.38) | NS |
| Word reading | |||
| Percent accuracy | 99.07 (1.81) | 98.17 (2.90) | .055 (.031) |
| Omissions | 0.56 (1.31) | 0.90 (2.17) | NS |
| Commissions | 0.38 (0.82) | 1.30 (1.78) | .003(.088) |
| Interference | |||
| Percent accuracy | 96.69 (5.33) | 92.44 (11.56) | .024(.047) |
| Omissions | 0.79 (1.61) | 2.88 (6.08) | .027(.044) |
| Commissions | 1.59 (2.86) | 2.48 (3.25) | .099 (.020) |
Fagerstrom Test For Nicotine Dependence.
Barratt Impulsiveness Scale (BIS-11).
Table 2.
Healthy controls vs early MJ onset vs late MJ onset one-way ANOVA results.
|
Variable Mean (SD) |
Healthy controls | Early MJ onset (before age 16) | Late MJ onset (age 16 or after) |
Sig 2-tailed p (η2) |
Sheffé comparisons sig 2-tailedp |
||
|---|---|---|---|---|---|---|---|
| E vs HC | L vs HC | E vs L | |||||
| N | 34 (21 M, 13 F) | 24 (21 M, 3 F) | 26 (21 M, 5 F) | – | – | – | – |
| Handedness | 32R, 2L | 21R, 3L | 25R, 1L | – | – | – | – |
| % Caucasian | 65.00% | 73.33% | 66.67% | – | – | – | – |
| Age | 24.47 (6.49) | 23.67 (7.26) | 24.27 (6.79) | NS | NS | NS | NS |
| Full Scale IQ (FSIQ) | 123.15 (10.94) | 115.38 (17.02) | 119.58 (12.93) | NS | NS | NS | NS |
| Past month days of alcohol use | 5.35 (5.56) | 5.92 (5.01) | 6.06 (6.05) | NS | NS | NS | NS |
| FTNDa | 0.00 (0.00) | 0.29 (0.91) | 0.08 (0.39) | NS | NS | NS | NS |
| MJ age of onset (years) | – | 14.17 (1.31) | 17.58 (1.94) | <.001(.521) | – | – | – |
| MJ smokes/week | – | 24.51 (21.25) | 13.53 (7.74) | .022(.109) | – | – | – |
| MJ grams/week | – | 14.57 (16.97) | 5.64 (4.54) | .016(.119) | – | – | – |
| MJ duration of use (years) | – | 9.50 (7.80) | 6.69 (5.68) | NS | – | – | – |
| Urinary THC concentration | – | 784.63 (1430.96) | 620.22 (916.38) | NS | – | – | – |
| BIS-11b |
Sig 1-tailed p (η2) |
Sheffé comparisons sig 1-tailedp | |||||
| Attention | 14.61 (0.56) | 16.81 (0.68) | 16.52 (0.63) | .012(.097) | .026 | .042 | NS |
| Motor | 19.58 (0.83) | 22.91 (1.01) | 22.36 (0.92) | .011(.099) | .023 | .045 | NS |
| Non-planning | 23.19 (0.85) | 26.67 (1.04) | 26.56 (0.96) | .006(.113) | .021 | .019 | NS |
| Total | 57.39 (1.75) | 66.38 (2.14) | 65.44 (1.96) | .001(.158) | .004 | .007 | NS |
| Stroop Color Word Test |
Sig 1-tailed p (η2) |
Sheffé comparisons sig 1-tailedp | |||||
| Color naming | |||||||
| Percent accuracy | 94.28 (5.68) | 91.59 (10.54) | 92.98 (8.02) | NS | NS | NS | NS |
| Omissions | 4.53 (5.79) | 7.38 (11.22) | 6.08 (8.86) | NS | NS | NS | NS |
| Commissions | 2.09 (2.31) | 2.71 (2.48) | 2.35 (2.33) | NS | NS | NS | NS |
| Word reading | |||||||
| Percent accuracy | 99.07 (1.81) | 97.95 (3.31) | 98.37 (2.51) | NS | NS | NS | NS |
| Omissions | 0.56 (1.31) | 1.04 (2.68) | 0.77 (1.61) | NS | NS | NS | NS |
| Commissions | 0.38 (0.82) | 1.42 (1.95) | 1.19 (1.63) | .011(.091) | .018 | NS | NS |
| Interference | |||||||
| Percent accuracy | 96.69 (5.33) | 88.88 (14.31) | 95.72 (7.06) | .003(.212) | .005 | NS | .019 |
| Omissions | 0.79 (1.61) | 4.42 (7.96) | 1.46 (3.14) | .007(.099) | .010 | NS | .046 |
| Commissions | 1.59 (2.86) | 3.46 (3.82) | 1.58 (2.34) | .021(.075) | .037 | NS | .048 |
Fagerstrom Test For Nicotine Dependence.
Barratt Impulsiveness Scale.
Comparisons between HCs and MJ smokers, as well as early and late onset MJ smokers also demonstrated that groups were well-matched for clinical state; no significant differences were noted between the groups for BDI, STAI, POMS, PANAS, HAM-A, YMRS, or MADRS scores (see Table 3). However, scores on the BIS-11 indicated significant differences between HCs and MJ smokers on all subscales (Attention, Motor, and Non-Planning) as well as Total BIS-11 scores (Table 1), demonstrating higher levels of impulsivity in MJ smokers. It is of note that while the three-group analyses indicated that both the early and late MJ onset groups differed significantly from the HC group, no between-group differences for early vs late MJ onset were noted for BIS-11 scores (Table 2).
Table 3.
Clinical rating scale results.
|
Variable Mean (SD) |
Healthy controls | MJ (whole Group) | Sig 2-tailedp | Early MJ onset (before age 16) | Late MJ onset (age 16 or after) | Sig 2-tailedp |
|---|---|---|---|---|---|---|
| HAM-Aa | 1.62 (2.30) | 1.844 (2.03) | NS | 1.61 (1.67) | 2.09 (2.37) | NS |
| MADRSb | 1.45 (1.28) | 1.97 (1.64) | NS | 2.00 (1.52) | 1.94 (1.77) | NS |
| YMRSc | 0.85 (1.14) | 1.53 (2.00) | NS | 1.29 (1.94) | 1.72 (2.08) | NS |
| BDId | 1.73 (2.64) | 2.52 (3.55) | NS | 2.05 (3.00) | 3.00 (4.04) | NS |
| POMSe | ||||||
| Vigor | 20.30 (4.37) | 19.27 (4.67) | NS | 18.91 (3.92) | 19.57 (5.31) | NS |
| Anger | 2.72 (3.70) | 4.59 (4.81) | NS | 3.65 (3.43) | 5.42 (5.71) | NS |
| Confusion | 4.31 (3.75) | 4.86 (2.74) | NS | 4.48 (2.41) | 5.19 (3.01) | NS |
| Tension | 5.17 (4.86) | 4.84 (3.51) | NS | 4.70 (3.17) | 4.96 (3.84) | NS |
| Fatigue | 3.83 (3.29) | 3.86 (2.96) | NS | 3.39 (2.62) | 4.27 (3.22) | NS |
| Depression | 3.27 (3.75) | 3.45 (4.49) | NS | 2.26 (2.60) | 4.50 (5.51) | NS |
| Total mood disturbance | −1.00 (16.93) | 2.33 (16.13) | NS | −0.43 (11.43) | 4.77 (19.27) | NS |
| PANASf | ||||||
| Positive | 32.46 (6.10) | 33.04 (6.93) | NS | 31.91 (7.69) | 34.04 (6.16) | NS |
| Negative | 11.18 (1.98) | 12.08 (3.27) | NS | 11.87 (3.09) | 12.27 (3.46) | NS |
| STAIg | ||||||
| Y1 (state) | 26.20 (4.05) | 27.48 (5.89) | NS | 27.79 (5.07) | 27.24 (6.64) | NS |
| Y2 (trait) | 30.20 (7.15) | 29.00 (6.67) | NS | 29.14 (6.01) | 28.88 (7.35) | NS |
Hamilton Anxiety Scale.
Montgomery-Asberg Depression Rating Scale.
Young Mania Rating Scale.
Beck Depression Inventory.
Profile of Mood States.
Positive and Negative Affect Schedule.
State-Trait Anxiety Inventory.
Interestingly, when MJ smokers were divided into early and late onset, the early onset MJ smokers reported higher frequency (smokes/week; F(1,46) = 5.655, p = .022, 2-tailed) and magnitude of MJ use (grams/week; F(1,46) = 6.203, p = .016, 2-tailed). In fact, the early onset group smoked nearly twice as often and over 2.5 times as much MJ as their late onset counterparts (Table 2). It is of note, however, that no between-group differences were detected for duration of MJ use.
3.2. Stroop Color Word Test performance results
Behavioral performance on the Stroop Color Word Test (Table 1) revealed that MJ smokers performed significantly more poorly relative to HCs during the Interference condition of the task, despite achieving similar percent accuracy during the Color Naming and Word Reading subtests. More specifically, during the Interference condition, MJ smokers demonstrated significantly increased omission errors (F(1,82) = 3.807, p = .027), or missed responses, and exhibited a trend for increased commission errors (F(1,82) = 1.678, p = .099), reflective of an inability to inhibit an inappropriate response. Together, these errors yielded lower percent accuracy scores in MJ smokers (F(1,82) = 4.016, p = .024) relative to HCs.
Separating the larger MJ smoking group into those with early vs late MJ onset revealed that the early onset smokers drove the between-group differences noted in task performance (Table 2), as they performed significantly worse relative to both the HCs and late onset smokers on the Interference subtest. Scheffé post hoc comparisons indicated that early onset smokers made significantly more errors of omission than both HCs (p = .010) and late onset smokers (p = .046); early onset smokers also made more errors of commission than both HCs (p = .037) and late onset smokers (p = .048). These errors contributed to significant differences in percent accuracy between early onset smokers relative to both HCs (p = .005) and late onset smokers (p = .019) on the Stroop Interference subtest. Interestingly, no significant differences in Stroop performance were detected between the late onset smokers and HCs.
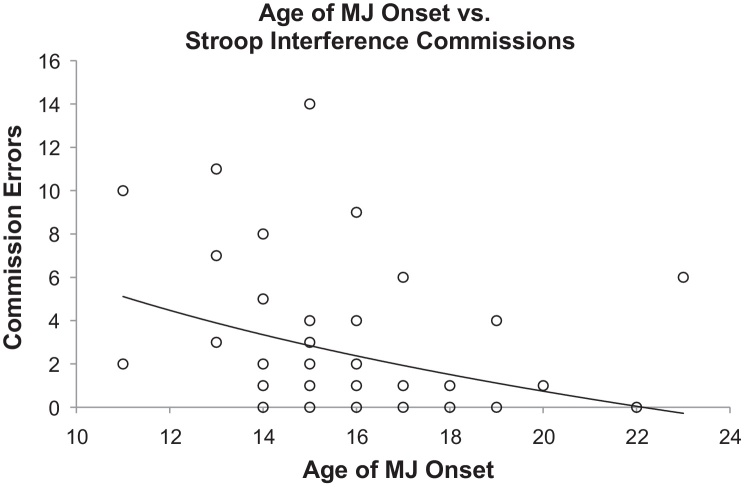
In order to further assess the role of these covariates, univariate regression analyses were performed for the variables that were significantly different between the early and late onset groups. Analyses indicated that earlier age of MJ onset (Fig. 1) as well as both higher frequency and increased magnitude of MJ use each individually predicted poorer performance on most measures of the Stroop Interference condition (Table 4). Multivariate modeling analyses could further dissect the overlap in variance attributable to these MJ use variables, but require far larger sample sizes than the current study provides.
Fig. 1.
Logarithmic regression analysis: age of onset of MJ use vs Stroop Interference commission errors. Within the MJ smokers, earlier age of onset was significantly associated with poorer performance on the Stroop Interference subtest, including increased commission errors, or incorrect responses (R2 = .076, F(1, 48) = 3.929, p = .027).
Table 4.
Univariate logarithmic regression analyses of MJ use demographics and Stroop interference performance.
| Stroop Color Word Test | R2 | F | Sig 1-tailed |
|---|---|---|---|
| Interference percent accuracy | |||
| Age of MJ onset | .067 | 3.433 | .035 |
| Smokes/week | .108 | 5.583 | .011 |
| Grams/week | .239 | 14.421 | <.001 |
| Interference omissions | |||
| Age of MJ onset | .041 | 2.067 | NS |
| Smokes/week | .057 | 2.2793 | .051 |
| Grams/week | .140 | 7.470 | .005 |
| Interference commissions | |||
| Age of MJ onset | .076 | 3.929 | .027 |
| Smokes/week | .167 | 9.237 | .002 |
| Grams/week | .288 | 18.582 | <.001 |
3.3. Stroop Color Word Test: fMRI results
fMRI analyses were conducted on 33 HCs and 49 MJ smokers (24 early onset, 25 late onset), as two subjects (1 HC, 1 late onset MJ smoker) had excessive motion and thus were excluded from imaging analyses. As shown in Table 5, single-group comparisons of Interference minus Color Naming activation revealed that while HCs demonstrated robust posterior activation in the right cingulate cortex, MJ smokers exhibited more anterior and diffuse activation throughout the cingulate, including bilateral cingulate regions. Interestingly, results also indicated that differences between HCs and MJ smokers were primarily attributable to the early onset MJ smokers who exhibited activation in the left anterior cingulate adjacent to the genu of the corpus callosum. Late onset smokers, however, demonstrated an activation pattern more similar to the HC group, with posterior right-hemisphere activation localized to the right cingulate cortex (Fig. 2).
Table 5.
Stroop activation: local maxima for single-group comparisons with cingulate cortex regional of interest (ROI).
|
Comparison group Region |
Cluster size (voxels) | x | y | z | SPM {t} |
Voxelp Uncorrected |
|---|---|---|---|---|---|---|
| 1-Sample t-tests against null | ||||||
| Healthy controls | ||||||
| Right middle cingulum, BA23 | 679 | 12 | −48 | 33 | 6.09 | <.001 |
| All MJ smokers | ||||||
| Left anterior cingulum, BA24 | 62 | −4 | 2 | 30 | 5.10 | <.001 |
| Left posterior cingulum, BA23 | 47 | −10 | −46 | 32 | 4.98 | <.001 |
| Middle frontal gyrus, BA10 | 62 | 4 | 50 | 14 | 4.94 | <.001 |
| Right middle cingulum | 69 | 16 | −39 | 40 | 4.71 | <.001 |
| Right middle cingulum | 11 | 0 | −14 | 31 | 4.64 | <.001 |
| Left anterior cingulum, BA32 | 59 | 0 | 34 | 22 | 4.61 | <.001 |
| Right middle cingulum, BA23 | 15 | 2 | −32 | 31 | 3.93 | <.001 |
| Left middle cingulum, BA31 | 13 | −2 | −32 | 40 | 3.83 | <.001 |
| Early MJ smokers | ||||||
| Left anterior cingulate, BA32 | 259 | −6 | 50 | −2 | 8.81 | <.001 |
| Late MJ smokers | ||||||
| Right middle cingulum, BA31 | 89 | 6 | −32 | 50 | 6.81 | <.001 |
Fig. 2.
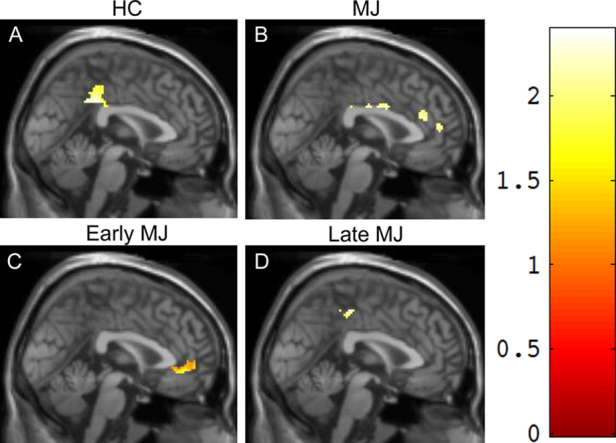
Stroop (interference-rest/fixation)–(color naming-rest/fixation) fMRI activation: single-group comparison. Single-group comparisons on the Stroop Interference condition: HCs (A) demonstrated robust posterior activation in the right cingulate, while MJ smokers (B) exhibited more diffuse activation throughout the cingulate. Within MJ smokers, the early onset group (C) demonstrated activation in the anterior region of the cingulate, adjacent to the genu of the corpus callosum, whereas late onset smokers (D) exhibited posterior activation in the right cingulate, more similar to the HC group.
4. Discussion
As hypothesized, MJ smokers performed significantly more poorly than HCs on the Interference condition of the Stroop Color Word Test, a frontally mediated task of executive function that assesses the ability to inhibit inappropriate responses. MJ smokers also reported significantly higher levels of impulsivity as measured by the BIS-11. Analyses designed to assess the potential impact of age of onset of MJ use revealed that those who initiated regular MJ use prior to age 16 (early onset) demonstrated significantly poorer performance compared to both HCs and those who began using MJ at age 16 or later (late onset). Interestingly, early onset smokers smoked, on average, nearly twice as often and more than 2.5 times as much MJ each week relative to the late MJ onset group. Regression analyses also indicated that earlier age of MJ onset, increased frequency and magnitude (grams/week) of MJ use were all individually predictive of worse task performance, specifically, lower percent accuracy and higher commission errors.
In addition, fMRI analyses revealed that MJ smokers demonstrated significantly different activation patterns during the Stroop Interference condition relative to HCs. While the HCs demonstrated a more focal, posterior pattern of activation, suggestive of increased neural efficiency, MJ smokers exhibited a more diffuse pattern in the anterior portion of the ROI. Similar to the behavioral results, which revealed that performance deficits in the MJ group were more pronounced in the early onset smokers, fMRI analyses also revealed that functional alterations in the MJ smokers were primarily driven by the early onset smokers. Relative to the HC group, late onset MJ smokers demonstrated a similar, focal posterior pattern of activation while early onset smokers exhibited a vastly more anterior pattern, localized to the anterior ACC. These findings suggest that earlier exposure to regular MJ use may result in greater vulnerability to behavioral and functional brain alterations relative to later exposure.
Data from the current study are consistent with previous work demonstrating poorer performance of inhibitory tasks in MJ smokers relative to HCs (Gruber and Yurgelun-Todd, 2005, Thames et al., 2014) and more specifically, those which have reported a significant inverse relationship between age of onset of MJ use and cognitive performance. For example, several studies have documented more pronounced neurocognitive deficits spanning a range of domains in those who begin smoking MJ during adolescence, including attention (Ehrenreich et al., 1999), visual search (Huestegge et al., 2002), IQ (Filbey et al., 2014, Meier et al., 2012, Pope et al., 2003), and executive function (Fontes et al., 2011, Gruber et al., 2012a, Solowij et al., 2012). Results from the current study also support and extend previous research, which has noted functional alterations in MJ smokers during inhibitory tasks (Battisti et al., 2010, Becker et al., 2010, Behan et al., 2014, Gruber et al., 2012b).
Analyses revealed that both frequency and magnitude of MJ use were independently predictive of poorer task performance, suggesting that increased MJ use appears to represent a trait characteristic specific to the early onset MJ smokers. Consequently, individuals with earlier MJ onset may collectively have an “additive vulnerability” – a relatively immature brain that is susceptible to the impact of MJ exposure, further compounded by an increased likelihood to engage in higher levels of MJ use than those with later exposure. This combination may help explain the relatively impaired behavioral performance and altered patterns of brain activation noted during inhibitory tasks in early MJ onset smokers relative to those with later onset. Taken together, study findings underscore the importance of independently assessing age of first regular MJ use as well as frequency and magnitude of use.
In the current study, behavioral impulsivity (frequently elevated in those who use substances) was significantly higher within the MJ group relative to HCs. Interestingly, however, no between-group differences were between the early and late onset MJ smokers. Nonetheless, as higher levels of reported impulsivity and poorer performance on inhibitory tasks have been well-documented in MJ users (Battisti et al., 2010, Gruber et al., 2012a, Solowij et al., 2012), these characteristics may reflect an important trait to be targeted for prevention. Prevention efforts must also target youth at a young age; it is now widely recognized that the brain develops well into adulthood and that the neural circuitry associated with inhibitory control undergoes significant development during adolescence (Giedd et al., 1999, Casey et al., 2005, Squeglia et al., 2009), leaving the brain particularly vulnerable during this time.
The current study provides a valuable contribution to the field for several reasons. First, results were derived from a relatively large sample of well-characterized, chronic MJ smokers who were thoroughly assessed for frequency, magnitude, and age of onset of MJ use. In addition, findings provide further evidence that executive function impairment in MJ smokers is accompanied by observable functional brain alterations, which differ between those with early and late MJ onset. Taken together, both behavioral and imaging results suggest that more pronounced difficulty with tasks requiring cognitive control is associated with earlier onset of MJ use. These findings are consistent with reports of altered brain activation patterns in early vs late MJ onset smoking groups during the performance of an inhibitory task (Gruber et al., 2012b); however, the current study demonstrates differences in task performance between the groups, not previously documented. This may be the result of increased power given the larger sample sizes presently included, but may also be attributable to the fact that the Stroop Interference condition is considered one of the most robust measures of inhibitory function, especially when responses are vocalized, as in the current study (MacLeod, 1991). Further, while findings demonstrated differences in task performance between those with early MJ onset and HCs, no between-group task differences were detected between the late MJ onset and HCs, suggesting a specific vulnerability of the early onset group. Given that the brain undergoes significant development during adolescence and emerging adulthood (Casey et al., 2005, Giedd et al., 1999, Gogtay et al., 2004) and that the frontal cortex is among the last of the brain regions to mature (Sowell et al., 1999), it is perhaps not surprising that individuals with earlier exposure to MJ have difficulty with tasks requiring frontal/executive function. Inconsistent reports of impaired cognitive function in MJ smokers may therefore be explained by studies that do not assess age of MJ onset independently. Given that individuals with late MJ onset performed similarly to HCs in the current study, and on a range of other cognitive measures in previous studies (Fontes et al., 2011, Gruber et al., 2012a, Pope et al., 2003), it is possible that including late MJ onset smokers’ cognitive performance scores with early onset MJ smokers’ may diminish significant results.
4.1. Limitations
While compelling, findings from the current study must be interpreted within the context of several limitations. First, while all subjects were required to abstain from MJ for a minimum of 12 h, we cannot exclude the possibility that some smokers may have used MJ within this time frame. It is of note, however, that no subject appeared altered or intoxicated, and all completed study visits without difficulty. Current methods to ensure subjects abstained from use have previously been used successfully (Gruber et al., 2013, Gruber et al., 2012a, Gruber et al., 2012b, Gruber et al., 2011) and as a result, it is likely that the 12-h abstinence minimum was followed. In addition, although the current study quantified frequency and magnitude of MJ use little is known about the potency of different strains of MJ used by participants. While unlikely to impact the current findings (as individuals from early and late MJ groups are recruited from similar geographic locations and report similar use), future research should examine the relationship between MJ potency and cognitive function. While some studies have utilized self-report estimates of potency (van der Pol et al., 2013), laboratory analyses of the constituents of MJ (i.e., THC, cannabidiol) are likely to provide insight into the differential effects of potency levels as well as the impact of specific constituents of MJ. Further, subjects in the current study spanned a wide age range (17–48) in comparison to other investigations. It is of note, however, that the groups were well-matched for age, and study findings are likely generalizable to a larger population.
Future research should clarify several areas regarding adolescent MJ use and the brain. First, while results demonstrated that age of onset of use was an important predictor of impairment, frequency and magnitude of MJ use also contributed significantly to study findings. Therefore, higher frequency and magnitude of MJ use, also noted in previous studies (Gruber et al., 2012a, Gruber et al., 2013), may be inextricably linked to earlier onset of MJ use, an important public health issue bearing further investigation. Additional studies should explore this relationship, as both increased frequency and magnitude of MJ use predicted worse cognitive performance to a greater degree than age of onset of MJ use. It is also important to examine how age of onset impacts executive function and functional brain changes in a group of occasional MJ smokers. Notably, the current study examined only chronic MJ smokers, the majority of whom reported smoking multiple times per day, and who had no history of other drug use or other Axis I pathology. Finally, IQ scores were significantly higher than average across all study groups, a finding reported in several previous studies including MJ smokers using a cross-sectional design (Pope and Yurgelun-Todd, 1996, Pope et al., 2001, Gruber and Yurgelun-Todd, 2005, Gruber et al., 2012a, Gruber et al., 2012b, Thames et al., 2014). Above average IQ scores in the current sample may reflect the geographic region used for recruitment, as the Boston area is surrounded by numerous universities, research centers, and hospitals. In addition, participants completed measures of estimated IQ using well-validated, normed assessments, commonly used for screening purposes but which may be more vulnerable to overestimating full scale IQ relative to comprehensive IQ assessments (e.g., WAIS-IV). Interestingly, despite significantly higher than average IQ scores across the groups and no between-group differences noted in IQ, MJ smokers overall demonstrated reduced cognitive performance on tasks requiring cognitive control/inhibitory function. This finding, consistent with previous investigations (Fontes et al., 2011, Gruber et al., 2012a), suggests that MJ use may differentially impact individual cognitive domains, with general IQ and other measures being relatively spared, while frontal/executive measures are subject to impairment. The current results, therefore, may not be generalizable to less frequent MJ smokers, those with comorbid psychiatric or substance use disorders, or those who exhibit lower IQ scores.
While some investigations have assessed MJ smokers after brief periods of abstinence and reported between group differences relative to non-MJ smokers (Bolla et al., 2002, Bolla et al., 2005, Hanson et al., 2010, Jacobus et al., 2014, Tapert et al., 2007), the question remains whether cognitive deficits and altered brain function may be partially or fully remediated after longer abstinence periods. Studies of former MJ smokers are needed to extend research and provide crucial information to shape prevention messages and public policy. Finally, as the current investigation utilized a cross-sectional design, it is not possible to determine “cause” from “effect.” Despite the strong association demonstrated between MJ use, reduced cognitive performance and altered patterns of brain activation, it is not possible to ascertain if these differences preceded MJ use, resulted from MJ use, or represent a combination of factors. Longitudinal studies designed to assess individuals prior to MJ use and follow them over time are needed to address this issue. These studies will also provide an opportunity to independently assess the impact of age of onset of MJ use from current MJ use patterns.
5. Conclusions
Given growing evidence suggesting that earlier onset of MJ use is related to increased cognitive deficits and neurobiologic alterations, the trend of increased MJ use among emerging adults and the increasing number of states with recreational and medical MJ available, it is critical to educate the nation regarding the potentially negative impact of MJ. As states consider legislature for both recreational and medical MJ use, it is imperative to determine safe guidelines regarding the impact of MJ on the brain, particularly during critical periods of neurodevelopment.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgment
The authors wish to thank Mr. Robert Baden for his administrative role, including grant preparation and submission.
Footnotes
Contributor Information
K.A. Sagar, Email: ksagar@mcean.harvard.edu.
M.K. Dahlgren, Email: dahlgren@mclean.harvard.edu.
A. Gönenç, Email: ag@mclean.harvard.edu.
M.T. Racine, Email: mracine@mclean.harvard.edu.
M.W. Dreman, Email: mdreman@mclean.harvard.edu.
S.A. Gruber, Email: gruber@mclean.harvard.edu.
References
- Batalla A., Bhattacharyya S., Yucel M., Fusar-Poli P., Crippa J.A., Nogue S., Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLOS ONE. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti R.A., Roodenrys S., Johnstone S.J., Pesa N., Hermens D.F., Solowij N. Chronic use of cannabis and poor neural efficiency in verbal memory ability. Psychopharmacology (Berl.) 2010;209:319–330. doi: 10.1007/s00213-010-1800-4. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Becker B., Wagner D., Gouzoulis-Mayfrank E., Spuentrup E., Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Behan B., Connolly C.G., Datwani S., Doucet M., Ivanovic J., Morioka R., Stone A., Watts R., Smyth B., Garavan H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bolla K.I., Brown K., Eldreth D., Tate K., Cadet J.L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla K.I., Eldreth D.A., Matochik J.A., Cadet J.L. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brady K.T., Myrick H., McElroy S. The relationship between substance use disorders, impulse control disorders, and pathological aggression. Am. J. Addict. 1998;7:221–230. [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Crane N.A., Schuster R.M., Fusar-Poli P., Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean R.D., Crane N.A., Masson B.J. Evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H., Rinn T., Kunert H.J., Moeller M.R., Poser W., Schilling L. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl.) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., Aslan S., Calhoun V.D., Spence J.S., Damaraju E., Capriahn A., Segall J. Long-term effects of marijuana use on the brain. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1415297111. pii:201415297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams B.W. Biometric Research Department, NY State Psychiatric Institute; New York: 1994. Structured Clinical Interview for Axis I DSM IV Disorders, Patient Edition (SCID-I/P) Version 2.0. [Google Scholar]
- Fontes M.A., Bolla K.I., Cunha P.J., Almeida P.P., Jungerman F., Laranjeira R.R., Lacerda A.L.T. Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos, Rapoport J.L. A Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S.A., Yurgelun-Todd D.A. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res. Cogn. Brain Res. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Gruber S.A., Rogowska J., Holcomb P., Soraci S., Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16:349–360. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- Gruber S.A., Silveri M.M., Dahlgren M.K., Yurgelun-Todd D.A. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp. Clin. Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S.A., Sagar K.A., Dahlgren M.K., Racine M., Lukas S.E. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S.A., Dahlgren M.K., Sagar K.A., Gönenç A., Killgore W.D.S. Age of onset of marijuana use impacts inhibitory processing. Neurosci. Lett. 2012;511:89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S.A., Dahlgren M.K., Sagar K.A., Gönenç A., Lukas S.E. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl.) 2013;231:1455–1465. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy S.M., Smith G.M., Bentler P.M. Consequences of adolescent drug use and personality factors on adult drug use. J. Drug Educ. 1994;24:109–132. doi: 10.2190/X4WU-BV3X-Q483-Y5BT. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hanson K.L., Winward J.L., Schweinsbrug A.D., Medina K.L., Brown S.A., Tapert S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heil S.H., Johson M.W., Higgins S.T., Bicknel W.K. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict. Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hester R., Nestor L., Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestegge L., Radach R., Kunert J.H., Heller D. Visual search in long-term cannabis users with early age of onset. Prog. Brain Res. 2002;140:377–394. doi: 10.1016/S0079-6123(02)40064-7. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Bava S., Cohen-Zion M., Mahmood O., Tapert S.F. Functional consequences of marijuana use in adolescents. Pharmacol. Biochem. Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L.M., Sorg S.F., Nguyen-Louie T.T., Tapert S.F. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J. Stud. Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.D., O’Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor: 2014. Monitoring the Future National Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Kann L., Kinchen S., Shanklin S., Flint K.H., Hawkins J., Harri W.A., Zaza S. Youth risk behavior surveillance – United States, 2013. Morb. Mortal. Wkly. Rep. 2014;63:2–47. [PubMed] [Google Scholar]
- Kempel P., Lampe K., Parnefjord R., Hennig J., Kunert H.J. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L., Strong D.R., Brown R.A. Evaluation of behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W.M., Zvolensky M.J., Pedulla C.M. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lisdahl K.M., Wright N.E., Kirchner-Medina C., Maple K.E., Shollengarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr. Addict. Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D.I., Cheetham A., Yucel M. Cannabis and adolescent brain development. Pharmacol. Ther. 2014 doi: 10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M. Half a century of research on the stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Luborsky L., O’Brien C.P., Woody G.E. An improved diagnostic instrument for substance abuse patients: the addiction severity index. J. Nerv. Ment. Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meier M.H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R.S., Moffitt T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nestor L., Hester R., Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pollock V., Cho D.W., Reker D., Volavka J. Profile of mood states: the factors and their physiological correlates. J. Nerv. Ment. Dis. 1979;167:612–614. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- Pope H.G., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. J. Am. Med. Assoc. 1996;21:521–527. [PubMed] [Google Scholar]
- Pope H.G., Gruber A.J., Hudson J.I., Huestis M.A., Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope H.G., Gruber A.J., Hudson J.I., Cohane G., Huestis M.A., Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Nagel B.J., Schweinsburg B.C., Park A., Theilmann R.J., Tapert S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Neuroimaging. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B., Leo G.I., Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1998;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Solowij N., Pesa N. Cognitive abnormalities and cannabis use. Rev. Bras. Psiquiatr. 2010;32:S31–S40. [PubMed] [Google Scholar]
- Solowij N., Jones K.A., Rozman M.E., Davis S.M., Ciarrochi J., Heaven P.C., Yucel M. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl.) 2012;219:575–586. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene P.R., Vagg P.R., Jacobs A.G. Consulting Psychologists Press; Palo Alto: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Squeglia L.M., Jacobus J., Nguyen-Louie T.T., Tapert S.F. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. doi: 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Jacobus J., Tapert S.F. The influence substance of substance use on adolescent brain development. Clin. EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- Tapert S.F., Schweinsburg A.D., Drummond S.P., Paulus M.P., Brown S.A., Yant T.T., Frank L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl.) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames A.D., Arbid N., Sayegh P. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict. Behav. 2014;39:994–999. doi: 10.1016/j.addbeh.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol P., Liebregts N., de Graff R., ten Have M., Korf D.J., van den Brink W., van Laar M. Mental health differences between frequent cannabis users with and without dependence and the general population. Addiction. 2013;108:1459–1469. doi: 10.1111/add.12196. [DOI] [PubMed] [Google Scholar]
- Vangsness L., Bry B.H., LaBouvie E.W. Impulsivity, negative expectancies, and marijuana use: a test of the acquired preparedness model. Addict. Behav. 2005;30:1071–1076. doi: 10.1016/j.addbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Vitaro F., Gendreau P.L., Tremblay R.E., Oligny P. Reactive and proactive aggression differentially predict later conduct problems. J. Child Psychol. Psychiatry. 1998;39:377–385. [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; New York, NY: 1981. Manual for the Wechsler Adult Intelligence Scale – Revised. [Google Scholar]
- Wechsler D. The Psychological Corporation, Hartcourt Brace and Company; San Antonio: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wrege J., Schmidt A., Walter A., Smieskova R., Bendfeldt K., Radue E.W., Borgwardt S. Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. Curr. Pharm. Des. 2014;20:2126–2137. doi: 10.2174/13816128113199990428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]