Abstract
Ipilimumab is a promising novel immunotherapy agent and is associated with a variety of immune-related adverse events (irAEs). The purpose of the study was to investigate the manifestations of irAEs on body imaging in advanced melanoma patients treated with ipilimumab. One-hundred forty-seven advanced melanoma patients (59 women, 88 men, median age: 64.5) treated with ipilimumab were studied. All patients had the baseline and at least one follow-up chest/abdomen/pelvis CT or PET/CT during therapy, which were reviewed by a consensus of two radiologists blinded to the clinical data. Findings indicative of individual types of irAEs were assessed, including thyroiditis, sarcoid-like lymphadenopathy, pneumonitis, hepatitis, pancreatitis, and colitis. Among the 147 patients, 46 (31%) had radiologically identified irAEs. Time interval from initiation of therapy to the development of irAEs was less than 3 months in 76% (35/46) of the patients [range: 0.2-9.1 months]. Clinical characteristics did not differ between patients with and without irAEs (P>0.18). Among the individual types of irAEs, colitis was most common (n=28; 19%), followed by sarcoid-like lymphadenopathy (n=8; 5%) and pneumonitis (n=8; 5%). Hepatitis (n=3), thyroiditis (n=2), and pancreatitis (n=1) were less common. The resolution of irAEs was noted in 32 among 36 patients (89%) with further follow-up scans, with a median time of 2.3 months after the detection of irAE. In conclusion, immune-related adverse events were noted on body imaging in 31% of melanoma patients treated with ipilimumab. Colitis was the most common, followed by sarcoid-like lymphadenopathy and pneumonitis. The results call for an increased awareness of irAEs, given the expanding role of cancer immunotherapy.
Keywords: Immunotherapy, immune-related adverse events, melanoma, ipilimumab, imaging
INTRODUCTION
Ipilimumab is an immune checkpoint inhibitor which blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4) and augments T-cell immune response against cancer cells (1-6). Following the demonstration of survival benefit and safety profile of ipilimumab in phase III clinical trials, it was approved by the U.S. Food and Drug Administration (FDA) in March of 2011 for the treatment of metastatic melanoma (1, 7). The success of ipilimumab in metastatic melanoma has led to the development of other immunotherapeutic agents such as the inhibitors of programmed cell-death receptor -1 (PD-1) and its ligand, PD-L1 (8-11), which has demonstrated marked clinical activity in advanced melanomas and other solid and hematologic malignancies, resulting in the recent FDA approvals of two different anti-PD-1 antibodies, pembrolizumab and nivolumab, for the treatment of patients with melanoma or squamous cell carcinoma of the lung (12-17).
Consistent with its mechanism of action as an immunomodulator, ipilimumab has unique side effects, which have been referred to as immune-related adverse events (irAEs; refs.18-21). The irAEs during ipilimumab therapy may involve various organs including colon, skin, liver, pancreas, as well as endocrine organs such as pituitary, thyroid, and adrenal glands (22). Most of the reports on irAEs are based on the results of phase II and phase III trials which used various doses of ipilimumab (0.3-10 mg/kg), with limited radiologic descriptions (23). The largest radiology series of irAEs included 81 patients treated with ipilimumab at a trial dose of 10 mg/kg and 38 patients treated in a trial of tremelimumab, another investigational agent that blocks CTLA-4 (21).
Imaging is a key component for monitoring patients during ipilimumab therapy, both for antitumor activity assessment and for work-up of immune-related toxicity, thus allowing the detection of radiologic manifestations of different types of irAEs. Many of the organ-specific irAEs can be diagnosed on cross-sectional imaging of the chest, abdomen, and pelvis. Early diagnosis of irAEs is essential for prompt patient management and adequate therapeutic decisions. The role of imaging in the identification and monitoring of irAEs is becoming more important in the clinical setting, given the recent accelerated approvals of immunotherapeutic agents for different types of tumors. However, the concept of irAEs and their currently limited radiologic descriptions present challenges for prompt and accurate imaging diagnosis of irAEs. It is therefore critical to systematically document the radiographic features of irAEs that can be identified on routine body imaging during ipilimumab therapy.
The purpose of this study is to investigate the frequency of radiographically-evident irAEs in patients with advanced melanoma treated with ipilimumab as a part of standard care, and describe the imaging profiles of organ-specific irAEs in correlation with clinical characteristics, based on a systematic review of longitudinal cross-sectional body imaging during therapy.
MATERIALS AND METHODS
Patients
The original cohort included 162 consecutive patients with advanced melanoma who were treated with ipilimumab monotherapy as part of the standard clinical care between April 2011 and September 2014 at the Dana-Farber Cancer Institute. Among the original cohort, 147 patients (59 women, 88 men, median age: 64.5 years) had baseline and at least one follow-up cross-sectional imaging studies (chest, abdomen, and pelvis CT or whole body 18F-fluoro-2-deoxy-D-glucose positron emission tomography/CT (FDG-PET/CT)) during therapy that were available for review, who were considered to be eligible for this radiographic study and were included in the study population. The remaining 15 patients were excluded due to a lack of available imaging studies for review. The histopathology of melanoma was confirmed in all patients. The standard clinical treatment included 4 cycles of ipilimumab at a dose of 3 mg/kg.
The demographics and clinical characteristics of the patients were obtained by review of medical records (performed by SHT and AK). This retrospective study was approved by the institutional review board with waiver for informed consent and was Health Insurance Portability and Accountability Act-compliant.
Cross-sectional Imaging and Analysis
CT scan of the chest, abdomen and pelvis utilized a 64-row MDCT scanner, with intravenous contrast agent unless medically contraindicated. Axial (5 mm thickness) and coronal (4 mm thickness) images were transferred to the Picture Archiving Communication System (PACS) (Centricity, GE Healthcare, Barrington, IL). Whole body PET imaging was performed approximately 60 min following intravenous administration of 18F-FDG. Non-contrast CT imaging was performed without breath-hold, and axial images (3.75-5 mm thickness) were transferred to PACS. Median time between the baseline scan and initiation of ipilimumab monotherapy was 0.7 months [range: 0-2.5 months]. Follow-up scans were performed per treating clinical providers’ discretion, without predefined intervals.
The images were reviewed by consensus of two board-certified radiologists (MN, NHR) with expertise in cancer imaging. A total of 748 CT and 326 PET/CT scans were reviewed (median number of scans per patient: 5). Radiologists were aware that the patients were treated with ipilimumab for advanced melanoma; however, the radiologists did not have access to other clinical details. For each patient, the baseline and follow-up scans were reviewed to identify the development of predefined imaging findings indicative of irAEs, based on the descriptions in the existing literature (21, 24-28).
The organ-specific irAEs evaluated in the present study included thyroiditis, sarcoid-like lymphadenopathy, pneumonitis, hepatitis, pancreatitis, and colitis. The imaging criteria for each of the irAEs are as follows; 1) thyroiditis: new enlargement of the thyroid gland with heterogeneous enhancement, or new diffuse FDG uptake on PET; 2) sarcoid-like lymphadenopathy: new bilateral symmetric mediastinal and hilar lymphadenopathy resembling typical sarcoidosis without evidence of infection, not suspicious for metastasis and occurring in the setting of response at other sites (21, 29-31); 3) pneumonitis: new consolidative or ground glass opacities with distributions and appearances that are not consistent with metastasis (24, 28); 4) hepatitis: hepatomegaly, heterogeneous parenchymal enhancement with low-attenuation areas, periportal/gall bladder edema (26); 5) pancreatitis: new focal or diffuse pancreatic enlargement with decreased enhancement and peripancreatic stranding, without a focal lesion suspicious for metastasis (32); 6) colitis: fluid-filled colon, mesenteric vessel engorgement, bowel wall thickening (> 4mm irrespective of distension), or increased mucosal enhancement on contrast-enhanced CT scan of the abdomen (27, 33). The cases of colitis were classified into two subtypes including: i) diffuse pancolitis, and ii) segmental colitis associated with diverticulosis (SCAD) restricted to a segment of colon with diverticulosis (27).
For each of the irAEs, a score was given by a consensus using a 5-point scale: 1=definitely not irAE, 2= probably not irAE, 3=equivocal, 4=probably irAE, and 5= definitely irAE. Cases with scores of 4 and 5 were considered as positive for irAEs. The date of the first scan that scored 4 or 5 for the irAEs was used to represent the onset of radiographically-evident irAEs. Subsequent follow-up scans, if available, were reviewed to assess resolution of the radiographic findings.
After the completion of the imaging review, cases with radiographically-evident irAEs were correlated with clinical, biochemical or histopathologic assessments, collected by review of medical records by SHT and AK.
Statistical analysis
Differences in demographics and clinical characteristics were compared between patients with and without radiographically-evident irAEs, using Fisher exact test for categorical variables and Wilcoxon test for continuous variables. Median time to development of radiographically-evident irAEs was calculated using the Kaplan-Meier method, and patients who did not develop radiologically identified irAE were censored at the time of last follow-up imaging.
All pvalues were based on a two-sided hypothesis. A pvalue of less than 0.05 was considered to be statistically significant.
RESULTS
Overall frequency and characteristics of radiographically-evident irAEs
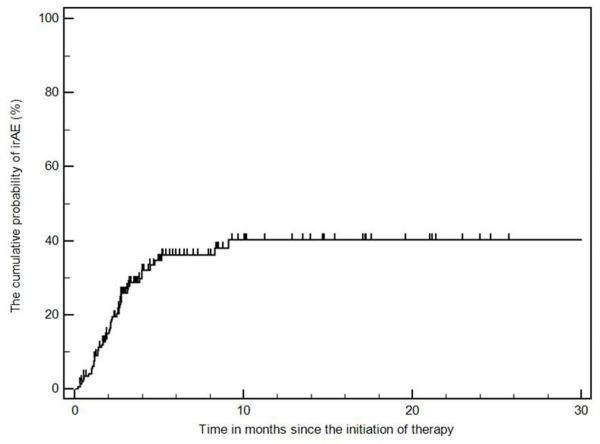
Among the 147 patients with advanced melanoma treated with ipilimumab, 46 patients (31%) developed radiographically-evident irAEs. Radiologic irAEs were noted within 3 months from the initiation of therapy in 35 (76%) among the 46 patients [range: 0.2-9.1 months] (Fig. 1). No statistically significant differences were observed between the 2 groups (p>0.18), including the median duration of ipilimumab therapy (p=0.61) (Table 1).
Fig. 1.
The cumulative probability of radiographically-evident immune-related adverse events (irAEs) during ipilimumab therapy.
Table 1.
Demographics and clinical characteristics
| Characteristics | With irAE (n=46) | Without irAE (n=101) |
Total (n=147) |
P value | |
|---|---|---|---|---|---|
| Sex | Male | 25 | 63 | 88 | 0.37 |
| Female | 21 | 38 | 59 | ||
| Median age (years) | 65.0 | 64.0 | 64.5 | 0.81 | |
| Race | White | 43 | 85 | 128 | 0.18 |
| Non-white | 3 | 16 | 19 | ||
| Smoking | Never | 22 | 47 | 69 | 0.92 |
| Former | 22 | 47 | 69 | ||
| Current | 2 | 7 | 9 | ||
| ECOG PS | 0 | 33 | 71 | 104 | 0.24 |
| 1 | 9 | 25 | 34 | ||
| 2 | 3 | 1 | 4 | ||
| Unknown | 1 | 4 | 5 | ||
|
Median duration of
ipilimumab therapy (months) |
8.9 | 8.0 | 8.1 | 0.61 | |
Further follow-up scans after the onset of radiographically-evident irAEs were available in 36 patients (89%). Eventual resolution of the radiographic findings was noted in 32 of the 36 patients; 22 of the 32 patients (69%) had resolution within 3 months [Median time from the onset to resolution: 2.3 months; range: 0.3-7.7 months].
Characteristics of individual types of organ-specific irAEs
Colitis was most common and was seen in 28 patients (19%), followed by sarcoid-like lymphadenopathy (n=8; 5%) and pneumonitis (n=8; 5%) (Table 2). Four patients with colitis had another organ involved by radiographically-evident irAE, including pneumonitis in 3 patients, and sarcoid-like lymphadenopathy in one patient. Among the total of 50 organ-specific irAEs, including 4 patients with colitis who also had another type of irAE, 43 (86%) irAEs were detected on CT alone, 6 (12%) were detected on PET/CT alone, and 1 (2%) were detected both on CT and PET/CT.
Table 2.
Organ-specific irAEs and the clinical characteristics
| Number of patients [%] | Months since therapy initiation (median, [range])* | |
|---|---|---|
| Colitis (total) | 28 (19%) | 1.9 [0.4-4.7] |
| Sarcoid-like lymphadenopathy | 8▲ (5%) | 3.2 [0.2-9.1] |
| Pneumonitis | 8◊ (5%) | 2.3 [1.1 – 8.3] |
| Hepatitis | 3 (2%) | 1.4 [0.3-2.7] |
| Thyroiditis | 2 (1%) | 4.3 [4.0-4.7] |
| Pancreatitis | 1 (0.6%) | 3.8 [3.8] |
Median and range represent those among the patients who had the events
Include one patient who also had colitis
Include 3 patients who also had colitis
Colitis
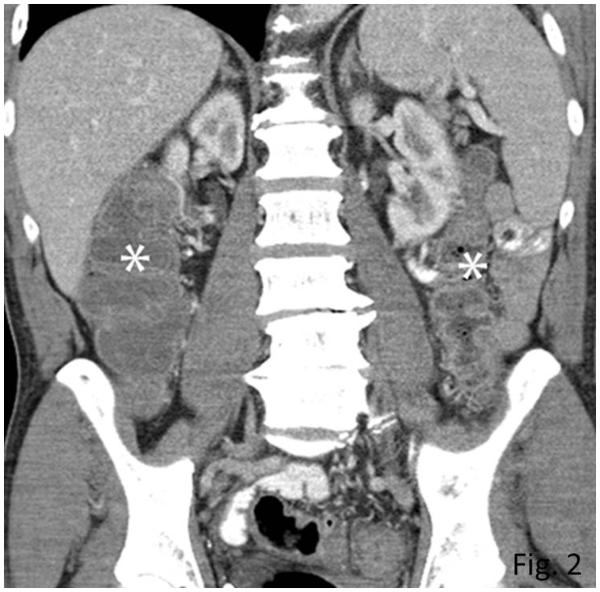
Among the 28 patients with radiographically-evident colitis, median interval from initiation of ipilimumab therapy to the development of colitis was 1.9 months [range: 0.4-4.7 months]. CT features of colitis included mesenteric hyperemia (n=26), bowel wall thickening (n=25), increased mucosal enhancement (n=21), and fluid-filled colon (n=20). Free fluid was also noted in 6 patients. Twenty-six patients had diffuse colitis pattern (Fig. 2) and two patients had SCAD pattern. Of the 28 patients, diarrhea (4-12 episodes/day) was noted in 25 patients, and 3 patients had non-specific abdominal pain without diarrhea. Colonoscopy and biopsy were performed in 22 patients, confirming colitis, which was thought to be consistent with ipilimumab-associated colitis given the clinical setting. Oral steroids were given as a treatment of colitis for 23 patients, and 11 of them also received infliximab. Surgery was performed in 3 patients with colonic perforation.
Fig. 2.
Colitis with a diffuse colitis pattern in a 64-year-old man with advanced melanoma treated with ipilimumab, presenting with diarrhea.
Coronal reformatted contrast-enhanced CT image of the abdomen obtained at 2.6 months after the initiation of ipilimumab treatment demonstrated a new fluid-filled dilated colon (asterisk) with mucosal hyperemia indicating diffuse colitis. Colonoscopic biopsy confirmed colonic inflammation with mucosal injury consistent with ipilimumab-associated colitis. Patient was treated with oral steroids followed by intravenous infliximab, leading to resolution of the findings on the follow-up scan at 1.8 months after the onset (data not shown).
Further follow-up scans were available in 21 patients. In 20 patients, the resolution of colitis was noted with a median interval from the onset to resolution of 1.8 months [range: 0.3-8.0 months]. In one patient, the findings of colitis continued at the last follow-up imaging performed 3 months after the onset.
Sarcoid-like lymphadenopathy
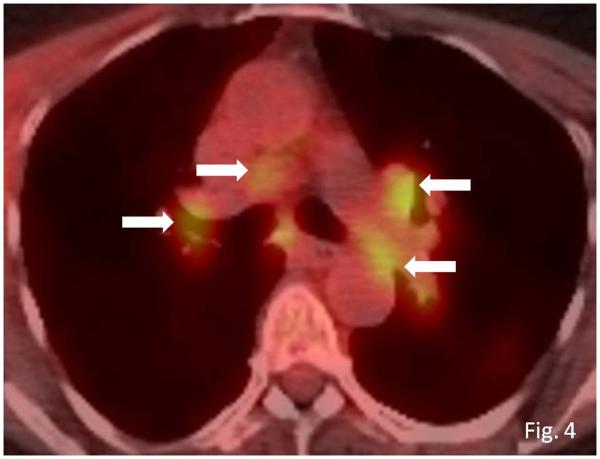
Sarcoid-like lymphadenopathy was seen in 8 patients, demonstrating newly enlarged mediastinal and hilar nodes (n=5), enlargement of preexisting nodes (n=1), and both new and enlarged nodes (n=2). The median interval from the initiation of ipilimumab therapy was 3.2 months [range: 0.2-9.1 months] (Figs. 3, 4). Coexistent pulmonary findings were also noted in 3 patients, including irregular nodular and parenchymal opacities with bilateral symmetric involvement predominantly in the upper and middle lungs (n=1), bilateral diffuse ground glass opacity with upper and middle lung predominance (n=1), and bilateral peripheral interstitial opacities in the lower lobes with pleural effusion (n=1). Coexistent intra-abdominal lymphadenopathy was noted in one patient.
Fig. 3.
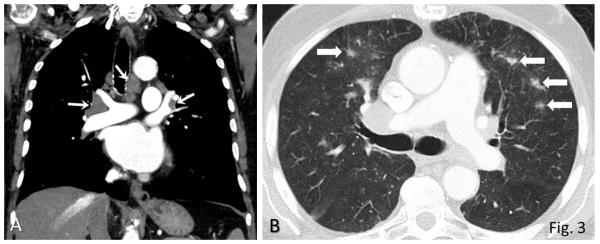
Sarcoid-like lymphadenopathy in an asymptomatic 81-year-old man with metastatic melanoma treated with ipilimumab.
A. Coronal reformatted contrast-enhanced chest CT performed at 4.9 months after the initiation of ipilimumab therapy demonstrated new bilateral symmetric mediastinal and hilar lymphadenopathy, resembling sarcoidosis.
B. Axial CT image of the lungs showed bilateral irregular and nodular parenchymal opacities in upper and middle lung predominance (arrows), with peribronchovascular involvement, which falls in the spectrum of lung parenchymal manifestations of pulmonary sarcoidosis.
Fig. 4.
Sarcoid-like lymphadenopathy in an asymptomatic 55-year-old woman with metastatic melanoma treated with ipilimumab.
Axial fused FDG-PET/CT images at 3 months of ipilimumab therapy demonstrate new FDG-avid mediastinal and bilateral hilar lymphadenopathy (arrows) mimicking sarcoidosis. A follow-up PET/CT performed 5 months later showed resolution of FDG-avid lymphadenopathy (data not shown).
Sarcoid-like lymphadenopathy was not associated with clinical signs or symptoms in any of the patients. None of the patients had history of sarcoidosis or hypercalcemia, or evaluation of serum angiotensin converting enzyme (ACE). Follow-up scans were available in 6 patients, demonstrating the resolution of lymphadenopathy in all of them, with a median interval to resolution of 3.1 months [range: 1.1-5.4 months].
Pneumonitis
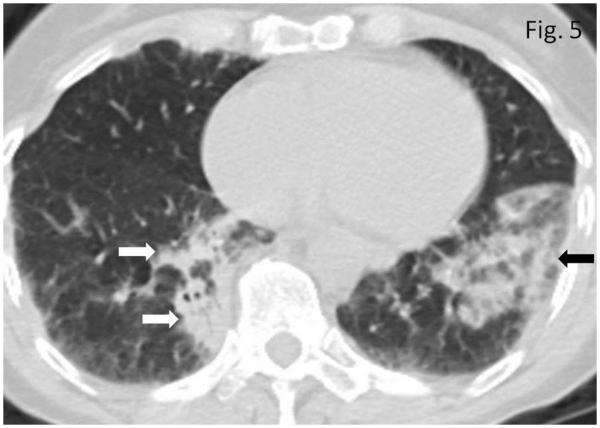
Pneumonitis was noted in 8 patients, with a median time from the initiation of therapy to the onset of 2.3 months [range: 1.1-8.3 months]. CT findings included bilateral consolidative and ground glass opacities predominantly in peripheral distribution, mimicking cryptogenic organizing pneumonia (COP) pattern (n=5) (Fig. 5), and GGO with interlobular septal thickening in basilar and peripheral distribution mimicking nonspecific interstitial pneumonia (NSIP) pattern (n=3). Four patients were symptomatic with cough at the time of radiographically-evident pneumonitis.
Fig. 5.
Pneumonitis in a 72-year-old woman with metastatic melanoma treated with ipilimumab. Axial CT image of the chest obtained at 4.0 months after the initiation of ipilimumab therapy demonstrates new bilateral consolidative and ground glass opacities predominantly in peripheral and lower distribution (arrows), mimicking cryptogenic organizing pneumonia (COP) pattern. Patient was symptomatic with cough at this time and was treated with oral steroids. Further follow-up CT scan performed 2.1 months after the onset demonstrated resolution of the findings (data not shown).
The radiologic findings of pneumonitis resolved in 5 patients, with a median interval to the resolution of 2.1 months [range: 0.5-5.6 months]. The findings persisted in 2 patients at the last follow-up imaging performed at 2.8 and 11.1 months after the onset.
Hepatitis
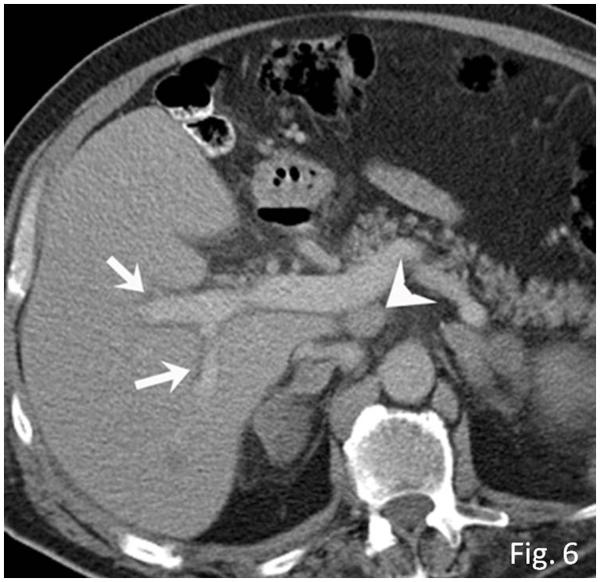
Hepatitis was radiologically detected in 3 patients, with heterogeneous parenchymal enhancement with low-attenuation areas, periportal and gallbladder edema, and ascites. Coexistent hepatomegaly was present in 2 patients (Fig. 6). None of these patients had other irAEs, gallstones, liver cirrhosis, renal or cardiac dysfunction, viral or preexisting autoimmune hepatitis, or exposure to other hepatotoxic drugs or alcohol.
Fig. 6.
Hepatitis in a 63-year-old man with metastatic melanoma treated with ipilimumab. Axial contrast-enhanced CT scan of the abdomen performed at 2.7 months of ipilimumab therapy demonstrates new periportal edema (arrows) with hepatomegaly and new periportal lymphadenopathy (arrowhead). Markedly elevated liver functions were noted, leading to liver biopsy, which revealed severe panlobular hepatitis with lymphoplasmacytic infiltrate and eosinophils, and foci of central vein damage and perivenular collapse, consistent with ipilimumab-associated hepatitis. The patient was treated with steroids and mycophenolate, and liver functions were normalized.
Hepatitis was clinically diagnosed in one of the 3 patients, whose liver function tests (LFTs) were markedly elevated, leading to liver biopsy. The biopsy specimen showed severe panlobular hepatitis with lymphoplasmacytic infiltrate and eosinophils, and foci of central vein damage and perivenular collapse, which represented the morphologic features for autoimmune hepatitis and were histologically thought to be consistent with ipilimumab-associated hepatitis given the clinical setting. The patient was treated with steroids and mycophenolate, leading to the subsequent normalization of liver function, however had no further follow-up imaging. The remaining 2 patients were clinically asymptomatic; one patient had grade I elevation of alkaline phosphatase (130 U/L; normal range: 36-118), with no further follow-up imaging. The other patient had grade 2 elevation of alkaline phosphatase (408U/L) and grade I elevation of SGOT (57 U/L; normal range: 9-30), and the radiologic findings persisted at the last follow-up imaging performed 4 weeks after the onset.
Thyroiditis
Thyroiditis was radiologically detected in 2 patients, demonstrating new diffuse FDG uptake on PET/CT, at 4.0 and 4.7 months of ipilimumab therapy. One patient had concurrent hypophysitis, clinically diagnosed with a decrease in serum thyroid stimulating hormone level and was treated with hormone replacement therapy, resulting in resolution of uptake 4.7 months after onset. The other patient had normal thyroid function tests and was not treated.
Pancreatitis
Pancreatitis was noted in one patient at 3.8 months of therapy, demonstrating pancreatic enlargement with a decreased enhancement and surrounding fat stranding on CT and an increased FDG uptake on PET. The patient was clinically asymptomatic for pancreatitis, however, the elevation of the serum amylase (10X) and lipase (32-fold) as noted at the time of positive imaging finding. The pancreatitis radiographically resolved 6.5 months after onset without any specific treatment. The patient also had immune-related hypophysitis diagnosed clinically 2 months prior to the onset of pancreatitis.
DISCUSSION
The irAEs were noted on body imaging in 31% (46/147) of patients with advanced melanoma treated with ipilimumab, and occurring within 3 months after the initiation of therapy in the majority of patients (76%, 35/46). Colitis was the most common organ-specific irAE, followed by sarcoid-like lymphadenopathy and pneumonitis. In most of the cases of irAEs (89%), the radiologic findings resolved on the subsequent follow-up imaging, at a median interval of 2.3 months. The high incidence of irAEs and their variable manifestations on body imaging involving different organs are provocative for the important role of imaging in diagnosis and monitoring of this emerging entity, in the new and expanding arena of cancer immunotherapy.
In the phase 3 trial of metastatic melanoma, the incidence of any irAE in 131 patients treated with ipilimumab monotherapy was 61% (1, 19), with dermatologic irAEs being the most common manifestation, occurring in 43.5% of patients. Dermatologic irAEs were not evaluated in the present study focusing on radiographic abnormalities, explaining the lower incidence of irAEs noted on body imaging (31%). On the other hand, the incidence of irAEs in the present study was higher than in the previous radiologic report by Bronstein and colleagues, which studied 119 patients (81 treated with ipilimumab and 38 treated with tremelimumab) and identified 20 patients (16.8%) with radiologic abnormalities potentially explained by irAEs (21). Several differences between the two studies could account for the difference. First, the 147 patients in the present study were all treated with ipilimumab monotherapy, while the Bronstein study included two subcohorts treated with different agents in different trial protocols. Second, the present study provided the predefined radiologic findings indicative of organ-specific irAEs in 6 different organs for radiology review, based on the descriptions of the reports focusing on the individual types of irAEs published between 2012-2014 (19, 20, 26-29, 34), which was not available at the time the report by Bronstein and colleagues, which was published in 2011 (21). The present study evaluated irAEs not described in the Bronstein study, including pneumonitis, hepatitis, and pancreatitis; the Bronstein study described hypophysitis, arthritis, myositis, and retroperitoneal fat opacities that are not included in the present study. The different approaches and different results are also indicative of the rapidly evolving knowledge of treatment effects and adverse events associated with cancer immunotherapy.
In 76% of the patients in the present study, radiographic irAEs were noted within 3 months after the initiation of therapy; this is consistent with the detailed analysis of the data from the phase 3 trial of ipilimumab by Weber and colleagues, which showed that a substantial number of irAEs occurred within the first 12 weeks of treatment (19). Radiologic findings of irAEs resolved in 89% of the patients with follow-up imaging in the current study, with a median interval of 2.3 months, which is in line with the time frame of 6-8 weeks reported by Weber and colleagues (19). There is a slightly longer time to resolution in our present study, which may be because the timing of resolution is subject to the timing of follow-up imaging in the present study, while the resolution of many clinical irAEs can be noted by clinical evaluations that occur more frequently.
Colitis was the most common organ-specific irAE, seen in 19% (28/147) of patients. The incidence of gastrointestinal irAEs varies between 31-46% in the clinical studies, with grade 3 or higher colitis occurring in 10-23% of patients (19, 22, 35, 36). In a report by Weber and colleagues, gastrointestinal irAEs were the second most common after dermatologic irAEs, noted in 29% (38/131) of patients, manifesting as diarrhea (27.5%; 36/131) and/or colitis (7.6%; 10/131; ref. 19). The incidence of colitis as irAE in the present study mostly falls within the ranges of incidence described in the prior clinical studies. The small differences can be explained by the differences between the definitions of radiographic colitis and the definitions of clinical gastrointestinal irAEs including the spectrum of diarrhea and colitis. Ipilimumab-associated colitis has been reported to typically develop 6-7 weeks after the initiation of therapy, and resolve within 6-8 weeks (19), which is consistent with our radiologic results, further confirming the clinical utility of the imaging evaluations used in the present study based on the previous report (27). Diffuse colitis was more common than SCAD, consistent with the prior report (27). Prompt diagnosis of colitis in patients treated with ipilimumab is critical because it can lead to serious complications such as bowel perforation if left untreated. Among a cohort of 643 patients in the phase 3 trial, 5 deaths were associated with colitis and its complications (19).
Sarcoid-like lymphadenopathy is an underreported ipilimumab-related irAE, though it is described in a few sporadic reports (25). The incidence of sarcoid-like lymphadenopathy in our study (5%) was similar to that reported by Bronstein and colleagues (6.7%, 8/119) (21). Three patients in our series also had concurrent pulmonary parenchymal changes that are within the spectrum seen in pulmonary sarcoidosis (37, 38), as described in a previous report (25). Some cases of sarcoid-like lymphadenopathy can be clinically silent and incidentally noted on imaging, as in the 8 cases in the present study, leading to under-documentation in the clinical literature. Because new lymphadenopathy also raises concern for metastatic diseases, the awareness of sarcoid-like lymphadenopathy as a manifestation of irAEs is essential in achieving accurate diagnosis and management, and in directing to adequate clinical testing, such as serum ACE level assessments. Sarcoid-like lymphadenopathy resolved in all the patients with follow-up scans in our study, further confirming the benign nature of the process.
Pneumonitis during ipilimumab therapy is described in a few clinical case reports and in a phase 2 trial conducted by the Eastern Cooperative Oncology Group, in which 7.5% (9/120) of the patients treated with ipilimumab monotherapy at a dose of 10 mg/kg developed pulmonary toxicity (24, 28, 39). However, their detailed radiologic description of this entity is limited. Among the 8 patients in the present study, the radiologic manifestations mimicked COP pattern in 5 patients as in the prior report (24), and the remaining 3 patients had findings resembling an NSIP pattern, which has been known to be associated with conventional agents such as methotrexate, and carmustine (40). The characterization of this irAE may be of increasing importance, given that anti-PD-1/PD-L1 antibodies are known to be associated with clinically significant, potentially lethal immune-related pneumonitis (41). The attention to the pulmonary findings described here may also be particularly important in the setting of combination therapy using ipilimumab plus anti-PD-1 antibodies or other agents, which are being tested (42-44). Further studies are needed to fully characterize the spectrum of drug-related pneumonitis during cancer therapy.
Hepatic irAEs related to CTLA-4 inhibition are less common compared to gastrointestinal or dermatologic irAEs in trials. A post hoc clinical review by Weber and colleagues identified 5 patients with grade ≥3 hepatic irAEs among 511 ipilimumab-treated patients (19). The relatively low incidence (2%) of hepatitis on imaging is similar to the clinical report given it focused on grade≥3 toxicity. Although less common, a potential severe clinical implication is noted in a prior study, which described one death attributed liver failure due to irAE during ipilimumab monotherapy (19), further emphasizing the clinical significance of prompt diagnosis.
Endocrine irAEs were relatively common after dermatologic and gastrointestinal irAEs in the phase 3 trial of ipilimumab, noted in 7.6% (10/131) of melanoma patients treated with ipilimumab monotherapy (1, 19). Hypothyroidism was seen in 2 patients (1.5%), which is similar to the incidence in the present study. The imaging features in 2 patients in the present study were new diffuse FDG uptake in the thyroid gland, which raises the possibility of under diagnosis among those without PET imaging.
Immune-related pancreatitis has been reported as a relatively rare manifestation of irAEs (45, 46). In a retrospective study of a cohort of 39 patients with uveal melanoma, one patient (2.5%) had ipilimumab- associated pancreatitis (46). Pancreatitis can be asymptomatic with an elevation of serum amylase and lipase alone, as in the case detected in the present study, or it can be associated with nonspecific symptoms (32). Imaging plays an important role in detecting this rare entity in the presence of the pertinent imaging findings somewhat simulating autoimmune pancreatitis in the relevant clinical setting.
The limitation of the present study include a retrospective design in the cohort treated at a single institution; however, this cohort is larger than the cohorts treated with single-agent ipilimumab in previous radiology or clinical studies (1, 19, 21, 46), and represents the first cohort of patients with advanced melanoma treated with ipilimumab monotherapy outside of clinical trials, undergoing systematic review of body imaging studies for irAEs. Because the patients were treated clinically without being enrolled in prospective trials, scans were performed as per clinical care provider’s discretion without predefined intervals, which limits the detailed analysis of sensitivity for irAEs across different image modalities. Although hypophysitis is a known endocrinologic irAE, it was not included in the present study focusing on 6 different types of irAEs noted on body imaging. The entity is well described in endocrinology literature (47-50), and a detailed neuroradiologic investigation is planned. Not all the cases of radiologically detected irAEs had histologic confirmation, which is often the case with cohorts of patients with advanced malignancy. Clinical confirmation was not obtained in some cases, which is partly because some irAEs, such as sarcoid-like lymphadenopathy, are known to be clinically silent. Of note, irAEs consist of a variety of entities involving different organs with emerging and evolving concepts, which led to the study design not requiring clinical confirmation for all positive cases. Instead, the predefined imaging features for each organ-specific irAE based on the published literature were used to guide the systematic radiology review. Given the emerging clinical and radiologic concept of irAEs, it is currently difficult to define the absolute “gold standard” for the diagnosis of irAEs; rather, the diagnosis often requires the interplay among clinicians, radiologists, and sometimes pathologists via their multidisciplinary interactions. Therefore, no formal assessments of sensitivity and specificity of radiological diagnosis of irAEs were performed.
In conclusion, radiographically-evident irAEs on body imaging were present in nearly one-third of patients with advanced melanoma treated with ipilimumab monotherapy as standard of care, based on the longitudinal systematic review of their imaging studies. Among the organ-specific irAEs, colitis was most common, followed by sarcoid-like lymphadenopathy and pneumonitis. The high incidence of irAEs and the imaging manifestations in various organs call for the awareness of this emerging entity, given the expanding roles and applications of cancer immunotherapy in clinical oncology practice.
Acknowledgments
Nishino M was supported by 1K23CA157631 (NCI).
Footnotes
Disclosures:
Tirumani, Ramaiya, Keraliya, Bailey: None
Ott and Nishino: Consultant, Bristol-Myers Squibb
Hodi: non-paid consultant to Bristol-Myers Squibb; received clinical trial support from Bristol-Myers Squibb; advisor and clinical trial support from Merck; advisor and clinical trial support from Genentech.
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 4.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–11. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Fisher DE. Adoptive transfer of antigen-specific CD4+ T cells in the treatment of metastatic melanoma. Nat Clin Pract Oncol. 2008;5:696–7. doi: 10.1038/ncponc1259. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 11.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino M, Cardarella S, Dahlberg SE, Araki T, Lydon C, Jackman DM, et al. Interstitial lung abnormalities in treatment-naive advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol. 2015;84:998–1004. doi: 10.1016/j.ejrad.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA Approval for Pembrolizumab. Available from URL: http://www.cancer.gov/cancertopics/druginfo/fda-pembrolizumab.
- 14.FDA approves Opdivo for advanced melanoma. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427716.htm.
- 15.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 18.O'Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol. 2011;197:W241–6. doi: 10.2214/AJR.10.6032. [DOI] [PubMed] [Google Scholar]
- 19.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–82. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 20.Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197:W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. ASCO Meeting Abstracts. 2011;29:8583. [Google Scholar]
- 23.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 24.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218:69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 25.Berthod G, Lazor R, Letovanec I, Romano E, Noirez L, Mazza Stalder J, et al. Pulmonary Sarcoid-Like Granulomatosis Induced by Ipilimumab. J Clin Oncol. 2012;30:e156–9. doi: 10.1200/JCO.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 26.Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071–7. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 27.Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, et al. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200:W468–74. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 28.Mis L, Clarke JM. Ipilimumab-Induced Pneumonitis: A Case Report. J Pharmacy Technol. 2013;29:94–8. [Google Scholar]
- 29.FDA approval of Nivolumab (Opdivo) for metastatic squamous non-small cell lung cancer. Food and Drug Administration 2015. Available from: http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm436566.htm.
- 30.Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 32.Di Giacomo AM, Danielli R, Guidoboni M, Calabró L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkpatrick ID, Greenberg HM. Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology. 2003;226:668–74. doi: 10.1148/radiol.2263011932. [DOI] [PubMed] [Google Scholar]
- 34.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocrine-Related Cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 37.Nishino M, Lee KS, Itoh H, Hatabu H. The spectrum of pulmonary sarcoidosis: variations of high-resolution CT findings and clues for specific diagnosis. Eur J Radiol. 2010;73:66–73. doi: 10.1016/j.ejrad.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 38.Nishino M, Itoh H, Hatabu H. A practical approach to high-resolution CT of diffuse lung disease. Eur J Radiol. 2014;83:6–19. doi: 10.1016/j.ejrad.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078–85. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 40.Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am. 2002;40:61–72. doi: 10.1016/s0033-8389(03)00109-x. [DOI] [PubMed] [Google Scholar]
- 41.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishino M, Patrick JL, Connors JM. Case 155: Lane-Hamilton syndrome. Radiology. 2010;254:985–8. doi: 10.1148/radiol.09082062. [DOI] [PubMed] [Google Scholar]
- 43.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–42. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barjaktarevic IZ, Qadir N, Suri A, Santamauro JT, Stover D. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest. 2013;143:858–61. doi: 10.1378/chest.12-1467. [DOI] [PubMed] [Google Scholar]
- 45.Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4:661–72. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaehler KC, Piel S, Livingstone E, Schilling B, Hauschild A, Schadendorf D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–98. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Min L, Hodi FS, Giobbie-Hurder A, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749–755. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albarel F, Gaudy C, Castinetti F, Ott PA, Luke JJ, Donahue H, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172:195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues BT, Otty Z, Sangla K, Shenoy VV. Ipilimumab-induced autoimmune hypophysitis: a differential for sellar mass lesions. Endocrinol Diabetes Metab Case Rep. 2014;2014:140098. doi: 10.1530/EDM-14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–95. doi: 10.1002/cncr.28282. [DOI] [PMC free article] [PubMed] [Google Scholar]