Abstract
PD-L1 expression in primary clear cell renal cell carcinoma (ccRCC) increases the likelihood of response to anti-PD-1 inhibition, but fails to identify all responders. We hypothesized that PD-L1 levels assessed in randomly selected areas of the primary tumors may not accurately reflect expression levels in metastatic lesions, which are the target of systemic therapy. Therefore, we compared PD-L1 expression in a series of primary ccRCC and their metastases. Tissue blocks from 53 primary ccRCCs and 76 corresponding metastases were retrieved. Areas with predominant and highest nuclear grade were selected. Slides were immunostained with a validated anti-PD-L1 antibody (405.9A11). Membranous expression in tumor cells was quantified using H-score. Expression in tumor-infiltrating mononuclear cells (TIMC) was quantified using a combined score. Discordant tumor cell PD-L1 staining between primary tumors and metastases was observed in 11/53 cases (20.8%). Overall, tumor cell PD-L1 levels were not different in primary tumors and metastases (p=0.51). Tumor cell PD-L1 positivity was associated with higher T stage (p=0.03) and higher Fuhrman Nuclear Grade (FNG) (p<0.01). Within individual lesions, PD-L1 positivity was heterogeneous and almost exclusively detected in high nuclear grade areas (p<0.001). No difference was found in PD-L1 levels in TIMCs between primary tumors and metastases (p=0.82).
Heterogeneity of PD-L1 expression in ccRCC suggests that its assessment as predictive biomarker for PD-1 blockade may require analysis of metastatic lesions. Notably, since PD-L1 expression was mostly detected in high nuclear grade areas, to avoid false negative results, these areas should be specifically selected for assessment.
Keywords: PD-L1, PD-1/PD-L1 inhibitors, renal cell carcinoma, clear cell, metastases, predictive biomarker, immunotherapy
Introduction
The most common type of renal cell carcinoma (RCC) is clear cell RCC (ccRCC), which represents >80% of cases, and accounts for 2–3% of all adult malignant neoplasms (1). Median survival for patients with metastatic disease with approved targeted therapies remains poor and ranges from 8 to 30 months according to prognostic risk groups (2), Therefore, more effective systemic therapies for the treatment of advanced RCC are needed (3). For more than two decades, ccRCC has been recognized as an immunogenic tumor and cytokine-based immunotherapy can produce durable responses in a small subset of patients (4–7).
Recent studies have demonstrated the role of the Programmed Death-1 (PD-1) T-cell co-receptor and its ligand PD-L1 (also known as B7-H1) in maintaining an immunosuppressive tumor microenvironment (8). The PD-1/PD-L1 pathway is known to be activated in many tumor types, including lung, ovarian, colorectal, breast, liver, head and neck, kidney, and bladder cancers and melanoma (9). PD-1 is mainly expressed on tumor-infiltrating lymphocytes, whereas its ligand PD-L1 is expressed on both hematopoietic cells (B, T, myeloid and dendritic cells) and tumor cells (10). There is evidence that similar to epithelial and stromal cells in normal tissues, tumor cells can express PD-L1 on the cell membrane in response to interferon gamma production by activated T cells. Thus, many tumors co-opt the natural physiology of the PD-1 pathway for tissue protection in the face of inflammation, to protect themselves from an antitumor immune response. In line with this hypothesis, it has been shown that tumors expressing PD-L1 are able to inhibit antitumoral T-cell immunity by binding PD-1 on T-cells (11).
It has been reported that PD-L1 is aberrantly expressed in human ccRCC and that patients with PD-L1-positive tumors display a higher risk of cancer-specific mortality (12–15). Currently, anti-PD-1 and anti-PD-L1 antibodies are actively being investigated in clinical development for metastatic ccRCC (8,10) and several datasets suggest that primary ccRCC tumors with PD-L1 positivity either on tumor cell membranes or inflammatory cells achieve better response to PD-1/PD-L1 targeting therapies (16–19). Although PD-L1 expression in primary ccRCC tissue increases the likelihood of response to PD-1 pathway inhibition, it fails to identify all responders. Moreover, many patients with PD-L1-positive tumors do not respond to this therapy. Developing biomarkers that reliably predict response will be essential for narrowing the application of PD-1 blockade to those patients most likely to benefit.
Clear cell RCC is characterized by intratumoral heterogeneity (20). We hypothesized that PD-L1 expression may vary significantly throughout the primary tumors (e.g. high nuclear grade versus low nuclear grade) and/or in the primary tumor versus the metastases and potentially constrain the predictive value of this biomarker. This knowledge is important to determine whether the development of optimal predictive models for PD-1/PD-L1 blockade can be conducted on primary tumor tissue or whether tissue from metastatic sites is likely to be more informative. For this reason, we performed an extensive analysis of PD-L1 expression in a series of primary ccRCCs and corresponding metastases (surgical resections). We assessed PD-L1 expression in both tumor cells and tumor-infiltrating immune cells.
Materials and Methods
Patients and samples
A cohort of 53 primary ccRCC tumors and 76 corresponding metastases from 53 patients, who had undergone surgical tumor resections, were selected from two institutions: Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center. Formalin-fixed paraffin-embedded (FFPE) tissue blocks from primary tumor and corresponding lymph node or distant metastases were retrieved. For each nephrectomy or metastasectomy specimen, all hematoxylin and eosin-stained slides containing tumor were reviewed by expert genitourinary pathologists (SS, EMG, MG). To address intratumoral morphologic heterogeneity, the nuclear grade was assessed in all slides using the criteria established by Fuhrman (21). For each specimen, both areas of highest nuclear grade, also known as Fuhrman nuclear grade (FNG), and areas of predominant nuclear grade were selected for analysis.
Immunohistochemistry
PD-L1 expression was evaluated by immunohistochemistry (IHC) using a mouse monoclonal anti-PD-L1 antibody (405.9A11) developed by Dr. Gordon Freeman (Boston, MA). The assay was validated using FFPE cell line controls known to be either positive or negative for PD-L1 expression by flow cytometry (22).
Four micron-thick tumor sections were stained with the anti-PD-L1 antibody (final concentration of 3.25ug/ml), on a Benchmark XT autostainer (Ventana Medical System, Tucson, AZ) with standard antigen retrieval (CC1 buffer, pH8.0, #950-124, Ventana). UltraView Universal DAB Detection kit (#760-500, Ventana) was used according to the manufacturer’s instruction. Counterstaining was performed as part of the automated staining protocol using hematoxylin (#760-2021, Ventana). After staining, slides were then washed in soapy water and distilled water, dehydrated in graded alcohol and xylene, mounted and cover slipped.
CD45 immunostaining was performed on adjacent four micron-thick tumor sections. Sections were initially deparaffinized, rehydrated and heated with a pressure cooker to 125°C for 30 seconds in citrate buffer for antigen retrieval and then incubated with peroxidase (Dako #S2003, Carpinteria, CA) and protein blocking reagents (Dako #X0909) each for 5 minutes. Sections were then incubated with anti-CD45 (1:100, Dako, clone 2B11+PD7/26) antibody for 1 hour at room temperature followed by incubation with the Dako EnVision+ System HRP-labeled polymer anti-mouse (Dako #K4001) for 30 minutes. All sections were developed using the DAB chromogen kit (Dako K3468) for 2 minutes and then lightly counterstained with hematoxylin.
Quantification of PD-L1 expression in tumor cell membranes and tumor-infiltrating mononuclear cells
Evaluation of PD-L1 expression in neoplastic cells and tumor-infiltrating mononuclear cells (TIMC) was independently performed by three pathologists (SS, MG and MC), blinded to clinical data.
Membranous PD-L1 expression in tumor cells was quantified using an H-score (23), which takes into consideration the percentage of positive tumor cells within each staining category (0 = negative, 1= weak, 2 = moderate, 3 = strong). In cases with focal positivity (<1%, positive tumor cells), the H score was calculated considering the positive tumor cell percentage equal to 1. A case was considered positive when any tumor cell membrane positivity was detected. In addition, in cases with any positivity in either the primary tumor or in the metastases, we recorded PD-L1 status (positive versus negative) in each nuclear grade component (1–4) present in the lesion.
The extent of TIMCs was evaluated on the basis of the immunoreactivity for CD45, a pan-leukocyte marker (24,25) and recorded as absent (0), focal (1), mild (2), moderate (3) or marked (4). The percentage of PD-L1-positive TIMCs was determined according to six categories (0%= 0, ≤5%=1, 6–25%=2, 26–50%=3, 51–75%=4 and >75%=5). PD-L1 expression in TIMCs was then quantified using an Immune Cells Adjusted Score, calculated by multiplying the extent of TIMCs by the “percentage of positive cells” category (26). Any score greater than zero was considered positive.
Statistical Analysis
The primary objective of this study was to characterize PD-L1 expression in primary ccRCC and their corresponding metastases, and to correlate the levels of expression with clinico-pathologic features. Patient and tumor characteristics were summarized descriptively. When several samples were available within one primary or multiple metastatic sites, an average was calculated for each case (similar results were obtained when considering a maximum or median value). Proportions of positive PD-L1 expression in matching primary and metastases from an individual case were compared with the exact McNemar test. Median H-score and Median Immune Cells Adjusted Score in matching primary and metastatic case were compared with the exact Wilcoxon signed rank test. Comparisons between PD-L1 expression and clinico-pathologic features were evaluated using Fisher’s exact test. All statistical computations were performed using Stata v.13.1 (StataCorp, College Station, TX, USA) and a p value (two-sided) <0.05 was considered statistically significant.
Results
Patient population and tumor tissue selection
We collected tissue samples from 53 primary clear cell RCCs and 76 matching metastases. In all cases, the metastatic lesions had been removed by surgical excision, providing sufficient and representative tumor tissue for analysis. ccRCCs are characterized by considerable Intratumoral morphologic heterogeneity with areas of low nuclear grade frequently intermixed with areas of high nuclear grade. In order to address the impact of this heterogeneity, for each primary or metastatic lesion, tumor tissue blocks containing both areas of highest nuclear grade, also known as Fuhrman nuclear grade (FNG), and areas of predominant nuclear grade were selected for analysis.
Metastatic sites included lung (n=20), bone (n=12), lymph node (n=11), soft tissues (n=9) adrenal gland (n=8), pleura (n=3), brain (n=2), thyroid (n=2) and others (n=9). While most primary tumors had only one matching metastasis, in 14 cases (26%), two or more metastatic lesions could be retrieved.
Patient characteristics are summarized in Table 1. Median age was 58 years (range 40–85). Pathologic T stages at diagnosis were T1/T2 in 18 patients and T3/T4 in 32 patients and unknown in 3 cases. No FNG I or II were identified in the cohort; 35 patients had FNG III and 18 had FNG IV.
Table 1.
Patient characteristics
| Characteristics | Total (n=53) | ||
|---|---|---|---|
| No. of Patients |
% | ||
| Gender | Male | 33 | 62.3 |
| Female | 20 | 37.7 | |
| Median age at primary surgery, years (range) | 58 (40–85) | ||
| T Stage | T1 | 4 | 7.5 |
| T2 | 14 | 26.4 | |
| T3 | 28 | 52.8 | |
| T4 | 4 | 7.5 | |
| Tx | 3 | 5.7 | |
| N Stage | N0 | 16 | 30.2 |
| N1 | 14 | 26.4 | |
| Nx | 23 | 43.4 | |
| Fuhrman Nuclear Grade | III | 35 | 66 |
| IV | 18 | 34 | |
| Number of metastatic sites analyzed per case | 1 | 39 | 73.6 |
| 2 | 10 | 18.9 | |
| 3–6 | 4 | 7.5 | |
Extent of discordant PD-L1 expression in primary tumors and metastases
Of the 53 cases analyzed, 17 cases (32%) presented PD-L1 tumor cell membrane positivity in the primary tumor and 12 cases (23%) presented PD-L1 tumor cell membrane positivity in the metastases (Table 2 and Figure 1A, B). The percentage of positive tumor cells ranged between [0–40%] in primary tumors, and [0–70%] in the metastases.
Table 2.
PD-L1 expression levels in primary tumors and metastases
| PD-L1 expression | PRIMARY | METASTASIS | P value | |
|---|---|---|---|---|
| Tumor Cells Membrane | Staining>0%: n (%) | 17 (32%) | 12 (23%) | p=0.23 |
| H-score: Median (range) | 1.3 (0, 85) | 1.5 (0, 170) | p=0.25 | |
| Tumor Infiltrating Immune Cells | Immune Cells Adjusted Score: Median (range) | 4 (0, 9) | 3 (1, 16) | p=0.82 |
Immune Cells Adjusted Score [0–20] = inflammatory extent*x percentage of positive immune cells **
inflammatory extent (absent= 0; focal= 1; mild=2; moderate= 3; marked= 4)
percentage of positive immune cells (0% = 0; ≤5% = 1; 6–25%= 2; 26–50%=3; 51–75%=4; >75%=5)
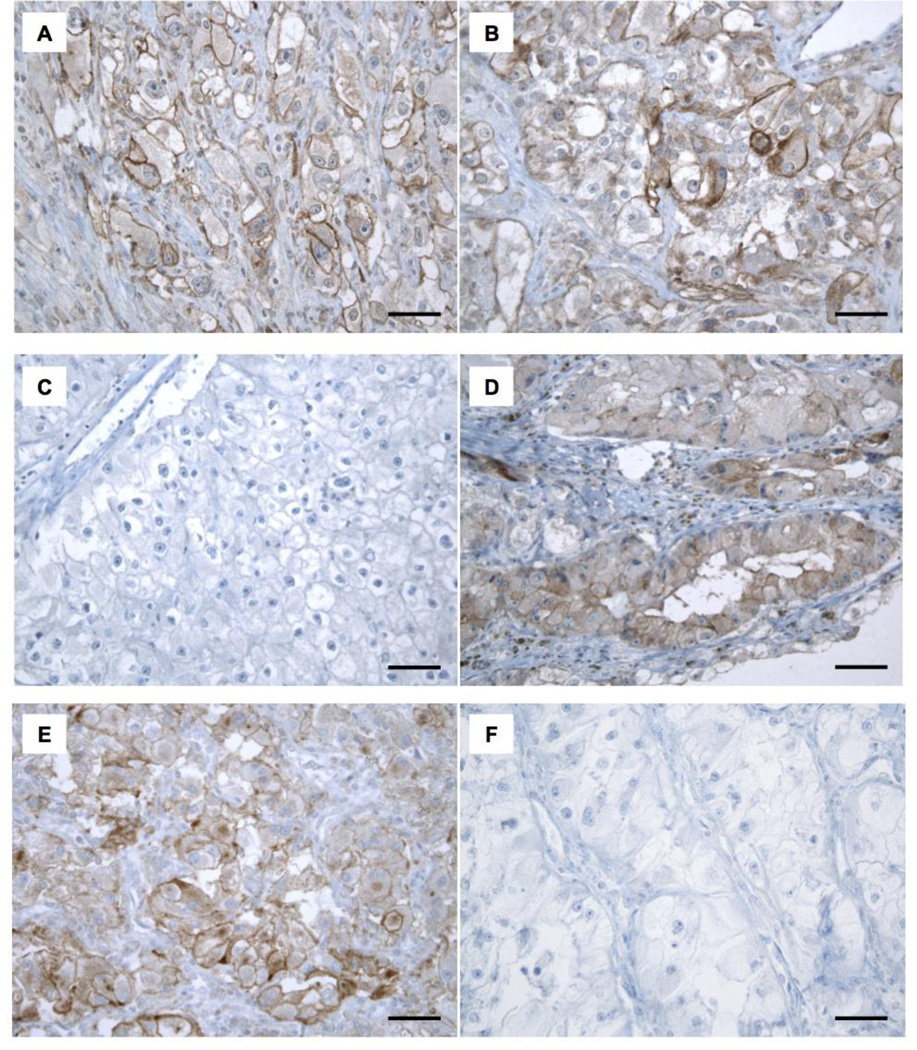
Figure 1. FFPE samples immunostained with anti-PD-L1 antibody (clone 9A11).
Representative images of three primary ccRCC tumors (A, C, E) and their corresponding metastases (B, D, F) immunostained for PD-L1. A, B. Membranous expression of PD-L1 in tumor cells is detected in both the primary tumor and the metastasis. C, D. Membranous expression of PD-L1 in tumor cells is only detected in the metastasis. E, F. Membranous expression of PD-L1 in tumor cells is only detected in the primary tumor. Scale bar: 50 µm
Discordant tumor cells PD-L1 staining between primary tumors and metastases was detected in 11 of 53 cases (20.8%, 95% CI: 10.8% −34.1%). Of the 36 cases with primary tumors that did not express PD-L1, 33 cases were also PD-L1-negative in the metastases. Of the 17 cases with primary tumors that expressed PD-L1 only 9 cases also expressed PD-L1 in the metastases (Table 3 and Figure 1C–F). Among the 11 discordant cases, 6 cases had less than 3-month time interval between the resection of primary tumor and the resection of the metastasis (Supplemental Table 1).
Table 3.
PD-L1 expression in primary tumors versus corresponding metastases
| Metastases | Total | |||
|---|---|---|---|---|
| PD-L1− | PD-L1+ | |||
| Primary Tumors | PD-L1− | 33 | 3 | 36 |
| PD-L1+ | 8 | 9 | 17 | |
| Total | 41 | 12 | 53 | |
It should be noted that several samples were characterized by low percentage (<5%) of PD-L1-positive tumor cells and only 6 cases (11%) showed ≥5% positive tumor cells in the primary tumor. Similarly, only 8 (15%) cases showed ≥ 5% positive tumor cells in the metastatic sites. Using the 5% cutoff, we observed discordant tumor cell PD-L1 staining between primary tumors and metastases in 6 of 53 cases (11.3%, 95% CI: % 4.3%–23.0%).
In the 20 cases with positive PD-L1 expression in the primary tumors and/or metastases, tumor cell PD-L1 levels (determined by an H-score) were not significantly different in primary tumors compared to the metastatic sites (median H-score: 1.3 [0, 85] versus 1.5 [0, 170], p=0.25) (Table 2).
All but one primary tumor and all metastases displayed PD-L1-positive TIMCs (range: 5% to 75%). PD-L1 expression levels in TIMCs assessed by median Immune Cells Adjusted Score was not significantly different in primary tumors and metastases (4 versus 3, p=0.82) (Table 2).
PD-L1 expression in multiple metastases from the same primary tumor
Among the 14 cases in which more than one metastatic lesion was analyzed, only one case (7%) was discordant for tumor cell PD-L1 positivity across the different metastases. Specifically, PD-L1 positivity was observed in a lung lesion but not in a pancreatic lesion. This case also did not present PD-L1 expression on the primary tumor. In the remaining 13 cases, all metastases were PD-L1 negative.
PD-L1 positivity is associated with poor pathologic features and is mostly restricted to high nuclear grade areas
We correlated PD-L1 expression with pathologic features within the cohort of 53 primary tumors (Table 4). We observed that tumor cell PD-L1 positivity was detected in 2 of 18 cases (11.1%) with T stage 1/2 compared to 14 of 32 cases (43.8%) with T stage 3/4, p=0.03. Furthermore, tumor cell PD-L1 positivity was more frequently detected in primary tumors with FNG IV (n=12) versus tumors with FNG III (n=5), p<0.01.
Table 4.
Primary tumor characteristics associated with PD-L1 positivity
| Characteristic | n | PD-L1+ | P-value | |
|---|---|---|---|---|
| T Stage | 1/2 | 18 | 2 (11.1%) | 0.031 |
| 3/4 | 32 | 14 (43.8%) | ||
| unknown | 3 | 1 (33.3%) | ||
| Fuhrman Nuclear Grade | III | 35 | 5 (14.3%) | <0.01 |
| IV | 18 | 12 (66.7%) | ||
Comparing T3/T4 to T1/T2
Pathologic evaluation revealed that in both primary tumors and metastases, PD-L1 positivity was heterogeneous and only present in a subset of tumor cells. Since our analysis was purposely conducted on multiple morphologically different tumor areas that included both the predominant and the highest nuclear grade (i.e. FNG), we further correlated PD-L1 expression with the distinct nuclear grade components detected within each primary or metastatic lesion. We found that PD-L1 expression was strongly associated with areas of nuclear grade 3 or 4 (i.e. high grade) (p<0.001) while areas of nuclear grade 1 or 2 (i.e. low grade) were negative in all but one lesion (Figure 2 and Supplemental Table 2). It should be noted that within the subset of PD-L1-positive cases, the coexistence of low nuclear grade (mostly PD-L1 negative) and high nuclear grade (PD-L1 positive) areas was observed in 18/20 (90%) primary tumors but only in 9/21 (43%) metastases. The vast majority of the remaining lesions (2 of 2 primaries and 10 of 12 metastases) were exclusively composed of high grade tumor cells. Therefore, intratumoral heterogeneity for PD-L1 expression was extensive in primary tumors but more limited in metastases (Supplemental Table 2).
Figure 2. PD-L1 positivity is detected in high-grade tumor areas.
a. Representative images of a primary ccRCC tumor with heterogeneous PD-L1 expression. Membranous expression of PD-L1 in tumor cells is negative in low nuclear grade areas (A) but present in high nuclear grade areas (B). Scale bar: 50 µm. b. Graphic representation of PD-L1 status in distinct nuclear grade areas within primary and metastatic lesions from PD-L1-positive cases. The height of each bar indicates the number of lesions that contain a given tumor grade component (i.e. Grade 1, Grade 2, Grade 3, Grade 4). PD-L1 positivity is indicated in red and PD-L1 negativity is indicated in blue.
Discussion
While systemic therapies targeting the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) axis and the mammalian target of rapamycin (mTOR) pathway represent major advances in the treatment of patients with mRCC, a plateau has been reached in terms of their impact on progression-free survival and overall survival (3). Very encouraging results have been obtained recently with new immunotherapy modalities that target immune checkpoints, including agents blocking the PD-1/PD-L1 pathway. It has been established that interaction of PD-1 with its ligands (PD-L1 and PD-L2) limits T-cell activation and there is evidence that chronic antigen exposure increases PD-L1 levels in immune cells within the tumor microenvironment, resulting in T-cell “exhaustion” and reduced immune control of tumor progression. Of note, cancer cells can also express PD-L1 and directly contribute to the inhibition of an antitumor immune attack. In this regard, PD-L1 expression has been investigated in several tumor types as both a prognostic biomarker and a potential predictive factor of response to therapeutic antibodies that block the PD-1/PD-L1 axis.
Studies from Thompson and colleagues first demonstrated that PD-L1 expression in RCC is associated with aggressive features such as higher TNM stage, tumor size or FNG and increased risk of cancer-specific mortality (12–15). In these reports, the expression of PD-L1 in either tumor cells or tumor-infiltrating immune cells was found to be an indicator of poor prognosis.
Initial clinical investigations of PD-1- and PD-L1-targeting antibodies in mRCC have raised high expectations and suggested that PD-L1 expression might be a useful biomarker of response to PD-1/PD-L1 inhibition. To date, several distinct clinical trials have shown that responses to PD-L1/PD-1 inhibition are more frequently observed among ccRCC patients whose tumors are positive for PD-L1 expression (16–19,27,28). However, it has become increasingly clear that IHC staining for PD-L1 in nephrectomy specimens fails to identify all responders to PD-1/PD-L1 blockade. Indeed, up to 18% of patients with PD-L1 negative tumors have been found to respond to the treatment (19), while many patients with PD-L1 positive tumors fail to respond (18). While there are several potential explanations for these results, it is possible that the predictive value of PD-L1 expression is negatively impacted by tumor heterogeneity. Predictive tissue biomarker research is usually conducted by analyzing the primary tumor because it is easier to obtain. However, given the significant tumor heterogeneity in ccRCC, nephrectomy specimens may not accurately reflect the biology of the metastatic tumors that are the target of the systemic therapy. In line with this hypothesis, we found discordant tumor cell PD-L1 staining between primary tumors and corresponding metastases in a high proportion of cases (~20%). In contrast, multiple metastases from the same patient presented limited discordance in PD-L1 expression (7%) in the relatively small number of samples that we analyzed (14 cases). Taken together, these data suggest that robust predictive models that include the assessment of PD-L1 expression in ccRCC tumor cells might require the analysis of tissue from metastatic lesions. This possibility should be tested in prospective clinical trials.
Our study also highlights the considerable intratumor heterogeneity of PD-L1 expression in ccRCC. We demonstrate for the first time that PD-L1-positive tumors (especially primary lesions) present considerably morphologic heterogeneity and harbor tumor areas of both low and high nuclear grade, with PD-L1 protein almost exclusively expressed in high grade areas. These findings have important implications for future predictive biomarker studies and imply that the random selection of tumor blocks for PD-L1 analysis might lead to false negative results. To avoid this possible bias, we recommend that in resected lesions characterized by morphologic heterogeneity, high nuclear grade areas should be specifically selected for assessment.
A recent study by Jilaveanu and colleagues explored PD-L1 expression in a cohort of 34 matched pairs of nephrectomy and metastatic tissue samples (29). The authors used an Automated Quantitative Analysis (AQUA) method on a tissue micro-array (TMA) consisting of four tissue cores per specimen. Similarly to our current work, they found that the correlation between tumor cell PD-L1 expression in matched primary and metastatic specimens was weak and the study highlighted PD-L1 staining heterogeneity within one specimen. In contrast to our results, however, the median AQUA score was higher in metastatic sites compared to primary specimens. Since our extensive analysis of whole tissue sections from both primary and metastatic tumors reveals that PD-L1 expression is highly heterogeneous and largely restricted to areas with aggressive pathologic features (i.e. high nuclear grade), it is possible that the analysis of only four tissue cores per lesion in a TMA is impacted by considerable selection bias.
Several papers have described PD-L1 expression in primary ccRCC, and the reported rate of positivity is highly variable and ranges from 15% to 66% (13–19,28–30). In the present study, we report a membranous tumor cell PD-L1-positivity rate of 32%, which is higher than the 23.9% rate previously reported by Thompson and colleagues (14). This difference can be ascribed to several factors, including the use of a different anti-PD-L1 antibody, the analysis of a metastatic patient population, the evaluation of multiple tumor blocks per primary tumor, and the fact that in our study a case was considered positive when any tumor cell positivity was detected, while in Thompson’s study cases with <5% tumor staining were considered negative. In this study, we decided to utilize any positivity as the cut-off for the following reasons: (i) the correlation between PD-L1 levels and inhibition of anticancer immunity is currently unknown and any level of PD-L1 protein detected by IHC might have significant biologic consequences; (ii) the optimal cut-off for PD-L1 expression as a biomarker of response to PD-1/PD-L1 inhibitors still needs to be established and recent clinical results show that responses can be achieved in patients whose tumors were considered negative using a 5% cut-off; (iii) pathologist-based evaluation is semi-quantitative and subjective, and the reproducibility of discerning 1% versus 5% PD-L1-positive tumor staining is questionable.
One major limitation of the PD-L1 staining reports published to date, including ours, is the variability in staining methodologies that utilize antibodies that are not commercially available, and thus prevent a direct comparison of their performance. Standardization of both staining procedures and scoring methods is warranted before PD-L1 can be widely used as predictive biomarker in the clinic.
Conclusions
Targeting the PD-1/PD-L1 interaction to reinvigorate the immune system is showing promising clinical efficacy in metastatic ccRCC and the ability to select patients that are more likely to benefit from this therapeutic approach relies on the development of predictive biomarkers such as PD-L1 expression. We report that discordant expression of PD-L1 between primary tumors and their metastases is detected in approximately 20% of cases suggesting that accurate assessment of PD-L1 as predictive biomarkers for PD-1 blockade in ccRCC may require the analysis of metastatic lesions. Moreover, we found that PD-L1 staining is almost exclusively observed in the high grade component of a tumor. This finding should guide pathologists to select appropriate tumor areas for PD-L1 immunohistochemical analysis.
Supplementary Material
Acknowledgements
This work was supported by the DF/HCC Kidney Cancer SPORE P50 CA101942-01 to SS, DFM, GJF, and TKC. Funded in part by the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute for TKC.
LA disclosed: consulting or advisory role to Pfizer, Novartis, Sanofi, Amgen; Research funding: Pfizer, Novartis. MBA disclosed: consulting or advisory role to Merck, BMS, Genentech, Novartis, GSK, Neostem, Alkermes, X4Pharma, Amgen; Expert testimony: BMS. FSH disclosed: research support from Bristol-Myers Squibb to institution; Consultant to Merck, Novartis; Clinical trial support to institution from Bristol-Myers Squibb, Merck, Genentech. TKC disclosed: consulting or advisory role to Pfizer, GSK, Novartis, Merck and Bayer; Research funding: Pfizer, GSK, Novartis, BMS, Merck, Exelixis, Roche, Astra Zeneca, Tracon. GJF has patents/pending royalties on the PD-1 pathway from Bristol-Myers-Squibb, Roche, Merck, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, and Novartis. GF has served on advisory boards for CoStim, Novartis, Roche, and Bristol-Myers-Squibb. SS serves as consultant for AstraZeneca, Merck, and Verastem.
Footnotes
Conflict of interest statement:
MC, MG, SC, EMG, APF, JS, IC, RSB, DFM: No disclosures.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Heng DYC, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albiges L, Choueiri T, Escudier B, Galsky M, George D, Hofmann F, et al. A Systematic Review of Sequencing and Combinations of Systemic Therapy in Metastatic Renal Cancer. Eur Urol. 2015;67:100–110. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 7.McDermott DF, Ghebremichael MS, Signoretti S, Margolin KA, Clark J, Sosman JA, et al. The high-dose aldesleukin (HD IL-2) “SELECT” trial in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol (Meeting Abstracts) 2010;28:4514. [Google Scholar]
- 8.Harshman LC, Choueiri TK, Drake C, Stephen Hodi F. Subverting the B7-H1/PD-1 Pathway in Advanced Melanoma and Kidney Cancer. Cancer J. 2014;20:272–280. doi: 10.1097/PPO.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott DF, Atkins MB. Immune therapy for kidney cancer: a second dawn? Semin Oncol. 2013;40:492–498. doi: 10.1053/j.seminoncol.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–2091. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri TK, Fishman MN, Escudier BJ, Kim JJ, Kluger HM, Stadler WM, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial. [cited 2014 Jun 19];J Clin Oncol. 2014 32:5s. [Internet]. Available from: http://meetinglibrary.asco.org/content/125914-144. [Google Scholar]
- 18.McDermott DF, Sznol M, Sosman JA, Soria J-C. Immune correlates and long term follow up of a phase Ia study of MPDL3280A, an engineered PD-L1 antibody, in patients with metastatic renal cell carcinoma (mRCC) Ann Oncol. 2014 Abstract 809O. [Google Scholar]
- 19.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camp RL, Rimm EB, Rimm DL. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer. 1999;86:2259–2265. doi: 10.1002/(sici)1097-0142(19991201)86:11<2259::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011;63:865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, et al. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998;158:565–572. doi: 10.1164/ajrccm.158.2.9705036. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25:2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake CG, McDermott DF, Sznol M, Choueiri TK, Kluger HM, Powderly JD, et al. Survival, safety, and response duration results of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in a phase I trial in patients with previously treated metastatic renal cell carcinoma (mRCC): Long-term patient follow-up. [cited 2014 Jul 31];J Clin Oncol. 2013 31 [Internet]. Available from: http://meetinglibrary.asco.org/content/113579-132. [Google Scholar]
- 28.Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) [cited 2014 Jul 18];J Clin Oncol. 2013 31 [Internet]. Available from: http://meetinglibrary.asco.org/content/113904-132. [Google Scholar]
- 29.Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, et al. PD-L1 Expression in Clear Cell Renal Cell Carcinoma: An Analysis of Nephrectomy and Sites of Metastases. J Cancer. 2014;5:166–172. doi: 10.7150/jca.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, et al. Correlation of PD-L1 Tumor Expression and Treatment Outcomes in Patients with Renal Cell Carcinoma Receiving Sunitinib or Pazopanib: Results from COMPARZ, a Randomized Controlled Trial. Clin Cancer Res. 2015;21:1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.