Abstract
Soy supplementation by breast cancer patients remains controversial. No controlled intervention studies have investigated the effects of soy supplementation on mammographic density in breast cancer patients. We conducted a double-blind, randomized, placebo-controlled intervention study in previously treated breast cancer patients (n=66) and high-risk women (n=29). We obtained digital mammograms and breast magnetic resonance imaging (MRI) scans at baseline and after 12 months of daily soy (50 mg isoflavones per day) (n=46) or placebo (n=49) tablet supplementation. The total breast area (MA) and the area of mammographic density (MD) on the mammogram was measured using a validated computer-assisted method, and mammographic density percent (MD% = 100 × MD/MA) was determined. A well-tested computer algorithm was used to quantitatively measure the total breast volume (TBV) and fibroglandular tissue volume (FGV) on the breast MRI, and the FGV percent (FGV% = 100 × FGV/TBV) was calculated. On the basis of plasma soy isoflavone levels, compliance was excellent. Small decreases in MD% measured by the ratios of month 12 to baseline levels, were seen in the soy (0.95) and the placebo (0.87) groups; these changes did not differ between the treatments (P=0.38). Small decreases in FGV% were also found in both the soy (0.90) and the placebo (0.92) groups; these changes also did not differ between the treatments (P=0.48). Results were comparable in breast cancer patients and high-risk women. We found no evidence that soy supplementation would decrease mammographic density and that MRI might be more sensitive to changes in density than mammography.
Introduction
Results from meta-analyses of observational epidemiologic studies have consistently shown an inverse relationship between regular dietary soy consumption and risk of breast cancer development (1-3). Pooled analyses of studies conducted in the United States and in Asia also showed a significant benefit in outcome among breast cancer patients who were regular soy consumers after breast cancer diagnosis (4). However, the mechanisms by which soy intake may favorably influence breast cancer risk and outcome remain elusive. Results from over 40 controlled short-term soy intervention studies do not show significant favorable reductions in circulating estrogen levels (5). The effects of soy supplementation on mammographic density from randomized controlled trials in healthy pre- and postmenopausal women are also not consistent (6). Lower postmenopausal estrogen levels and lower mammographic density at all ages have been associated with lower risk of breast cancer development (7, 8).
Despite the recognized utility of mammographic density as an intermediate biomarker of breast cancer, mammographic density may not be sufficiently sensitive due to the 2-dimensional projection of the breast, particularly in studies of postmenopausal women where density levels may not be sufficiently high to demonstrate a change. Measurements using magnetic resonance imaging (MRI), which allows the 3-dimensional characterization of fibroglandular tissue volume (FGV) may be more discriminatory and informative than mammographic density (9, 10). In studies of breast density and tamoxifen, significant changes were found mainly in premenopausal or younger (age <50) women but not in postmenopausal or older women (11-14). We are aware of one study that found a statistically significant effect of tamoxifen on breast density in postmenopausal women (15). No significant reductions in breast density were found in 7 of the 8 studies on raloxifene that were conducted in postmenopausal women (16). In the small study conducted by Eng-Wang and colleagues, daily raloxifene for 1-2 years in premenopausal women was not associated with a significant change in mammographic density but they found a significant 17% reduction based on MRI (17). We are not aware of other intervention studies that have monitored changes in breast tissue density using measures from both MRI and mammograms.
The primary objective of this intervention study was to determine the effects of daily soy vs placebo tablets for 12 months using both MRI density and mammographic density as measures of breast tissue density. Because MRI provides strong soft tissue contrast between fibroglandular and fatty tissues with a three-dimensional coverage of the entire breast (9), we hypothesized that breast MRI may offer more precise measurements of breast and fibroglandular tissue volumes, providing a more reproducible and sensitive assessment of changes in breast tissue.
Materials and Methods
Study subjects
The study population consisted of women aged 30 to 75 years who were a) diagnosed with ductal carcinoma in situ or invasive breast cancer and completed medical treatment including tamoxifen or an aromatase inhibitor (AI) at least 6 months earlier and were without evidence of recurrence; or b) breast cancer patients who did not wish to take endocrine therapy (i.e., tamoxifen, AI) or were not eligible for it (hormone receptor negative breast cancer); or c) other high-risk women with a five-year Gail risk >1.7%, or were a known BRCA1/BRCA2 mutation carriers, or had a family history consistent with hereditary breast cancer or had a prior biopsy exhibiting atypical hyperplasia. We excluded women who showed any suspicious changes in their baseline mammograms, or were regular soy consumers (i.e., once per week of soy food, soy supplements, or other products) or had a known food allergy such as to soy or nuts or were current users of hormone therapy. Subjects were recruited from the University of Southern California (USC) Norris Cancer Center and the Los Angeles County (LAC)+USC Medical Center as well as from the Army of Women (18). Recruitment for the study commenced in September 2010 and ended in December 2012 (field activities ended in January 2014).
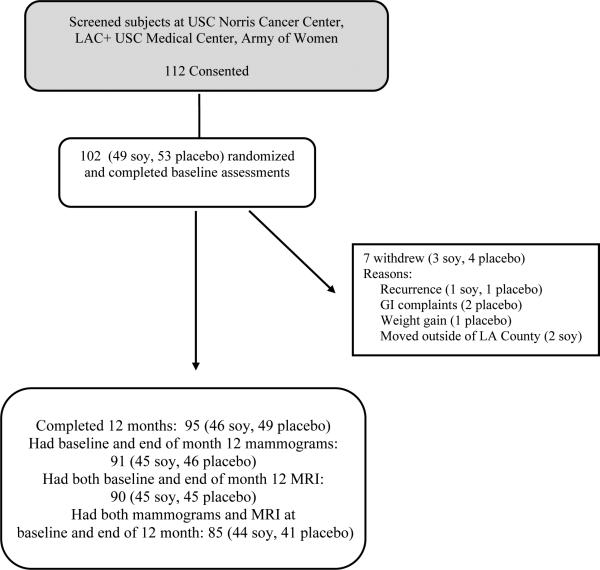
Our plan was to recruit 100 women to the study. In total, we recruited 112 volunteers but 10 withdrew before randomization (Figure 1). Of the 102 patients enrolled in the study, 7 (3 soy,4 placebo) later withdrew because of: recurrence (1 soy,1 placebo); gastrointestinal complaints (2 placebo); weight gain (1 placebo); or had moved outside of Los Angeles County (2 soy). Mammograms and MRIs were available on 95 subjects who completed the 12 months of intervention. However, 4 subjects (1 soy, 3 placebo) had mammograms but did not have MRIs and 5 subjects (1 soy, 4 placebo) had MRIs but did not have mammograms. Since an important part of this study is a comparison of breast density based on MRI and mammograms, we present below results on 85 participants (44 soy, 41 placebo) who had both MRI and mammogram results at both baseline and at the end of 12 months of study.
Figure 1.
CONSORT diagram for study design and availability of mammograms and MRIs at baseline and after 12 months of intervention
Study drugs
The soy/placebo tablet we used was prepared by MeriCal (www.merical.com), one of the nutritional industry's formulation and custom packaging specialists that is licensed with the FDA and the California Board of Pharmacy. The soy ingredient in the soy tablets was Novasoy 400 (lot number 0606051) from Archer Daniels Midland Company that is distributed through B&D Nutritional Ingredients. The placebo material was microcrystalin cellulose. Each soy tablet contained 50 mg isoflavones (27.72% total aglycone isoflavones – 12.79% daidzein, 11.57% genistein, 3.36% glycitein). The tablet weight of the soy/placebo pill was 600 mg per pill. The study tablets were released to staff at the USC Pharmacy which worked closely with the study team. Each pill bottle contained a one month supply (30 tablets). Participants were given 3 bottles of tablets at each quarterly visit and instructed to take one tablet per day at the same time of day for 12 months. Participants were asked to return pill bottles at the quarterly clinic visits and the number of tablets taken per month deduced from pills remaining was recorded.
Baseline assessment and data and sample collection
A baseline questionnaire that asked about menstrual, reproductive and menopausal factors, family history of cancer and personal medical history was administered to participants. Body weight, waist and hip measurements, and blood samples were obtained at baseline and every 3 months (i.e., at months 3, 6, 9, and 12) of the intervention using methods we have used in previous intervention studies (19, 20). At each follow-up visit, we obtained updated information on medical history and use of non-hormonal medications. A diagnostic digital mammogram was obtained at baseline and at the end of intervention (month 12) at the Norris Cancer Center or at LAC +USC Medical Center. Participants were also asked to have 2 breast MRI scans without gadolinium contrast, one at baseline and one at the end of intervention. Women were included if their baseline mammograms were considered negative or showed benign findings based on BI-RADS category (21). The MRI was done after the mammogram (usually on the same day) in the Radiology Department at USC Health Care Consultation Center. We obtained simultaneously bilateral breast MRI without contrast using a 1.5 T scanner with a dedicated phase array breast coil in prone position. Bilateral sagittal T1 images with and without fat suppression were obtained at 3 mm intervals.
Mammographic density measurement
Digital mammograms of the contralateral breast (of breast cancer patients) or the opposite side to their dominant arm (for high-risk women) at baseline and at the completion of 12 months of intervention were assessed for mammographic density by Dr. Ursin using the USC Madena method, a validated computer-assisted quantitative technique (22) that we have used extensively in previous studies (23, 24). Briefly, this method used the digitized craniocaudal mammographic image. A reader trained by Dr. Ursin first quantified the total breast area (MA) using a computerized outlining tool. Dr. Ursin then used a thresholding technique to quantify the area of dense tissue (MD) within the breast. The total number of pixels in the digitized image that constituted the total area and the dense area was calculated and the ratio of these two values expressed as a percentage (mammographic density percent, MD% = 100 × MD/MA) was computed. Both mammograms from each subject were read in the same batch set. Dr. Ursin read the mammograms in batches of about 50. She was blinded to all characteristics of the study participants including the treatment group and the order of collection of the mammograms.
Magnetic Resonance Imaging (MRI)
MRI of the unaffected breast (for breast cancer cases) or the breast on the opposite side to their dominant arm (for high-risk women) at baseline and at the completion of 12 months of intervention were assessed for the total breast volume (TBV) and the fibroglandular tissue volume (FGV) in the laboratory of Dr. Su using procedures they have developed and tested extensively (9, 25, 26). The FGV% was obtained as the ratio of the FGV to the TBV × 100. In brief, computer-assisted algorithms were used to segment the breast and the fibroglandular tissue. The breast segmentation procedures consisted of: (1) defining the lateral boundaries and removing the chest wall, (2) identifying the chest wall muscle for further exclusion, and (3) excluding the nipple and skin. After these procedures were completed, the TBV was calculated. For fibroglandular tissue segmentation, a robust bias-field correction method and K-means clustering were used to separate fibroglandular from fatty tissues, typically using a total cluster number of K=6, the lower three intensity clusters identified fibroglandular tissue and the upper three intensity clusters fatty tissue. After the fibroglandular tissue was segmented, the volume was calculated. Although the above-described procedures were fully automatic, the operator needed to define the beginning and ending slices for breast segmentation. Since the study objective was to compare the change in the same subject, the analyzed breast region in baseline and follow-up MRI needed to be consistent by carefully matching the shape of breast tissues on the beginning and the ending slices in the baseline and follow-up MRI. Typically 40-45 slices were selected as the breast region for segmentation. To ensure consistency, the operator analyzed the 2 MRIs from the same subject in one sitting, but blinded as to the order of the MRI or the treatment group.
Blood isoflavone measurement
Plasma levels of the major soy isoflavones (genistein, daidzein), and the important metabolite of daidzein (equol) were quantified after enzymatic deconjugation using a validated isotope dilution electrospray tandem mass spectrometric method (27) we have published previously (28). The limits of detection for genistein, daidzein, and equol were 20, 3, and 20 nmol/L, respectively, and the intra- and inter-assay precision was 1-6% for analysis of 10 μl aliquots of spiked plasma samples.
Statistical analysis
The distributions of both MD% and FGV% were markedly skewed with long tails to the right. We investigated the effect of soy vs placebo capsules using logarithmic and square-root transformation to obtain more normal distributions of these variables, and in both situations the square-root transformation gave a clearly more symmetric distribution of these variables. This transformation was used in all calculations involving MA, MD, MD%, TBV, FGV, or FGV%. The results of these calculations were converted back to the standard scales for presentation.
Treatment group comparisons on demographic and other baseline characteristics were compared using chi-square tests for categorical variables and t tests for continuous variables. Pearson correlations between MD% and FGV% were computed and their statistical significance was calculated based on Fisher's z transformation. We used analysis of variance and analysis of covariance methods to assess the relationships between age, body mass index, menopausal status and other variables and baseline MD% and FGV%. The primary outcomes were the change in MD% and FGV% from baseline to end of month 12 for the soy and placebo groups: change was calculated as the ratio of the month 12 to the baseline measurements and mean values were calculated using transformed values to give equal weight to results above and below unity. The calculations were made on the square-root scale and then converted to original scale by squaring the mean values obtained using the square root scale. Mean levels of MD% and FGV% at baseline and end of month 12 were compared between treatments using the Wilcoxon ranksum test. The change in MD% and FGV% within treatment were compared using the signed ranks test. The differences in the change in MD% and FGV% were also compared between treatments using the ranksum tests. Approximate confidence intervals were computed using the results from t-tests assuming equal variances. With a sample size of 50 women in the soy and placebo arm, a two-sided alpha of 0.05, we would have 80% power to observe differences of 3.2% up to 8.4% in FGV% differences if the standard deviation was 5% and 15%, respectively. These observed differences would be changed to 3.1% and 9.4% if we had 20% dropout rate (or a sample size of 40 women in each group).
Results
The baseline characteristics of evaluable women randomized to soy (n=44) and placebo (n=41) tablets were similar; treatment groups did not differ statistically significantly in age, race/ethnicity, education, age at menarche, parity, body mass index, menopausal status, years since menopause or history of breast cancer. Among the participants who had a history of breast cancer (29 soy vs 29 placebo), there were no differences in tumor characteristics of previous breast cancer (stage at diagnosis, hormone receptor status, histology, breast surgery type, chemotherapy) and the average number of years between breast cancer diagnosis or completion of chemotherapy and entry into the current trial (Table 1).
Table 1.
Characteristics of study participants
| Soy (N=44) | Placebo (N=41) | P value | |
|---|---|---|---|
| Mean Age (Standard deviation, SD) | 57.6 (8.1) | 55.0 (7.6) | 0.14 |
| Race | |||
| Non-Hispanic White | 24 (54.6%) | 25 (61.0%) | |
| Hispanic | 16 (36.4%) | 11 (26.8%) | |
| African American | 4 (9.1%) | 3 (7.3%) | |
| Asian American | 0 | 2 (4.9%) | 0.39 |
| Education | |||
| high school or less | 15 (34.1%) | 8 (19.5%) | |
| Some college | 10 (22.7%) | 8 (19.5%) | |
| College graduate or higher | 19 (43.2%) | 25 (61.0%) | 0.24 |
| Mean age menarche (SD) | 13.0 (1.6) | 12.8 (1.6) | 0.68 |
| Mean number of births (SD) | 2.0 (1.4) | 1.8 (1.4) | 0.63 |
| Body mass index(BMI), kg/m2 (baseline) | 28.3 (6.3) | 27.3 (5.0) | 0.44 |
| Menopausal status | |||
| Pre | 7 (15.9%) | 12 (29.3%) | |
| Post | 37 (84.1%) | 29 (70.7%) | 0.14 |
| Postmenopausal | |||
| Surgical | 12 (32.4%) | 11 (37.9%) | |
| Natural | 20 (54.1%) | 14 (48.3%) | |
| After cancer | 5 (13.5%) | 4 (13.8%) | 0.88 |
| Years postmenopausal prior to entry into trial | |||
| ≤5 years | 7 (18.9%) | 11 (37.9%) | |
| 6-10 years | 13 (35.1%) | 4 (13.8%) | |
| 11-20 years | 11 (29.7%) | 10 (34.5%) | |
| >20 years | 6 (16.2%) | 4 (13.8%) | 0.16 |
| History of breast cancer | |||
| Yes | 29 (65.9%) | 29 (70.7%) | |
| No | 15 (34.1%) | 12 (29.3%) | 0.63 |
| Stage at diagnosis1 | |||
| 0 | 6 (20.7%) | 10 (34.5%) | |
| I | 8 (27.6%) | 4 (13.8%) | |
| IIA/IIB | 11 (37.9%) | 9 (31.0%) | |
| IIIABC | 4 (13.8%) | 6 (20.7%) | 0.40 |
| Estrogen receptor (ER) status1,2 | |||
| ER positive (+) | 10 (34.5%) | 12 (41.4%) | |
| ER negative (−) | 17 (58.6%) | 11 (37.9%) | |
| Unknown | 2 (6.9%) | 6 (20.7%) | 0.18 |
| Progesterone receptor (PR) status1, 3 | |||
| PR positive (+) | 8 (27.6%) | 9 (31.0%) | |
| PR negative (−) | 18 (62.1%) | 13 (44.8%) | |
| Unknown | 3 (10.3%) | 7 (24.1%) | 0.29 |
| ER/PR combined1 | |||
| ER+/PR+ | 8 (27.6%) | 9 (31.0%) | |
| ER+/PR− | 1 (3.4%) | 2 (6.9%) | |
| ER−/PR− | 17 (58.6%) | 11(37.9%) | |
| Unknown | 3 (10.3%) | 7 (24.1%) | 0.35 |
| HER2 status1 | |||
| HER2+ | 6 (20.7%) | 4 (13.8%) | |
| HER2− | 18 (62.1%) | 13 (44.8%) | |
| Unknown | 5 (17.2%) | 12 (41.4%) | 0.13 |
| Histology1 | |||
| Ductal carcinoma in situ | 6 (20.7%) | 10 (34.5%) | |
| Ductal invasive | 21 (72.4%) | 16 (55.2%) | |
| Lobular carcinoma in situ | 0 | 1 (3.4%) | |
| Lobular invasive | 0 | 1 (3.4%) | |
| Other invasive | 2 (6.9%) | 1 (3.4%) | 0.40 |
| Surgery1 | |||
| Mastectomy | 10 (34.5%) | 9 (31.0%) | |
| Lumpectomy | 17 (58.6%) | 19 (65.5%) | |
| Excision | 2 (6.9%) | 1 (3.4%) | 0.78 |
| Median number of months between breast cancer and entry into trial1 | |||
| ≤54 months | 15 (51.7%) | 14 (48.3%) | |
| >54 months | 14 (48.3%) | 15 (51.7%) | 0.79 |
| Median number of months between completion of chemotherapy/endocrine treatment and entry into trial1,4 | |||
| ≤ 12 months | 14 (48.3%) | 15 (51.7%) | |
| >12 months | 15 (51.7%) | 14 (48.3%) | 0.79 |
Among 58 participants with a history of breast cancer
Unknown ER status: 6 had DCIS; 1 had LCIS, and 1 had invasive ductal carcinoma which was diagnosed in 1994 and ER result was missing.
Unknown PR status: 8 had DCIS, 1 had LCIS; and 1 had invasive ductal carcinoma which was diagnosed in 1994. Although PR measurement was ordered, the result was not recorded on the path report.
Among participants in the >12 months category, 8 of 15 in the soy group and 9 of the 14 in the placebo group completed chemotherapy/endocrine treatment 4 or more years prior to entry into trial.
Soy/placebo tablets were well tolerated with minimal adverse events. During the 12 months of intervention, a total of 10 and 15 adverse events were reported in the soy and placebo groups, respectively. They included breast pain/bruising (3 soy, 7 placebo), hair loss (1 soy, 1 placebo), hot flashes/sweat (1 soy, 1 placebo), diarrhea (1 soy, 0 placebo), nausea (1 soy, 0 placebo), lymphedema (0 soy, 1 placebo), and others. They were all grade I or II events based on the NCI CTCAE version 3.0.
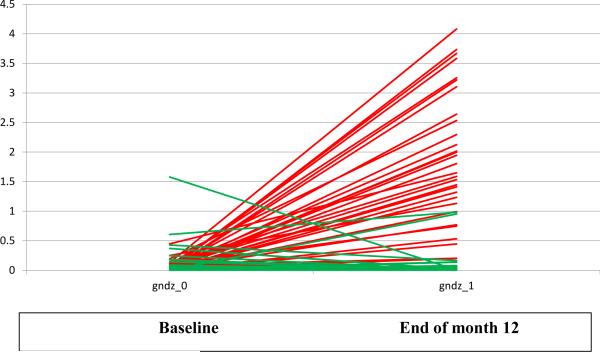
To assess compliance with the soy tablets, plasma samples were compared before and after treatment for all participants (Figure 2). Baseline mean levels of plasma soy isoflavone (genistein and daidzien combined) (pmol/μl) were low and did not differ between soy (0.05, 95% CI 0.02-0.07) and placebo (0.12, 95% CI 0.03-0.21) groups (P=0.11). In association with the intervention, the mean plasma concentrations of soy isoflavone levels increased significantly in the soy group (1.37, 95% CI 0.98-1.76) but remained low in the placebo group (0.07, 95% CI 0.01-0.14); the changes differed significantly between the groups (P<0.0001). Few subjects at baseline (2 soy and 2 placebo) or at the end of month 12 (7 soy and 1 placebo) produced equol, a metabolite reduced from daidzein and postulated by some to account for the benefits of soy consumption (29). Compliance was also assessed by counting tablets that were returned and found to be higher than 90% among both placebo and soy tablet groups (data not shown).
Figure 2.
Plasma soy isoflavone (genistein and daidzein combined) at baseline and after 12 months of intervention (Red= soy group; green= placebo group) (gndz_0: genistein and daidzein combined at baseline; gndz_1: genistein and daidzein combined at the end of month 12)
Baseline breast area, dense area, and MD% did not differ between soy (n=44) and placebo (n=41) groups (P>0.05) (Table 2, top). After 12 months of intervention, breast density area increased in the soy group but decreased in the placebo group, but the changes in breast density area did not differ between the two groups (P=0.23). The ratios of MD% at month 12 to baseline decreased in both soy (0.95, P=0.28) and placebo groups (0.87, P=0.011), but there was no significant difference in the changes in MD% between the two groups (P=0.38).
Table 2.
Mean and 95% confidence interval (95% CI) of mammographic total breast area (MA), dense area (MD) and mammographic density percent (MD%) in the soy and placebo group at baseline and at the end of 12 months of soy intervention
| Measure | Treatment | N | Baseline mean (95% CI) | P value between treatments at baseline | Month 12 mean (95% CI) | Ratio month 12 / baseline mean | P value-ratio within treatments | P value-ratio between treatments |
|---|---|---|---|---|---|---|---|---|
| All subjects | ||||||||
| MA | Soy | 44 | 741.7 (371.3, 1238.9) | 0.45 | 780.0 (391.8, 1300.5) | 1.03 (0.98, 1.08) | 0.58 | 0.48 |
| Placebo | 41 | 729.9 (390.7, 1174.3) | 775.9 (421.5, 1237.7) | 1.08 (1.00, 1.17) | 0.15 | |||
| MD | Soy | 44 | 80.8 (37.5, 140.4) | 0.18 | 81.7 (37.3, 143.5) | 0.98 (0.86, 1.11) | 0.50 | 0.23 |
| Placebo | 41 | 142.0 (68.6, 241.7) | 137.1 (67.3, 231.6) | 0.94 (0.80, 1.10) | 0.031 | |||
| MD% | Soy | 44 | 13.3 (9.4, 17.8) | 0.066 | 12.7 (9.0, 17.0) | 0.95 (0.84, 1.07) | 0.28 | 0.38 |
| Placebo | 41 | 19.9 (14.6, 26.0) | 18.3 (13.3, 24.1) | 0.87 (0.78, 0.98) | 0.011 | |||
|
Breast cancer patients | ||||||||
| MA | Soy | 29 | 834.0 (342.5, 1540.6) | 0.56 | 869.4 (356.2, 1608.0) | 1.01 (0.94, 1.08) | 0.48 | 0.10 |
| Placebo | 29 | 833.6 (367.1, 1488.8) | 909.3 (414.4, 1596.3) | 1.12 (1.00, 1.25) | 0.103 | |||
| MD | Soy | 29 | 77.1 (26.8, 153.2) | 0.24 | 84.9 (28.1, 172.3) | 0.97 (0.83, 1.13) | 0.86 | 0.51 |
| Placebo | 29 | 130.2 (49.6, 249.0) | 133.1 (52.3, 250.7) | 1.02 (0.84, 1.24) | 0.34 | |||
| MD% | Soy | 29 | 11.8 (7.4, 17.3) | 0.13 | 11.7 (7.4, 17.1) | 0.97 (0.84, 1.11) | 0.87 | 0.44 |
| Placebo | 29 | 17.0 (12.1, 22.7) | 16.0 (11.2, 21.7) | 0.91 (0.80, 1.04) | 0.16 | |||
Comparable patterns of results were observed when we repeated these analyses restricted to the participants who had a history of breast cancer (29 soy, 29 placebo). Baseline breast area, dense area, and MD% did not differ between the soy and placebo groups (all P>0.05) (Table 2, bottom). MD% decreased non-significantly in the soy and placebo groups and the differences in the changes did not differ between the two groups (P=0.44). Similar patterns of results were also obtained when we repeated the analyses by menopausal status (data not shown).
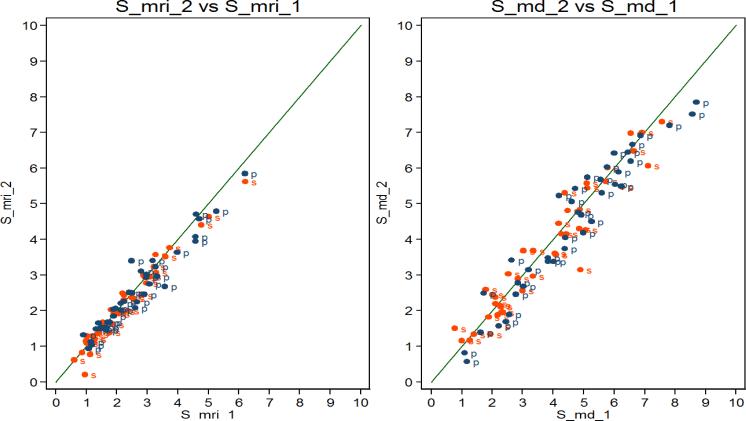
Figure 3a shows the MD% at the end of month 12 plotted against the MD% at baseline. The correlation was 0.93 when we plotted results on a square-root scale and was 0.92 without transformation.
Figure 3.
(a) Baseline mammographic density percent (MD%) on square-root scale (S_md_1) (square-root scale mammographic density percent at baseline) (x-axis) vs end of 12-month MD% (S_md_2) (square-root scale mammographic density percent at 12-month) (y-axis); and (b) Baseline FGV% on square-root scale (S_mri_1) (square-root scale fibroglandular volume percent at baseline) (x-axis) vs end of 12-month FGV% (S_mri_2) (square-root scale fibroglandular volume percent at 12-month) (y-axis) (s=soy group; p=placebo group).
TBV, FGV, and FGV% were compared before and after 12 months of intervention in the soy (n=44) and placebo groups (n=41). Baseline levels of TBV and FGV% did not differ between treatment groups (P>0.05, Table 3 top) but baseline FGV was lower in the soy than in the placebo group (P=0.033). After 12 months of intervention, statistically nonsignificant declines in FGV% were observed in the soy group (0.90, P=0.22) and in the placebo group (0.92, P=0.056). These changes in FGV% did not differ between the soy and placebo groups (P=0.48). We repeated these analyses among the participants who had a history of breast cancer (29 soy, 29 placebo) (Table 3, bottom). Reductions in FGV% (0.84, P=0.07) were observed in the soy group and placebo group (0.90, P=0.11); these differences were not significantly different. Similar patterns of results were also obtained when we repeated the analyses by menopausal status (data not shown). Soy supplementation had no significant effects on breast density (MD% or FGV%) when we compared the 12-month breast density (MD% or FGV%) in the soy and placebo groups with adjustment for potential confounders including age, race, BMI, baseline breast density and 12-month blood isoflavone levels (data not shown).
Table 3.
Mean and 95% confidence interval (95% CI) of total breast volume (TBV) (cm3), fibroglandular tissue volume (FGV), and FGV% in the soy and placebo groups at baseline and at the end of 12 months of intervention
| Measure | Treatment | N | Baseline mean (95% CI) | P value between treatments at baseline | Month 12 mean (95% CI) | Ratio month 12 / baseline mean | P value-ratio within treatments | P value-ratio between treatments |
|---|---|---|---|---|---|---|---|---|
| All subjects | ||||||||
| TBV | Soy | 44 | 868.0 (725.9, 1022.7) | 0.92 | 866.7 (722.5, 1024.0) | 1.00 (0.97, 1.03) | 0.57 | 0.65 |
| Placebo | 41 | 826.9 (728.7, 931.3) | 824.4 (722.8, 932.7) | 0.99 (0.96, 1.03) | 0.96 | |||
| FGV | Soy | 44 | 37.4 (26.7, 50.0) | 0.033 | 35.2 (25.1, 47.0) | 0.90 (0.78, 1.04) | 0.14 | 0.74 |
| Placebo | 41 | 52.9 (39.1, 68.8) | 49.6 (36.4, 64.8) | 0.91 (0.82, 1.01) | 0.051 | |||
| FGV% | Soy | 44 | 4.8 (3.4, 6.6) | 0.095 | 4.5 (3.2, 6.1) | 0.90 (0.77, 1.06) | 0.22 | 0.48 |
| Placebo | 41 | 7.1 (5.0, 9.6) | 6.6 (4.7, 8.8) | 0.92 (0.82, 1.02) | 0.056 | |||
|
Breast cancer patients | ||||||||
| TBV | Soy | 29 | 849.6 (672.2, 1047.8) | 0.79 | 846.6 (664.3, 1051.0) | 0.99 (0.95, 1.03) | 0.33 | 0.47 |
| Placebo | 29 | 844.4 (741.0, 954.6) | 847.5 (737.5, 965.1) | 1.00 (0.97, 1.03) | 0.96 | |||
| FGV | Soy | 29 | 32.0 (22.4, 43.4) | 0.13 | 28.6 (19.6, 39.4) | 0.83 (0.67, 1.03) | 0.028 | 0.69 |
| Placebo | 29 | 45.0 (30.8, 61.9) | 42.0 (28.4, 58.3) | 0.90 (0.78, 1.03) | 0.14 | |||
| FGV% | Soy | 29 | 4.3 (2.8, 6.1) | 0.31 | 3.9 (2.5, 5.7) | 0.84 (0.66, 1.06) | 0.068 | 0.91 |
| Placebo | 29 | 5.6 (3.8, 7.8) | 5.1 (3.6, 6.9) | 0.90 (0.78, 1.04) | 0.11 | |||
The correlation between FGV% at the end of month 12 versus FGV% at baseline was 0.96 when plotted on a square-root scale (Figure 3b) (correlation was 0.96 without transformation).
Baseline MD% was significantly correlated with baseline FGV% (R2=0.62) among the 85 participants who had both exams. FGV% was 3.3-fold higher in younger (age <50 years old) than older (aged >60 years) women (P<0.0001), 2-fold higher in premenopausal than postmenopausal women (P=0.011), and 2-fold higher in women with lower BMI (<25 kg/m2) than those with higher BMI (>30 kg/m2) (P=0.0002) after adjustment for age. Similar patterns of differences in MD% were observed; MD% was 2.1-fold higher in younger than older women (P=0.021), 1.8-fold (P=0.012) higher in premenopausal than postmenopausal women, and 3.3-fold (P<0.0001) higher in women with lower BMI than those with higher BMI. After further adjustment for age the difference in MD% by menopausal status diminished and was no longer statistically significant (P=0.23) but the difference in MD% by BMI was essentially unchanged and remained statistically significant (P<0.0001) (Table 4).
Table 4.
Mean and 95% confidence interval (95% CI), fibroglandular volume percent (FGV%) and mammographic density percent (MD%) for 85 subjects at baseline
| N | FGV% (95% CI) | Age adjusted FGV% (95% CI) | MD% (95% CI) | Age adjusted MD% (95% CI) | |
|---|---|---|---|---|---|
| Age | |||||
| <50 | 17 | 12.05 (8.49, 16.24) | 23.97 (15.86, 33.74) | ||
| 50-59 | 34 | 5.88 (4.12, 7.95) | 18.36 (13.24, 24.30) | ||
| ≥60 | 34 | 3.64 (2,29, 5.30) | 11.35 (7.42, 16.12) | ||
| P trend | <0.0001 | 0.021 | |||
| Menopausal status | |||||
| Premenopausal | 19 | 12.65 (9.24, 16.59) | 10.80 (7.36, 14.89) | 25.41 (17.44, 34.89) | 22.31 (14.11, 32.37) |
| Postmenopausal | 66 | 4.43 (3.33, 5.66) | 5.30 (3.86, 6.98) | 14.08 (10.81, 17.78) | 15.58 (11.39, 20.43) |
| P value | <0.001 | 0.011 | 0.012 | 0.23 | |
| Body mass index (kg/m2) | |||||
| <25 | 35 | 8.99 (6.75, 11.54) | 9.82 (7.67, 12.23) | 25.17 (19.63, 31.41) | 26.26 (20.73, 32.44) |
| 25-<30 | 24 | 5.11 (3.16, 7.52) | 5.77 (3.85, 8.07) | 15.86 (10.72, 22.01) | 16.60 (11.45, 22.70) |
| ≥30 | 26 | 3.29 (1.83, 5.19) | 4.14 (2.57, 6.07) | 7.73 (4.41, 11.98) | 8.64 (5.14, 13.06) |
| P trend | 0.001 | 0.0002 | <0.0001 | <0.0001 |
Discussion
To our knowledge, this is the first double-blind, randomized intervention study to report on the effects of soy vs placebo supplementation on longitudinal changes in both MD% and FGV% in breast cancer patients and high-risk women. On the basis of plasma isoflavone levels, compliance in the soy intervention group was excellent and there was no contamination in the placebo arm. The correlation between baseline MD% and FGV% was high. Small decreases in MD% and FGV% were found in both the soy (0.95 and 0.90, respectively) and in the placebo (0.87 and 0.92, respectively) groups. These declines did not differ between treatment in all subjects, in breast cancer patients or high-risk women, or in pre- or post-menopausal women and likely reflected declines of fibroglandular tissue associated with aging. Although previous studies have suggested that the effect of soy may be more effective in equol producers (30); there were few equol producers in our study to conduct meaningful analyses. These findings do not support our hypothesis that soy isoflavone supplementation would decrease breast tissue density and that the three-dimensional nature of MRI technology might overcome some of the limitations of mammography, allowing more accurate and precise assessment of fibroglandular volume.
Eng-Wong et al (17) studied the effects of raloxifene on breast density calculated by a semiquantitative thresholding technique and MRI-breast volume in premenopausal women. They found no significant change in breast density calculated based on mammograms whereas MRI-breast volume decreased significantly during the 3 years of raloxifene treatment; the median percent reduction was −17% in year 1 and −9% in year 3. In most studies the effect of tamoxifen and breast density reduction has been much clearer in premenopausal women than in postmenopausal women (11, 12). Since over 70% of the study participants in our study were postmenopausal women, it is plausible the overall null findings in our study is due to MD% and FGV% in our participants not being elevated enough to detect a change but our findings were comparable in both pre- and postmenopausal women (data not shown). Although there are known limitations in the measurement of MD% and FGV%, we have confidence in our assessments as the baseline MD% and FGV% were highly correlated (R2=0.62, P<0.001), compatible with correlations reported in other studies (31, 32). In the study which compared three different mammographic measures of volumetric breast density to MRI, the R2 for single-energy x-ray absorptiometry (SCA), Quantra and Volpara compared with MRI was 0.78, 0.51, and 0.73, respectively (32). We also observed significant correlations between MD% as well as FGV% and variables including age, menopausal status, and BMI, well-established determinants of breast tissue density (33, 34).
Previous intervention studies that were conducted to evaluate the effects of soy isoflavones on mammographic density in heathy women have been negative. In a meta-analysis of randomized controlled trials in healthy premenopausal (n=519) and postmenopausal (n=592) women, there was no overall effect of soy isoflavones (6). The amount soy isoflavones used in these studies varied: three studies used <50 mg, 5 studies used 50 to <100 mg, 1 study used >100 mg and the isoflavones were supplemented in tablets (35-39), protein powder (40) or foods (41). The duration of intervention was for 6 months to 1 year (35, 37, 40), 2 years (36, 42) or three years (38, 39). The mean difference in MD% was 0.69% for all women combined (−1.10% in postmenopausal women and 1.83% in premenopausal women) (6). No significant differences in mammographic density between soy and placebo groups were reported in two other trials that supplemented soy isoflavones or placebo for 10 months (32 soy vs 34 placebo) (43) and 12 months (65 soy vs 65 placebo) (44).
Several intervention studies have been conducted in breast cancer patients or high-risk women to investigate the short-term effects of soy on breast tissue changes. In one study, breast cancer patients and high-risk women took soy supplements (235 mg isoflavones or placebo) for 6 months (45) and in three pre-surgical studies, participants took soy supplements or soy protein an average of 14 to 22 days between diagnosis and surgery (46-48). No significant changes in cell proliferation between treatment groups were found in these studies.
Several reasons may explain the discrepancies between findings in clinical studies compared to observational epidemiologic studies. First, whole soy and traditional soy foods were studied in the observational studies whereas soybean components and processed ingredients were used in the clinical studies. The nutritional value of whole soybeans and minimally processed traditional fermented and nonfermented soy foods likely differed from components of the soybeans and soy tablets (49). Timing of soy intervention (such as in later adult life or after menopause) may be too late. Several studies suggest that earlier life soy exposure may confer the strongest benefit in relation to breast cancer risk (50). Finally, it is also plausible that soy isoflavones do not affect breast cancer via breast tissue density.
Several strengths and limitations of this study should be mentioned. This study was conducted in the catchment area of the Norris Cancer Center and LAC+USC Medical Center and about half of the participants were non-whites. Participants were blinded to the treatment group to which they were randomly assigned. We had an objective serological marker of compliance in this study and participants were compliant. Because it has been suggested that evaluation of longitudinal changes in breast tissue density may not be sufficiently sensitive (17), MRI assessments were included in this investigation. The comparable results we observed using MD% and FGV% suggest that it is unlikely that we have missed a true effect. However, there are several study limitations. The sample size of this study was modest and included a heterogeneous group of women, including pre- and postmenopausal women, who had a history of breast cancer or were high-risk based on their personal history and family history of breast cancer. Because of logistical reasons, we were not able to time the breast imaging with menses among premenopausal women. While it has been reported that breast density varied across the menstrual cycle(51), longitudinal data (52) and results from recent studies (53-55) suggest that the magnitude of variation is likely small. In addition, although our study was designed with 80% power to observe 3.1% up to 9.4% in breast density differences with 40 women in each study arm (see page 8), the observed changes in MD% and FGV% were much smaller (<1%) than what we had hypothesized based on results from previous studies (17).
In summary, in this randomized, double-blinded placebo-controlled intervention study in breast cancer patients or high-risk women, soy capsule intervention for 12 months did not have any beneficial or adverse effects on breast tissue changes based on FGV% and MD%.
Acknowledgment
We are grateful to all the study participants for their contributions and support. We thank Wendy Cheng, Cynthia Acosta, and June Yashiki, and the entire data collection team for their contribution and recruitment advice from Diana Chingos throughout the study. We thank Dr. Daniel Doerge and his laboratory for conducting the soy isoflavone measurements.
Grant Support: This work was supported by a translational grant from the California Breast Cancer Research Program (15OB-0162) (Wu, Tseng, Hovanessian-Larsen, Hawes), and an USC Cancer Center Core Support grant (2P30 CA14089-26) (Wu) and a NIEHS grant (P30 ES07048) (Wu).
Footnotes
Clinical Trial Registration: Clinicaltrials.gov Identifier: NCT01219075
Disclosure of Potential Conflict of Interest: None
References
- 1.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315–23. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 3.Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013;8:e81968. doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nechuta SJ, Caan BJ, Chen WY, Flatt SW, Lu W, Patterson RE, et al. The After Breast Cancer Pooling Project: rationale, methodology, and breast cancer survivor characteristics. Cancer Causes Control. 2011;22:1319–31. doi: 10.1007/s10552-011-9805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–40. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–60. doi: 10.1093/humupd/dmq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, et al. Mammographic densities and breast cancer risk. Breast Dis. 1998;10:113–26. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 8.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 9.Nie K, Chen JH, Chan S, Chau MK, Yu HJ, Bahri S, et al. Development of a quantitative method for analysis of breast density based on three-dimensional breast MRI. Med Phys. 2008;35:5253–62. doi: 10.1118/1.3002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie K, Chang D, Chen JH, Hsu CC, Nalcioglu O, Su MY. Quantitative analysis of breast parenchymal patterns using 3D fibroglandular tissues segmented based on MRI. Med Phys. 2010;37:217–26. doi: 10.1118/1.3271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 13.Brisson J, Brisson B, Cote G, Maunsell E, Berube S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–5. [PubMed] [Google Scholar]
- 14.Konez O, Goyal M, Reaven RE. Can tamoxifen cause a significant mammographic density change in breast parenchyma? Clinical imaging. 2001;25:303–8. doi: 10.1016/s0899-7071(01)00329-1. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863–6. [PubMed] [Google Scholar]
- 16.Lienart V, Carly B, Kang X, Guzy L, Sajovitz AM, Liebens F. Effect of preventive hormonal therapy on breast density: a systematic qualitative review. TheScientific World Journal. 2014;2014:942386. doi: 10.1155/2014/942386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng-Wong J, Orzano-Birgani J, Chow CK, Venzon D, Yao J, Galbo CE, et al. Effect of raloxifene on mammographic density and breast magnetic resonance imaging in premenopausal women at increased risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1696–701. doi: 10.1158/1055-9965.EPI-07-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love S. An efficient resource to accelerate research into the cause and prevention of breast cancer: The Love/Avon Army of Women. Cancer Epidemiol Biomarkers Prev. 2013;22:471. [Google Scholar]
- 19.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu AH, Stanczyk FZ, Martinez C, Tseng CC, Hendrich S, Murphy P, et al. A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am J Clin Nutr. 2005;81:1133–41. doi: 10.1093/ajcn/81.5.1133. [DOI] [PubMed] [Google Scholar]
- 21.Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99:386–95. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 22.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–7. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Ursin G, Pike MC, Spicer DV, Porrath SA, Reitherman RW. Can mammographic densities predict effects of tamoxifen on the breast? J Natl Cancer Inst. 1996;88:128–9. doi: 10.1093/jnci/88.2.128-a. [DOI] [PubMed] [Google Scholar]
- 24.Wu AH, Ursin G, Koh WP, Wang R, Yuan JM, Khoo KS, et al. Green tea, soy, and mammographic density in singapore chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17:3358–65. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JH, Chang YC, Chang D, Wang YT, Nie K, Chang RF, et al. Reduction of breast density following tamoxifen treatment evaluated by 3-D MRI: preliminary study. Magnetic resonance imaging. 2011;29:91–8. doi: 10.1016/j.mri.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin M, Chen JH, Wang X, Chan S, Chen S, Su MY. Template-based automatic breast segmentation on MRI by excluding the chest region. Med Phys. 2013;40:122301. doi: 10.1118/1.4828837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:139–45. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 28.Wu AH, Pike MC, Williams LD, Spicer D, Tseng CC, Churchwell MI, et al. Tamoxifen, soy, and lifestyle factors in Asian American women with breast cancer. J Clin Oncol. 2007;25:3024–30. doi: 10.1200/JCO.2006.10.5023. [DOI] [PubMed] [Google Scholar]
- 29.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 30.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:1664S–7S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khazen M, Warren RM, Boggis CR, Bryant EC, Reed S, Warsi I, et al. A pilot study of compositional analysis of the breast and estimation of breast mammographic density using three-dimensional T1-weighted magnetic resonance imaging. Cancer Epidemiol Biomarkers Prev. 2008;17:2268–74. doi: 10.1158/1055-9965.EPI-07-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D, et al. Agreement of mammographic measures of volumetric breast density to MRI. PLoS One. 2013;8:e81653. doi: 10.1371/journal.pone.0081653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie K, Su MY, Chau MK, Chan S, Nguyen H, Tseng T, et al. Age- and race-dependence of the fibroglandular breast density analyzed on 3D MRI. Med Phys. 2010;37:2770–6. doi: 10.1118/1.3426317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, et al. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004;6:R170–9. doi: 10.1186/bcr773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, et al. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981–6. doi: 10.3945/jn.108.102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- 38.Powles TJ, Howell A, Evans DG, McCloskey EV, Ashley S, Greenhalgh R, et al. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. 2008;14:6–12. doi: 10.1258/mi.2007.007033. [DOI] [PubMed] [Google Scholar]
- 39.Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787–96. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- 40.Verheus M, van Gils CH, Kreijkamp-Kaspers S, Kok L, Peeters PH, Grobbee DE, et al. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632–8. doi: 10.1158/1055-9965.EPI-08-0344. [DOI] [PubMed] [Google Scholar]
- 41.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–94. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 42.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, et al. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–44. [PubMed] [Google Scholar]
- 43.Delmanto A, Nahas-Neto J, Traiman P, Uemura G, Pessoa EC, Nahas EA. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Menopause. 2013;20:1049–54. doi: 10.1097/GME.0b013e3182850270. [DOI] [PubMed] [Google Scholar]
- 44.Colacurci N, De Franciscis P, Atlante M, Mancino P, Monti M, Volpini G, et al. Endometrial, breast and liver safety of soy isoflavones plus Lactobacillus sporogenes in post-menopausal women. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2013;29:209–12. doi: 10.3109/09513590.2012.738724. [DOI] [PubMed] [Google Scholar]
- 45.Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila) 2012;5:309–19. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–24. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 47.Sartippour MR, Rao JY, Apple S, Wu D, Henning S, Wang H, et al. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr Cancer. 2004;49:59–65. doi: 10.1207/s15327914nc4901_8. [DOI] [PubMed] [Google Scholar]
- 48.Shike M, Doane AS, Russo L, Cabal R, Reis-Filo J, Gerald W, et al. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein MA, Nahin RL, Messina MJ, Rader JI, Thompson LU, Badger TM, et al. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J Nutr. 2010;140:1192S–204S. doi: 10.3945/jn.110.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messina M, Hilakivi-Clarke L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer. 2009;61:792–8. doi: 10.1080/01635580903285015. [DOI] [PubMed] [Google Scholar]
- 51.White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, et al. Variation in mammographic breast density by time in menstrual cycle among women aged 40-49 years. J Natl Cancer Inst. 1998;90:906–10. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
- 52.Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2001;10:141–2. [PubMed] [Google Scholar]
- 53.Morrow M, Chatterton RT, Jr., Rademaker AW, Hou N, Jordan VC, Hendrick RE, et al. A prospective study of variability in mammographic density during the menstrual cycle. Breast Cancer Res Treat. 2010;121:565–74. doi: 10.1007/s10549-009-0496-9. [DOI] [PubMed] [Google Scholar]
- 54.Hovhannisyan G, Chow L, Schlosser A, Yaffe MJ, Boyd NF, Martin LJ. Differences in measured mammographic density in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2009;18:1993–9. doi: 10.1158/1055-9965.EPI-09-0074. [DOI] [PubMed] [Google Scholar]
- 55.Chen JH, Chen WP, Chan S, Yeh DC, Su MY, McLaren CE. Correlation of endogenous hormonal levels, fibroglandular tissue volume and percent density measured using 3D MRI during one menstrual cycle. Ann Oncol. 2013;24:2329–35. doi: 10.1093/annonc/mdt158. [DOI] [PMC free article] [PubMed] [Google Scholar]