Abstract
Esophageal adenocarcinoma (EAC) is an increasingly common disease with a dismal 5-year survival rate of 10-15%. In the first systematic evaluation of the PD-1 pathway in EAC, we identify expression of PD-L2 in cancer cells in 51.7% of EACs. Epithelial PD-L1 was expressed on only 2% of cases, though PD-L1+ immune cells were observed in 18% of EACs. We also evaluated expression in the precursor lesion of EAC, Barrett's Esophagus (BE), which emerges following gastric reflux-induced esophageal inflammation, and found PD-L2 expression in BE but not in non-BE esophagitis. As the progression from squamous esophagitis to BE is accompanied by a transition from a Th1 to Th2 immune response, we hypothesized that the Th2 cytokines IL4/IL13 could contribute to PD-L2 induction. We confirmed that these cytokines can augment PD-L2 expression in EAC cell lines. These results suggest that the inflammatory environment in BE and EAC may contribute to the expression of PD-L2. Furthermore, the potential for PD-1 receptor blockade to be effective in EACs with epithelial PD-L2 or immune cell PD-L1 expression should be evaluated in clinical trials.
Keywords: esophageal adenocarcinoma, Barrett's esophagus, Th2 response, PD-L2, PD-1 pathway
Introduction
The incidence of esophageal adenocarcinoma (EAC) has increased dramatically in the Western world in the last decades (1). Five-year survival rates are 10-15%, and treatment is largely reliant upon minimally effective cytotoxic chemotherapy (2). There have been multiple attempts to use molecularly targeted agents, but to date only the monoclonal antibody trastuzumab has proven effective, when used in the ~15% of patients with HER2 overexpression (3). There is a pressing need for new therapies.
As EACs harbor a high somatic mutation burden (4) and develop in a background of chronic inflammation caused by gastric reflux, EACs are potentially immunogenic tumors and therefore promising candidates for immunotherapy. In response to gastric reflux, the lower esophagus often undergoes metaplasia to an intestinalized epithelium, Barrett's esophagus (BE). Barrett's metaplasia is associated with a change from an acute (Th1 type) immune response accompanied with IFNγ expression (5, 6) to a Th2 type chronic inflammation with production of IL4/IL13, a transition that potentially induces an immunosuppressive, tumor-promoting environment.
Among the most promising targets in cancer immunology is the programmed cell death protein 1 (PD-1) pathway. PD-1 is a negative co-stimulatory receptor expressed primarily on activated T cells. The interaction of PD-1 with its ligands, programmed cell death ligand 1 or 2 (PD-L1 or PD-L2) inhibits T-cell activation (7). Expression of PD-L1 on cancer cells and immune cells can inhibit T-cell antitumor response and permit neoplastic growth. Expression of these ligands thus serves as a tool exploited by cancers to avoid immune clearance. PD-1 and PD-L1 inhibitors in melanoma, lung, and renal cancer have shown marked response rates with durable clinical responses (8). However, the PD-1 pathway has not been systematically evaluated in EAC.
Characterization of the inflammatory state is a critical initial step towards rational development of immunotherapy for EAC. Here, we evaluated PD-1, PD-L1, and PD-L2 expression in a series of 354 EACs. These studies revealed expression of PD-L2 or PD-L1 in a majority of tumors, raising immediate hypotheses regarding the role of PD-1 pathway activation in EAC and the potential of PD-1-blocking drugs in this deadly disease.
Materials and methods
Patient series
Tumor tissue microarrays (TMA) were constructed from 354 EACs from the University of Pittsburgh. Detailed clinical and pathologic data are summarized in Table 1.
Table 1.
Clinical and Pathological Characteristics of Esophageal adenocarcinomas in relation to PD-1, PD-L1 and PD-L2 expression
| All Cases N (%) | PD-L2+ Cases ≥ 1 core, N (%) | P | PD-L2+ Cases all cores, N (%) | P | PD-L1+ Cases N (%) | P | PD-1+ Cases N (%) | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| All Cases | 354 (100) | 183/354 (51.7) | 70/354 (19.8) | 62/344 (18.0) | 212/349 (60.7) | |||||
| Sex | Male | 292 (82.5) | 149 (51.0) | 57 (19.5) | 51 (17.7) | 176 (61.3) | ||||
| Female | 62 (17.5) | 34 (54.8) | 0.862 | 13 (21.0) | 0.861 | 11 (18.1) | 1.000 | 36 (58.1) | 0.668 | |
| Age | (median, IQR) | 67 (60-75) | 67 | 0.152 | 67.7 | 0.395 | 68.2 | 0.192 | 67 | 0.405 |
| Body Mass Index | (median, IQR) | 27.7 (25.1-31.6) | 28.4 | 0.507 | 28.5 | 0.639 | 29.1 | 0.737 | 28.7 | 0.576 |
| History of smoking | No | 101 (28.9) | 52 (51.5) | 23 (22.8) | 18 (18.4) | 57 (56.4) | ||||
| Yes | 249 (71.1) | 130 (52.2) | 0.902 | 47 (18.9) | 0.461 | 44 (18.2) | 1.000 | 152 (62) | 0.336 | |
| History of GERD | No | 92 (26.2) | 42 (45.7) | 13 (14.1) | 25 (26.9) | 62 (67.4) | ||||
| Yes | 258 (73.8) | 139 (53.9) | 0.175 | 55 (21.3) | 0.167 | 37 (15.0) | 0.017 | 159 (60.7) | 0.211 | |
| History of Barrett's Esophagus | No | 110 (31.1) | 49 (44.5) | 14 (12.7) | 27 (24.8) | 75 (68.2) | ||||
| Yes | 244 (68.9) | 134 (54.9) | 0.071 | 56 (23.0) | 0.030 | 35 (14.9) | 0.034 | 137 (57.3) | 0.059 | |
| Charlson Comorbidity Index | Low (CCI 0-1) | 134 (37.8) | 75 (56) | 27 (20.1) | 26 (19.7) | 80 (60.6) | ||||
| Intermediate (CCI 2-5) | 151 (42.7) | 73 (48.3) | 30 (19.9) | 26 (17.9) | 90 (60) | |||||
| High (CCI >5) | 69 (19.5) | 35 (50.7) | 0.43 | 13 (18.8) | 1 | 10 (14.9) | 0.731 | 42 (62.7) | 0.948 | |
| Tumor Location | Mid/proximal esophagus | 7 (2.0) | 4 (57.1) | 2 (28.6) | 2 (33.3) | 4 (66.7) | ||||
| Distal esophagus | 96 (27.4) | 50 (52.1) | 23 (24.0) | 16 (172) | 54 (18.3) | |||||
| GE Junction | 247 (70.6) | 126 (51.0) | 0.661 | 44 (17.8) | 0.301 | 44 (18.3) | 0.612 | 151 (62.1) | 0.679 | |
| Tumor Stage (AJCC7) | T1 | 138 (39.1) | 88 (63.8) | 42 (30.4) | 22 (17.1) | 56 (42.1) | ||||
| T2 | 36 (10.2) | 17 (47.2) | 5 (13.9) | 7 (20) | 23 (63.9) | |||||
| T3 | 174 (49.3) | 75 (43.1) | 22 (12.6) | 32 (18.5) | 131 (75.7) | |||||
| T4 | 5 (1.4) | 2 (40) | 0.003 | 1 (20) | 0.001 | 1 (20) | 0.950 | 1 (20) | <0.001 | |
| Tumor Size | (median, IQR) | 3.7 (2.4-5.5) | 3.78 | 0.040 | 3.4 | 0.014 | 4.5 | 0.186 | 4.5 | <0.001 |
| Tumor Grade | Well differentiated | 24 (6.8) | 22 (91.7) | 13 (54.2) | 1 (4.2) | 5 (20.8) | ||||
| Moderately differentiated | 145 (41.0) | 78 (53.8) | 33 (22.8) | 23 (16.5) | 80 (56.7) | |||||
| Poorly differentiated | 185 (52.2) | 83 (44.9) | <0.001 | 24 (13.0) | <0.001 | 38 (21.0) | 0.105 | 127 (69) | <0.001 | |
| Node Stage (AJCC7) | N0 | 140 (39.7) | 79 (56.4) | 36 (25.7) | 20 (15.3) | 51 (37.8) | ||||
| N1 | 80 (22.7) | 39 (48.8) | 12 (15) | 12 (15) | 54 (66.7) | |||||
| N2 | 62 (17.6) | 26 (41.9) | 10 (16.1) | 12 (19.4) | 44 (71) | |||||
| N3 | 71 (20) | 38 (53.5) | 0.263 | 12 (16.9) | 0.185 | 17 (24.3) | 0.379 | 62 (88.6) | <0.001 | |
| Metastasis Stage | M0 | 341 (96.6) | 177 (51.9) | 69 (20.2) | 59 (17.8) | 203 (60.2) | ||||
| M1 | 12 (3.4) | 5 (41.7) | 0.485 | 1 (8) | 0.473 | 3 (25) | 0.460 | 8 (72.2) | 0.537 | |
| Final AJCC7 Stage Group | Stage I | 116 (33.0) | 74 (63.80 | 36 (31.0) | 15 (14.0) | 41 (36.9) | ||||
| Stage II | 53 (15.0) | 22 (41.5) | 7 (13.2) | 12 (22.6) | 27 (50.9) | |||||
| Stage III | 171 (48.6) | 52 (47.3) | 26 (15.2) | 31 (18.2) | 134 (77.9) | |||||
| Stage IV | 12 (3.4) | 5 (41.7) | 0.009 | 1 (8.3) | 0.003 | 3 (25) | 0.505 | 8 (72.7) | <0.001 |
PD-L2+, >1 core: 1 or more cores have moderate to strong epithelial PD-L2 expression. PD-L2+, all cores: all evaluated cores have moderate to strong epithelial PD-L2 expression. PD-L1+: any positive immune cell. PD-1+: any positive lymphocyte. P-values<0.05 are considered statistically significant. ns: non-significant. IQR: interquartile range. GERD: gastro-esophageal reflux disease. AJCC: American joint committee on cancer.
Immunohistochemistry
PD-1, PD-L1, PD-L2 and PD-L1/CD68 double-staining were performed as previously described (9, 10) (described in detail in Supplementary Methods). PD-L1/CD163 double-staining was performed with anti-CD163 (Neomarkers). PD-L1 was considered positive if ≥5% of tumor cells had membranous staining or any positive immune cell. PD-L2 expression was considered positive when ≥50% of tumor cells on TMAs or ≥10% of tumor cells on whole-tissue slides had moderate-strong PD-L2 staining in the cytoplasm and/or membrane.
Cell lines
EAC cell lines OE19, OACM5.1C, ESO26, KYAE-1, and FLO-1 were purchased from the European Collection of cell cultures and authenticated by SNP arrays (2012). OE33 cells were purchased from Sigma (St. Louis, MO) and MKN7 cells from the Broad Institute and characterized within the Cancer Cell Line Encyclopedia project. Only low-passage cell aliquots of original stocks were used for experiments.
IL4/IL13 treatment
Cells were stimulated with human recombinant IL4 (BD Pharmingen), IL13 (Peprotech) or medium for 4, 8, and 24 hours. STAT6 expression and phosphorylation were determined by Western blot (anti-pSTAT6, BD Biosciences; anti-STAT6 Santa Cruz Biotechnology).
STAT6 knockdown
Transfection of siRNA against STAT6 or a non-targeting control (ON-TARGETplus, Dharmacon) was performed using Lipofectamine RNAiMAX (Invitrogen).
Flow cytometry
Cell lines
Adherent cells were harvested with EDTA and stained with anti-human PD-L2 antibody or isotype-control (PE, BioLegend).
Xenografts
EAC biopsies were implanted into the flanks of nude mice. At the time of passage mice were sacrificed and tumors were cut into pieces and incubated in collagenase-containing buffer. Single-cell suspensions were stained with anti–PD-L2 antibody, isotype-control, and anti-EpCAM (Pacific Blue, Biolegend). PD-L2 levels were determined with Western blot and flow cytometry.
Statistical analyses
Associations with clinical and pathological characteristics were analyzed using the Student t-test, Pearson's chi-square, or Fisher's Exact test. Associations with overall survival were analyzed using the Kaplan-Meier method, log-rank test and the multivariate Cox proportional hazards model. All p-values are two sided. A p-value <0.05 was considered statistically significant.
Results
Esophageal adenocarcinomas commonly express PD-L2 in cancer epithelial cells
We assayed PD-1, PD-L1, and PD-L2 expression in TMAs containing cores from 354 EACs using antibodies optimized for formalin-fixed paraffin-embedded (FFPE) tissues (9, 10). The PD-L2 assay was recently validated on FFPE human lymphoid tissues (9). Clinical and pathologic characteristics of the study population are listed in Table 1.
A strong majority (289/354, 81.6%) of EACs showed evidence of at least weak epithelial PD-L2 expression in 1 of the cores. In 51.7% (183/354) of EACs, we observed moderate-strong PD-L2 epithelial expression in at least 1 core. In 19.8% (70/354) of EACs, all evaluated cores had moderate-strong PD-L2 staining (Figure 1). Immune cells were negative for PD-L2. Due to the high frequency of weak epithelial PD-L2 expression, only moderate-strong PD-L2 expression was considered positive for further analyses.
Figure 1.

IHC staining of FFPE esophageal tissues in tissue microarrays (20×). Staining with (A) anti-PD-1 antibody showing PD-1+ TILs; (B) anti-PD-L1 antibody showing PD-L1+ immune cells; (C) negative PD-L2 staining, (D) weak PD-L2 staining in tumor epithelium scored as 1+, (E) moderate PD-L2 staining scored as 2+, and (F) strong PD-L2 staining scored as 3+. High magnification images to show cytologic details for PD-1 and PD-L1.
PD-L1 and PD-1 expression in stromal inflammatory cells
PD-L1+ tumor cells were observed in 1.7% (6/344) of EACs. PD-L1+ inflammatory cells, however, were observed in 18% (62/344) of EACs (Table 1). Morphologically, PD-L1+ inflammatory cells appeared to be macrophages, which was confirmed by PD-L1 double-staining with macrophage markers CD68 and CD163 on whole-tumor sections of 16 PD-L1+ EACs (data not shown). PD-1+ tumor-infiltrating lymphocytes (TIL) were identified in 59.8% (215/349) of EACs with 1-181 PD-1+ TILs identified per core when present.
Co-expression of PD-L2, PD-L1, and PD-1
While PD-L2 and PD-L1 expression was not mutually exclusive, tumors with PD-L2 expression in all evaluated core were less likely to possess PD-L1+ immune cells (P=0.045). Both PD-L2+ and PD-L1+ tumors had a higher average number of PD-1+ TILs compared to tumors without PD-L2 or PD-L1 expression (7.2 vs 3.7, P=0.052 and 12.6 vs 2.5, P<0.001 respectively). In 15.5% (53/343) of EACs, no PD-L2+, PD-L1+, or PD-1+ cells were observed.
TMA results were validated using immunohistochemistry (IHC) on whole-tissue slides from 45 tumors. We confirmed expression of PD-L2 in 27/30 cases that expressed PD-L2 on the TMA. Tumors with PD-L2 expression in all evaluated cores had a larger percentage of PD-L2+ cells (70-100% of tumor cells positive) compared to tumors with 1 or 2 positive cores (30-60% of tumor cells positive). Moreover, 6/14 tumors lacking evident PD-L2 expression on TMAs showed focal regions with PD-L2 expression in whole tissue slides, suggesting that PD-L2 expression may be more frequent than suggested by the TMA results. Similarly, evaluation of whole-tumor slices showed PD-L1+ immune cells in 16/45 (35.6%) tumors, of which 7/16 were not identified on the TMAs. In 5/16 EACs, PD-L1+ immune cells were observed only at the tumor border, while in the remaining 11 patients PD-L1+ cells infiltrated the tumor.
Validation of IHC results
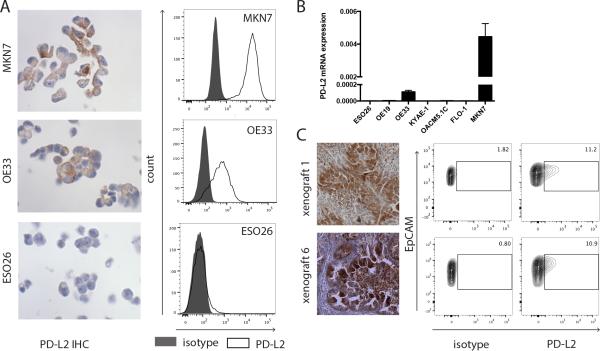
As this is the first study to report predominant PD-L2 expression in epithelial cancer cells, we next sought to validate our IHC results. We queried PD-L2 epithelial expression in gastro-esophageal cell lines OE19, ESO26, OE33, OACM5.1C, FLO-1, KYAE-1, and MKN7 by IHC, and identified PD-L2 positivity in OE33 and MKN7 lines (Figure 2A). These IHC results were concordant with flow cytometry using a distinct PD-L2 antibody and with mRNA expression (Figure 2A-B). As an additional validation, we identified two EAC patient-derived xenografts with evidence of epithelial PD-L2 expression in the primary tissue and xenograft by IHC. Using fresh xenograft tissue, we confirmed PD-L2 expression by immunoblotting (Supplemental Figure S1) and flow cytometry, which identified EpCAM/PD-L2 double-positive cells, further validating the IHC findings (Figure 2C).
Figure 2.
Validation of IHC results with flow cytometry. A. IHC and flow cytometry results of PD-L2-expressing OE33 and MKN7 cells, and PD-L2 non-expressing ESO26 cells (black, isotype; white, PD-L2). B. PD-L2 mRNA expression in 7 gastro-esophageal cell lines. Data are depicted as mean + standard deviation. C. IHC staining of 2 EAC biopsies with anti-PD-L2 antibody (20×). Staining was scored strong positive for both tumors. Tumor biopsies were implanted in the flanks of nude mice. At time of passage tumors were disaggregated into single-cell suspensions and analyzed. Flow cytometry shows co-expression of EpCAM and PD-L2 in 11.9% and 10.2% of EpCAM+ cells. PE-isotype was used as control. A representative experiment of 2 independent experiments is shown.
PD-L2 expression is detected at the transition of reflux esophagitis to Barrett's esophagus and can be induced by IL4/IL13
We hypothesized that PD-L2 epithelial expression may also occur in BE, the precursor to EAC. We evaluated PD-L2 expression via IHC in samples with BE (n=21) and reflux esophagitis without BE (n=14) (Figure 3A). While no esophagitis samples exhibited PD-L2 expression, 42.8% (9/21) of BE cases (complete and incomplete intestinal metaplasia, high- and low-grade dysplasia) showed PD-L2 epithelial expression. PD-L1 expression in epithelial or immune cells was not observed.
Figure 3.
PD-L2 expression is detected at the transition of reflux esophagitis to Barrett's Esophagus and can be induced by IL4/IL13 A. IHC staining with hematoxylin and (20×) and anti-PD-L2 antibody (20×) shows PD-L2 expression in Barrett's esophagus tissues with complete intestinal metaplasia (1) and incomplete intestinal metaplasia (2) and not in reflux esophagitis tissues. Induction of PD-L2 mRNA (B) and protein expression (C) of IL4- and IL13-treated MKN7, OE33 and FLO-1 cells. A representative experiment is shown ( n= 3-5). mRNA expression data are depicted as mean + standard deviation. Flow cytometry: black, isotype; white and dashed line, PD-L2 expression 24 hours after adding medium; grey, PD-L2 expression 24 hours after IL4 treatment.
Acquisition of PD-L2 expression with the development of BE raised the hypothesis that IL4/IL13 expression which accompanies the transition to BE can contribute to PD-L2 induction. We tested this hypothesis in vitro. Treatment of PD-L2+ cell lines OE33 and MKN7 with exogenous IL4 and IL13 induced STAT6 phosporylation and an increase in PD-L2 mRNA (Figure 3B). For OE33, an increase in protein expression was observed by flow cytometry (Figure 3C). In PD-L2 non-expressing cell lines, IL4 or IL13 increases PD-L2 mRNA expression in FLO-1 cells (Figure 3B) but not in ESO26 cells (data not shown.) In order to test if constitutive PD-L2 expression is dependent upon IL4/IL13/STAT6 signaling, we knocked down STAT6 in the PD-L2-expressing cell lines OE33 and MKN7, which did not lead to a change in PD-L2 mRNA or protein expression (data not shown). These data suggest that Th2-related cytokines can induce PD-L2 expression in EAC cells but that other factors likely also influence the expression of this protein.
Clinical and pathologic associations of PD-1 pathway member expression in EAC
We next evaluated clinical correlates of PD-1, PD-L1, and PD-L2 expression. In tumors where all cores showed PD-L2 expression, PD-L2 expression was associated with early-stage (P=0.003), smaller tumor size (P=0.014), and a well-differentiated grade (P<0.001) (Table 1). Moreover, cancers with consistent PD-L2 expression were more likely to have histologic evidence of BE with intestinal metaplasia (P=0.030). In contrast, PD-L1 expression was significantly enriched in those tumors lacking evidence of BE (P=0.034) or lacking a clinical history of gastro-esophageal reflux disease (P=0.017). Tumors with PD-1+ TILs had higher tumor stage (P<0.001), were more frequently poorly differentiated (P<0.001), and a trend towards absence of histologically confirmed BE (P=0.056). PD-1 positivity correlated with an increased mortality (univariate Cox regression HR=1.89 (95% CI 1.38-2.6), P<0.000; Supplemental Figure S2). However, the negative association between PD-1 and mortality was lost after adjustment for other prognostic factors. PD-L2 expression showed a trend towards an improved outcome (univariate Cox regression HR= 0.75; 95% CI 0.54-1.03; P=0.078). For PD-L1 expression, no association to survival was observed.
Discussion
EAC is a highly lethal disease that lacks effective systemic treatment, making exploration of immunotherapy targets of clear importance. This study represents the first systematic effort to characterize expression and clinical correlations of the PD-1 pathway in EAC. Our results show that the vast majority of EACs harbor expression of at least one member of this pathway: expression of PD-1 on lymphoid cells or of ligands PD-L2 on tumor cells or PD-L1 on immune cells. A limitation of our data was the use of TMAs. Given the heterogeneous expression of these markers, our results may underestimate the frequency of expression of these markers in EAC. Furthermore, we evaluated only tumors that have not been treated with chemotherapy or chemoradiation. Nonetheless, our data support the testing of PD-1 pathway inhibitors in EAC. The presence of PD-L2 expression may mark a scenario in which inhibition of PD-1, a receptor for both PD-L1 and PD-L2, may have more efficacy than targeting PD-L1.
A surprising finding in our study was our observation of common PD-L2 expression in esophageal epithelial cells. While PD-L1 expression has been described in epithelial cancer cells of various lineages (8, 11), predominant PD-L2 expression has been recorded only for primary mediastinal large B-cell lymphoma (9). Although PD-L2 expression has been described in esophageal squamous cell carcinomas (12), cervical carcinomas (13), and hepatocellular carcinomas (14), which was in co-occurrence with PDL1 epithelial expression. Epstein-Barr Virus-positive gastric cancers (15) show mRNA expression of both PD-L1 and PD-L2, which occurs in the setting of 9p24.1 amplification, the locus containing both of these genes. However, these amplifications have been seen only rarely in EAC (4).
These data raise the question of what induces epithelial PD-L2 expression in the absence of PD-L1 co-expression in EAC. In macrophages and dendritic cells PD-L2 transcription is regulated by IL4/IL13/STAT6 signaling (16, 17). Notably, EACs develop in a background of chronic inflammation and typically emerge from BE, a tissue with a documented Th2-skewed inflammatory state with increased IL4/IL13 expression (5, 6). PD-L2 epithelial expression in EAC and BE may indeed be a consequence of IL4/IL13 expression in the immune microenvironment (18).
The finding of PD-L2 expression in EACs and BE suggests that PD-L2 may be a component of a larger chronic inflammatory microenvironment that facilitates tumor survival. Recent studies have shown that Th2 polarization in esophageal cancer is associated with the infiltration of myeloid-derived suppressor cells (MDSC) and M2-polarized macrophages (18, 19), suggesting the presence of other immune evasion mechanisms beyond the PD-1 pathway. Future studies will need to evaluate the implications of strong cytoplasmic PD-L2 expression in many EAC samples and to determine whether the PD-L2 expression we have observed in EAC functions to mediate immune evasion and specifically predicts response to PD-1 blockade. The role of PD-L2 in inhibiting T-cell responses is controversial. Although PD-L1 and PD-L2 show structural similarity, each ligand has alternative secondary receptors, RGMb for PD-L2 (20) and CD80 for PD-L1 (21). While a number of studies show an inhibitory role for PD-L2 (22, 23), others suggest that PD-L2 can stimulate T-cell proliferation (24) via a PD-1-receptor independent mechanism, potentially involving a distinct PD-L2 binding partner.
Beyond PD-L2 expression, we also identified PD-L1+ immune cells in 18%-36% of EACs. PD-L1 expression may predominate in tumors with a different inflammatory state than PD-L2-predominant tumors. PD-L1 expression is strongly induced by Th1 cytokine IFNγ while PD-L2 is only weakly induced (17), raising the hypothesis that PD-L1+ tumors emerge in the setting of a Th1-type acute inflammation. These findings raise the question as to why EACs harbor distinct checkpoint inhibitors. While all the tumors in this dataset carry a clinical diagnosis of EAC, there is likely heterogeneity within our collection. Indeed, these tumors may comprise a combination of true esophageal tumors emerging from a BE precursor and other tumors which emerged directly from proximal gastric mucosa at the gastro-esophageal junction. The predilection for PD-L1 expression in resection samples without evidence of BE in our dataset suggests the hypothesis that those tumors that do not emerge from BE may have a distinct inflammatory environment. However, because PD-L1+ tumors were also larger and more advanced compared to the PD-L2-expressing tumors in our dataset, we cannot exclude the possibility that the absence of BE in these samples may simply reflect overgrowth by the tumor.
Considerable additional work will be needed in the coming years to explore the nature of distinct inflammatory states in EAC, and their mechanisms for evading immune attack. Nonetheless, these data demonstrate for the first time that the majority of EACs show evidence of PD-1 pathway activity. These data suggest that exploration of PD-1 inhibition is warranted in this disease and that studies will need to evaluate potential capacity of PD-1 and both PD-L1 and PD-L2 to serve as biomarkers of response. Furthermore, in PD-L2+ tumors, the presence of secondary mechanisms of immune evasion, such as the presence of M2 macrophages, may also impact response to therapies, potentially leading to tumors in which combination immunotherapeutic approaches may ultimately be required.
Supplementary Material
Acknowledgement
The project described was supported by the National Cancer Institute K07CA511613 (K.S.N.), P01 CA098101 (A.J.B.), the Center of Immune Oncology at Dana-Farber Cancer Institute (X.L. and S.J.R.) and Target Cancer Foundation (A.J.B and M.S.G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. S.D. is financially supported by the Dutch Cancer Society (VU 2012-535).
Financial support:
The project described was supported by the Dutch Cancer Society (VU 2012-535) (S.D.), the National Cancer Institute K07CA511613 (K.S.N.), P01 CA098101 (A.J.B.), the Center of Immune Oncology at Dana-Farber Cancer Institute (X.L. and S.J.R.), R01AI089955 (G.J.F) and Target Cancer Foundation (A.J.B and M.S.G).
Footnotes
Conflicts of interest:
G.F. receives patent licensing fees on the PD-1 pathway from Bristol-Myers-Squibb, Roche, Merck, EMD-Serrono, Boehringer-Ingelheim, Amplimmune/AstraZeneca, and Novartis. G.F. has served on scientific advisory boards for CoStim, Novartis, Roche, and Bristol-Myers-Squibb.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England journal of medicine. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nature genetics. 2013;45:478–86. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002;51:316–22. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moons LM, Kusters JG, Bultman E, Kuipers EJ, van Dekken H, Tra WM, et al. Barrett's oesophagus is characterized by a predominantly humoral inflammatory response. The Journal of pathology. 2005;207:269–76. doi: 10.1002/path.1847. [DOI] [PubMed] [Google Scholar]
- 7.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi M, Roemer MG, Chapuy B, Liao X, Sun H, Pinkus GS, et al. Expression of Programmed Cell Death 1 Ligand 2 (PD-L2) Is a Distinguishing Feature of Primary Mediastinal (Thymic) Large B-cell Lymphoma and Associated With PDCD1LG2 Copy Gain. The American journal of surgical pathology. 2014;38:1715–23. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. Journal of immunology. 2003;170:1257–66. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 12.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 13.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6341–7. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 14.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World journal of gastroenterology : WJG. 2011;17:3322–9. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Research N, Analysis Working Group: Dana-Farber Cancer I, Institute for Systems B, University of Southern C, Memorial Sloan Kettering Cancer C, Agency BCC et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014 [Google Scholar]
- 16.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. The Journal of clinical investigation. 2011;121:3100–8. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5336–41. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Wu Y, Su Z, Amoah Barnie P, Jiao Z, Bie Q, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PloS one. 2014;9:e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer immunology, immunotherapy : CII. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, et al. RGMb is a novel binding partner for PD L2 and its engagement with PD-L2 promotes respiratory tolerance. The Journal of experimental medicine. 2014;211:943–59. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, et al. Regulation of T cell activation and tolerance by PDL2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11695–700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. The Journal of experimental medicine. 2003;197:1083–91. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davison JM, Ellis ST, Foxwell TJ, Luketich JD, Gibson MK, Kuan SF, et al. MUC2 expression is an adverse prognostic factor in superficial gastroesophageal adenocarcinomas. Human pathology. 2014;45:540–8. doi: 10.1016/j.humpath.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.