Abstract
The protozoan Sarcocystis neurona is an important cause of severe clinical disease of horses (called equine protozoal myeloencephalitis, EPM), marine mammals, companion animals, and several species of wildlife animals in the Americas. The Virginia opossum (Didelphis virginiana) is its definitive host in the USA and other animals act as intermediate or aberrant hosts. Samples of tongue and heart from 35 bobcats hunted for fur and food from Mississippi State, USA in February, 2014 were used for the present study. Muscles were examined for Sarcocystis infection by microscopic examination of either unfixed muscle squash preparations or pepsin digests, by histopathology of fixed samples, and by molecular methods. Sarcocystis-like bradyzoites were found in digests of 14 hearts and 10 tongues of 35 bobcats. In histological sections, sarcocysts were found in 26 of 35 bobcats; all appeared relatively thin-walled similar to S. felis sarcocysts under light microscope at 1000x magnification. S. neurona-like sarcocysts having thickened villar tips were seen in unstained muscle squash of tongue of two bobcats and PCR-DNA sequencing identified them definitively as S. neurona-like parasite. DNA extracted from bradyzoites obtained from tongue and heart muscle digests was analyzed by PCR-DNA sequencing at the ITS1 locus. Results indicated the presence of S. neurona-like parasite in 26 of 35 samples. ITS1 sequences identical to S. dayspi were identified in 3 bobcats, 2 of which were also co-infected with S. neurona-like parasite. The high prevalence of sarcocysts in bobcat tissues suggested an efficient sylvatic cycle of Sarcocystis spp. in the remote regions of Mississippi State with the bobcat as a relevant intermediate host.
Keywords: Bobcat (Lynx rufus), S. neurona, S. felis, S. dayspi, Sarcocysts, PCR-DNA sequencing
1. Introduction
Sarcocystis neurona is an unusual species within the genus Sarcocystis because of its wide host range. It causes fatal disease in horses (equine protozoal myeloencephalitis, EPM), marine and terrestrial mammals, including companion animals (Barbosa et al., 2015; Dubey et al., 2015a). Opossums (Didelphis virginiana, D. albiventris) are the definitive host and domestic cats (Felis catus), skunk (Mephitis mephitis), armadillo (Dasypus novemcinctus), raccoons (Procyon lotor), and sea otters (Enhydra lutris kenyoni) are proven intermediate hosts of S. neurona (Dubey et al., 2015a). Here we report the Sarcocystis spp. infection in bobcats (Lynx rufus) trapped from remote areas of Mississippi State.
2. Materials and methods
2.1. Sample collection
Samples from 35 bobcats were collected in February 2014. Samples were collected from remote areas in three Mississippi State counties, Claiborne (25), Warren (6), and Jefferson (4). The bobcats (22 males, 13 females) were older than 18 months and were trapped for fur and food. Samples were protected from freezing to minimize repeated freeze-thaw that causes structural damage in the tissue histology and ultrastructure. Long exposure of samples in open condition deteriorates the quality of tissues for bioassay and microscopy examination; however 19 to 5 days elapsed between sampling and examination. Samples of the heart and tongue were submitted to the Animal Parasitic Diseases Laboratory (APDL), United States Department of Agriculture Beltsville, Maryland for protozoal testing. All hunting, trapping, and sampling activities were conducted under permits assigned by Mississippi Department of Wildlife, Fisheries, and Parks.
2.2. Light microscope examination
At APDL, samples of myocardium and tongue from individual bobcats were processed separately. Pieces of tongue (5 × 2 cm) were fixed in 10% buffered neutral formalin for histological examination. Tissue samples were cut into sections (2.5 × 0.7 cm), embedded in paraffin, and sectioned 5 μm thick. The sections were stained with hematoxylin and eosin (H and E) and observed under the microscope. The number of sarcocysts per tissue section was recorded.
The conventional histological examination of tissue sections is very insensitive for the detection of protozoal tissue cysts. To increase the sensitivity of detecting bradyzoites, tissue sections were treated with pepsin to dissolve the sarcocyst wall and release bradyzoites for microscopic identification (Dubey et al., 1989). Thirty grams of myocardium and tongue from individual bobcats were homogenized and digested in acidic pepsin solution separately, as described previously (Dubey, 2010). Drops (50 μl) of digest were screened under the light microscope at 400x magnification for the presence of Sarcocystis-like bradyzoites. The remaining digested samples were centrifuged, washed, and stored at −80 °C for molecular assays.
2.4. DNA extraction
An aliquot (500μl) of digested suspension of myocardium and tongue of all 35 bobcats were subjected separately for DNA isolation using DNeasy Blood and Tissue Kit (Qiagen, Inc., Valencia, CA, USA) according to manufacturer instructions. DNA quantification and quality were determined by Thermo Scientific NanoDrop Lite Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
2.5. PCR amplification
The presence of S. neurona DNA was detected using previously published ITS1500 primers (ITS1500FE: TTCTCTTGTGTGTGCCCCTAC; ITS1500RE: TGCGTCCTTCATCGTTGCGC; ITS1500FI: CAAAATGAACGTGTCTATGTGTGA; ITS1500RI: GAGCCAAGACATCCATTGCT) that specifically amplify a ~500 bp region of the ITS1 locus in either S. neurona or S. falcatula (Miller et al., 2009). Briefly, the primary PCR amplifications were performed in 50 μl total reaction volume containing 3 μl of template DNA, 20 pmol of primer pair; ITS1500FE and ITS1500RE, and 1x Taq PCR Master Mix Kit (Qiagen, Inc., Valencia, CA) in a thermal cycler (Veriti® Thermal Cycler, Applied Biosystems, Foster City, CA). The thermal cycler conditions was set at initial denaturation at 95 °C for 5 min; 35 cycles of amplification (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s) and final extension at 72 °C for 10 min. Nested PCR was carried out using primer pair ITS1500FI and ITS1500RI and 2μl product of primary PCR as a template in 50 μl total reaction volume. Same thermal cycler conditions were used in both PCR reactions. Appropriate positive (S. neurona and S. falcatula) and negative (H2O) controls were included in all the batches respectively. Amplified PCR products were run on 2.5% (w/v) agarose gel with ethidium bromide stain and visualized by using Gel Logic 212 Imaging Systems (Eastman Kodak Company, Rochester, NY). S. falcatula is phylogenetically related to S. neurona but these parasites are biologically distinct; S. falcatula is an avian pathogen and is not infective to horses, whereas S. neurona is an equine pathogen (Dubey et al., 2015a).
2.6. DNA sequencing and phylogenetic analysis
For characterization of Sarcocystis spp., single PCR amplicons of ITS1 locus (~500 bp) were excised and purified using QIAquick Gel Extraction (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's recommendation. Individual purified PCR products of all positive samples of bobcats and two positive control samples were sent to Macrogen Corporation (Rockville, MD, USA) for direct sequencing using the same primer pair to obtain both forward and reverse reads. Sequence chromatograms were read, edited, and analyzed using the program Geneious version 8.0.4 (Biomatters Ltd., Auckland, NZ). The sequences were aligned and compared with the previously published sequences in NCBI by BLASTn analysis.
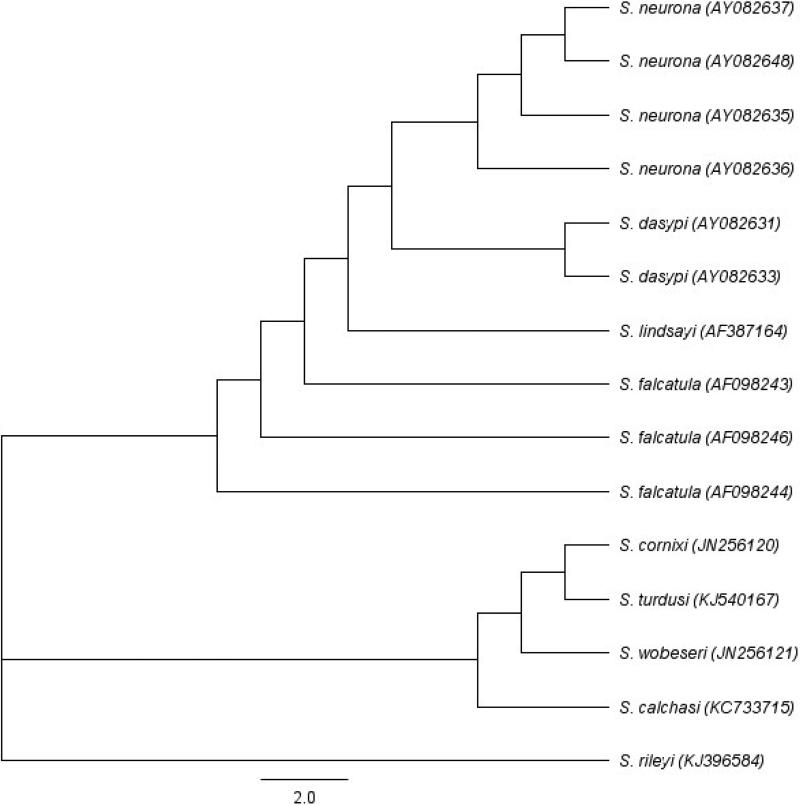
Neighbor-joining tree was constructed using sequences of related Sarcocystis species in Geneious version 8.0.4 using the Tamura-Nei genetic distance model to show phylogenetic relationship among various species especially four Sarcocystis spp. (S. neurona, S. falcatula, S. dasypi and S. lindsayi) that employ opossums as their definitive host. Input sequences were the ITS1 regions of various genera retrieved from GenBank with accession number.
3. Results
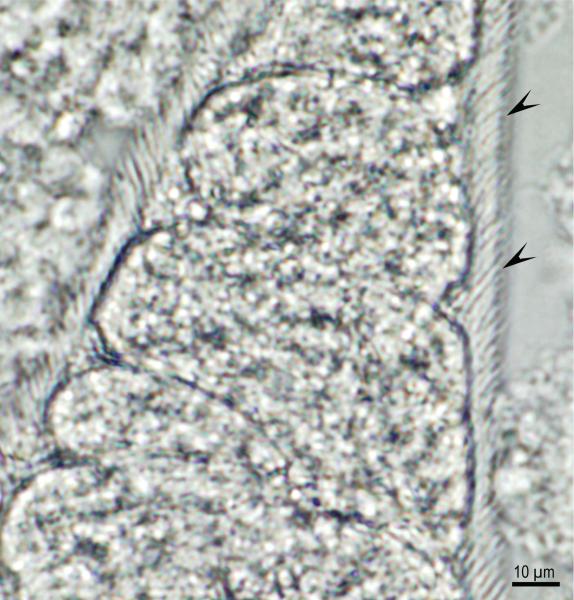
Sarcocystis-like bradyzoites were found in pepsin digests of 14 hearts and 10 tongues, and sarcocysts were found in histological sections of tongues of 26 (18 male and 8 female) of 35 bobcats (Table 1). The density of sarcocysts ranged from one to seven per section (average 2.2/section). All sarcocysts examined using light microscopy at 1000x magnification were relatively thin-walled and appeared morphologically similar to S. felis sarcocysts (Dubey et al., 1992; Dubey et al., 2015b). In two bobcats, S. neurona–like sarcocysts possessing thickened villar tips were identified in tongue muscle squash by light microscopy (Fig. 1). However, attempts to locate these sarcocysts by TEM were unsuccessful (data not shown). To determine whether the observed sarcocysts were due to infection by S. neurona, DNA was extracted from the hearts and tongues of all 35 bobcats and PCR amplified using ITS1500 primers to specifically detect S. neurona or S. falcatula, two Sarcocystis spp. of the North American opossum (Fig. 2). The ITS1 locus was amplified from the DNA of 17 hearts and 18 tongues of bobcats (Table 1). DNA sequencing of the ITS1 PCR amplicons identified unambiguous sequences in 27 of 35 bobcats; 24 bobcats had ITS1 sequences with 100% identity to S. neurona ITS1 sequence. One bobcat tissue harbor DNA that amplified ITS1 sequences identical to published S. dasypi ITS1 sequence, and two bobcats had ITS1 sequences identical to sequence of S. dasypi and S. neurona suggesting that both were co-infected with S. neurona-like and S. dasypi-like parasites. (Table 1). Multiple sequence alignment of the ITS1 region established that S. dasypi (AY082633) is distinguishable from S. neurona (AY082637) by five single nucleotide polymorphisms (SNP); at nucleotide position 876 (S. neurona has an A; S. dasypi has a G). The other nucleotide differences between these two sequences uses, W (A or T), R (A or G), R (A or G) and W (A or T), indicating uncertainty or a potential of two different nucleotides at particular site. Phylogenetic analysis also inferred that S. dasypi shared very close relationship to S. neurona (Fig. 2).
Table 1.
Microscopic examination, PCR amplification and sequence analysis of Sarcocystis species infection in bobcats (n=35) from Mississippi, USA
| Stage/primers | Samples | Positive bobcat # | Total positive | |
|---|---|---|---|---|
| Microscopy | Bradyzoites | Heart digest | 3, 4, 8, 9, 10, 12, 13, 21, 24, 25, 26, 27, 29, 31 | 14 |
| Bradyzoites | Tongue digest | 8, 16, 17, 18, 20, 24, 31, 32, 34, 35 | 10 | |
| Sarcocysts | Tongue H&E stained section | 1(4)a, 3(1), 8(1), 9(4), 10(2), 11(1), 12(7), 13(2), 16(2), 17(4), 18(2), 19(2), 20(3), 21(1), 23(6), 24(1), 25(1), 26(2), 27(1), 29-32(1), 33(2), 34(3), 35(1) | 26 | |
| PCR amplification | ITS1500 (S. neurona, S. falcatula,S. dasypi, S. lindsayi ITS1 locus, ~500bp) | Heart digest | 1, 2, 3, 8, 11, 13, 17, 23, 24, 25, 27, 28, 29, 31, 32, 33, 34 | 17 |
| Tongue digest | 6, 7, 8, 9, 11, 15, 17, 18, 20, 21, 22, 24, 25, 26, 29, 30, 31, 35 | 18 | ||
| Sequence analysis | ITS1500 (S. neurona Positive) | Heart digest | 2, 3, 8, 11, 13, 17*, 23, 24, 25, 27*, 28*, 29, 32, 33, 34 | 15 |
| Tongue digest | 6*, 7, 8, 9, 11, 15, 18*, 20*, 21*, 22, 24, 26, 29, 30, 35* | 15 | ||
| ITS1500 (S. dasypi Positive) | Heart digest | 31 | 1 | |
| Tongue digest | 17, 25* | 2 |
Indicates poor quality sequence
Fig. 1.
Sarcocystis neurona sarcocyst in squash of tongue of bobcat #32. Note dense areas on villar protrusions (arrowheads). Unstained.
Fig. 2.
Neighbor-joining tree based on ITS1 sequences of Sarcocystis species. Phylogenetic tree was constructed in Geneious version 8.0.4 using the Tamura-Nei genetic distance model. Input sequences, including accession numbers, of various taxon were retrieved from NCBI GenBank. The four Sarcocystis spp. (S. neurona, S. falcatula, S. dasypi and S. lindsayi) that employ opossums as their definitive host were grouped together. S. dasypi was inferred to share an especially very close relationship to S. neurona
4. Discussion
Sarcocysts in tongue squash of two bobcats were structurally similar to sarcocysts of S. neurona (Dubey et al., 2015a). The DNA extracted from the muscle tissues of these two bobcats was confirmed to be that of S. neurona-like parasite. PCR-DNA sequencing performed on the muscle tissues of an additional 33 bobcats from rural regions in Mississippi State identified a high prevalence of infection with S. neurona-like parasite.
Until the discovery of the sarcocyst stage of S. neurona in domestic cats and their experimental transmission to opossums in 2000 (Dubey et al., 2000), S. felis was thought to be the only Sarcocystis species infecting felids, both domestic and wild (Dubey et al., 1992). Sarcocystis felis was named for the sarcocysts found in bobcats from Arkansas. Based on the morphology of their sarcocysts, S. felis is thought to parasitize lions, cheetahs, and domestic cats (reviewed in Dubey et al., 2015b). However, the life cycle of S. felis is unknown, and the domestic cat represents only an intermediate host, not the definitive host (Dubey et al., 1992). With the recognition that the domestic cat is also an intermediate host for S. neurona (Dubey et al., 2000; Butcher et al., 2002), and the observation that natural cases of S. neurona can cause encephalitis in cats (Bisby et al., 2010; Dubey et al., 2003; Dubey et al., 2015a), there is a necessity to distinguish between S. neurona and S. felis infections using specific molecular markers. Earlier phylogenetic reports using 18S rDNA sequences indicated that these two parasites are closely related, however, they can be differentiated using the more resolved ITS1 locus (Elsheikha et al., 2006; Gillis et al., 2003). The ITS1 region shows much higher level of sequence divergence in comparison to 18S rRNA and 28S rRNA loci and has been used in several studies to discriminate very closely related Sarcocystis species. PCR-DNA sequence analysis using highly specific ITSI500 primers can diagnose infections in felids by S. neurona versus S. felis (Miller et al., 2009). The present study supports the widespread infection of bobcats from rural Mississippi with S. neurona-like parasite. Whether it is likewise the case in other bobcat populations throughout the United States has yet to be examined.
The S. dasypi described from the nine-banded armadillo (Dasypus novemcinctus) isclosely related with S. neurona. Howells et al. (1975) named S. dasypi and S. diminuta for the armadillo from Brazil. Lindsay et al. (1996) reported that S. dasypi, S. diminuta-like sarcocysts, and another undetermined species can infect armadillos throughout the USA. These observations were made before the recognition of armadillos as natural and experimental hosts of S. neurona (Cheadle et al., 2001; Tanhauser et al., 2001). Indeed, sarcocysts found in 30 of 48 armadillos in Florida and DNA extracted from infected muscles of six armadillos was identified unequivocally as S. neurona by molecular PCR (Tanhauser et al., 2001). DeLucia et al.(2002) reported S. dasypi prevalence in 38 of 63 and S. diminuta in 6 of 63 armadillo's skeletal muscle from Florida by light microscopic examination; however, because neither TEM nor PCR-DNA sequencing was performed, it is not possible to confirm whether the Sarcocystis species present in the armadillos from Florida were S. dasypi or S. neurona. Subsequently, Cheadle et al. (2001) deposited an ITS1 sequence (AY082633) into NCBI GenBank from an infected armadillo as that of S. dasypi, however, laboratory raised opossums fed naturally infected armadillo muscle excreted S. neurona-like sporocysts, and a foal fed these sporocysts developed clinical disease simulating EPM (Cheadle et al., 2001). Thus, it remains uncertain whether the parasite was S. neurona or S. dasypi. Results of the present study in bobcats indicate that S. dasypi–like parasites were present in three bobcats; whether the parasite is a variant of S. neurona or is actually S. dasypi is unknown. The ITS1 marker alone may not be sensitive enough to distinguish S. neurona and S. dasypi because there is a confirmed single nucleotide polymorphism at nt 876 (S. neurona has an A; S. dasypi has a G). Because there are no archived specimens of S. dasypi, the true identity of S. dasypi may never be known. Limited TEM studies indicate that S. dasypi and S. neurona are structurally similar (Lindsay et al., 1996; Dubey et al., 2001b).
Bobcats in the present study were from a very remote wilderness area of Mississippi. The high prevalence of S. neurona-like parasite (26 of 35) infecting bobcats from this area is intriguing. Bobcats are strict carnivores, so exactly how they become infected with Sarcocystis spp. is enigmatic. Drinking water contaminated with sporocysts is one potential source of infection. Alternatively, consumption of the definitive hosts is also a possibility. For example, 19 of 72 D. virginiana from Mississippi were previously found to harbor S. neurona sporocysts (Dubey et al., 2001a) so it is also possible that bobcats prey on opossum or eat dead opossum and are conceivably exposed to millions of sporocysts trapped in the lamina propria of the small intestine of opossums. In either scenario, the high prevalence of S. neurona-like parasite in bobcats indicates a very efficient sylvatic cycle.
Highlights.
Sarcocystis neurona–like sarcocysts possessing thickened villar tips were identified in two bobcats tongue muscle squash under light microscope.
26 of 35 bobcats were found to harbor S. neurona-like DNA in their tissues by PCR-DNA sequencing at ITS1.
One bobcat was infected with S. dasypi-like parasite and two bobcats were co-infected with S. neurona-like and S. dasypi-like parasites detected by PCR-DNA sequencing at ITS1.
The high prevalence of sarcocysts in bobcat tissues suggested an efficient sylvatic cycle of Sarcocystis spp.
Acknowledgements
R. Calero-Bernal is a postdoctoral fellow (Ref. PO12010) funded by the Department of Employment and Innovation of the Regional Government of Extremadura (Spain) and the European Social Fund. This study was financially support in part by the Intramural Research Program of the NIH and NIAID. M.E.G. is a scholar of the Canadian Institute for Advanced Research Integrated Microbial Biodiversity Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbosa L, Johnson CK, Lambourn DM, Gibson AK, Haman KH, Huggins JL, Sweeny AR, Sundar N, Raverty SA, Grigg ME. Encephalitis in an unexpectedly broad range of marine mammals from the northeastern pacific ocean. Int. J. Parasitol. 2015 doi: 10.1016/j.ijpara.2015.02.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby TM, Holman PJ, Pitoc GA, Packer RA, Thompson CA, Raskin RE. Sarcocystis sp. encephalomyelitis in a cat. Vet. Clin. Pathol. 2010;39:105–112. doi: 10.1111/j.1939-165X.2009.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher M, Lakritz J, Halaney A, Branson K, Gupta GD, Kreeger J, Marsh AE. Experimental inoculation of domestic cats (Felis domesticus) with Sarcocystis neurona or S. neurona-like merozoites. Vet. Parasitol. 2002;107:1–14. doi: 10.1016/s0304-4017(02)00107-3. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Tanhauser SM, Dame JB, Sellon DC, Hines M, Ginn PE, MacKay RJ, Greiner EC. The nine-banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona. Int. J. Parasitol. 2001;31:330–335. doi: 10.1016/s0020-7519(01)00177-1. [DOI] [PubMed] [Google Scholar]
- DeLucia PM, Cheadle MA, Greiner EC. Prevalence of Sarcocystis sarcocysts in nine-banded armadillos (Dasypusnovemcinctus) from Florida. Vet. Parasitol. 2002;103:203–205. doi: 10.1016/s0304-4017(01)00594-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Fayer R. Sarcocystosis of Animals and Man. CRC Press; Boca Raton, Florida: 1989. p. 215. [Google Scholar]
- Dubey JP, Hamir AN, Kirkpatrick CE, Todd KS, Rupprecht CE. Sarcocystis felis sp. n. (Protozoa: Sarcocystidae) from the bobcat (Felis rufus). J. Helminthol. Soc. Wash. 1992;59:227–229. [Google Scholar]
- Dubey JP, Saville WJA, Lindsay DS, Stich RW, Stanek JF, Speer CA, Rosenthal BM, Njoku CJ, Kwok OCH, Shen SK, Reed SM. Completion of the life cycle of Sarcocystis neurona. J. Parasitol. 2000;86:1276–1280. doi: 10.1645/0022-3395(2000)086[1276:COTLCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Black SS, Rickard LG, Rosenthal BM, Lindsay DS, Shen SK, Kwok OCH, Hurst G, Rashmir-Raven A. Prevalence of Sarcocystis neurona sporocysts in opossums (Didelphis virginiana) from rural Mississippi. Vet. Parasitol. 2001a;95:283–293. doi: 10.1016/s0304-4017(00)00394-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Fritz D, Speer CA. Structure of Sarcocystis neurona sarcocysts. J. Parasitol. 2001b;87:1323–1327. doi: 10.1645/0022-3395(2001)087[1323:SOSNS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Benson J, Larson MA. Clinical Sarcocystis neurona encephalomyelitis in a domestic cat following routine surgery. Vet. Parasitol. 2003;112:261–267. doi: 10.1016/s0304-4017(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of Animals and Humans. 2nd ed. CRC Press; Boca Raton, FL: 2010. p. 313. [Google Scholar]
- Dubey JP, Howe DK, Furr M, Saville WJ, Marsh AE, Reed SM, Grigg ME. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 2015a doi: 10.1016/j.vetpar.2015.01.026. doi:10.1016/j.vetpar.2015.01.026-In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of Animals and Humans. 2nd ed. CRC Press; Boca Raton, Florida: 2015b. In press. [Google Scholar]
- Elsheikha HM, Soltan DM, el-Garhy MF. Inference of molecular phylogeny of Sarcocystis felis (Sarcocystidae) from cats based on nuclear-encoded ribosomal gene sequences. J. Egypt. Soc. Parasitol. 2006;36:441–453. [PubMed] [Google Scholar]
- Gillis KD, MacKay RJ, Yowell CA, Levy JK, Greiner EC, Dame JB, Cheadle MA, Hernandez J, Massey ET. Naturally occurring Sarcocystis infection in domestic cats (Felis catus). Int. J. Parasitol. 2003;33:877–883. doi: 10.1016/s0020-7519(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Howells RE, Carvalho ADV, Mello MN, Rangel NM. Morphological and histochemical observations on Sarcocystis from the nine-banded armadillo, Dasypus novemcinctus. Ann. Trop. Med. Parasitol. 1975;69:463–474. [Google Scholar]
- Lindsay DS, McKown R, Upton SJ, McAllister CT, Toivio-Kinnucan MA, Veatch JK, Blagburn BL. Prevalence and identity of Sarcocystis infections in armadillos (Dasypus novemcinctus). J. Parasitol. 1996;82:518–520. [PubMed] [Google Scholar]
- Miller MA, Barr BC, Nordhausen R, James ER, Magargal SL, Murray M, Conrad PA, Toy-Choutka S, Jessup DA, Grigg ME. Ultrastructural and molecular confirmation of the development of Sarcocystis neurona tissue cysts in the central nervous system of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 2009;39:1363–1372. doi: 10.1016/j.ijpara.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhauser SM, Cheadle MA, Massey ET, Mayer BA, Schroeder DE, Dame JB, Greiner EC, MacKay RJ. The nine-banded armadillo (Dasypus novemcinctus) is naturally infected with Sarcocystis neurona. Int. J. Parasitol. 2001;31:325–329. doi: 10.1016/s0020-7519(01)00178-3. [DOI] [PubMed] [Google Scholar]