Abstract
Background
Risk factors for obstructive sleep apnea (OSA) and development of subsequent cardiovascular (CV) complications differ by sex. We hypothesize that the relationship between OSA and high sensitivity troponin T (hs-TnT), cardiac structure, and CV outcomes differs by sex.
Methods and Results
752 men and 893 women free of CV disease participating in both the Atherosclerosis Risk in the Communities and the Sleep Heart Health Studies were included. All participants (mean age 62.5±5.5 years) underwent polysomnography and measurement of hs-TnT. OSA severity was defined using established clinical categories. Subjects were followed for 13.6±3.2 years for incident coronary disease, heart failure, and CV and all-cause mortality. Surviving subjects underwent an echocardiography after 15.2±0.8 years. OSA was independently associated with hs-TnT among women (p=0.03) but not in men (p=0.94). Similarly, OSA was associated with incident HF or death in women (p=0.01) but not men (p=0.10). This association was no longer significant after adjusting for hs-TnT (p=0.09). Among surviving participants without an incident CV event, OSA assessed in mid-life was independently associated with higher left ventricle mass index only among women (p=0.001).
Conclusions
Sex-specific differences exist in the relationship between OSA and CV disease. OSA, assessed in mid-life, is independently associated with higher levels of concomitantly measured hs-TnT among women but not men, in whom other comorbidities associated with OSA may play a more important role. During 13-year follow-up, OSA was associated with incident HF or death only among women, and among those without an incident event, was independently associated with LV hypertrophy only in women.
Keywords: Sleep disorders, Troponin T, Sex, Echocardiography, Heart Failure
Background
Obstructive sleep apnea (OSA) affects at least 2–6% of the U.S. population1 with a higher prevalence in men compared to women by 2:1 in population based studies and up to 8:1 in referral populations.2,3 Epidemiologic studies suggest an association between OSA and both coronary heart disease (CHD) and heart failure (HF).4 Indeed, OSA severity is positively associated with higher levels of hs-TnT, a powerful risk factor for incident HF, after adjusting for multiple potential confounders in community dwelling persons free of CHD or HF.5 Existing cross-sectional studies have also suggested an association between OSA and both left ventricular hypertrophy6,7 and right ventricular hypertrophy.8 However, the association between clinical risk factors, such as BMI, and OSA differ by sex.9 Epidemiologic studies also suggest sex-based differences in the association between OSA and cardiovascular outcomes.4,10 Whether the relationship between OSA severity and subclinical myocardial injury reflected in hs-TnT levels varies by sex is not known. Furthermore, whether the impact of OSA in mid-life on subsequent HF risk and on cardiac structure and function in late-life varies by sex is not known.
We used data from a well phenotyped cohort participating in both the Atherosclerosis Risk in Communities (ARIC) Study and the Sleep Heart Health Study (SHHS) to determine whether sex-based differences exist in the relationship between OSA and cardiovascular disease. Specifically, we hypothesized that sex-specific differences in the pathophysiology of OSA would result in significant sex-based differences in the association of OSA severity measured in mid-life with subclinical myocardial injury as measured by hs-TnT levels, with incident HF, and with late-life cardiac structure and function.
Methods
Population
Briefly, ARIC is a prospective epidemiologic cohort study that enrolled 15,792 middle-aged subjects between 1987 and 1989, and was designed to investigate the etiology and natural history of clinical and subclinical atherosclerosis.11 Between 1996 and 1998, surviving subjects underwent a fourth visit at which time blood samples were obtained from which hs-TnT, NT-proBNP, and hs-CRP were measured.12 The SHHS was a prospective cohort study conducted between 1995–1998 that recruited 6,441 patients older than 40 years from 9 U.S. cohorts, including 1,920 subjects from ARIC.11 Treatment for OSA, including oral devices, CPAP or other continuous airway pressure device, or tracheostomy, as well as home oxygen therapy were exclusion criteria for recruitment.13 Participants underwent overnight home polysomnography and lung function tests.13 The SHHS visit and the fourth ARIC visit were performed independently of each other during the same three-year period. Therefore, assessments of OSA severity and clinical and laboratory values were not performed at the same time (median difference of ARIC visit relative to the SHHS visit 172 days [interquartile range −51 to 387 days]).
A total of 916 men and 974 women participated in both the ARIC Study visit 4 and the SHHS. All participants underwent overnight home polysomnography and measurement of serum biomarkers, and were free of prevalent coronary heart disease (CHD) or heart failure (HF) at baseline assessment as previously described in detail.5 Subjects were considered to have HF or CHD if they reported prevalent HF or CHD at ARIC visit 1 or had incident HF or CHD between ARIC visit 1 and the later of ARIC visit 4 or the SHHS visit based on ARIC cohort surveillance criteria.14,15
All demographics, clinical characteristics, and laboratory values were obtained from ARIC visit 4 exam while pulmonary function test results were obtained from the SHHS visit. Subjects with prevalent HF or CHD at the later of either ARIC visit 4 or SHHS visit were excluded.5 From participants that underwent both ARIC and SHHS visits, 139 males and 61 females were excluded because of prevalent CHD or HF and additional 41 men and 34 women were excluded due to missing hs-TnT data, leaving 752 men and 893 women included in this analysis (Supplemental Figure 1).
Polysomnography
All participants underwent overnight unattended polysomnography which was centrally scored as previously described.5,16 OSA severity was assessed with the apnea hypopnea index (AHI) which included all apneas and hypopneas accompanied by at least a 4% drop in oxygen saturation.17 The severity of OSA was defined using conventional clinical categories: none (AHI≤5), mild (AHI >5 to ≤15), moderate (AHI >15 to ≤30), and severe (AHI>30).
Cardiac Biomarkers
Blood samples were taken at the time of ARIC visit 4 and plasma was stored centrally at −80°C. Hs-TnT was measured using a highly sensitive assay (Elecsys Troponin T, Roche diagnostics, Indianapolis, IN).19 NT-proBNP was measured using electrochemiluminescent immunoassay (Roche Diagnostics).18 Hs-CRP was assessed by immunoturbidimetric CRP-Latex high sensitivity assay from Denka Seiken using Hitachi 911 analyzer according to the manufacturer’s protocol.18
Echocardiography
Between 2011 and 2013, surviving ARIC participants were invited for a fifth visit at which time echocardiography was performed.19 Of 1,328 surviving participants, 906 subjects free of HF or CHD elected to participate and underwent echocardiography by a standardized protocol as previously described.19 LV and RV size and systolic and diastolic function measurements were assessed according to the recommendations of the American Society of Echocardiography (ASE).20,21 LV mass was calculated from LV linear dimensions and indexed to body surface area as recommended by ASE guidelines.20 To account for body size LV mass was indexed (LV mass index -LVMI) by dividing LVM to height2.7. LVMI was used to define LV hypertrophy as >49 g/m2.7 in men or >45 g/m2.7 in women.
Clinical Outcomes
ARIC participants were followed for all-cause mortality, incident CHD and incident HF as previously described.5 Incident HF was defined as the occurrence of a hospitalization with an ICD-9 discharge code 428 in any position or a death certificate with either an ICD-9 code 428 in any position or an ICD-10 code 150 in any position.14 Incident CHD was defined as a definite or probable hospitalized MI based on committee adjudication of abstracted hospitalization records including chest pain symptoms, ECGs, and cardiac enzymes or an adjudicated definite CHD death based on chest pain symptoms, cause of death from death certificate, associated hospitalization records and medical history as previously described in detail.15 Death was ascertained based in annual phone call follow-up or through health department death certificate files.14
Statistical Analysis
OSA was modeled categorically using the clinically defined thresholds noted above. As the distribution of hs-TnT was heavily skewed and could not be transformed to achieve normality, hs-TnT was modeled as an ordinal categorical variable using 5 categories based on our population’s hs-TnT distribution as previously described.5 As the population distribution of hs-TnT is known to vary by sex,22,23 we employed sex-specific hs-TnT categories: the first category was defined by undetectable values based on the limit of quantification (5 ng/L), the fifth category was defined by the 90th percentile (≥14 ng/L for males and ≥8 ng/L for females), and the remainder of participants were divided into tertiles (for males: 5–6, 7–9, and 10–13 ng/L; for females: 5, 6, and 7 ng/L). We performed additional sensitivity analysis using the assay limit of measurement of 3 ng/L as the upper limit for the first category of hs-TnT level (see Supplemental Data). We also explored whether sex-based differences existed in the association between OSA severity and NT-proBNP or hs-CRP levels. NT-proBNP and hs-CRP were modeled linearly using logarithmic transformed values. The NT-proBNP limit of detection was 5 pg/mL and subjects with undetectable values were assigned a value of 2.5 pg/mL.
The relationship between OSA and hs-TnT was assessed using univariate and multivariable ordinal regression with hs-TnT category. The associations between OSA and either NT-proBNP or hs-CRP were assessed using linear regression models. In order to assess the need for sex-specific models, we considered a fully adjusted model in which sex-by-covariate interaction terms were added for all covariates, thus allowing all beta coefficients to vary by sex. We compared this to a simplified model containing no interaction terms via a likelihood ratio test.24 We repeated the likelihood ratio test for each biomarker. As several demographic, clinical, and metabolic variables associated with OSA may act as either confounders or/and mediators of the OSA-biomarker relationship, we employed three additive multivariable ordinal logistic regression models adjusting for sequentially more variables. Model covariates were selected based on a priori knowledge and variables significantly associated with the predictor variable of interest in univariate analysis. Model 1 adjusted for age and BMI; Model 2 additionally adjusted for clinical covariates demonstrating a differential association with AHI by sex and possible confounders: history of hypertension (defined as systolic blood pressure >140 mmHg; diastolic blood pressure>80 mmHg or taking antihypertensive medications), history of diabetes (defined as fasting blood glucose >126mg/dl or on medication for diabetes), systolic blood pressure and smoking status; Model 3 additionally adjusted for alcohol intake, pulmonary function tests (FEV1 and FVC), history of chronic lung disease, estimated glomerular filtrated rate (eGFR) and blood levels of fasting insulin, total cholesterol, LDL, HDL and triglycerides. Analyses were repeated using waist circumference instead of BMI to account for body fat distribution. A sensitivity analysis was performed restricted to participants with only 1-year difference between ARIC visit 4 and the SHHS visit to explore the impact of the time interval between polysomnography and hs-TnT assessment on the model estimates (n=1,019).
To assess the relationship between OSA and time to subsequent HF or death, we used sex-specific unadjusted Cox-proportional hazard models. Further analysis was performed after adjustment for hs-TnT to assess the potential role of hs-TnT as a mediator of the association between OSA and incident HF. This was done by assessing the reduction in magnitude of the beta coefficient for the association between OSA and the composite outcome after subsequent adjustment for hs-TnT. Finally, an additional model further adjusted for the same variables as in Model 2 above assessed at the time of polysomnography.
Among participants attending ARIC Visit 5 free of cardiovascular diseases, including HF, we assessed the association between OSA severity measured at mid-life and echocardiographic measures of LV and RV structure and function when elderly. As many clinical risk factors could behave as either confounders or mediators, we used sex-specific univariate and multivariable regression models adjusting for the same covariates as in Model 2 above, but assessed at both timepoints (at polysomnography and at echocardiography), in addition to the use of statins, beta-blockers, RAS inhibitors and mineralocorticoid receptor antagonists at both timepoints, and self-reported use of CPAP at the time of the echocardiography. Measures from both timepoints were included as the change in status over time could affect the association between OSA and cardiac structure. Finally, we repeated the Cox proportional hazard models to assess the association among women and men of OSA severity with the composite endpoint of all-cause mortality, incident HF, incident CHD (a known risk factor of HF that has been associated with OSA,4 and LV hypertrophy. For this composite endpoint, multivariable analysis adjusted for use of statins, beta-blockers, RAS inhibitors and mineralocorticoid receptor antagonists at both the timepoints and for self-reported CPAP use at Visit 5. All analyses were performed using STATA 12.1 (StataCorp LP. 2009. Texas).
Results
Demographic, clinical characteristics, and cardiac biomarker levels by sex and by OSA category are summarized in Table 1. In men, more severe OSA was associated with older age, higher BMI, higher systolic and diastolic blood pressure and higher FEV1/FVC ratio. In addition to previous associations noted in men, among women more severe OSA was also associated with a higher prevalence of diabetes, hypertension, and current smoking.
Table 1.
Baseline demographics, clinical characteristics, and cardiac biomarker levels by OSA categories for each sex. F and M indicate the hs-TnT category thresholds for males and females respectively. P for trend is based on OSA categories number as a linear term. For binary categorical variables we used a chi-squared test for linear trend.
| Overall N=1645 |
Men (n=752): OSA Severity
|
P for trend |
Women (n=893): OSA Severity
|
P for trend |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None (n=312) |
Mild (n=267) |
Moderate (n=108) |
Severe (n=65) |
None (n=592) |
Mild (n=208) |
Moderate (n=58) |
Severe (n=35) |
||||
| Age (years) | 62.5 (5.5) | 62.5 (5.7) | 63.5 (5.3) | 63.8 (5.4) | 64.9 (4.9) | <0.001 | 61.4 (5.4) | 63.0 (5.4) | 62.6 (5.0) | 63.3 (6.0) | <0.001 |
| White n(%) | 1630 (99) | 312 (100) | 259 (97) | 108 (100) | 64(98) | 0.31 | 590 (99.7) | 205 (99) | 57 (98) | 35 (100) | 0.27 |
| Co-morbidities | |||||||||||
| Hypertension | 618 (38) | 104 (33) | 101 (38) | 36 (33) | 34(52) | 0.03 | 195 (33) | 94 (45) | 34 (59) | 20 (57) | <0.001 |
| Diabetes | 179 (11) | 32 (10) | 47 (18) | 14 (13) | 10(15) | 0.18 | 33 (6) | 26 (12) | 10 (17) | 7 (20) | <0.001 |
| Prior stroke | 23 (1.4) | 3 (1) | 5 (1.9) | 2 (1.9) | 1 (1.5) | 0.50 | 7 (1.2) | 3 (1.4) | 1 (1.7) | 1 (2.9) | 0.41 |
| Atrial fib/flutter | 12 (0.7) | 4 (1.3) | 2 (0.7) | 1 (0.9) | 0 | 0.35 | 3 (0.5) | 1 (0.5) | 0 | 1 (2.9) | 0.36 |
| COPD | 119 (7) | 19 (6) | 23 (9) | 5 (5) | 3 (5) | 0.63 | 47 (8) | 16 (8) | 6 (10) | 0 (0) | 0.41 |
| Asthma | 122 (7) | 20 (6) | 18 (7) | 15 (14) | 4 (6) | 0.21 | 42 (7) | 19 (9) | 3 (5) | 1 (3) | 0.58 |
| Smoking | |||||||||||
| Current | 169 (10) | 41 (13) | 16(6) | 11 (10) | 6 (9) | 0.17 | 77 (13) | 13 (6) | 3 (5) | 2 (6) | 0.004 |
| Former | 773 (47) | 178 (57) | 164 (61) | 68 (63) | 40 (62) | 0.26 | 209 (35) | 82 (39) | 22 (38) | 10 (29) | 0.95 |
| BMI (kg/m2) | 28.6 (5.0) | 27.5 (3.9) | 29.0 (3.9) | 30.5 (4.5) | 32.3 (4.1) | <0.001 | 26.8 (4.5) | 30.4 (5.6) | 34.1 (6.0) | 35.4 (5.9) | <0.001 |
| SBP (mmHg) | 125 (18) | 124 (18) | 127 (17) | 127 (18) | 128 (15) | 0.03 | 123 (17) | 129 (18) | 130 (18) | 132 (18) | <0.001 |
| DBP (mmHg) | 71 (9) | 71 (9) | 72 (9) | 73 (10) | 73 (9) | 0.03 | 69 (9) | 71 (10) | 72 (10) | 72 (12) | 0.004 |
| FEV1/FVC (%) | 74 (7) | 72 (8) | 73 (7) | 73 (9) | 74 (6) | 0.011 | 75 (6) | 76 (5) | 76 (5) | 78 (4) | <0.001 |
| eGFR (mL/min/1.73 m2) | 83.3 (13.1) | 84.0 (12.3) | 83.1 (13.0) | 83.2 (12.9) | 81.8 (11.5) | 0.21 | 83.9 (13.3) | 81.9 (13.8) | 84.4 (13.4) | 80.2 (13.4) | 0.11 |
| Hs-TnT (ng/L) | 0.006† | <0.001† | |||||||||
| Undetectable (< 5 ng/L) | 853 (52) | 106 (34) | 77 (29) | 40 (37) | 16 (25) | 432 (73) | 133 (64) | 32 (55) | 17 (49) | ||
| M: 5 – 6 ng/L F: 5 ng/L |
253 (15) | 78 (25) | 59 (22) | 16 (15) | 14 (22) | 55 (9) | 21 (10) | 7 (12) | 3 (9) | ||
| M: 7 – 9 ng/L F: 6 ng/L |
217 (13) | 71 (23) | 57 (21) | 22 (20) | 13 (20) | 34 (6) | 11 (5) | 7 (12) | 2 (6) | ||
| M: 10 – 13 ng/L F: 7 ng/L |
134 (8) | 32 (10) | 42 (16) | 19 (18) | 6 (9) | 18 (3) | 10 (5) | 2 (3) | 5 (14) | ||
| M:≥ 14 ng/L F: ≥8 ng/L |
188 (11) | 25 (8) | 32 (12) | 11 (10) | 16 (25) | 53 (9) | 33 (16) | 10 (17) | 8 (23) | ||
Based in non-parametric trend test
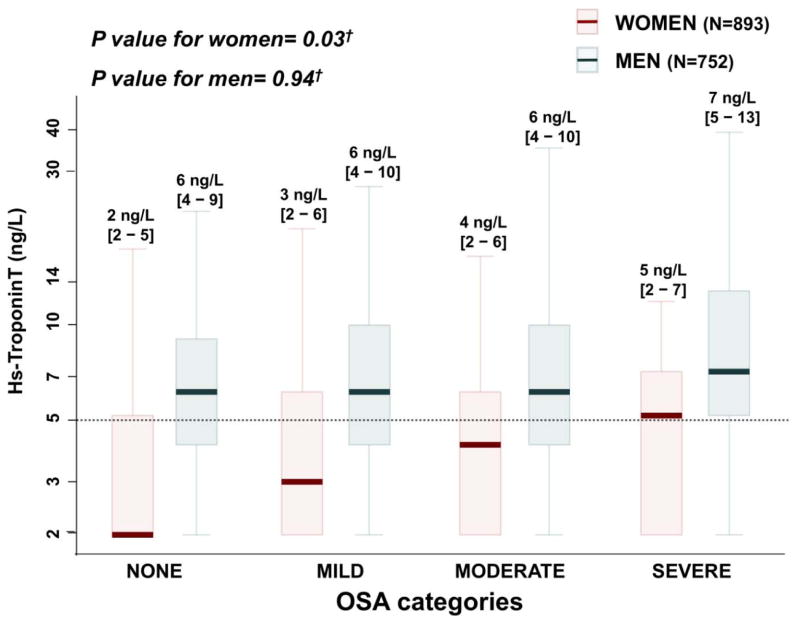
Men demonstrated higher hs-TnT levels than women across OSA categories. The fully adjusted model for the association between OSA severity and hs-TnT levels with sex-specific regression coefficients demonstrated significantly better model fit compared to the model with sex-independent coefficients (likelihood ratio test p value=0.009). This improvement in model fit included a significant interaction between sex and OSA (p=0.04). Therefore, we analyzed the relationship between OSA and hs-TnT separately by sex. Based on unadjusted ordinal logistic regression, a change from a lower OSA category to the next more severe category was associated with an odds ratio for being in a higher hs-TnT category of 1.48 [95% CI: 1.25–1.74, p<0.001] in women and 1.20 [95% CI: 1.04–1.37, p=0.01] in men (interaction p=0.05). In women, the relationship between OSA and hs-TnT remained significant in multivariable ordinal logistic regression models adjusting for age and BMI (OR: 1.34 [1.11 – 1.63], p = 0.003; Supplemental Table 1), age, BMI, hypertension, diabetes, systolic blood pressure, and smoking status (OR: 1.30 [1.07 – 1.58], p = 0.009), and after full multivariable adjustment (OR: 1.25 [1.02 – 1.52], p = 0.03, Figure 1). However, in men, the relationship between AHI and hs-TnT was noticeably attenuated and not significant after adjusting for age and BMI (OR: 0.98 [0.84 – 1.14], p = 0.80; Figure 1 and Supplemental Table 1). Similar results to the primary analysis were found when the analysis was repeated in the following sensitivity analyses: (1) adjusting for waist circumference instead of BMI; (2) using 3 ng/L as the limit of measurement of hs-TnT, instead of 5 ng/L (Supplemental Tables 2 and 3); (3) restricting the population to participants with OSA and ns-TnT measurement performed within 1-year of each other (551 women and 467 men Supplemental Table 4); and (4) excluding 14 participants missing complete data for all covariates (885 women and 746 men; data not shown).
Figure 1.
Box-and-whisker plot of hs-TnT levels among OSA categories stratified by sex. Hs-TnT is shown using a logarithmic scale from row hs-TnT values. Values under the limit of measurement (3 ng/L) are assigned to a value of 2 ng/L. Values below the limit of quantification (5 ng/L) –marked as a dotted line in the figure- are included in the first of the five hs-TnT categories. †Multivariable ordinal logistic regression adjusted by age, BMI, smoking status, alcohol intake, hypertension, diabetes, chronic lung disease, pulmonary function tests, eGFR, systolic and diastolic blood pressure and blood levels of total cholesterol, LDL, HDL, triglycerides and insulin (model 3).
As described previously,5 there was no significant association between OSA severity and NT-proBNP levels after adjusting for age, sex, and BSA, nor did sex modify this association (p for interaction = 0.36). OSA severity was not significantly associated with hs-CRP levels in unadjusted analysis or after multivariable adjustment (Supplemental Table 5). Similarly, no effect modification of sex on the relationship between OSA severity and hs-CRP level was observed (p for interaction = 0.84).
After 13.6±3.2 years of clinical follow-up, 210 men and 154 women died or suffered incident HF. In the unadjusted analysis, OSA severity was significantly associated with incident HF or death in women but not in men (Table 2; hazard ratio for moderate/severe vs none/mild OSA of 1.26 [1.05 – 1.50] and 1.12 [0.98 – 1.29] respectively, Figure 2). This association among women was no longer significant after adjusting for hs-TnT level at Visit 4 (p=0.07). Mediation analysis suggested that hsTn-T level at the time of OSA assessment accounted for approximately 30% of the association between OSA and incident HF or death among women.
Table 2.
Cox Hazard Ratios for incident cardiovascular outcomes and death.
| MEN | WOMEN | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Events/Subjects at risk | Hazard Ratio [95% Confidence Interval] | Number of Events/Subjects at risk | Hazard Ratio [95% Confidence Interval] | |||||
| Unadjusted | Adjusted by hs-TnT | Fully Adjusted* | Unadjusted | Adjusted by hs-TnT | Fully Adjusted* | |||
| Heart Failure | 83/737 | 1.22 [0.98 – 1.51] | 1.12 [0.90 – 1.39] | 0.97 [0.77 – 1.22] | 72/879 | 1.41 [1.10 – 1.80] | 1.27 [0.99 – 1.63] | 0.94 [0.69 – 1.28] |
| Composite Heart Failure or Death | 170/737 | 1.12 [0.98 – 1.29] | 1.07 [0.93 – 1.23] | 0.98 [0.84 – 1.13] | 118/879 | 1.26 [1.05 – 1.50] | 1.17 [0.98 – 1.41] | 0.96 [0.77 – 1.20] |
| Combined HF/CHD/Death/LVH | 341/737 | 1.10 [0.99 – 1.22] | 1.05 [0.94 – 1.17] | 1.01† [0.83 – 1.23] | 280/879 | 1.49 [1.31 – 1.69] | 1.42 [1.25 – 1.62] | 1.33† [1.03 – 1.74] |
Fully adjusted models are adjusted by age, BMI, prevalent hypertension and diabetes, systolic blood pressure, and smoking status at the time of the polysomnography and the time of the echocardiography.
The full adjusted model for the composite outcome of HF/CHD/Death/LVH also adjusted for the use of statins, beta-blockers, RAS inhibitors and mineralocorticoid receptor antagonists at both polysolmnography and echocardiography, and for self-reported CPAP use at the time of the echocardiography.
Figure 2.
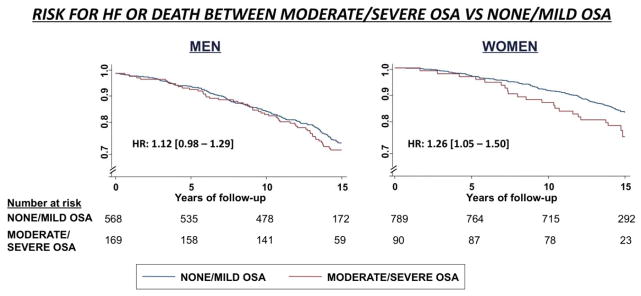
Kaplan-Meier survival curves for the risk of HF or death of moderate/severe OSA vs none/mild OSA stratify by sex. Shown hazard ratios are showing the linear unadjusted association among all OSA categories.
Among surviving subjects free of CVD –including HF – at the initiation of the fifth ARIC visit in 2011, 371 men and 535 women underwent echocardiography (Supplemental Figure 1). The associations between OSA severity and echocardiographic measurements at Visit 5 for men and women are shown in Supplemental Table 6. In women, OSA severity was independently associated with higher LVMI while in men this association was not significant after adjustment for potential confounders (Figure 3). OSA severity was not associated with impairments in LV function, RV size, or RV function in either men or women at Visit 5. In women, but not men, OSA severity was associated with the composite outcome of incident HF, CHD, death, or LV hypertrophy (p<0.0001 and p=0.08, respectively), even in fully adjusted models (Table 2; p=0.03 and p=0.93, respectively). Consistent results were found when the analysis was repeated excluding participants with missing information for any covariate (n=2; data not shown).
Figure 3.
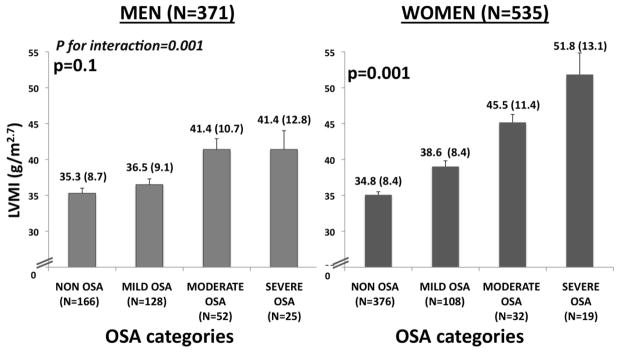
Left ventricular mass index (LVMI) among OSA categories stratified by sex. P values are based in multivariable linear regression adjusted by age, BMI, hypertension, diabetes, smoking status, systolic blood pressure, and self-reported use of statins, beta-blockers, angiotensin-blockers, or mineralocorticoid blockers assessed at both time points (the polysomnography and the echocardiography times) and by self-reported use of CPAP at the time of the echocardiography.
Discussion
Although sex differences for CVD risk factors have been well-described,4,10 there have been inconsistent data regarding the relationship of OSA to CV outcomes in men as compared to women.25 This study confirms prior research that has shown that compared to women, men have a higher prevalence of OSA and hypertension, as well higher levels of hs-TnT, a biomarker of subclinical myocardial injury. However, in this cohort of middle aged community dwelling individuals without prevalent cardiovascular disease, we demonstrate that greater OSA severity is more strongly positively associated with higher hs-TnT in women compared to men, and this relationship remained robust after adjusting for multiple potential confounders including BMI and metabolic variables in women but not men. We further report the novel finding that after 13.6±3.2 years of follow-up, OSA severity was associated with incident HF in women but not men, and this was partially accounted for by hsTnT level. Furthermore, among surviving participants without prevalent CVD, OSA assessed in mid-life was significantly and independently associated with LV hypertrophy in women but not men, additionally supporting an adverse effect of OSA on cardiovascular risk in middle-aged women.
Elevated hs-TnT is a powerful risk factor for incident HF,18 and we have previously shown that while this association exists among all OSA categories, it is strongest in persons with severe OSA.5 Therefore, our finding of an independent cross-sectional association between OSA severity and hs-TnT level among women but not men suggests that, in mid-life, the risk of HF associated with OSA would be higher in women compared to men. Indeed, in time-to-event analysis, OSA severity was associated with incident HF or death among women but not men, and hs-TnT level appeared to account for nearly one-third of this association. We did not observe an association between OSA severity and HF risk among women independent of other confounders, possibly due to lack of power as a result of the small proportion of women in the most severe OSA categories (only 3.6% with severe OSA) and lower overall event rates. Indeed, among participants without an incident CV event at the end of follow-up, in women but not men OSA severity was independently associated with higher LVMI and LVH, an important intermediate phenotype for HF.26 Similarly, among women, but not men, OSA severity was robustly and independently associated with the composite of mortality, incident cardiovascular disease (HF or CHD), or LVH at the end of follow-up even after full multivariable adjustment.
Existing data regarding the impact of sex on the relationship between OSA and cardiovascular outcomes is controversial. Although some data suggest that women may experience a greater risk of death associated with OSA compared to men,25,27 findings from the overall SHHS suggest that OSA severity may be associated more strongly with mortality10 and incident HF4 in men compared to women. However, this data was based on several distinct cohorts which varied greatly in age; overall, the combined sample was younger and experienced lower overall event rates as compared to the sample in ARIC.10
The stronger associations we observed with OSA among women compared to men may reflect sex differences in the pathobiology of OSA, differences in the response to cardiovascular stressors, and/or differences in the contribution of OSA to myocardial injury, hypertrophy, and clinical events relative to other cardiovascular comorbidities. Women are more likely to have REM-dominant OSA, the sleep state when sympathetic activity is highest and nocturnal ischemia may be greatest.28 Indeed women demonstrate a greater heart rate response associated with arousals compared to men.29 Additionally, women with OSA may display greater endothelial dysfunction than men with OSA.30 Cardiac adaptations to hemodynamic stress also appear to differ by sex. Women with OSA demonstrate a higher propensity to develop pulmonary hypertension.31 While the cross-sectional association between prevalent hypertension and OSA appears stronger in men than women, which may reflect greater duration of exposure to OSA among men,32 the prospective association of OSA with incident hypertension may be stronger in women than in men, as shown in a sub-analysis of the SHHS data.33 Importantly, women demonstrate a greater left ventricular hypertrophic response to pressure overload due to systemic hypertension,34 a pattern of cardiac remodeling that has been associated with detectable high sensitivity troponin.35
Sex differences in time of development of OSA may also contribute to the observed sex-based differences. Men develop OSA earlier in life, while women tend to develop OSA after menopause.9 Hs-TnT may be an earlier and more sensitive biomarker of cardiac injury by OSA than LVH. After an initial stage in which hs-TnT is elevated, the degree of cardiac ischemic injury in response to the intermittent hypoxemia and sympathetic nervous system surges of OSA may enhance the underlying collateral circulation and mitigate the OSA-associated hs-TnT elevation.36 Indeed, in men, this may happen earlier in life which would be consistent with the observation of an association between OSA and high sensitive troponin I in a younger cohort with an larger proportion of men, although they did not perform a sex-specific analyses.37 Although speculative, this early presentation combined with a longer lifetime exposure to OSA may therefore provide some protection from OSA related injury. Finally, survival bias may influence the observed associations of mid-life OSA with late-life LV hypertrophy but would not be expected to influence the association of OSA with incident cardiovascular events.
The association between OSA severity and other measurements of cardiac structure and function is controversial, with some - but not all - studies describing associations with LV diastolic function6,7,8 and RV hypertrophy.8 Measures of RV hypertrophy were not available in our study and the high prevalence of diastolic dysfunction among the elderly may have limited our ability to detect an association with mid-life OSA severity. In addition, many existing studies had relatively small sample size, and differences in study populations with respect to OSA-associated comorbidities may contribute to the differences among the studies’ results. Most of these studies were cross-sectional in design, with OSA assessed coincident with echocardiography. Population survivor selection during the follow-up period between OSA assessment and echocardiography in our study may have biased our analysis toward the null.
Several limitations of this analysis should be noted. Ninety-nine percent of our study population was white and we were, therefore, not able to study any race-specific differences. In addition, our results may not be generalizable to other populations. Our analysis with hs-TnT was cross-sectional in design and precludes conclusions regarding causality. While multiple additive multivariable models were employed in an attempt to optimally control for potential confounding of the relationship between OSA severity and hs-TnT levels, residual confounding cannot be excluded. Conversely, many potential confounders may also act as mediators between OSA and hs-TnT level, potentially leading to greater Type 2 error in our additive multivariable models. Hs-TnT was not measured coincident with polysomnography, with a median difference between the two measurements of 172 days [interquartile range −51 to 387 days]). However, prior studies suggest that sleep apnea classification remains largely stable over several months to years.38,39 Additionally, a sensitivity analysis restricting the population to those with sleep variables and soluble biomarkers ascertained within 1 year of each other demonstrated concordant results with the primary analysis. Although we cannot exclude the possibility that changes in OSA status over the time biased the associations with echocardiographic findings, the association between OSA and LVMI remained robust after all adjustments. Furthermore, the higher mortality rates in observed in more severe OSA categories would have biased our findings towards the null. The follow-up analysis was limited by the low number of events among the highest OSA categories, especially among women, limiting our power to find an independent association. However, we included LV abnormal remodeling as a surrogate of CV outcome due to the strong association between LV hypertrophy and risk of HF and mortality. Finally, survivor bias may be one explanation for the lack of association between OSA and LVMI in men. Limited data were available on the use of CPAP, and we were unable to account for the potential impact of CPAP use on the sex-specific relationship between OSA and clinical outcomes. However, no participants were using CPAP at the time of polysolmnography and therefore CPAP use did not confound our analysis of sex differences in the association of OSA with hs-TnT. Additionally, we were able account for self-reported CPAP use in the analysis of sex-based differences in the association of OSA with cardiac structure and function.
Conclusions
Important sex-based differences exist in the association between OSA and CV disease. To date, the role of OSA as a cardiovascular risk factor in women has been unclear. This study provides evidence from multiple convergent and complementary analyses-including analysis of a strong biomarker for cardiovascular disease, longitudinal data on incident HF and death, and prospectively obtained echocardiographic data- that support a significant role of OSA in contributing to incident CVD and cardiac remodeling in women, and in fact, suggests that these associations are stronger than those observed in middle aged to elderly male community dwelling individuals without prevalent cardiovascular disease. Our finding of a stronger association of OSA with cardiovascular disease in women highlights the importance of screening for OSA in women as well as men.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources: This work was supported by National Heart, Lung, and Blood Institute cooperative agreements NHLBI-HC-11-08 (Brigham and Women’s Hospital), U01HL53940 (University of Washington), U01HL63463 (Brigham and Women’s Hospital), and U01HL53934 (University of Minnesota). Dr. Shah is supported in part by K08-HL-116792 and a grant from the American Heart Association (14CRP20380422). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Disclosures: Dr. Redline reports research support from ResMed Inc and ResMed Foundation. Dr. Ballantyne reports research support from Roche. Dr Shah reports research support from Novartis, Gilead, and Actelion. The remaining authors report no relevant financial conflicts.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Quintana-Gallego E, Carmon-Bernal C, Capote F, Sánchez-Armengol A, Botebol-Benhamou G, Polo-Padillo, Castillo-Gomez J. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb D, Yenokyan G, Newman A, O’Connor G, Punjabi N, Quan S, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective Study of Obstructie Sleep Apnea and Incident Coronary Heart Disease and Heart Failure. The Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Querejeta Roca G, Redline S, Clagget B, Punjabi N, Ballantyne CM, Solomon SD, Shah AM. Sleep apnea is associated with subclinical myocardial injury in the community. Am J Respir Crit Care Med. 2013;188:1460–5. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chami HA, Devereux RB, Gottdiener JS, Mehra R, Roman MJ, Benjamin EJ, Gottlieb DJ. Left ventricular morphology and systolic function in sleep-disordered breathing: The Sleep Heart Health. Circulation. 2008;117:2599–2607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niroumand M, Kupperstein R, Sasson Z, Hanly P. Impact of obstructive sleep apnea in left ventricular mass and diastolic function. Am J Resp Crit Care Med. 2001;163:1632–6. doi: 10.1164/ajrccm.163.7.2007014. [DOI] [PubMed] [Google Scholar]
- 8.Guidry UC, Mendes LA, Evans JC, Levy D, O’Connor GT, Larson MG, Gottlieb DJ, Benjamin EJ. Echocardiographic features of the right heart in the sleep-disordered breathing. Am J Resp Crit Care Med. 2001;164:933–8. doi: 10.1164/ajrccm.164.6.2001092. [DOI] [PubMed] [Google Scholar]
- 9.Lin C, Davidson T. Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–96. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Saunders J, Nambi V, de Lemos J, Chambles L, Virani S, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac Troponin T Measured by a Highly Sensitive Assay Predicts Coronary Heart Disease, Heart Failure, and Mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet SM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 14.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of Coronary Heart Disease Incidence with Carotid Arterial Wall Thickness and Major Risk Factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 16.Redline S, Sanders MH, Lind BK, Quan SF, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley P. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 17.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 18.Saunders J, Nambi V, de Lemos J, Chambles L, Virani S, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac Troponin T Measured by a Highly Sensitive Assay Predicts Coronary Heart Disease, Heart Failure, and Mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities study. Circulation Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flaschkampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Rudski LG, Lai WW, Afilalo J, Hua L, Hanschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB1. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Keller T, Ojeda F, Zeller T, Wild PS, Tzikas S, Sinning CR, Peetz D, Münzel T, Blankenberg S, Lackner KJ. Defining a reference population to determine the 99th percentile of a contemporary sensitive cardiac troponin I assay. Int J Cardiol. 2013;167:1423–9. doi: 10.1016/j.ijcard.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Eggers KM, Jaffe AS, Lind L, Venge P, Lindahl B. Value of Cardiac Troponin I Cutoff Concentrations below the 99th Percentile for Clinical Decision-Making. Clin Chem. 2008;55:85–92. doi: 10.1373/clinchem.2007.101683. [DOI] [PubMed] [Google Scholar]
- 24.Wilks S. The large-sample distribution of the likelihood ratio for testing composite hypotheses. Ann Math Stat. 1938:60–62. [Google Scholar]
- 25.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53 (Suppl 3):S16–9. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse Left Ventricular Remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail. 2014;2:512–22. doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 28.Koo BB. Cerebral response to obstructive apnea: the times they are a-changin’. Sleep Breath. 2012;16:269–270. doi: 10.1007/s11325-011-0533-x. [DOI] [PubMed] [Google Scholar]
- 29.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, Fogel RB, Malhotra A, White DP. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep in humans. J Physil. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–1120. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 31.Minai OA, Ricaurte B, Kaw R, Hammel J, Mansour M, McCarthy K, Golish JA, Stoller JK. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300–1306. doi: 10.1016/j.amjcard.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JE, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport D, Redline S, Resnick HE, Samet J, Shahar E. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. AJRCCJ. 2009;179:1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 35.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of Troponin T Detected With a Highly Sensitive Assay and Cardiac Structure and Mortality Risk in the General Population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steiner S, Schueller PO, Schulze V, Strauer BE. Occurrence of Coronary Collateral Vessels in Patients With Sleep Apnea and Total Coronary Occlusion. Chest. 2010;137:516–520. doi: 10.1378/chest.09-1136. [DOI] [PubMed] [Google Scholar]
- 37.Einvik G1, Røsjø H1, Randby A1, Namtvedt SK1, Hrubos-Strøm H2, Brynildsen J1, Somers VK, Omland T. Severity of obstructive sleep apnea is associated with cardiac troponin I concentrations in a community-based sample: data from the Akershus Sleep Apnea Project. Sleep. 2014;37:1111–6. doi: 10.5665/sleep.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, Redline S, Sanders M, Young T for the Sleep Heart Health Study research group. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography--the Sleep Heart Health Study. [corrected] Sleep. 2002;25:843–849. [PubMed] [Google Scholar]
- 39.Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.