Summary
Centriole duplication is coordinated such that a single round of duplication occurs during each cell cycle. Disruption of this synchrony causes defects including supernumerary centrosomes in cancer and perturbed ciliary signaling [1–5]. To preserve the normal number of centrioles, the level, localization, and post-translational modification of centriole proteins is regulated so that when centriole protein expression and/or activity is increased, centrioles self-assemble. Assembly is initiated by the formation of the cartwheel structure that comprises the base of centrioles [6–11]. SAS-6 constitutes the cartwheel and SAS-6 levels remain low until centriole assembly is initiated at S-phase onset [3, 12, 13]. Cep135 physically links to SAS-6 near the site of microtubule nucleation and binds to CPAP for triplet microtubule formation [13, 14]. We identify two distinct protein isoforms of Cep135 that antagonize each other to modulate centriole duplication: full length Cep135 (Cep135full) promotes new assembly while a short isoform, Cep135mini, represses it. Cep135mini represses centriole duplication by limiting the centriolar localization of Cep135full binding proteins (SAS-6 and CPAP) and the pericentriolar localization of γ-tubulin. The Cep135 isoforms exhibit distinct and complementary centrosomal localization during the cell cycle. Cep135mini protein decreases from centrosomes upon anaphase onset. We suggest that the decrease in Cep135mini from centrosomes promotes centriole assembly. The repression of centriole duplication by a splice isoform of a protein that normally promotes it serves as a novel mechanism to limit centriole duplication.
Keywords: centriole, microtubule, centrosome, Cep135, alternative splicing
Results and Discussion
Cep135 is a microcephaly associated (MCPH8), cartwheel protein that promotes centriole assembly and stability [9, 13, 15–19]. The protein contains a coiled-coil domain and conserved regions near the N-terminus and the C-terminus, respectively (Figure 1A). The Cep135 N-terminus binds to microtubules and CPAP [20, 21]. The Cep135 C-terminus binds to the cartwheel protein SAS-6 and maintains it at the cartwheel in Chlamydomonas [22]. Thus, Cep135 links the cartwheel with centriole triplet microtubule assembly, an early event in centriole biogenesis [13]. How Cep135 activity is regulated remains unclear [3].
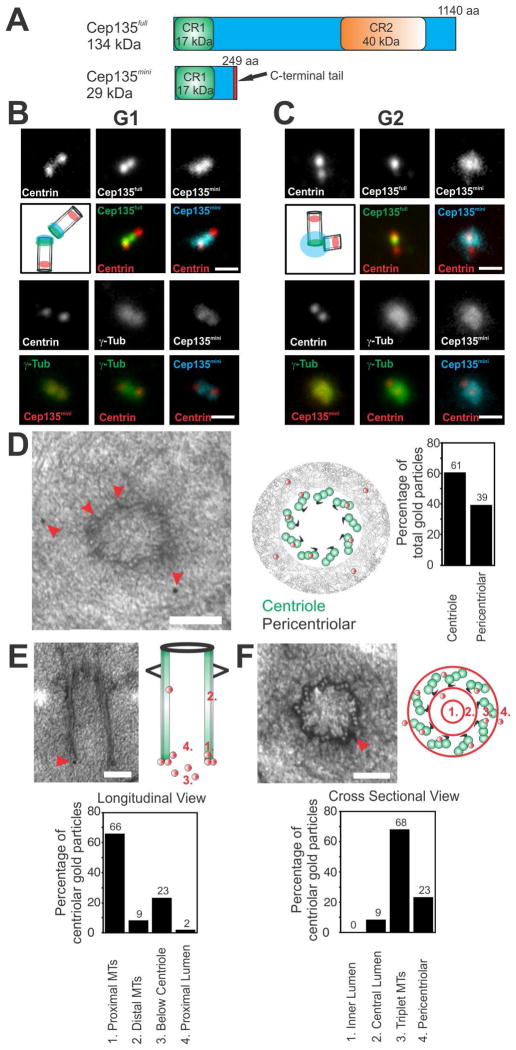
Figure 1. Cep135 isoforms exhibit unique localization patterns.
(A) Cep135 protein isoforms. CR, conserved region. Red, C-terminal tail is divergent from Cep135full. (B) Localization of Centrin (α-Centrin, red), γ-tubulin (α-γ-Tub; green), Cep135full (α-Cep135full, green), and Cep135mini (Alexa488-α-Cep135mini, cyan and red) during G1. Cep135full and Cep135mini localize to the proximal end of G1 centrioles. Scale bar, 1.0 μm. (C) During G2, Cep135mini localizes to centrioles and PCM. Scale bar, 1.0 μm. (D) Immuno-EM localization of Cep135mini to the centriolar microtubules and the PCM (n=152 gold particles for 33 centrioles). (E and F) Cep135mini localizes to the proximal centriole triplet microtubules. (n=47 gold particles). (D–F) The relative distribution of gold particles was quantified for localization to the centrioles and the PCM, centriolar longitudinal sections, and centriolar cross sections, respectively. Red arrowheads denote gold localization. Scale bar, 100 nm.
A short isoform of Cep135 localizes to centrioles and the PCM
We identified an alternative splice isoform of human Cep135 (Figures 1A and S1A; Cep135mini). Intron 5 is retained in the mRNA of this short isoform causing translation read-through into the intron thereby adding 16 amino acids followed by a stop codon to generate Cep135mini. The 29 kDa Cep135mini isoform contains the Cep135 N-terminus required for microtubule binding but lacks the predicted CPAP and SAS-6 binding domains found in the 134 kDa Cep135full isoform [13, 16]. Both Cep135full and Cep135mini transcripts are detected in U2OS, RPE1, and HeLa cells (Figure S1B; data not shown).
Affinity purified antibodies that specifically recognize the Cep135mini isoform were generated using two peptides containing amino acid sequences specific to Cep135mini’s divergent C-terminus (Figures 1, S1). Cep135mini localizes to centrosomes in RPE1, U2OS, and HeLa cells (Figures 1B–F, S1; data not shown). Similar localization is found in cells expressing fluorescent protein fusions to Cep135mini (Figure S1I). Cep135mini’s localization pattern changes through the cell cycle. During G1-phase of the cell cycle, Cep135mini localizes predominantly to the proximal-end of centrioles but is slightly spread compared to Cep135full (Figures 1B, S4C–F), while in G2 it localizes both to centrioles and to the pericentriolar material (PCM) as judged by co-staining with γ-tubulin (Figures 1B, C and S4D–F). Cep135full localization to the PCM was not observed in either G1 or G2 (Figures 1B, C and S1I) [16, 20, 23–25]. Immuno-EM localization also places Cep135mini at the proximal end of the centriole co-incident with the triplet microtubules and the PCM (Figures 1D–F). Thus, the Cep135 protein isoforms exhibit distinct localization patterns at the centrosome.
Cep135full and Cep135mini have opposing functions in centriole duplication
The unique localization patterns of the two Cep135 isoforms suggest that Cep135full and Cep135mini have distinct functions. To determine how Cep135full and Cep135mini expression impacts centriole duplication, we knocked down or over expressed these isoforms in U2OS cells and quantified the frequency of centriole over- and under-duplication [26]. Cep135full knockdown significantly increases the number of cells with under-duplicated centrioles (Figures 2A, B) while Cep135full over expression increases the number of cells with over-duplicated centrioles (Figure 2C). These results confirm prior studies showing that Cep135full promotes centriole assembly and that its levels must be controlled to limit centriole over-duplication [9, 13]. Conversely, Cep135mini knockdown increases the number of cells with over-duplicated centrioles (Figure 2A, B) while over expression of Cep135mini causes an increase in cells with under-duplicated centrioles (Figure 2C). These results suggest that Cep135mini represses centriole assembly. Cep135mini knockdown was confirmed by measuring transcript and protein levels (Figure S1F,H and S2B,C). All phenotypes were rescued by exogenous expression of siRNA impervious mutants (Figure S2D). Finally, the Cep135full and Cep135mini knockdown had a minimal impact on the cell cycle (Figure S2E); suggesting, the phenotypes are not due to changes in the cell cycle or in aberrant cell divisions. We did observe a low level of multipolar mitoses upon Cep135mini knockdown as is expected with centrosome amplification (data not shown). Together, the knockdown and over expression experiments support a model in which Cep135mini, in contrast to Cep135full, negatively regulates centriole and centrosome duplication.
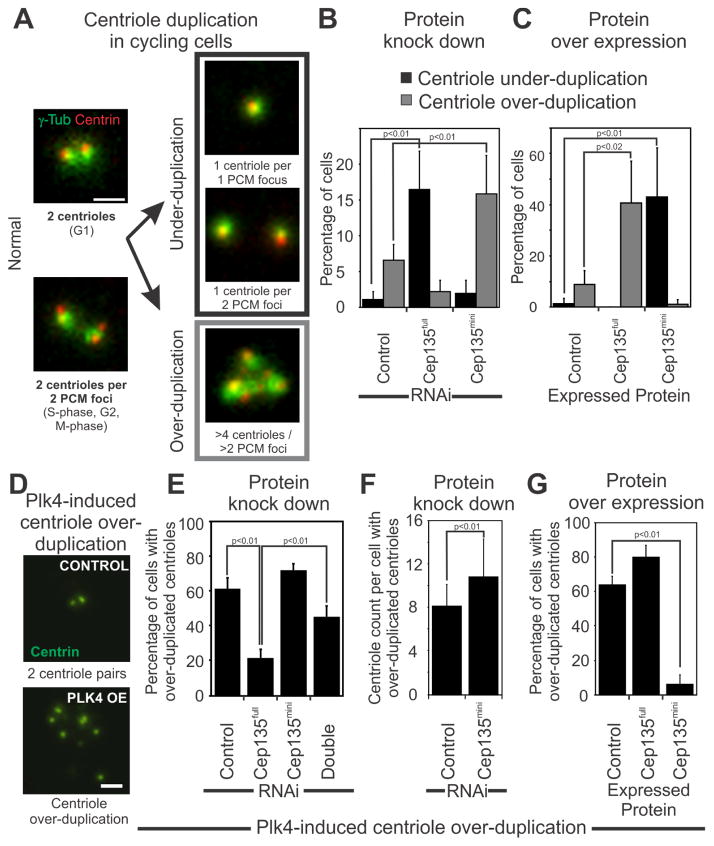
Figure 2. Cep135mini inhibits centriole duplication.
(A) Centrioles (Centrin, red) are visualized relative to PCM (γ-tubulin, green) to quantify centriole under- and over-duplication in cycling U2OS cells. Normal centriole number is characterized as having either two centrioles per PCM focus or closely positioned foci during G1 or two centrioles per PCM focus during S-, G2-, and M-phase of the cell cycle. Scale bar, 1 μm. (B) Cep135full and Cep135mini depletion inhibits and promotes centriole duplication, respectively. Cep135full knockdown causes an increase in cells with under-duplicated centrioles (1±1% versus 16±6%). Cep135mini knockdown causes an increase in centriole over-duplication (7±2% versus 16±5%). Mean±SD represent five separate experiments for >500 cells for each condition. (C) Exogenous Cep135full and Cep135mini over expression induces an increase in centriole over-duplication (9±5% versus 41±16%) and an increase in centriole under-duplication (2±2% versus 43±19%), respectively. Mean±SD represents three separate experiments. (D) Plk4 over expression causes centriole amplification (Centrin-GFP, green) in 61±6% of S-phase arrested RPE1 cells. Scale bar, 1 μm. (E) Cep135full knockdown reduces the number of cells with amplified centrioles (61±6% versus 22±5%) while Cep135mini knockdown marginally increases the number of cells with amplified centrioles (61±6% versus 72±4%). Knockdown of both isoforms returned the cells to near control levels of centriole over-duplication (45±6%). (F) Cep135mini depletion increases the number of centrioles per cell (8±2 versus 11±4 centrioles per cell). (G) Over expression of Cep135full causes a moderate increase in the number of cells with amplified centrioles (64±5% versus 80±7%) while Cep135mini over expression represses centriole amplification (64±5% versus 6±6%). Mean±SD represents three separate experiments.
To determine whether Cep135full and Cep135mini perturbations affect centriole duplication independent of the cell cycle, we next examined whether these isoforms modulate aberrant Plk4-induced centriole amplification in S-phase arrested RPE1 cells [8–11]. 61% of control cells that over express Plk4 over-duplicate their centrioles (Figures 2D, E). Cep135full knockdown blocks Plk4-induced centriole over-duplication (Figures 2E) while Cep135full over expression moderately augments centriole over-duplication (Figure 2G, [9, 13]). In contrast, Cep135mini knockdown moderately increases over-duplication and also increases the total number of centrioles per cell (Figure 2F), while Cep135mini over expression dramatically reduces the number of cells with over-duplicated centrioles (Figure 2G). Concurrent knockdown of both isoforms partially rescues the loss in centriole over-duplication that is seen in Cep135full only knockdown (Figure 2E). A nearly identical trend was observed in S-phase arrested U2OS cells that normally over-duplicate their centrioles (Figure S2A). Collectively, these results suggest that the balance between the Cep135 isoforms is important for the normal homeostasis of centriole numbers and that Cep135mini counteracts Cep135full function in promoting centriole duplication.
Cep135mini expression limits the centriolar levels of essential assembly factors
The negative effect of Cep135mini on centriole duplication led us to ask whether Cep135mini disrupts the ability of Cep135full to promote centriole duplication. A simple explanation is that Cep135mini functions as a dominant negative molecule to remove Cep135full from the centriole. Over expression of either Cep135 isoform does not disrupt the localization of the other (Figure S3A) suggesting that Cep135full is able to localize to centrioles even when Cep135mini levels are high.
An alternative possibility is that over expressed Cep135mini disrupts centriole duplication by binding to Cep135full and precluding its association with its binding partners (SAS-6 and CPAP). To explore the potential interaction between the Cep135 isoforms, we took advantage of the ectopic cytoplasmic foci that Cep135 forms upon over expression [21, 27]. Such foci are presumably the result of oligomerization of Cep135 protein. When both isoforms are expressed in RPE1 cells, ectopic foci that are not associated with centrosomes contain both Cep135full and Cep135mini. This suggests that Cep135mini is competent to associate with Cep135full (Figure S3B).
We next asked whether Cep135mini affects the localization of Cep135full binding proteins that function in centriole duplication (SAS-6 and CPAP). SAS-6 dependent cartwheel assembly initiates centriole biogenesis [12, 28–30]. Over expression of Cep135full and Cep135mini decreases SAS-6 levels at centrioles by 48% and 44%, respectively (Figure 3A). The reduced SAS-6 levels are consistent with the loss of SAS-6 from centrioles in Chlamydomonas bld10 (Cep135) mutants and suggests that the interplay between SAS-6 and Cep135full is important for new centriole biogenesis [21, 22]. Overexpressed Cep135full protein binds SAS-6 and might sequester SAS-6 from the centriole (Figure S3C). In contrast, we speculate that Cep135mini, which only weakly and variably interacts with SAS-6, promotes a cartwheel conformation that disrupts SAS-6 localization to the centriole (Figure S3C). We next assessed whether the centriolar localization of the Cep135full binding protein and centriole duplication factor, CPAP, is affected by Cep135 isoform levels [9, 21, 31]. Cep135full over expression modestly reduces centriolar CPAP localization (Figure 3B), which we predict is because expressed Cep135full binds to and sequesters CPAP to the cytoplasm (Figure S3D). Conversely, Cep135mini, which does not interact with CPAP, exhibits a potent inhibitory effect on CPAP localization (Figure S3D). This may allow Cep135mini to selectively inhibit centriole duplication. Collectively, our data suggest that Cep135mini prevents centriole duplication by limiting Cep135full-interacting, centriole assembly factors from associating with the centriole.
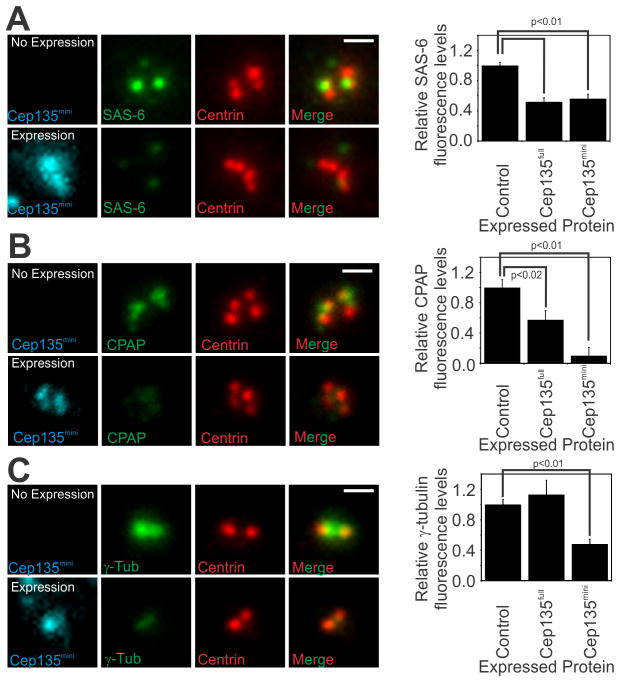
Figure 3. Cep135mini expression displaces SAS-6, CPAP, and γ-tubulin from centrioles and centrosomes.
(A) Exogenous Cep135full and Cep135mini expression in S-phase arrested RPE1 cells decreases SAS-6 localization to centrioles. Exogenous Cep135full and Cep135mini expression causes a 48% and 44% decrease in SAS-6 levels, respectively. Upper panels depict SAS-6 (green) in non-transfected cells and lower panels show SAS-6 in Cep135mini transfected cells. Centrin (red) levels were not affected by expression of either protein. (B) Cep135mini expression in S-phase arrested RPE1 cells decreases CPAP localization to centrioles. Cep135mini expression causes a 90% decrease in CPAP levels. Cep135full expression causes an intermediate 43% decrease in CPAP levels. Upper panels depict CPAP (green) in non-transfected cells and lower panels show CPAP in Cep135mini transfected cells. (C) Exogenous Cep135mini, but not Cep135full, expression in cycling RPE1 cells causes a 52% decrease in γ-tubulin levels. Upper panels depict γ-tubulin (green) in non-transfected cells and lower panels show γ-tubulin in Cep135mini transfected cells. (A–C) Mean±SEM represents at least three separate experiments. Scale bar, 1 μm.
The PCM network of proteins surrounding centrioles is organized into distinct functional domains [32–35]. The PCM is required for centriole biogenesis and CPAP is important for PCM organization [28, 36–38]. The PCM establishes a nucleation domain from which nascent centrioles are built, beginning with the cartwheel [32–35]. Over expression of Cep135mini, but not Cep135full, causes a 56% decrease in γ-tubulin from the PCM (Figure 3C). Thus, Cep135mini over expression causes loss of both centriolar (SAS-6 and CPAP) and PCM (γ-tubulin) components suggesting that it regulates multiple facets of centriole and centrosome biogenesis.
Cep135 isoforms localize in a cell cycle dependent manner
The antagonistic functions of the Cep135 isoforms suggests that their centriolar levels and localization are modulated through the cell cycle to promote centriole assembly only during G1/S-phase of the cell cycle. To test this, we staged cycling cells and examined the centriolar level of the Cep135 isoforms. Cep135full levels are high during centriole duplication at the G1/S-phase boundary (Figure 4A). Effectively one half of the total Cep135full protein is predicted to be associated with each G1 centriole or new centriole pair following S-phase. These levels increase by 15% through metaphase of mitosis (Figure 4A) and then increase further (11%) by telophase (Figure 4C). Consistent with a role for Cep135mini in repressing centriole assembly, Cep135mini protein levels are lowest during G1/S when centrioles duplicate. After centriole duplication, the centriolar levels of Cep135mini increase and peak at metaphase of mitosis. Because Cep135mini over expression decreases γ-tubulin levels at the centrosome (Figure 3C), the high mitotic Cep135mini levels are inconsistent with the high γ-tubulin levels found at mitotic centrosomes [39]. Perhaps the Cep135mini over expression results represents non-physiological Cep135mini activity. Alternatively, Cep135mini may normally control γ-tubulin recruitment to the centrosome. In summary, at G1/S, Cep135full levels are high and Cep135mini levels are low to promote new centriole biogenesis while Cep135mini increases through the remainder of the cell cycle until metaphase, perhaps to repress promiscuous centriole duplication and PCM expansion.
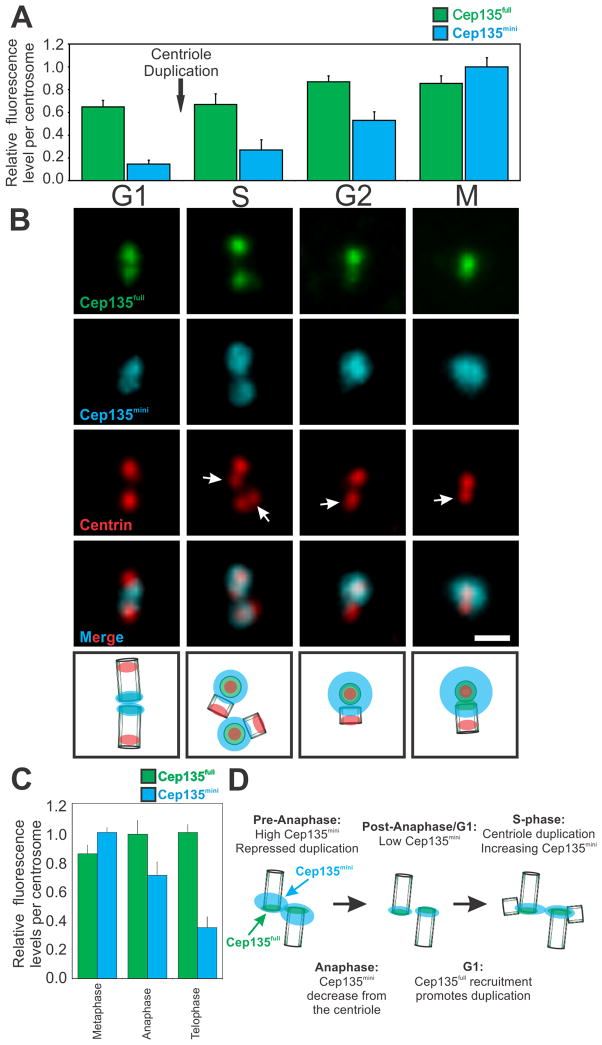
Figure 4. Cep135mini levels are controlled through the cell cycle.
(A) Cep135full (green) and Cep135mini (cyan) exhibit differential protein levels at centrioles and centrosomes through the cell cycle. Normalized fluorescence levels were quantified per centrosome (single G1 centriole or S phase, G2, or M centriole pair. The fluorescence of the two G1 centrioles and four S-phase centrioles (two centriole pairs) was normalized by halving the total fluorescence. Mean±SEM represents three separate experiments of >20 cells per condition. (B) Cep135full (α-Cep135full, green) and Cep135mini (Alexa488 labeled α-Cep135mini, cyan) exhibit distinct and dynamic localization relative to centrioles (Centrin, red). Scale bar, 1 μm. (C) Cep135full levels slightly increase during anaphase and telophase while Cep135mini levels drop sharply at anaphase and telophase. Mean±SEM of three separate experiments of >20 cells per condition. (D) Model of Cep135 isoform regulation of centriole duplication.
Centrin localizes to the distal end of centrioles, allowing us to determine the sub-centrosomal localization of the Cep135 isoforms during the cell cycle (Figures 4B and S4C; [40]). As expected, Cep135full localizes at the proximal end of the two centrioles during G1 and at the mother centrioles during S-phase. Consistent with previous reports, Cep135full is not detectable at daughter centrioles until G2 [35]. Maturation of the daughter centriole is accompanied by a slow accumulation of Cep135full so that protein levels are once again high by the G1/S-phase boundary of the following cell cycle. Additionally, we propose that Cep135full at the mother centriole is important for nucleation of the daughter centriole as is shown for SAS-6 [41, 42].
Unlike Cep135full, Cep135mini localizes to the proximal end of the centriole during G1 and, upon progression into the cell cycle, becomes increasingly associated with the PCM (Figures 4B and S4D–F). This redistribution of Cep135mini to the PCM may position Cep135mini to suppress Cep135full-dependent centriole biogenesis by limiting Cep135full’s association with its binding partners. However, the function of the unique localization between the two Cep135 isoforms remains to be discovered. Upon transit from metaphase into the subsequent G1, Cep135mini levels drop. Consistent with the Cep135mini protein decrease, Cep135mini transcript levels also decrease during mitosis (Figure S4A). We suggest this decrease permits centriole assembly.
Conclusions
We discovered a Cep135 splice isoform, Cep135mini, which limits centriole duplication (Figure 4D). Cep135mini is a centrosome localized protein whose levels are regulated through the cell cycle and play an inhibitory role in centriole assembly. Following centriole duplication, Cep135mini levels accumulate to restrict centriole duplication. By surrounding the Cep135full-containing mother centriole, Cep135mini might block the assembly of new centrioles by limiting the ability of SAS-6, CPAP, and the PCM (γ-tubulin) to promote centriole biogenesis. The negative regulation of centriole duplication by a splice isoform of a protein that normally promotes it is a potent and novel mechanism to limit centriole duplication.
Supplementary Material
Acknowledgments
We thank Christina Clarissa and Courtney Ozzello for immuno-EM imaging expertise, Dr. Iain Cheeseman for vectors, Dr. Brian Tsou for the Plk4 overexpressing cells and discussions, Dr. Tang K. Tang for α-Cep135full antibodies and GFP constructs and Dr. Pierre Gönczy for GFP constructs. Thank you to Drs. Lee Niswander, Alex Stemm-Wolf, Jennifer Deluca, Brian Mitchell, Richard Davis, Mark Winey, and Debbie Klos-Derhing for comments and helpful discussions on the manuscript. CGP is supported by NIGMS (GM099820), the Pew Biomedical Scholars Program, and the Boettcher Foundation.
Abbreviations
- IEM
immuno-electron microscopy
- PCM
pericentriolar material
- TEM
transmission electron microscopy
Footnotes
Supplemental information includes four figures and Supplemental Experimental Procedures.
Author Contributions
KDD designed and performed experiments, DGS, BAB, MEP, LRH, and THG performed experiments, DFG designed and produced macros for image analysis, and CGP designed and performed experiments and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PloS one. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonczy P. Towards a molecular architecture of centriole assembly. Nature reviews Molecular cell biology. 2012;13:425–435. doi: 10.1038/nrm3373. [DOI] [PubMed] [Google Scholar]
- 4.Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godinho SA, Picone R, Burute M, Dagher R, Su Y, Leung CT, Polyak K, Brugge JS, Thery M, Pellman D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature. 2014;510:167–171. doi: 10.1038/nature13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigg EA. Centrosome duplication: of rules and licenses. Trends in cell biology. 2007;17:215–221. doi: 10.1016/j.tcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nature cell biology. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nature cell biology. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 9.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Developmental cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Pearson CG, Winey M. Plk4/SAK/ZYG-1 in the regulation of centriole duplication. F1000 biology reports. 2010;2:58. doi: 10.3410/B2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P. Regulated HsSAS-6 Levels Ensure Formation of a Single Procentriole per Centriole during the Centrosome Duplication Cycle. Developmental cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YC, Chang CW, Hsu WB, Tang CJ, Lin YN, Chou EJ, Wu CT, Tang TK. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. The EMBO journal. 2013 doi: 10.1038/emboj.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatzopoulos GN, Erat MC, Cutts E, Rogala KB, Slater LM, Stansfeld PJ, Vakonakis I. Structural analysis of the G-box domain of the microcephaly protein CPAP suggests a role in centriole architecture. Structure. 2013;21:2069–2077. doi: 10.1016/j.str.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayless BA, Giddings TH, Jr, Winey M, Pearson CG. Bld10/Cep135 stabilizes basal bodies to resist cilia-generated forces. Molecular biology of the cell. 2012;23:4820–4832. doi: 10.1091/mbc.E12-08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, Bettencourt-Dias M. Stepwise evolution of the centriole-assembly pathway. Journal of cell science. 2010;123:1414–1426. doi: 10.1242/jcs.064931. [DOI] [PubMed] [Google Scholar]
- 17.Hiraki M, Nakazawa Y, Kamiya R, Hirono M. Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr Biol. 2007;17:1778–1783. doi: 10.1016/j.cub.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Jerka-Dziadosz M, Gogendeau D, Klotz C, Cohen J, Beisson J, Koll F. Basal body duplication in Paramecium: the key role of Bld10 in assembly and stability of the cartwheel. Cytoskeleton (Hoboken, N J. 2010;67:161–171. doi: 10.1002/cm.20433. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura K, Lefebvre PA, Kamiya R, Hirono M. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. The Journal of cell biology. 2004;165:663–671. doi: 10.1083/jcb.200402022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho-Santos Z, Machado P, Alvarez-Martins I, Gouveia SM, Jana SC, Duarte P, Amado T, Branco P, Freitas MC, Silva ST, et al. BLD10/CEP135 Is a Microtubule-Associated Protein that Controls the Formation of the Flagellum Central Microtubule Pair. Developmental cell. 2012;23:412–424. doi: 10.1016/j.devcel.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Lin YC, Chang CW, Hsu WB, Tang CJ, Lin YN, Chou EJ, Wu CT, Tang TK. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. The EMBO journal. 2013;32:1141–1154. doi: 10.1038/emboj.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazawa Y, Hiraki M, Kamiya R, Hirono M. SAS-6 is a Cartwheel Protein that Establishes the 9-Fold Symmetry of the Centriole. Curr Biol. 2007;17:2169–2174. doi: 10.1016/j.cub.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Lee S, Chang J, Rhee K. A novel function of CEP135 as a platform protein of C-NAP1 for its centriolar localization. Experimental cell research. 2008;314:3692–3700. doi: 10.1016/j.yexcr.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Mottier-Pavie V, Megraw TL. Drosophila bld10 is a centriolar protein that regulates centriole, basal body, and motile cilium assembly. Molecular biology of the cell. 2009;20:2605–2614. doi: 10.1091/mbc.E08-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R. Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. The Journal of cell biology. 2002;156:87–99. doi: 10.1083/jcb.200108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nature cell biology. 2008;10:322–328. doi: 10.1038/ncb1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryu JH, Essner R, Ohta T, Kuriyama R. Filamentous polymers induced by overexpression of a novel centrosomal protein, Cep135. Microscopy research and technique. 2000;49:478–486. doi: 10.1002/(SICI)1097-0029(20000601)49:5<478::AID-JEMT10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Dammermann A, Muller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K. Centriole assembly requires both centriolar and pericentriolar material proteins. Developmental cell. 2004;7:815–829. doi: 10.1016/j.devcel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nature cell biology. 2005;7:115–125. doi: 10.1038/ncb1220. [DOI] [PubMed] [Google Scholar]
- 30.Pelletier L, O’Toole E, Schwager A, Hyman AA, Muller-Reichert T. Centriole assembly in Caenorhabditis elegans. Nature. 2006;444:619–623. doi: 10.1038/nature05318. [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa D, Kohlmaier G, Keller D, Strnad P, Balestra FR, Fluckiger I, Gonczy P. Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. Journal of cell science. 2011;124:3884–3893. doi: 10.1242/jcs.089888. [DOI] [PubMed] [Google Scholar]
- 32.Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA. Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nature cell biology. 2012;14:1159–1168. doi: 10.1038/ncb2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu J, Glover DM. Structured illumination of the interface between centriole and peri-centriolar material. Open biology. 2012;2:120104. doi: 10.1098/rsob.120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nature cell biology. 2012;14:1148–1158. doi: 10.1038/ncb2591. [DOI] [PubMed] [Google Scholar]
- 35.Sonnen KF, Schermelleh L, Leonhardt H, Nigg EA. 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biology open. 2012;1:965–976. doi: 10.1242/bio.20122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dammermann A, Maddox PS, Desai A, Oegema K. SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. The Journal of cell biology. 2008;180:771–785. doi: 10.1083/jcb.200709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkham M, Muller-Reichert T, Oegema K, Grill S, Hyman AA. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112:575–587. doi: 10.1016/s0092-8674(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 38.Gopalakrishnan J, Mennella V, Blachon S, Zhai B, Smith AH, Megraw TL, Nicastro D, Gygi SP, Agard DA, Avidor-Reiss T. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nature communications. 2011;2:359. doi: 10.1038/ncomms1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Current topics in developmental biology. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 40.Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. Journal of cell science. 1996;109(Pt 13):3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- 41.Keller D, Orpinell M, Olivier N, Wachsmuth M, Mahen R, Wyss R, Hachet V, Ellenberg J, Manley S, Gonczy P. Mechanisms of HsSAS-6 assembly promoting centriole formation in human cells. The Journal of cell biology. 2014;204:697–712. doi: 10.1083/jcb.201307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong CS, Kim M, Yang TT, Liao JC, Tsou MF. SAS-6 assembly templated by the lumen of cartwheel-less centrioles precedes centriole duplication. Developmental cell. 2014;30:238–245. doi: 10.1016/j.devcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.