SUMMARY
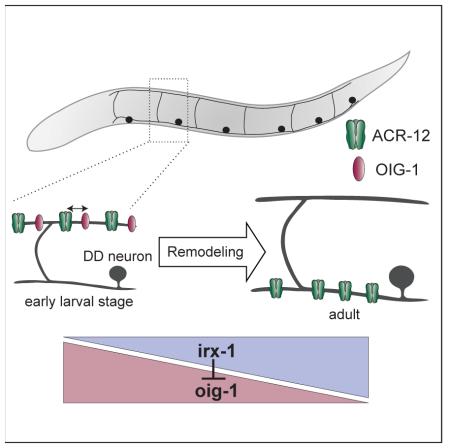
Neural circuits are actively remodeled during brain development but the molecular mechanisms that trigger circuit refinement are poorly understood. Here we describe a transcriptional program in C. elegans that regulates expression of an Ig domain protein, OIG-1, to control the timing of synaptic remodeling. DD GABAergic neurons reverse polarity during larval development by exchanging the locations of pre- and postsynaptic components. In newly born larvae, DDs receive cholinergic inputs in the dorsal nerve cord. These inputs are switched to the ventral side by the end of the first larval (L1) stage. VD class GABAergic neurons are generated in the late L1 and are postsynaptic to cholinergic neurons in the dorsal nerve cord but do not remodel. We investigated remodeling of the postsynaptic apparatus in DD and VD neurons using targeted expression of the acetylcholine receptor (AChR) subunit, ACR-12::GFP. We determined that OIG-1 antagonizes the relocation of ACR-12 from the dorsal side in L1 DD neurons. During the L1/L2 transition, OIG-1 is down-regulated in DD neurons by the transcription factor, IRX-1/Iroquois, allowing the repositioning of synaptic inputs to the ventral side. In VD class neurons, which normally do not remodel, the transcription factor UNC-55/COUP-TF turns off IRX-1, thus maintaining high levels of OIG-1 to block the removal of dorsally-located ACR-12 receptors. OIG-1 is secreted from GABA neurons but its anti-plasticity function is cell-autonomous but may not require secretion. Our study provides a novel mechanism by which synaptic remodeling is set in motion through regulated expression of an Ig domain protein.
RESULTS AND DISCUSSION
GABAergic DD motor neurons remodel postsynaptic components during larval development
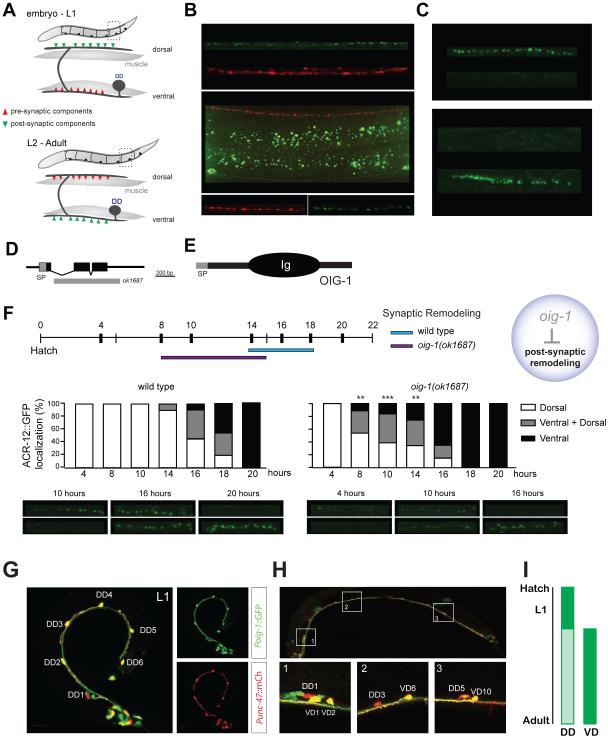
Motor neurons located in the ventral nerve cord drive locomotion in C. elegans. Sinusoidal waves are generated by cholinergic motor neurons that signal at dyadic synapses to excite contraction of ipsilateral body muscles while simultaneously stimulating GABA neurons to induce muscle relaxation on the contralateral side (Figure S1A) [1, 2]. We have previously shown that transgenic expression of a functional ionotropic acetylcholine receptor (iAChR) subunit ACR-12::GFP (green fluorescent protein) in GABAergic motor neurons marks these connections with punctate clusters that are closely apposed to cholinergic presynaptic regions labeled with mCherry::RAB-3 (Figures S1B and S1C) [3]. Reconstruction of the DD motor circuit by serial section electron microscopy indicated that cholinergic inputs to DD neurons are switched from dorsal to ventral locations late in the first larval (L1) stage [4]. To confirm this observation, we used the flp-13 promoter to express both ACR-12::GFP and mCherry::RAB-3 in DD neurons. In this case, ACR-12::GFP clusters are confined to the dorsal side whereas mCherry::RAB-3-labeled synaptic vesicles are limited to the ventral nerve cord in early L1 larvae (Figures 1A-C, top). By the adult stage, this configuration is reversed with ACR-12::GFP puncta on the ventral side and mCherry::RAB-3 restricted to presynaptic inputs to dorsal muscles (Figures 1A-C, bottom). The repositioning of ACR-12::GFP from dorsal to ventral locations was mimicked by another iAChR subunit, UNC-29::GFP, which shows robust expression in GABA neurons (Figure S1D-E) [5]. These results confirm that DD remodeling involves a polarity reversal with presynaptic and postsynaptic components switching places at opposite ends of a morphologically intact GABAergic neuron.
Figure 1. OIG-1 inhibits postsynaptic remodeling of DD motor neurons.
A. Embryonic DD motor neurons innervate ventral muscles and extend commissures to the dorsal side for input from cholinergic motor neurons. Toward the end of the L1 larval stage, presynaptic vesicles (red) and postsynaptic acetylcholine receptors (AChRs) (green) switch locations as DDs remodel.
B. In a newly hatched L1 larva, RAB-3::mCherry (red) marks DD synapses with ventral muscle and ACR-12::GFP (green) labels postsynaptic DD regions in the dorsal nerve cord. In an L4 larva, presynaptic RAB-3::mCherry (red) labels DD inputs to dorsal muscles and ACR-12::GFP (green) is restricted to ventral DD postsynaptic locations. Asterisk denotes gut autofluorescence. Scale bars are 5 μm.
C. ACR-12::GFP AChR subunits are dorsally localized in early L1 DD motor neurons but are strictly ventral by the L4 larval stage. Scale bars are 5 μm.
D. The oig-1 gene includes three exons (black boxes) with a canonical N-terminal signal peptide (SP) sequence. Exons 2 and 3 are deleted in oig-1(ok1687).
E. The OIG-1 protein includes an N-terminal signal peptide (SP) and a single immunoglobulin (Ig) domain.
F. Postsynaptic remodeling is precocious in oig-1(ok1687). Wild-type DD neurons remodel 14 - 18 hours after hatching whereas oig-1 mutant DD neurons remodel 8 - 16 hours post-hatching. Quantification and representative images of DD remodeling are shown at the bottom. The x-axis denotes time since hatching (hour). L1 larvae were binned according to the distribution of Pflp-13::ACR-12::GFP puncta as dorsal only (white), ventral only (black) or dorsal and ventral (gray). **, p<0.005, ***, p<0.0005, vs wild-type (dorsal only vs dorsal + ventral & ventral only) (n = 20 for each time point), Fisher’s Exact test. Scale bars are 2μm.
G. In L1 larvae, Poig-1::GFP is highly expressed in all six DD neurons as shown by colocalization of Poig-1::GFP (green) and Punc-47::mCherry (red). Scale bars are 10 μm.
H. By the adult stage, Poig-1::GFP is not detected in DD motor neurons but is strongly expressed in VD motor neurons. Insets show representative examples of adjacent DD and VD neurons with differential Poig-1::GFP expression. Scale bars are 20 μm.
I. Schematic denoting periods of strong Poig-1::GFP expression (dark green) in developing DD and VD neurons.
In principle, remodeling of the postsynaptic domain could occur either by translocation of existing receptor complexes from the dorsal to the ventral side, or by elimination of dorsal receptors and concomitant synthesis of new receptor subunits that assume a ventral position. To distinguish between these possibilities, we used laser microsurgery to sever the commissural process of the DD1 neuron in the early L1 when ACR-12-containing iAChRs are restricted to the dorsal side (Figure S1F). We then monitored the appearance of ACR-12::GFP in the ventral DD1 process and found that ventral ACR-12::GFP clusters were indistinguishable from those in mock-axotomized animals, suggesting that an intact commissural connection between the dorsal and ventral DD processes is not required for postsynaptic remodeling (Figure S1G). These results indicate that ACR-12 receptor translocation from the dorsal to the ventral side is not essential for remodeling and provide evidence that a primary contribution to the ventral receptor pool occurs through de novo ACR-12 synthesis.
An Immunoglobulin superfamily (IgSF) protein, OIG-1, antagonizes postsynaptic remodeling of GABAergic motor neurons
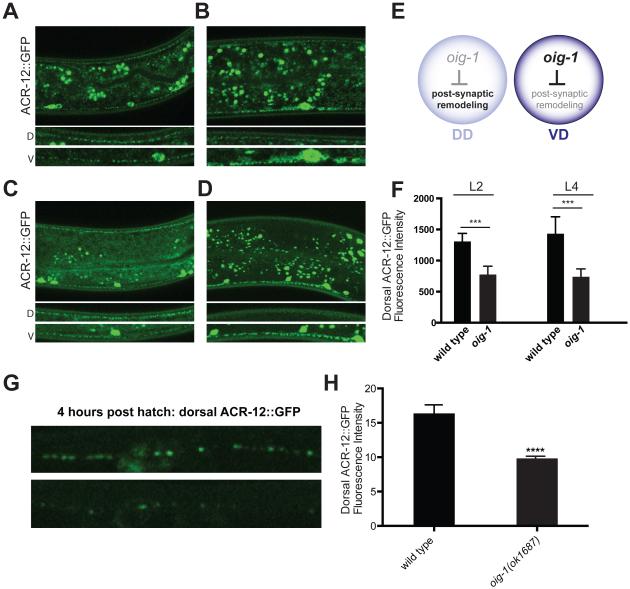
We used cell-specific microarray analysis to detect strong expression of a transcript encoding a short single-Ig domain protein, OIG-1, in early L1 DD motor neurons (See Supplemental Experimental Methods) (Supplemental File 1, GEO accession number GSE71618) (Figures 1D, E). A canonical N-terminal signal peptide predicts that the mature OIG-1 protein (137 amino acids in length) is secreted [6]. Because recent work established that a closely related paralog, OIG-4, stabilizes iAChR complexes in C. elegans body muscle cells [7], we wondered if OIG-1 might exert a similar role and thus potentially retard the dissociation of ACR-12 receptor complexes in remodeling GABA neurons. To address this question, we monitored Pflp-13::ACR-12::GFP localization in the null allele, oig-1(ok1687) (Figure 1D), and observed that DD postsynaptic remodeling was initiated significantly earlier than in the wild-type (8-16 hours vs. 14-18 hours post-hatching) (Figure 1F, top) with the precocious removal of dorsal ACR-12::GFP puncta coinciding with their early appearance on the ventral side (Figure 1F, bottom). This result suggests that OIG-1 normally functions to antagonize the relocation of ACR-12 in L1 stage DD motor neurons (Figure 1F). This model also predicts, however, that OIG-1 expression should be down-regulated by the late L1 stage to allow the normal onset of DD remodeling. To test this hypothesis, we used a GFP reporter gene that includes the oig-1 upstream region (Poig-1::GFP) to confirm expression in DD motor neurons in early L1 larvae (Figure 1G) [6, 8]. As development proceeds, the Poig-1::GFP signal declines in DD motor neurons but shows strong expression in VD motor neurons beginning soon after their birth at the end of the L1 stage (Figure 1H). This temporal pattern of expression (Figure 1I) prompted us to ask if OIG-1 is also necessary to prevent the dorsal to ventral translocation of the ACR-12 receptor in VD neurons, which normally do not remodel. Indeed, oig-1 mutants showed fewer dorsal ACR-12::GFP puncta than wild-type in both L2 and L4 larval stages (Figures 2A-F) and a reciprocal relative increase in ventral ACR-12::GFP (Figures S2G). This result suggests that oig-1 mutant VD motor neurons undergo ectopic remodeling (e.g., removal of dorsal ACR-12::GFP puncta with reassembly on the ventral side). Notably, oig-1 mutant animals showed significantly less dorsal ACR-12::GFP than wild-type before remodeling (i.e., 4 hr post hatch), and an overall lower level of ACR-12::GFP in L4 larvae, suggesting that OIG-1 might have an additional role of stabilizing the initial clusters of ACR-12::GFP (Figures 2G-H, Figures S2F-G).
Figure 2. OIG-1 inhibits postsynaptic remodeling of VD motor neurons.
A, B. Representative images of Punc-47::ACR-12::GFP in L2 wild-type and oig-1(ok1687) larvae showing ACR-12::GFP puncta in both dorsal (D) and ventral (V) nerve cords. In oig-1 mutants, dorsal ACR-12::GFP is significantly reduced compared to wild type by the L2 stage. Asterisk denotes gut autofluorescence. Scale bars are 20 μm.
C, D Representative images of Punc-47::ACR-12::GFP puncta in L4 wild-type and oig-1(ok1687) larvae. Insets (bottom) show ACR-12::GFP puncta in dorsal (D) and ventral (V) nerve cords. Note depletion of dorsal ACR-12::GFP puncta in oig-1(ok1687). Scale bars are 20 μm.
E. Model depicting oig-1 expression in DD and VD motor neurons (L2 – adult) and negative regulation of postsynaptic remodeling.
F. Quantification of dorsal ACR-12::GFP fluorescence intensity comparing wild-type and oig-1(ok1687) L2 and L4 larvae. ***, p<0.001, Student’s t test, n > 15 for each group. Error bars represent SD.
G. Representative images of dorsal ACR-12::GFP puncta in wild-type and oig-1 mutant L1 animals (4 hours post hatch). Scale bar is 5μm. Error bars represent SEM.
H. Quantification of ACR-12::GFP localization in the dorsal nerve cord detects a weaker signal in oig-1 mutants than in wild-type at 4 hours post hatch. ****, p<0.0001 vs wild-type. n=10 for each group, student’s t test. Error bars represent SEM.
A transcriptional switch regulates expression of OIG-1 to control postsynaptic remodeling in GABAergic neurons
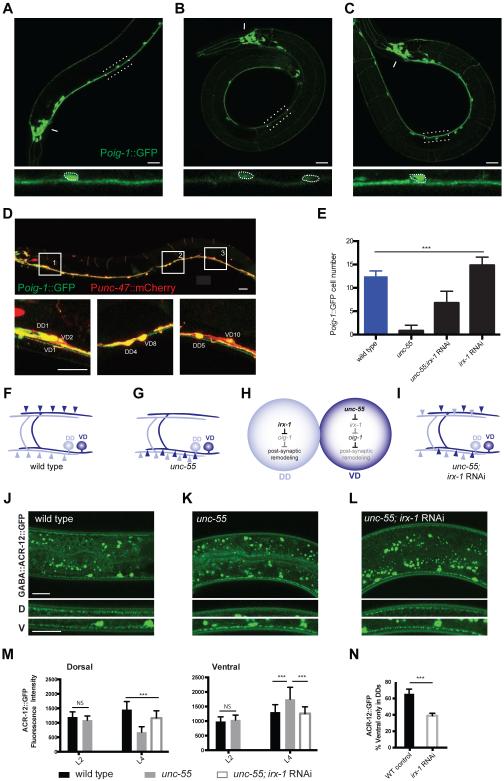
The strong expression of OIG-1 in VD neurons resembles that of the COUP-TF family transcription factor, UNC-55, which has been previously shown to block presynaptic remodeling in VD neurons [9-12]. We thus asked if OIG-1 is a downstream target of UNC-55. The Poig-1::GFP signal is significantly weaker in unc-55 mutant VD motor neurons but is unaffected in other Poig-1::GFP-positive neurons in the head region (Figures 3A, B, E). Because UNC-55 is likely to function as a transcriptional repressor [10, 12], we reasoned that this effect should depend on an intermediate target in the unc-55 pathway. An obvious candidate for this role is the Iroquois family homeodomain transcription factor irx-1, which is up-regulated in unc-55 mutant VD motor neurons [12]. Consistent with this model, treatment of unc-55 mutant animals with irx-1 RNAi restores Poig-1::GFP expression to VD motor neurons (Figures 3C, E). irx-1 RNAi also results in ectopic expression of Poig-1::GFP in late larval and adult DD motor neurons (Figures 3D, E). These data point to related genetic pathways in which irx-1 antagonizes oig-1 expression in DD motor neurons, while unc-55 blocks irx-1 expression in VD motor neurons to prevent negative regulation of oig-1.
Figure 3. A transcriptional cascade involving UNC-55/COUP-TF and IRX-1/Iroquois regulates oig-1 expression in GABA neurons.
All panels depict adults, dorsal is up and anterior to left. A, B, C. Poig-1::GFP is highly expressed in wild-type VD motor neurons in the ventral nerve cord and in a subset of head neurons (arrow). In unc-55 mutants, Poig-1::GFP is decreased in VD neurons but is maintained in head neurons (arrow). irx-1 RNAi restores Poig-1::GFP expression to ventral cord motor neurons. Insets (bottom) feature enlarged and straightened segments of the ventral nerve cord. Dotted circles denote Poig-1::GFP-expressing ventral cord neurons. Asterisk denotes gut autofluorescence. Scale bars are 20 μm for A-C.
D. RNAi knockdown of irx-1 restores Poig-1::GFP expression to DD neurons in wild-type adults; Poig-1::GFP is maintained in the VDs. Inserts (1, 2, 3) show adjacent DD and VD neurons expressing Poig-1::GFP. Punc-47::mCherry marks GABAergic motor neurons and was merged with Poig-1::GFP images to produce yellow overlays. Scale bars are 20 μm.
E. Quantification of Poig-1::GFP expression in adult ventral cord GABAergic motor neurons. Poig-1::GFP is expressed in all 13 VD motor neurons in the wild-type but is rarely detected in the ventral cord of unc-55 mutants. Poig-1::GFP expression is partially restored in irx-1 RNAi-treated unc-55 mutants and is ectopically expressed in adult DD neurons with irx-1 RNAi of wild-type animals. ***, p<0.001. One-way ANOVA followed by Tukey multiple comparison test, n > 30 for each group. Error bars represent SD.
F, G In wild-type adults, DD postsynaptic ACh receptors (light blue) are located on the ventral side, whereas VD postsynaptic receptors (dark blue) are located dorsally. In unc-55 mutants, postsynaptic ACh receptors are ectopically relocated to the ventral side in VD motor neurons.
H. Model: IRX-1 is expressed in DD motor neurons to promote postsynaptic remodeling by inhibiting OIG-1 expression. IRX-1 is repressed by UNC-55 in VD motor neurons to prevent ectopic remodeling of postsynaptic components to the ventral side.
I. irx-1 RNAi suppresses postsynaptic remodeling of both DD neurons and ectopically remodeled VD neurons in unc-55 mutants.
J - L. GABA::ACR-12::GFP (or Punc-47::ACR-12::GFP) puncta are detected in both dorsal and ventral nerve cords of wild-type L4 larval animals due to contributions of VD (dorsal) and DD (ventral) neurons (J) .In unc-55 mutants, GABA::ACR-12::GFP puncta are largely ventral (K) but are relocated to the dorsal side in animals treated with irx-1 RNAi (L). Scale bars are 20 μm for J-L.
M. Quantification of dorsal and ventral GABA::ACR-12::GFP fluorescence intensity for wild-type (black), unc-55 (grey) and unc-55;irx-1 RNAi (white) at L2 and L4 stages. In L2 larvae, the distribution of ACR-12::GFP puncta in the dorsal nerve cord does not differ between unc-55 vs wild-type indicating that the initial assembly of the VD postsynaptic apparatus is not perturbed in unc-55 animals. In contrast, in L4 larvae, unc-55 mutant animals show significantly fewer dorsal ACR-12::GFP puncta than wild-type; this ectopic remodeling effect was blocked by irx-1 RNAi. ***, p < 0.001, one way ANOVA, n > 15 for each group. Error bars represent SD.
N. irx-1 RNAi knockdown delays DD postsynaptic remodeling. By 27 hours after egg laying (16 hours post hatch), 66 ± 6 % of wild-type (WT) control larvae have completed DD remodeling (i.e., show ventral GABA::ACR-12::GFP only) whereas only 39 ± 3% of irx-1 knockdown animals have completed DD remodeling, ***, p < 0.001, (n = 113), Fisher’s exact test. Error bars represent SD.
We confirmed the roles of these regulatory cascades in postsynaptic remodeling with additional genetic experiments. In wild-type adults, expression of ACR-12::GFP with the unc-47 GABA neuron promoter results in comparable levels of postsynaptic ACR-12::GFP clusters on dorsal (VD) and ventral (DD) sides (Figure 3F, J, M). At the L2 stage, unc-55 mutants show a similar distribution of ACR-12::GFP (Figure 3M). Later, in L4 larvae however, ACR-12::GFP puncta are largely localized to the ventral side of unc-55 mutants (Figures 3G-M, Figures S2F-G). This result suggests that unc-55 mutant VD neurons initially establish postsynaptic ACR-12 receptor domains in the dorsal nerve cord as in the wild type, but then reposition these ACR-12::GFP puncta to the ventral side as predicted by our model (Figure 3G). This ectopic postsynaptic remodeling effect in VD neurons can be reversed by global RNAi knock down of irx-1 (Figures 3I, L, M). Moreover, the ACR-12::GFP remodeling phenotype of unc-55;oig-1 double mutants is not more severe than that of oig-1 and shows a slightly weaker phenotype than unc-55 (Figure S2G). We interpret these findings to indicate that oig-1 is the principal downstream effector of unc-55 in a pathway that blocks postsynaptic remodeling in VD neurons. To ask if irx-1 is also required for postsynaptic remodeling in DD motor neurons, we used RNAi to downregulate irx-1 expression in wild-type animals expressing Pflp-13::ACR-12::GFP. More than half (66%) of control L1 larvae showed strictly ventral ACR-12::GFP puncta by 27 hours after egg-laying whereas significantly fewer (39%) of irx-1 RNAi-treated animals completed postsynaptic remodeling (Figure 3N). The partial remodeling of DD neurons could result from either inefficient RNAi knockdown of irx-1 or the parallel function of another transcriptionally regulated pathway in the DD remodeling program [13]. In any case, the delay in DD remodeling in irx-1-RNAi-treated animals requires oig-1 activity (Figure S2E). Finally, we confirmed that irx-1 function is cell autonomous in DD and VD neurons using cell-specific RNAi (Figure S2A-D). Taken together, our results demonstrate that postsynaptic remodeling in GABA neurons is modulated by the opposing roles of UNC-55 and IRX-1 in the regulation of OIG-1 expression (Figures 3H and S2H). These findings parallel earlier results showing that UNC-55 and IRX-1 also control presynaptic GABA neuron plasticity [12, 13] and thus suggest that this genetic pathway orchestrates the overall remodeling program (see below).
OIG-1 functions in GABA neurons to block postsynaptic remodeling
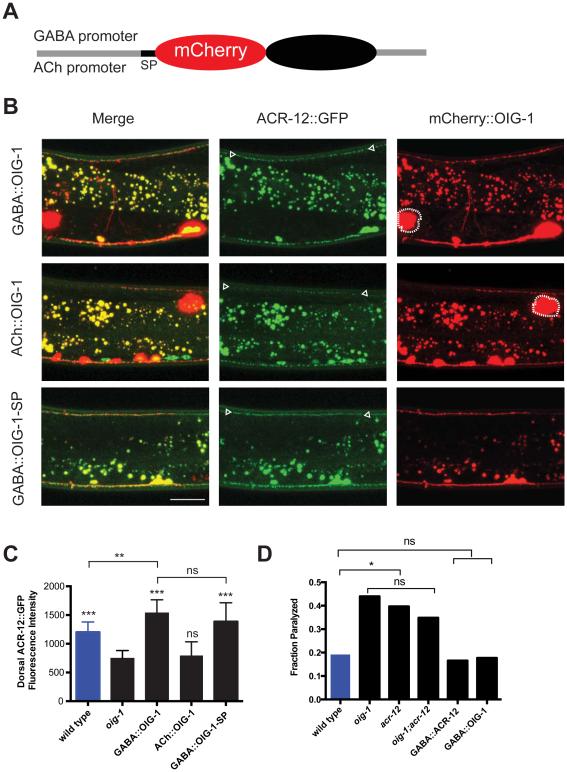
Although OIG-1 is strongly expressed in DD and VD neurons, the OIG-1 protein is predicted to be secreted, and thus could potentially function as an extracellular antagonist of postsynaptic remodeling in a non-autonomous fashion. To test for this possibility, we used transgenic reporters in which mCherry was inserted immediately after the signal peptide (SP) to label the OIG-1 protein (Figure 4A). Expression of the mCherry::OIG-1 construct with the GABA-neuron specific unc-25 promoter (GABA::OIG-1) resulted in bright punctate mCherry signals along both ventral and dorsal nerve cords (Figure 4B top). Similar results were obtained using the native promoter driving OIG-1 fused to superfolder GFP (Figure S3A, B). A robust mCherry signal in coelomocytes, macrophage-like cells in the body cavity, confirms that mCherry::OIG-1 is secreted (Figure 4B top). mCherry::OIG-1 expression in the GABAergic neurons of oig-1 mutant animals restored dorsal Punc-47::ACR-12::GFP to a wild-type level, thus demonstrating that the mCherry::OIG-1 fusion protein is functional and that expression of OIG-1 in GABA neurons is sufficient to rescue the Oig-1 postsynaptic remodeling defect (Figures 4B top and 4C). Secretion of mCherry::OIG-1 from neighboring cholinergic neurons (ACh::OIG-1), however, did not rescue the Oig-1 phenotype (Figures 4B middle and 4C). This result indicates either that OIG-1 function is cell autonomous and requires expression in GABA neurons or that the secreted form of OIG-1 is not actively involved in postsynaptic remodeling.
Figure 4. Cell-autonomous expression of OIG-1 blocks postsynaptic remodeling in GABA neurons.
A. Schematic of OIG-1 fusion protein. mCherry was inserted immediately after the OIG-1 signal peptide (SP) and fused to upstream promoters for expression in either GABA or ACh (cholinergic) motor neurons in the ventral nerve cord.
B. All panels show young adults, anterior to left, dorsal is up. Expression of mCherry::OIG-1 in GABA neurons with the unc-25 promoter (GABA::OIG-1) restores dorsal ACR-12::GFP puncta (arrowheads) to an oig-1 mutant. mCherry::OIG-1 is detected in both the ventral and dorsal nerve cords and is secreted as indicated by mCherry-labeled coelomocytes (dotted outline) in the body cavity. mCherry::OIG-1 expression in cholinergic motor neurons with the acr-2 promoter (ACh::OIG-1) does not result in significant restoration of ACR-12::GFP puncta to the dorsal nerve cord (arrowheads) although mCherry-labeled coelomocytes (dotted outline) are indicative of secretion. GABA neuron expression of a non-secreted version of mCherry::OIG-1 (GABA::OIG-1-SP) restores dorsal ACR-12::GFP (arrowheads), suggesting that secretion of the OIG-1 protein may not be required for its function in GABA neuron remodeling. Asterisk denotes gut autofluorescence. Scale bar is 20 μm.
C. Quantification of dorsal ACR-12::GFP fluorescence intensity in young adults. oig-1 mutants (black) show reduced dorsal ACR-12::GFP signal vs wild-type (blue). Expression of mCherry::OIG-1 in GABA neurons but not in cholinergic motor neurons restores dorsal ACR-12::GFP to wild-type levels. GABA neuron expression of OIG-1-SP, the non-secreted form of mCherry::OIG-1, also rescues oig-1. ***, p<0.0001 vs oig-1, **, p<0.001 vs wild type, ns (p>0.33), one way ANOVA followed by Tukey multiple comparison test, n > 15 for each experimental group. Error bars represent SD.
D. acr-12(ok367) and oig-1(ok1687) mutants show locomotory defects that depend on GABA motor neuron function. The number of immobilized L4 larvae at the end of a 10-minute swimming assay was determined by direct observation. oig-1(ok1687) and acr-12(ok367) mutants showed a higher fraction of immobilized animals than wild-type. The swimming defect can be rescued by expression of ACR-12 (GABA::ACR-12) and OIG-1 (GABA::OIG-1) specifically in GABA neurons. *, p<0.05 vs wild-type. ns, p>0.05, n>60 for each experimental group. All comparisons by Fisher’s exact test.
To distinguish between these possibilities, we generated a mCherry::OIG-1 protein that excludes the N-terminal signal peptide, thus preventing secretion, and expressed it in GABA neurons [GABA::OIG-1-SP] (See Experimental Procedures). Transgenic oig-1(ok1687) animals expressing GABA::OIG-1-SP showed strong mCherry puncta in GABA neuron processes in both the dorsal and ventral nerve cords (Figure 4B bottom). As predicted for a non-secreted form of OIG-1, coelomocytes were not labeled with mCherry in this strain; however, ACR-12::GFP was restored to the dorsal nerve cord indicating strong rescue of the Oig-1 postsynaptic remodeling defect (Figures 4B bottom and 4C). We note that transgenic expression of OIG-1 and OIG-1-SP appears to elevate ACR-12::GFP levels in comparison to wild type perhaps due to the overall role of OIG-1 in stabilizing ACR-12::GFP clusters (Figure 4C). We used a live animal antibody labeling method [14] to further investigate OIG-1 secretion in each situation. The external cell membranes of GABA neurons showed strong immunostaining in animals expressing full length OIG-1, but no extracellular signal was detected in animals expressing OIG-1-SP (Figure S3C). While we cannot exclude the possibility OIG-1-SP may reach the extracellular environment at low levels that are below the threshold of detection in our experiments, our evidence points to an alternative model in which postsynaptic remodeling does not require the secreted form of OIG-1, but instead involves an intracellular OIG-1 function in GABA neurons. In an additional experiment to define a location for OIG-1 function, we used mosaic expression of a low-copy number GABA::mCherry::OIG-1 transgene to show that localization of mCherry::OIG-1 puncta to the dorsal nerve cord is correlated with the restoration of dorsal ACR-12::GFP puncta in an oig-1 mutant (Figure S3E-F). This result points to a local role for OIG-1 in antagonizing the removal of ACR-12 receptors by the remodeling program. We note, however, that mCherry::OIG-1 and ACR-12::GFP do not overlap in the dorsal nerve cord, but instead adopt a striking pattern of alternating mCherry and GFP puncta (Figure S3G). This finding argues against the idea that OIG-1 stabilizes ACR-12-containing iACh receptors by direct interaction at the synapse and favors an alternative model potentially involving additional components (Figure S4F). The proposed role for OIG-1 in postsynaptic remodeling is further reinforced by our findings that oig-1 and acr-12 mutants display similar locomotory defects that depend on GABA neuron dysfunction (Figure 4D).
OIG-1 inhibits presynaptic remodeling in GABAergic motor neurons
Having shown that OIG-1 antagonizes postsynaptic remodeling, we next asked if OIG-1 also regulates the location of presynaptic proteins in the remodeling program. In the wild type, the presynaptic marker SNB-1::GFP switches from the ventral to the dorsal side in remodeling DD motor neurons, while VD motor neurons synapse with ventral muscles throughout life. These patterns of expression produce a mature GABAergic motor circuit with SNB-1::GFP puncta in both the dorsal (DD) and ventral (VD) nerve cords (Figure S4A). In unc-55 mutants, however, VD motor neurons undergo ectopic remodeling resulting in the net depletion of ventral SNB-1::GFP puncta (Figure S4C and S4D) [9, 12, 15]. Ventral SNB-1::GFP puncta in the GABAergic circuit are also reduced in oig-1(ok1687) versus the wild type (Figure S4B), suggesting that presynaptic components are ectopically remodeled in oig-1 mutant VD motor neurons. Our data are consistent with the observation that DD motor neurons show precocious presynaptic remodeling in oig-1 mutant L1 larvae and that this effect is rescued by cell autonomous OIG-1 function [16]. It is notable, however, that oig-1 mutants retain a greater number of ventral SNB-1::GFP puncta than observed in unc-55, which suggests that ectopic presynaptic remodeling in oig-1 mutants is incomplete and substantially less severe (Figure S4D). This difference indicates that UNC-55 likely controls an additional parallel-acting pathway involving irx-1 that regulates presynaptic remodeling (Figure S4E) [12].
Although synaptic refinement is crucial to the creation of a mature nervous system, it may be equally important to maintain the architecture of established circuits by tightly controlling the activation of remodeling pathways. Our results show that the opposing roles of the conserved transcription factors IRX-1/Iroquois and UNC-55/COUP-TF orchestrate both the timing and location of synaptic remodeling in the C. elegans GABA motor neuron circuit (Figure S2H). irx-1 antagonizes oig-1 expression in late L1 stage DD neurons to permit the disassembly of the postsynaptic apparatus by the remodeling program. This inhibition of oig-1 is blocked in the VDs by unc-55, which turns off irx-1 and thus maintains high levels of OIG-1 to preserve dorsal clusters of iACh receptors (Figure S2H). This negative regulatory pathway appears to function in concert with the PITX homeodomain transcription factor UNC-30, which promotes oig-1 expression [16] (Figure S4F). However, the role of unc-30 is likely complex because it is also required for DD expression of irx-1 [12].
IgSF proteins perform central roles in fundamental aspects of neuronal development, including cell migration, growth cone guidance and synapse formation and function. IgSF proteins may act as cell adhesion molecules (CAMs), secreted ligands or auxiliary subunits that facilitate the function of specific receptors [6, 17-20]. In the case of OIG-1, our work suggests that OIG-1 protein inhibits the disassembly of the ACR-12 receptor complex in a mechanism that opposes remodeling of the postsynaptic region. Given our finding that a non-secreted form of OIG-1 (OIG-1-SP) is functional (Figure 4B, C), we suggest that OIG-1 might exert this effect before entering the secretory pathway [21] such that OIG-1-SP could potentially interact with its normal physiological targets. Our results have established a key role for OIG-1 in a mechanism that regulates the relocation of a postsynaptic iAChR from the dorsal to ventral arms of remodeling GABAergic neurons. We also detected a relatively minor function for OIG-1 in the redistribution of a presynaptic component in the opposite direction (Figure S4A-D). The origin of this effect is unclear but could indicate that the removal of the postsynaptic apparatus facilitates assembly of presynaptic components in the same location. By comparison, unc-55 exerts a strong negative effect on the ectopic relocation of SNB-1 in VD neurons [12], perhaps indicating that other effectors regulated by unc-55 serve parallel roles in presynaptic remodeling (Figure S4E). We have shown that the IgSF protein OIG-1 antagonizes developmental remodeling of postsynaptic iAChRs in the processes of GABAergic neurons. The molecular mechanism underlying this effect and the components of the remodeling program that OIG-1 opposes are important subjects for future studies.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Miller lab and V. Budnik for critical reading of the manuscript and helpful discussions, K. Howell, J. White and O. Hobert for sharing information before publication, Chris Lambert for technical assistance, Y. Jin for strain juIs223 and E. Lundquist for Pstr-1::GFP. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This work was supported by National Institutes of Health grants to DMM (R01NS081259), CVG (R01NS077929) and MMF (R01NS064263). SH is partially supported by the Vanderbilt International Scholar Program. AP is supported by an NIH predoctoral NRSA (F31DA038399). Experiments were performed in the VMC Flow Cytometry Shared Resource and by VANTAGE (supported by NIH grants, P30 CA68485, DK058404, P30 EY08126, G20 RR030956).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
SH generated transgenic lines, collected confocal images and quantified results for oig-1, irx-1 and unc-55 mutant phenotypes. AP generated transgenic lines, collected confocal images and quantified results for oig-1 mutant effects. RM collected and analyzed DD microarray data. AP, CVG and DT conducted laser ablation experiments. MHC, IMH and SH performed the swimming assays. MMF and DMM designed and interpreted experiments and wrote the final document.
REFERENCES
- 1.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond, B, Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 2.Zhen M, Samuel AD. C. elegans locomotion: small circuits, complex functions. Current Opinion in Neurobiology. 2015;33:117–126. doi: 10.1016/j.conb.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Petrash HA, Philbrook A, Haburcak M, Barbagallo B, Francis MM. ACR-12 Ionotropic Acetylcholine Receptor Complexes Regulate Inhibitory Motor Neuron Activity in Caenorhabditis elegans. Journal of Neuroscience. 2013;33:5524–5532. doi: 10.1523/JNEUROSCI.4384-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J, Albertson D, Anness M. Connectivity changes in a class of motoneurone during the development of a nematode. Nature. 1978 doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- 5.Spencer WC, Zeller G, Watson JD, Henz SR, Watkins KL, Mcwhirter RD, Petersen S, Sreedharan VT, Widmer C, Jo J, et al. A spatial and temporal map of C. elegans gene expression. Genome Research. 2011;21:325–341. doi: 10.1101/gr.114595.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurelio O, Aurelio O, Hall DH, Hobert O. Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science. 2002;295:686–690. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]
- 7.Rapti G, Bessereau J-L, Richmond J. A single immunoglobulin-domain protein required for clustering acetylcholine receptors in C. elegans. EMBO J. 2011;30:706–718. doi: 10.1038/emboj.2010.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinar H, Keles S, Jin Y. Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Curr Biol. 2005;15:340–346. doi: 10.1016/j.cub.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Walthall WW, Plunkett JA. Genetic transformation of the synaptic pattern of a motoneuron class in Caenorhabditis elegans. J Neurosci. 1995;15:1035–1043. doi: 10.1523/JNEUROSCI.15-02-01035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan G, Kim K, Li C, Walthall WW. Convergent genetic programs regulate similarities and differences between related motor neuron classes in Caenorhabditis elegans. Developmental Biology. 2005;280:494–503. doi: 10.1016/j.ydbio.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Zhou HM, Walthall WW. UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans. J Neurosci. 1998;18:10438–10444. doi: 10.1523/JNEUROSCI.18-24-10438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen SC, Watson JD, Richmond JE, Sarov M, Walthall WW, Miller DM. A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. Journal of Neuroscience. 2011;31:15362–15375. doi: 10.1523/JNEUROSCI.3181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson-Peer KL, Bai J, Hu Z, Kaplan JM. HBL-1 Patterns Synaptic Remodeling in C. elegans. Neuron. 2012;73:453–465. doi: 10.1016/j.neuron.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottschalk A, Schafer WR. Visualization of integral and peripheral cell surface proteins in live Caenorhabditis elegans. Journal of Neuroscience Methods. 2006;154:68–79. doi: 10.1016/j.jneumeth.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- 16.Howell K, White JG, Hobert O. Spatiotemporal control of a novel synaptic organizer molecule. Nature. 2015:1–15. doi: 10.1038/nature14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 18.Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951. doi: 10.1126/science.1145727. [DOI] [PubMed] [Google Scholar]
- 19.Woo J, Kwon S-K, Nam J, Choi S, Takahashi H, Krueger D, Park J, Lee Y, Bae JY, Lee D, et al. The adhesion protein IgSF9b is coupled to neuroligin 2 via S-SCAM to promote inhibitory synapse development. The Journal of Cell Biology. 2013;201:929–944. doi: 10.1083/jcb.201209132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3:a001727–a001727. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ast T, Cohen G, Schuldiner M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell. 2013;152:1134–1145. doi: 10.1016/j.cell.2013.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.