Abstract
Objectives
Physical activity reduces mobility impairments in elders. We examined the association of physical activity on risk of subjective and objective physical function in adults with and at risk for osteoarthritis (OA).
Methods
Adults aged≥60years from the longitudinal Osteoarthritis Initiative(OAI), a prospective observational study of knee OA, were classified by sex-specific quartiles of Physical Activity Score for the Elderly (PASE) scores.Using linear mixed models, we assessed 6-year data on self-reported health, gait speed, Late-Life Disability Index(LLDI) and chair stand.
Results
Of 2,252 subjects, mean age ranged from 66-70 years. Within each quartile, physical component(PCS) of the Short Form-12 and gait speed decreased from baseline to follow-up in both sexes (all p<0.001),yet the overall changes across PASE quartiles between these two time points were no different(p=0.40 and 0.69, males and females, respectively).Decline in PCS occurred in the younger age group, but rates of change between quartiles over time were no different in any outcomes in either sex.LLDI scores declined in the 70+ age group.Adjusting for knee extensor strength reduced the strength of association.
Discussion
Higher physical activity is associated with maintained physical function, and is mediated by muscle strength highlighting the importance of encouraging physical activity in older adults with and at risk for osteoarthritis.
Keywords: Exercise, Disability, Strength, Elderly
INTRODUCTION
Osteoarthritis (OA) is a leading cause of functional impairment1 and is increasingly observed in an aging population2. In elders, the observed risk of impairment and disability occurs partly due to sex-specific changes in body composition3 but also because of complex changes in joint cartilage and bone, as well as other articular and periarticular tissues4. This leads to declining mobility, increasing risk of falls5, and dependency on others for assistance. Understanding the interplay between groups with and at risk for osteoarthritis may provide important answers to incident disability and its time course that is observed in clinical practice.
While disability and impairment are common outcomes of the aging process, there is a critical need to identify key factors that slow impending functional decline and preserve activities of daily living. Providers routinely recommend physical activity (PA) in patients as a key element to healthy aging6. In fact, physical activity is recommended at all ages7, and is relevant in older adults since it has been strongly associated with improved physical function8, gait speed9, muscle strength7, and cardiometabolic variables10, 11. Preserved performance on such measures leads to enhanced quality of life12, mobility, reduced institutionalization13, and mortality14.
PA is a well established non-pharmacological treatment that reduces pain from OA15, which can favorably improve quality of life and physical function. Commonly, clinicians are reluctant to encourage older patients about engaging in regular and sustained physical activity primarily because they fear injury in addition to the unclear consequences on long-term physical function16. Whether ‘too much’ physical activity is detrimental also requires examination. The aim of this study was to assess the association of high levels of self-reported physical activity on functional measures in an older adult population with and at risk for osteoarthritis and to determine whether the rate of change of these measures differs over time.
METHODS
We performed a secondary analysis of data from the Osteoarthritis Initiative (OAI), a multi-center, longitudinal, prospective observational study of adults with osteoarthritis which began in 2004. The central purpose of this study was to examine the natural history of knee osteoarthritis in community-dwelling adults. There were four clinical sites: Baltimore, MD; Pawtuckett, RI; Pittsburgh, PA; and Columbus, OH.
Data and procedure manuals are available online at http://oai.epi-ucsf.edu. Briefly, participants were recruited through mailings, advertisements, and community meetings. Eligibility was determined by telephone interview, and subjects attended a screening clinic visit if eligible. Exclusion criteria consisted of: rheumatoid arthritis; severe joint space narrowing; bilateral total knee replacements; inability to undergo an MRI; unable to provide blood samples; comorbidity preventing study participation; other research participation and unwillingness to sign an informed consent. The enrollment clinic visit collected baseline demographic and questionnaire data and physical assessments within a six-week period. The study recruited subjects aged 45-79 years, an equal number in each sex, and all ethnic groups. Individuals who were unlikely to be residing in the area for at least 3 years were also excluded. Funding was provided by a public-private partnership of the National Institute of Arthritis, Musculoskeletal and Skin Diseases and private industry. OAI had a separate IRB approval process. Our local Institutional Review Board deemed the study exempt for research purposes.
Study Population
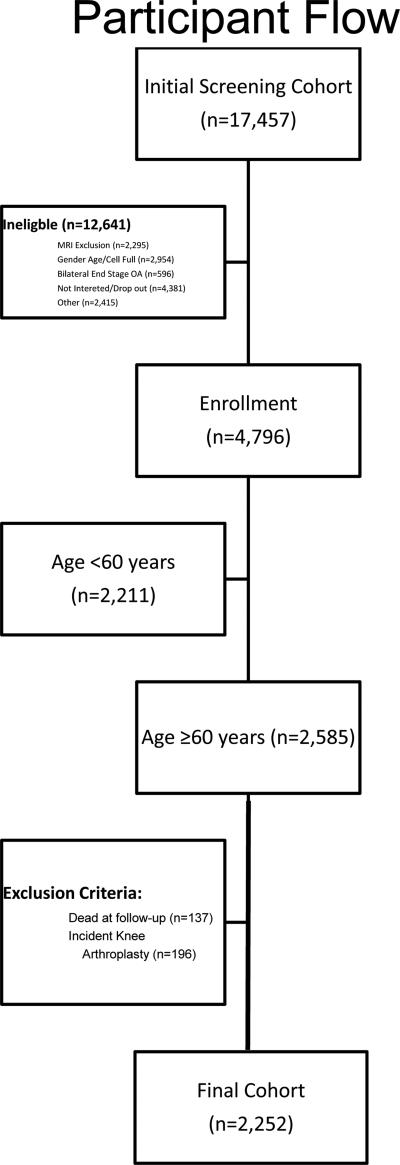
At baseline, subjects were classified into three cohorts: clinically significant knee osteoarthritis at risk of disease progression (progression cohort); high risk of developing clinically significant knee OA (incident cohort); and controls. Progression subjects complained of frequent knee symptoms and radiographic tibiofemoral knee OA in at least one native, non-replaced knee. Incident cohort was free of baseline symptomatic knee OA, but had established risk factors including the presence of Heberden's nodes in both hands; increased weight; previous knee operation; previous knee injury; family history of end-stage osteoarthritis; and pain in the knee on most days of the preceding month. Control patients did not have pain nor radiographic findings or risk factors for OA. After limiting our subjects to those aged ≥60years, we excluded those with incident total knee arthroplasty (n=196) and those who died at follow-up (n=137) to allow the ascertainment of the progression of osteoarthritis in the absence of surgical intervention for the knee. We deliberately excluded participants <60years since this population is known to have a lesser degree of functional impairment and disability17 and increased capacity for homeostasis18. The final cohort consisted of 2,252 subjects (Figure 1). Data was collected as part of this study at baseline, and yearly intervals up to six years.
Figure 1. Participant Flow among 17,457 screened in the Osteoarthritis Initiative Protocol.
Patient flow is demonstrated from initial telephone screen to cohort included in this study. Abbreviations: BMI – body mass index; MRI – magnetic resonance imaging; OA – osteoarthritis; PASE – physical activity for the elderly survey; SF – short form; WC – waist circumference
Study Measures
Demographic, medical, and social characteristics were collected via self-reported questionnaire. Age at the initial visit was considered age at baseline in years. We categorized subjects according to age range (60-69years and ≥70years). Marital status was dichotomized as ‘married’ or ‘single’, with the latter consisting of widow, divorced, separated or never married. We categorized education as follows: high school (graduated or not); attended college; college graduate; graduate level. Ever smokers were considered patients who smoked >100 cigarettes in their life. Self-reported knee pain was assessed using the Western Ontario and McMaster University OA Index (WOMAC) Pain Scale on a 5-point Likert scale, ranging from 0-20. Subjects with x-ray defined knee osteoarthritis were considered to have knee OA. Co-morbidity was assessed using the Charlson co-morbidity index19. Knee extensor strength was measured with patients sitting in a Good Strength chair with their back supported, and the knee joint at a 60° angle measured by a goniometer for transducer placement. Each participant performed two practice trials at 50% effort, performed after a 15-20minute warm-up session. A transducer was placed behind the participant's leg, centered behind the leg, with the bottom 2cm above the calcaneous. The leg was strapped and the participant was instructed to do three trials at maximum effort. Measurements were indicated in newtons (N) and full details of the study protocol are available online at http://oai.epi-ucsf.org. Maximum knee strength was considered the greater of the left or right knee extensor strength, in newtons.
Primary Predictor
We assessed physical activity using the 26-item in-person Physical Activity Scale for the Elderly (PASE)20, which is a measure of occupational, household, and leisure activities during a one-week period in older adults. The leisure activities require participants to self report the number of days per week, and hours per day of performing an activity. All study assessments were performed by self-reported questionnaire. A greater score indicates greater level of activity. There is no minimal clinically important difference score available for PASE for clinicians to use when determining patient response to treatment and to guide clinical decision-making, although there are minimally detectable changes based on 40 individuals with hip osteoarthritis21. This validation study defined a minimal detectable change of 87 points for total PASE score. Population normative data are available for those ≤70years (142.9points), and those >70 years (110.8points)22.
Outcome Measures
We identified both objective and subjective measures of physical function. Gait speed (m/s) was measured using the 20m walk test, a validated measure of functional status in people with knee osteoarthritis22. Participants walked 20m in an unobstructed corridor at their usual walking speed and were timed. Long-term disability was measured using the validated Late-Life Function and Disability Instrument (LLDI)23 which focuses on functional limitations and frequency limitations based on a wide variety of life tasks. Functional limitations focus on instrumental and management domain scores, while frequency limitations focus on personal and social role domains. These domains parallel Nagi's disablement framework24 on disability in community-dwelling adults. A person's inability to perform daily activities reflects functional limitations, while frequency limitations characterize the inability to engage in social environments and major life tasks. Higher scores correlate with higher functional levels (less disability) and is scored on a 0-100 scale. The scale corresponds to both the physical functioning subscale of the SF-36 and London Handicap Scale25. The Short-Form 12 (SF12) is an easily administered, self-reported, valid, and reliable, measure of a person's perceived health status26 comprised of Physical and Mental component scores assessed on a Likert scale. For the purposes of this study focusing on physical function, we represent only the physical component score (PCS). A score of 50 is the mean of the general population. Chair stand test is a validated measure of leg strength27 measured using a straight-backed chair without arms, with the seat height of 45cm, placed against a wall for stability. Participants were asked to fold their arms, stand up as quickly as they can five times, rising until they are in a fully standing position. The test was timed and measured to the hundredth of a second.
Statistical Analysis
We stratified PASE by sex-specific quartiles [males: <97, 98-144, 145-187, >188; females: <85, 86-124, 125-170, >171]. Our univariate analysis assessed differences between the four quartiles of PASE (low, 25-50, 50-75, >75% percentile (high)) on all baseline characteristics. Data was presented as means ± standard deviations or counts (percent). One-way ANOVA assessed differences among categories.
Separate models were performed by sex as functional decline differs by sex with age17 as we demonstrated in previous analyses. Linear mixed models tested these associations including both PASE quartile and time-main effects as well as PASE quartiles*time interaction terms. In this way, both differences at baseline and 6 years could be examined along with differences in change over time between the PASE quartiles. Data was available at a number of times points including baseline, 12, 24, 36, 48, 60 and 72 months for SF-12 and gait speed, and only at six-years for LLDI. Unadjusted models were used to estimate baseline and 6-year means for each outcome. For each model, we performed: 1) within PASE quartile, a comparison of mean outcome at baseline and 6-years; 2) within time-point, a comparison of mean outcome across all categories; 4) mean change from baseline to 6-years, a comparison across all PASE quartiles. Each of these tests were performed by creating appropriate contrasts of model parameter estimates from the unadjusted models. Linear mixed models adjusting for age, education, race, cohort type (incidence, progression, control), Charlson co-morbidity score 19, and smoking status were fit. Within these models, we compared both the main effect of PASE quartile (representing differences between PASE quartiles at baseline) as well as the interaction between PASE quartiles and time (representing differences between PASE quartiles in change over time). We additionally incorporated knee extensor strength in our models as a surrogate for sarcopenia in our modeling28. We fit the adjusted models and also stratified by age group based on our previous analysis that suggested differences in physical function based on age17, 29. Sensitivity analyses compared baseline characteristics in those with and without missing data. Multicollinearity was assessed using variance inflation factor and a value greater than 5.0 was considered collinear. As an exploratory analysis, we performed sex-specific analysis by cohort type (progression and incidence only). Data was analyzed using STATA version 12 (STATACorp, College Station, TX). A p-value <0.05 was considered statistically significant.
RESULTS
Mean age ranged from 66.8 to 70.1 years in males, and 65.8 to 68.8 years in females. Of the 2,252 subjects, 1,397 were females. Baseline characteristics of each sex-specific cohort are presented in Table 1. Generally, covariates were different amongst PASE quartile in females than in males. Subjects with incomplete data (n=333) had higher WOMAC scores, slower gait speeds and less yearly income in both males and females than those included in our cohort (Appendix). Table 2 outlines the unadjusted subjective and objective outcomes according to PASE quartile by sex. Trends suggest that the highest quartile of PASE in both males and females had higher LLDI frequency scores, but was significant only for limitations in females. Gait speed, chair stand, and SF-12 physical function scores decreased with PASE quartile, although there were no significant differences among the change in score in the four quartiles between baseline and follow-up scores.
Table #1.
Overall Baseline Characteristics of Cohort (n=2252)
| Males (n=842) | Females (n=1,397) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 PASE | Q2 PASE | Q3 PASE | Q4 PASE | P-value | Q1 PASE | Q2 PASE | Q3 PASE | Q4 PASE | P-value | |
| <97 N=214 | 98-144 N=210 | 145-187 N=209 | >188 N=209 | <85 N=355 | 86-124 N=347 | 125-170 N=351 | >171 N=344 | |||
| Age | 70.1 ± 5.3 | 69.9±5.2 | 68.8±4.9 | 66.8±5.3 | <0.001 | 68.8±5.6 | 68.4±5.2 | 67.7±5.1 | 65.8±4.9 | <0.001 |
| Education Status | ||||||||||
| < High School | 44 (20.8) | 27 (12.9) | 32 (14.4) | 18 (8.6) | 88 (24.8) | 91 (25.6) | 71 (20.0) | 105 (29.6) | ||
| Some College | 33 (15.6) | 31 (14.8) | 33 (15.9) | 41 (19.6) | 0.04 | 87 (25.1) | 90 (25.9) | 68 (19.6) | 102 (29.4) | 0.02 |
| College | 42 (19.8) | 55 (26.2) | 40 (19.2) | 54 (25.8) | 78 (22.4) | 108 (31.0) | 52 (14.9) | 110 (31.6) | ||
| >College | 93 (43.9) | 97 (46.2) | 103 (49.5) | 96 (45.9) | 65 (19.1) | 113 (33.1) | 42 (12.3) | 121 (35.5) | ||
| Yearly Income | ||||||||||
| >$50,000 | 121 (58.7) | 135 (65.9) | 127 (64.8) | 147 (72.4) | 0.04 | 147 (43.9) | 144 (43.5) | 150 (45.3) | 172 (55.0) | 0.01 |
| Marital Status | ||||||||||
| Married | 166 (78.3) | 175 (83.3) | 165 (79.3) | 176 (84.2) | 0.32 | 181 (51.1) | 209 (60.2) | 209 (60.1) | 196 (57.5) | 0.05 |
| Race | ||||||||||
| White | 165 (77.1) | 190 (90.5) | 182 (87.1) | 187 (89.5) | 248 (70.1) | 279 (80.4) | 299 (85.2) | 287 (83.4) | ||
| Black | 42 (19.6) | 16 (7.6) | 25 (12.0) | 15 (7.2) | 0.002 | 93 (26.3) | 63 (18.2) | 46 (13.1) | 45 (13.1) | <0.001 |
| Asian | 3 (1.4) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 8 (2.3) | --- | 3 (0.9) | 3 (0.9) | ||
| Charlson Score | 0.60 ± 1.08 | 0.60 ± 1.05 | 0.53 ± 0.99 | 0.54 ± 1.08 | <0.001 | 0.46±0.88 | 0.41±0.85 | 0.37±0.70 | 0.28±0.67 | 0.03 |
| Baseline WOMAC Right | 10.9 ± 12.5 | 9.2 ± 12.0 | 8.2 ± 11.0 | 8.9 ± 11.2 | 0.007 | 15.4 ± 16.9 | 12.9±15.6 | 10.9±13.1 | 10.9±13.0 | <0.001 |
| Baseline WOMAC Left | 11.7 ± 15.0 | 9.8±13.3 | 7.9±12.7 | 8.8 ± 13.5 | 0.03 | 14.5±17.3 | 11.7±15.3 | 10.0±13.8 | 11.1±14.5 | 0.001 |
| Ever Smoker | 115 (54.3) | 123 (58.9) | 111 (54.4) | 111 (53.4) | 0.67 | 166 (47.2) | 153 (44.5) | 175 (50.9) | 159 (46.8) | 0.41 |
| # Medications | 4.24 ± 2.77 | 3.79 ± 2.41 | 3.63 ± 2.30 | 3.32 ± 2.20 | 0.004 | 4.29±2.56 | 3.88±2.41 | 3.66±2.40 | 3.50±2.33 | <0.001 |
| Body mass index | 28.9±3.7 | 28.8±4.1 | 28.6±4.0 | 28.3±3.7 | 0.46 | 29.0±5.3 | 27.9±4.8 | 27.5±4.5 | 27.8±4.8 | <0.001 |
| Maximum Knee Extensor strength (N) | 411.2±108.9 | 424.1±107.1 | 434.2±109.0 | 463.0±110.8 | <0.001 | 262.4±82.2 | 283.4±77.3 | 284.3±73.5 | 302.5±77.7 | <0.001 |
| Cohort Allocation | ||||||||||
| Incidence | 143 (66.8) | 153 (72.9) | 153 (73.2) | 150 (71.8) | 235 (66.2) | 256 (73.8) | 270 (76.9) | 262 (76.2) | ||
| Progression | 69 (32.2) | 54 (25.7) | 50 (23.9) | 53 (25.4) | 0.31 | 118 (33.2) | 91 (26.2) | 76 (21.7) | 76 (22.1) | 0.001 |
| Control | 2 (0.93) | 3 (1.4) | 6 (2.9) | 6 (2.9) | 2 (0.6) | -- | 5 (1.4) | 6 (1.7) | ||
All values are represented as mean ± standard deviation, or count (%).
Abbreviations: BMI – body mass index; Q – quartile; WOMAC – Western Ontario McMaster Universities Arthritis Index
p-values represent analysis of variance between four quartile categories in each sex
At baseline, there were 13 subjects without baseline PASE scores
Table 2.
Sex-Specific Unadjusted outcomes of Functional Outcomes by Physical Activity Quartile
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 PASE | Q2 PASE | Q3 PASE | Q4 PASE | P-valueB | Q1 PASE | Q2 PASE | Q3 PASE | Q4 PASE | P-valueB | |
| Quartile Score | <97 N=214 | 98-144 N=210 | 145-187 N=209 | >188 N=209 | <85 N=355 | 86-124 N=347 | 125-170 N=351 | >171 N=344 | ||
| SF-12 Physical | ||||||||||
| Baseline | 47.2±9.0 | 48.5±8.6 | 50.6±7.6 | 51.2±6.9 | <0.001 | 44.7±10.9 | 48.7±8.7 | 50.0±8.0 | 50.3±8.1 | <0.001 |
| Follow-up | 45.4±9.4 | 46.5±8.7 | 47.1±9.3 | 48.7±9.1 | 0.01 | 42.8±11.3 | 45.5±9.9 | 47.4±9.0 | 48.6±9.2 | <0.001 |
| P-valueA | <0.001 | <0.001 | <0.001 | <0.001 | 0.78C | <0.001 | <0.001 | <0.001 | <0.001 | 0.37C |
| SF-12 Mental | ||||||||||
| Baseline | 55.6±9.0 | 55.7±8.6 | 55.4±7.6 | 55.4±6.9 | 0.94 | 54.3±10.9 | 54.0±8.7 | 54.4±8.0 | 54.5±8.1 | 0.88 |
| Follow-up | 55.6±7.5 | 54.9±7.2 | 55.4±7.3 | 55.8±7.5 | 0.72 | 53.7±9.3 | 54.5±8.5 | 54.6±7.5 | 54.0±8.8 | 0.54 |
| P-valueA | 0.18 | 0.24 | 0.83 | 0.40 | 0.38C | 0.39 | 0.82 | 0.95 | 0.29 | 0.86C |
| Gait Speed | ||||||||||
| Baseline | 1.27 ± 0.22 | 1.30 ±0.21 | 1.35±0.20 | 1.37±0.17 | <0.001 | 1.19 ±0.22 | 1.26±0.21 | 1.30±0.19 | 1.32±0.21 | <0.001 |
| Follow-up | 1.20 ±0.21 | 1.26±0.19 | 1.29±0.22 | 1.32±0.19 | <0.001 | 1.15±0.21 | 1.21±0.21 | 1.24±0.20 | 1.27±0.22 | <0.001 |
| p-valueA | <0.001 | <0.001 | <0.001 | <0.001 | 0.40C | <0.001 | <0.001 | <0.001 | <0.001 | 0.69C |
| LLDI | ||||||||||
| Frequency | 51.7±5.0 | 52.6±5.8 | 54.6±6.1 | 55.4±6.0 | <0.001 | 54.2±6.0 | 56.2±6.0 | 56.8±6.1 | 57.7±6.7 | <0.001 |
| Limitation | 81.1±15.6 | 81.5±15.8 | 82.6±15.7 | 84.9±14.6 | 0.10 | 76.3±15.5 | 79.6±15.5 | 81.5±13.9 | 83.0±14.9 | <0.001 |
| Chair Stand | ||||||||||
| Baseline | 0.46±0.14 | 0.49±0.15 | 0.52±0.15 | 0.51±0.13 | <0.001 | 0.44±0.14 | 0.47±0.13 | 0.48±0.13 | 0.49±0.13 | <0.001 |
| Follow-up | 0.47±0.13 | 0.52±0.17 | 0.53±0.13 | 0.54±0.15 | <0.001 | 0.46±0.14 | 0.48±0.14 | 0.51±0.14 | 0.53±0.17 | <0.001 |
| P-valueA | 0.44 | 0.02 | 0.94 | 0.06 | 0.26C | 0.04 | 0.18 | 0.29 | 0.006 | 0.17C |
All values represented are means ± standard deviation or count (%)
p-values within PASE quartiles represent significance of change from baseline to follow-up.
p-values represent overall test of difference in means between PASE quartiles
P-values represent differences in change from baseline to follow-up between PASE quartiles
PASE – Physical Activity Score for the Elderly
LLDI – Late-life function & Disability Index
Q – Quartile
SF-12 – Short form 12
Multivariable modeling is presented in Table 3 and 4. We used knee extensor strength as a surrogate for sarcopenia in our models and found that the estimates were reduced among all outcome variables, although general trends were similar (data not shown). Estimated means for the adjusted models are plotted over time (Figure 2 & 3). In males, higher PASE scores, as represented by increasing quartiles, were associated with higher SF-12 PCS scores, gait speed and LLDI-frequency scores. The decline in PCS scores and chair stand speed, occurred earlier in the 60-70 year age group than in the 70+ year age group. Rates of change (time*PASE quartile interaction) in all models were similar by age cohort , although change in gait speed slope differed in both age groups. In females, declines in PCS occured in both age categories as did gait speed. LLDI scores appeared to decline in the 70+ year age group, while chair stand speeds were higher earlier in life. No collinearity was observed in any of our models other than with Race which consistently had a variance inflation factor greater than 5.0. In our exploratory analysis, notable differences were by PASE quartile in females across all outcomes in the progression cohort, and seen only in PCS and LLDI scores in the incident cohort (Appendix 2). Generally there were no significant changes in the outcomes over time by PASE quartile (time × PASE quartile interaction).
Table 3.
Multivariable Regression Analysis of Primary Outcome Measures of Quality of Life, Lower Extremity Function and Late Life Disability (n=2,210) – MALES
| MALES | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 43.3 [34.9:51.6] | 1.49 [1.27:1.70] | 56.3 [52.9-59.6] | 100.0 [91.2:108.9] | 0.42 [0.28:0.57] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50 percentile PASE | 0.90 [−0.45:2.25] | 0.07 [−0.03:0.04] | 0.35 [−0.18:0.87] | 0.02 [−1.39:−1.43] | 0.03 [0.004:0.05] |
| 50-75th percentile PASE | 1.43 [0.06:2.79] | 0.05 [0.02:0.09] | 2.21 [1.68:2.74] | −0.15 [−1.56:1.26] | 0.04 [0.01:0.06] |
| High PASE | 1.90 [0.52:3.28] | 0.05 [0.02:0.09] | 2.74 [2.21:3.28] | 0.76 [−0.66:2.19] | 0.02 [−0.001:0.05] |
| Time | −0.58 [−0.76:−0.40] | −0.015 [−0.02:−0.012] | -- | -- | 0.0003 [−0.003:0.003] |
| Time * Low PASE | Ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | 0.14 [−0.11:0.40] | 0.004 [−0.001:0.08] | -- | -- | 0.002 [−0.002:0.006] |
| Time * 50-75percentile PASE | −0.07 [−0.33:0.18] | 0.001 [−0.003:0.006] | -- | -- | 0.0011 [−0.003:0.005] |
| Time * High PASE | 0.11 [−0.14:0.36] | 0.006 [0.001:0.01] | -- | -- | 0.003 [−0.001:0.007] |
| Age 60-70 years | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 36.7 [31.6:41.8] | 1.15 [1.02:1.28] | 50.4 [48.4:52.5] | 73.8 [68.4:79.2] | 0.30 [0.20:0.40] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50percentile PASE | 2.07 [0.18:3.97] | 0.01 [−0.04:0.06] | 0.83 [0.06:1.60] | 3.26 [1.24:5.29] | 0.05 [0.02:0.09] |
| 50-75percentile PASE | 2.16 [0.34:3.98] | 0.04 [−0.01:0.09] | 2.80 [2.07:3.52] | 2.79 [0.89:4.70] | 0.04 [0.01:0.08] |
| High PASE | 3.24 [1.52:4.97] | 0.05 [0.01:0.09] | 2.70 [2.00:3.39] | 3.28 [1.46:5.10] | 0.02 [−0.01:0.05] |
| Time | −0.52 [−0.79:−0.26] | −0.01 [−0.02:−0.01] | -- | -- | 0.0003 [−0.004:0.005] |
| Time * Low PASE | ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | 0.27 [−0.10:0.65] | 0.010 [0.004:0.017] | -- | -- | 0.009 [0.002:0.015] |
| Time * 50-75percentile PASE | −0.021 [−0.38:0.34] | 0.0024 [−0.004:0.009] | -- | -- | −0.0009 [−0.007:0.005] |
| Time * High PASE | 0.23 [−0.11:0.57] | 0.008 [0.002:0.014] | -- | -- | 0.004 [−0.002:0.09] |
| Age 70+ years | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 28.1 [17.0:39.2] | 0.97 [0.70:1.24] | 38.8 [34.1:43.6] | 54.9 [42.2:67.8] | 0.28 [0.11:0.45] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50percentile PASE | −0.32 [−2.29:1.65] | 0.003 [−0.05:0.05] | −0.05 [−0.78:0.67] | −3.84 [−5.70:−2.34] | 0.004 [0.03:0.04] |
| 50-75percentile PASE | 0.59 [−1.47:2.65] | 0.06 [0.01:0.12] | 1.77 [1.00:2.54] | −2.99 [−5.07:−0.91] | 0.03 [−0.006:0.06] |
| High PASE | 0.40 [−1.85:2.64] | 0.07 [0.02:0.13] | 3.33 [2.50:4.15] | −0.39 [−1.85:−2.62] | 0.04 [0.004:0.07] |
| Time | −0.63 [−0.87:−0.39] | −0.02 [−0.02:−0.01] | -- | -- | 0.002 [−0.004:0.004] |
| Time * Low PASE | Ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | 0.05 [−0.28:0.39] | −0.002 [−0.008:0.004] | -- | -- | −0.003 [−0.009:0.002] |
| Time * 50-75percentile PASE | −0.16 [−0.51:0.20] | −0.001 [−0.007:0.006] | -- | -- | 0.004 [−0.002:0.009] |
| Time * High PASE | −0.18 [−0.55:0.19] | 0.002 [−0.005:0.008] | -- | -- | 0.0015 [−0.004:0.007] |
All linear mixed models are adjusted for age, education, race, cohort type (incidence, progression, control), Charlson co-morbidity score, smoking status, and maximum knee strength. Referent category is low quartile of Physical Activity Score for the Elderly (PASE) for each sex. Time-dependent co-variates are included in time × PASE quartile interaction. Boldfaced items indicate p<0.05. LLDI was only available at 6-year follow-up thereby no time interaction term model was considered for this outcome measure. Abbreviations: CI – Confidence Intervals; LLDI – Late-Life Functional and Disability Index; MCS – Mental Component Score; PASE – Physical Activity Score for the Elderly; PCS – Physical Component Score; SF – Short Form
Table 4.
Multivariable Regression Analysis of Primary Outcome Measures of Quality of Life, Lower Extremity Function and Late Life Disability (n=2,210) – Females by age with strength
| FEMALES | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 42.5 [35.3:49.5] | 1.53 [1.36:1.69] | 55.4 [52.6-58.1] | 76.2 [69.8:82.7] | 0.45 [0.34-0.55] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50percentile PASE | 2.21 [1.05:3.38] | 0.06 [0.03:0.08] | 1.69 [1.23:2.14] | 2.32 [1.24:3.40] | 0.01 [−0.003:0.03] |
| 50-75percentile PASE | 3.52 [2.35:4.69] | 0.08 [0.05:0.11] | 2.22 [1.76:2.68] | 3.65 [2.57:4.73] | 0.02 [0.007:0.04] |
| High PASE | 3.68 [2.48:4.87] | 0.07 [0.05:0.10] | 3.13 [2.67:3.60] | 5.13 [4.03:6.22] | 0.03 [0.01:0.04] |
| Time | −0.50 [−0.65:−0.35] | −0.013 [−0.016:−0.01] | -- | -- | 0.002 [−0.003:0.004] |
| Time * Low PASE | ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | −0.20 [−0.40:−0.001] | 0.005 [−0.003:0.004] | -- | -- | 0.0006 [−0.003:0.004] |
| Time * 50-75percentile PASE | −0.003 [−0.20:0.20] | 0.002 [−0.002:0.006] | -- | -- | 0.0008 [−0.0023:0.004] |
| Time * High PASE | 0.08 [−0.12:0.28] | 0.002 [−0.002:0.005] | -- | -- | 0.002 [−0.0013:0.005] |
| Age 60-70 years | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 35.4 [30.6:40.3] | 1.06 [0.95:1.17] | 50.4 [48.3:52.5] | 55.0 [50.3:59.8] | 0.32 [0.25:0.40] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50percentile PASE | 2.34 [0.83:3.85] | 0.07 [0.03:0.10] | 1.29 [0.67:1.91] | 1.89 [0.47:3.32] | 0.02 [−0.002:0.05] |
| 50-75percentile PASE | 3.65 [2.16:5.14] | 0.08 [0.05:0.12] | 1.37 [0.76:1.99] | 3.51 [2.10:4.97] | 0.03 [0.01:0.05] |
| High PASE | 3.52 [2.07:4.98] | 0.08 [0.05:0.11] | 2.26 [1.66:2.85] | 4.65 [3.30:6.01] | 0.04 [0.01:0.06] |
| Time | −0.39 [−0.59:−0.19] | −0.01 [−0.013:−0.006] | -- | -- | 0.002 [−0.001:0.005] |
| Time * Low PASE | Ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | −0.20 [−0.47:0.07] | 0.0004 [−0.005:0.005] | -- | -- | 0.003 [−0.001:0.008] |
| Time * 50-75percentile PASE | 0.02 [−0.25:0.28] | −0.0003 [−0.005:0.004] | -- | -- | 0.001 [−0.003:0.005] |
| Time * High PASE | 0.04 [−0.22:0.29] | 0.002 [−0.003:0.007] | -- | -- | 0.004 [0.00003:0.008] |
| Age 70+ years | SF-12 PCS β (95% CI) | Gait Speed β (95% CI) | LLDI-Frequency β (95% CI) | LLDI-Limitations β (95% CI) | Chair Stand β (95% CI) |
|---|---|---|---|---|---|
| Intercept | 38.5 [31.6:45.4] | 0.90 [0.74:1.06] | 60.7 [58.1:63.1] | 77.7 [72.5:83.9] | 0.33 [0.23:0.42] |
| Low PASE | Ref | Ref | Ref | Ref | Ref |
| 25-50percentile PASE | 2.13 [0.29:3.97] | 0.04 [−0.003:0.09] | 1.89 [1.24:2.54] | 2.53 [0.90:4.17] | 0.007 [−0.019:−0.03] |
| 50-75percentile PASE | 3.50 [1.58:5.42] | 0.07 [0.03:0.12] | 3.21 [2.55:3.88] | 3.95 [2.27:5.62] | 0.02 [−0.008:0.05] |
| High PASE | 4.71 [2.61:6.81] | 0.09 [0.04:0.14] | 4.65 [3.93-5.37] | 7.11 [5.30:8.91] | 0.02 [−0.01:0.05] |
| Time | −0.63 [−0.85:−0.42] | −0.018 [−0.022:−0.014] | -- | -- | 0.0014 [−0.002:0.005] |
| Time * Low PASE | Ref | Ref | -- | -- | Ref |
| Time * 25-50percentile PASE | −0.23 [−0.53:0.08] | 0.0003 [−0.006:0.006] | -- | -- | −0.004 [−0.009:0.0007] |
| Time * 50-75percentile PASE | −0.09 [−0.40:0.23] | 0.004 [−0.002:0.01] | -- | -- | 0.001 [−0.004:0.006] |
| Time * High PASE | 0.024 [−0.31:0.36] | −0.004 [−0.011:0.003] | -- | -- | −0.005 [−0.01:0.00002] |
All linear mixed models are adjusted for age, education, race, cohort type (incidence, progression, control), Charlson co-morbidity score, maximum knee strength, smoking status. Referent category is low quartile of Physical Activity Score for the Elderly (PASE) for each sex. Time-dependent co-variates are included in time × PASE quartile interaction. Boldfaced items indicate p<0.05. LLDI was only available at 6-year follow-up thereby no time interaction term model was considered for this outcome measure. Abbreviations: CI – Confidence Intervals; LLDI – Late-Life Functional and Disability Index; MCS – Mental Component Score; PASE – Physical Activity Score for the Elderly; PCS –Physical Component Score; SF – Short Form
Figure 2.
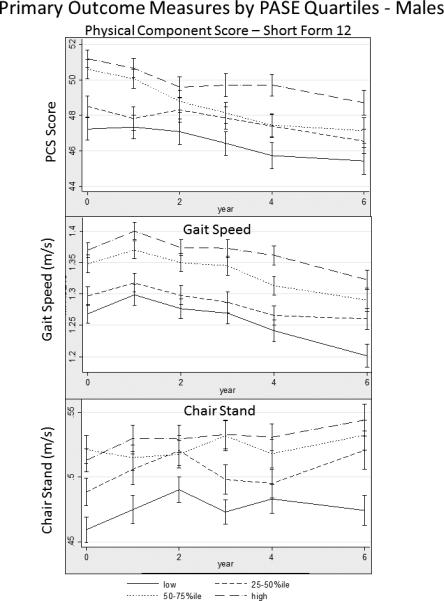
Time-trends of Primary Outcome Measures Among Older Adult Participants in the Osteoarthritis Initiative in Males
Figure 3.
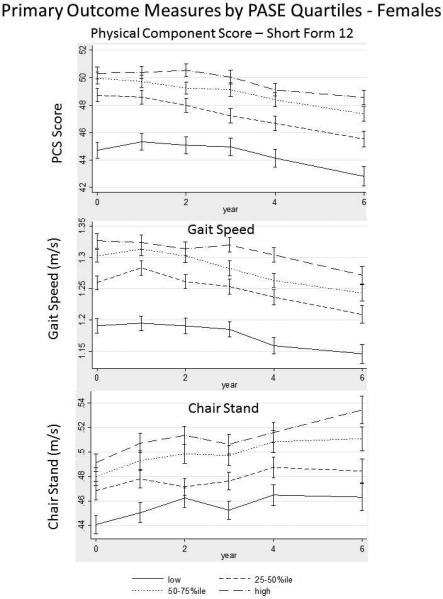
Time-trends of Primary Outcome Measures Among Older Adult Participants in the Osteoarthritis Initiative in Females
DISCUSSION
Higher self-reported physical activity levels are associated with higher subjective and objective measures of physical function in both males and females in this population with and at risk for osteoarthritis. Our data highlight the importance of encouraging high levels of physical activity in older adults.
Our results confirm the importance of physical activity on longitudinal changes in both objective (gait speed) and subjective (LLDI) functional measures in older adults. Gait speed, in particular, is a marker of disability that is associated with functional decline and mortality30. Specifically in a population at risk for osteoarthritis, the results suggest recommending exercise to reduce risk of onset of disability. Importantly, we demonstrated an effect of activity level on physical function. Previous reports in older adults suggested that there may be a ceiling effect of physical activity, and overuse was associated with detrimental outcomes including mortality31, 32. Our results suggest that patients should not be deterred from engaging in higher levels of activity. We do caution the reader that the overall PASE score does not discriminate between aerobic and anaerobic activity, or intensity, all of which impact cardiovascular and musculoskeletal systems in different manners. As such, our results provide a prelude of further study of the association of different types of physical activity and their degree of magnitude on the primary outcome of physical function.
We introduced a time × PASE quartile interaction term in our models and surprisingly found that rates of change were no different by age category. We believe that the observed changes in scores (in all our outcome measures), is likely reflected by the specifics of this population, that the magnitude of such declines occur earlier in life. This was further validated in our exploratory results stratified by cohort type where we expected changes over time, particularly in the progression cohort as they had risk factors for osteoarthritis.
Interestingly, our data confirms previous sex-specific impact of muscle mass and strength on long-term physical function by our group33. While males have higher muscle strength, after multivariable adjustment, there was attenuation of the results observed, suggesting that physical activity in males is likely, in part, mediated through muscle strength. While sarcopenia is known to impact physical function28, and may be present in subjects with OA, this modulation of our results requires further exploration.
Physical activity appears to play a mediating role in the relationship with physical function. Our results suggest that high levels of self-reported physical activity may be associated with lower long-term disability scores, in both sexes. The mechanisms that explain this phenomenon are thought to be on the biological level. Physical activity is a known surrogate for cardiovascular fitness34 may dampen pro-inflammatory cytokines, including IL-1, IL-6, and TNF-a35, all of which may lead to homeostatic derangements leading to frailty and subsequently disability18, 36. Joints of subjects with osteoarthritis37, 38 may exhibit a similar inflammatory milieu commonly implicated in the aforementioned disorders. While these biomarkers would be helpful in understanding the potential mechanisms of frailty and disability, they were unavailable for analysis in this dataset, but could be the subject of future investigation.
We caution the reader that our estimates may in fact be conservative. Our cohort was relatively young (mean age ~68years) and thus longer follow-up may be needed to observe the changes in physical function observed with aging. Disability and frailty often are preceded by compensated functional decline17, data that would not be reflected in our findings. Second, participants were ambulatory, community-based adults, which may not be fully representative of the general older adult population. Third, the degree of co-morbidity was modest, implying a healthier population. Fourth, while our estimates are statistically significant, it is unclear whether our results are of clinical significance. Lastly, we purposely created quartiles of PASE to allow us to categorize whether intermediate categories of activity levels were any different than higher categories. We acknowledge that differences may be introduced simply because categories reflect different points in the distribution and thus we presented our data using PASE as a continuous variable as well.
Other limitations in this study exist. The OAI dataset was designed specifically to examine longitudinal outcomes of OA; our analysis may not have coincided with the primary scope of the study design. Both males and females not included had a higher degree of comorbidity and lower functional status at baseline, thereby possibly underestimating the true effect observed in our results. Our study results were also at risk for possible over-adjustment but we deliberately presented unadjusted data to show the similarities after accounting for these a priori variables. While race was a highly collinear variable, ethnicity and education can impact both physical activity39, 40 and disability41, 42 and hence was included in the model.
CONCLUSION
In older adults, higher levels of physical activity are associated with higher self-reported and objective functional measures. Encouraging patients with OA to be physically active should be strongly considered to improve joint pain and overall walking performance.
Footnotes
DISCLOSURES/ CONFLICTS OF INTEREST: Supported in part by the Department of Medicine and the Dartmouth Centers for Health and Aging. Dr. Batsis was the recipient of the 2014 American Geriatrics Society/Merck New Investigator Award
REFERENCES
- 1.Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I: Effects of comorbid medical conditions. J Clin Epidemiol. 1994;47:809–815. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 2012;4:S10–19. doi: 10.1016/j.pmrj.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Tanamas SK, Wluka AE, Davies-Tuck M, et al. Association of weight gain with incident knee pain, stiffness, and functional difficulties: a longitudinal study. Arthritis Care Res (Hoboken) 2013;65:34–43. doi: 10.1002/acr.21745. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 6.Eden KB, Orleans CT, Mulrow CD, Pender NJ, Teutsch SM. Does counseling by clinicians improve physical activity? A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:208–215. doi: 10.7326/0003-4819-137-3-200208060-00015. [DOI] [PubMed] [Google Scholar]
- 7.American College of Sports M. Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 8.McGuire KA, Ross R. Incidental physical activity is positively associated with cardiorespiratory fitness. Med Sci Sports Exerc. 2011;43:2189–2194. doi: 10.1249/MSS.0b013e31821e4ff2. [DOI] [PubMed] [Google Scholar]
- 9.Lopopolo RB, Greco M, Sullivan D, Craik RL, Mangione KK. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: a meta-analysis. Phys Ther. 2006;86:520–540. [PubMed] [Google Scholar]
- 10.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001;56:23–35. doi: 10.1093/gerona/56.suppl_2.23. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 13.Wolinsky FD, Stump TE, Clark DO. Antecedents and consequences of physical activity and exercise among older adults. Gerontologist. 1995;35:451–462. doi: 10.1093/geront/35.4.451. [DOI] [PubMed] [Google Scholar]
- 14.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 15.Uthman OA, van der Windt DA, Jordan JL, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morey MC, Sullivan RJ., Jr. Medical assessment for health advocacy and practical strategies for exercise initiation. Am J Prev Med. 2003;25:204–208. doi: 10.1016/s0749-3797(03)00180-6. [DOI] [PubMed] [Google Scholar]
- 17.Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health. 1997;87:378–383. doi: 10.2105/ajph.87.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Svege I, Kolle E, Risberg MA. Reliability and validity of the Physical Activity Scale for the Elderly (PASE) in patients with hip osteoarthritis. BMC Musculoskelet Disord. 2012;13:26. doi: 10.1186/1471-2474-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 23.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52:1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 24.Nagi SZ. A Study in the Evaluation of Disability and Rehabilitation Potential: Concepts, Methods, and Procedures. Am J Public Health Nations Health. 1964;54:1568–1579. doi: 10.2105/ajph.54.9.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwood RH, Rogers A, Dickinson E, Ebrahim S. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Qual Health Care. 1994;3:11–16. doi: 10.1136/qshc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware J, Kosinski M, Keller S. The Mental Health Summary Scales. The Health Institute, New England Medical Center; Boston, MA: 1995. How to Score the SF-12 Physical. [Google Scholar]
- 27.Meretta BM, Whitney SL, Marchetti GF, Sparto PJ, Muirhead RJ. The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J Vestib Res. 2006;16:233–243. [PubMed] [Google Scholar]
- 28.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batsis JA, Zbehlik AJ, Barre LK, Mackenzie TA, Bartels SJ. The impact of waist circumference on function and physical activity in older adults: longitudinal observational data from the osteoarthritis initiative. Nutr J. 2014;13:81. doi: 10.1186/1475-2891-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart. 2014;100:1037–1042. doi: 10.1136/heartjnl-2013-305304. [DOI] [PubMed] [Google Scholar]
- 32.Mons U, Hahmann H, Brenner H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart. 2014;100:1043–1049. doi: 10.1136/heartjnl-2013-305242. [DOI] [PubMed] [Google Scholar]
- 33.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Lopez-Jimenez F. Normal weight obesity and functional outcomes in older adults. European journal of internal medicine. 2014 doi: 10.1016/j.ejim.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Rheaume C, Arsenault BJ, Dumas MP, et al. Contributions of cardiorespiratory fitness and visceral adiposity to six-year changes in cardiometabolic risk markers in apparently healthy men and women. J Clin Endocrinol Metab. 2011;96:1462–1468. doi: 10.1210/jc.2010-2432. [DOI] [PubMed] [Google Scholar]
- 35.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Cur Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374–3377. doi: 10.1002/art.24881. [DOI] [PubMed] [Google Scholar]
- 38.Sturmer T, Brenner H, Koenig W, Gunther KP. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis. 2004;63:200–205. doi: 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease C, Prevention Prevalence of regular physical activity among adults--United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56:1209–1212. [PubMed] [Google Scholar]
- 40.Shaw BA, Spokane LS. Examining the association between education level and physical activity changes during early old age. J Aging Health. 2008;20:767–787. doi: 10.1177/0898264308321081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory PC, Szanton SL, Xue QL, Tian J, Thorpe RJ, Fried LP. Education predicts incidence of preclinical mobility disability in initially high-functioning older women. The Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2011;66:577–581. doi: 10.1093/gerona/glr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams ED, Tillin T, Whincup P, Forouhi NG, Chaturvedi N. Ethnic differences in disability prevalence and their determinants studied over a 20-year period: a cohort study. PLoS One. 2012;7:e45602. doi: 10.1371/journal.pone.0045602. [DOI] [PMC free article] [PubMed] [Google Scholar]