Abstract
Authentication and quality assessment of Cordyceps sinensis, a precious and pricey natural product that offers a variety of health benefits is highly significant. To identify effective chemical markers, authentic C. sinensis was thoroughly screened by using HPLC-MS/MS. In addition to many previously reported ingredients, two glycosides, i.e. cyclo-Ala-Leu-rhamnose and Phe-o-glucose were detected for the first time in this material. Six ingredients detected, including cordycepin, D-mannitol, Phe, Phe-o-glucose, cyclo-Gly-Pro, and cyclo-Ala-Leu-rhamnose, were selected as a collection of chemical markers. An HPLC-MS/MS method was developed to simultaneously quantify them with sensitivity and specificity. The method had limits of detection ranging from 0.008 μg mL−1 for cordycepin to 0.75 μg mL−1 for cyclo-Gly-Pro. Recovery was found between 96% and 103% in all tests. To evaluate the effectiveness of the marker collection proposed, 5 authentic C. sinensis samples and 5 samples of its substitutes were analyzed. Cordycepin, D-mannitol, and Phe were found present in all samples. The contents ranged from 0.0076 to 0.029% (w/w) for cordycepin, 0.33 to 18.9 % for mannitol, and 0.0013 to 0.642% for Phe. Interestingly, the two glycosides, Phe-o-glucose and cyclo-Ala-Leu-rhamnose were detected only in authentic C. sinensis samples. These results indicated that the proposed protocol based on HPLC-MS/MS quantification of the markers might have a great potential in authentication and quality assessment of C. sinensis.
Keywords: Chemical markers, Cordyceps sinensis, Cordycepin, Glycosides, Authentication, HPLC-MS
Graphical abstract
Introduction
Cordyceps sinensis is a precious and very pricey natural material that offers many health benefits and has been used for a long time in traditional Oriental medicine in order to treat fatigue, respiratory diseases, renal dysfunction, arrhythmias and other heart diseases, etc.[1–5]. Lab studies have shown that extracts of Cordyceps exhibit pharmacological effects, including antifungal, antibacterial, anticancer, anti-inflammatory, and antioxidant [6–11]. In addition to its therapeutic use, C. sinensis is widely used as a folk tonic food or an invigorant in Asia. Due to a very limited availability of C. sinensis, various cultivated or cultured substitutes are produced [3–5, 12–13]. Studies comparing C. sinensis with its substitutes in regards to their chemical compositions and medicinal effects have been receiving a great amount of research interest [14–17]. Several active ingredients such as cordycepin (i.e. 3′-deoxyadenosine), nucleosides, and polysaccharides were suggested as markers of C. sinensis for quality control purposes [3, 18–20].
As a biological hybrid of larva and parasitic fungus, C. sinensis contains a complex enzymatic system and many ingredients of medicinal value. Nucleosides are believed to be an important group of bioactive components in Cordyceps [3]. Cordycepin, was first isolated from cultured Cordyceps militaris, a related species of C. sinensis commonly used as a substitute. It has been shown to exhibit potent antitumor and antimicrobial activities [8, 11, 21]. Polysaccharides were detected at high levels in Cordyceps. Their anticancer effects were found to come from an enhancement of body’s immune system instead of direct cytocidal action [4]. In addition, amino acids, sterols, fatty acids and cyclic peptides were also detected in Cordyceps [1–5]. To date, various methods have been developed for analysis of bioactive components in C. sinensis [18, 20]. Most of them were based on thin layer chromatography (TLC), high performance liquid chromatography (HPLC), and capillary electrophoresis (CE) [22–26]. While easy to assess and to perform, these methods lack the capability of chemical structure identification, which in some cases may produce false results, particular when analyzing such a complex sample as C. sinensis. LC [27–28] and CE [29] methods with mass spectrometric detection for determination of nucleosides and their bases in Cordyceps samples were also reported.
The aim of the present study was to develop a facile and effective protocol based on HPLC-MS/MS quantification for authentication and quality assessment of C. sinensis. The authentic material was thoroughly screened in order to identify the ingredients that might serve as effective markers. Special attention has been given to cyclic peptides and glycosides since C. sinensis contains a very complex enzymatic system. Peptide cyclization and glycosylation are mainly enzymatic processes in a form of co-translational and post-translational modification. Based on the medicinal significance and detectability of the ingredients identified in C. sinensis, a set of compounds were chosen as a collection of chemical markers. An HPLC-MS/MS method was then developed and validated for simultaneous quantification of the target markers. The effectiveness of the HPLC-MS/MS based protocol for authentication and quality assessment of C. sinensis was demonstrated by analyzing 5 samples of the authentic natural product and 5 samples of its substitutes.
Experimental section
Reagents and Materials
Cordycepin, D-mannitol, Cyclo-Gly-Pro, Cyclo-Ala-Leu, phenylalanine, HPLC grade methanol, and formic acid were purchased from Sigma-Aldrich (St. Louise, MO, USA). Other chemicals used were of analytical grade. Milli-Q water (Millipore, Bedford, MA) was used throughout the work.
Cordyceps samples
A total of 10 samples were analyzed in this study. These included 5 authentic C. sinensis samples, classified as caterpillar host and mycelium of C. sinensis which were collected in different regions of China. Five samples of Cordyceps extract products (in the form of tablets) were purchased from local health supplements stores in the US. The sample information is summarized in Table 1.
Table 1.
Cordyceps Samples analyzed in the study*
| Sample Number | Sample name | Origin | Date of collection or Batch number |
|---|---|---|---|
| 1 | Caterpillar host of C. sinensis | Qinghai Province, China | 05/11/2014 |
| 2 | Mycelium of C. sinensis | Qinghai Province, China | 05/11/2014 |
| 3 | Caterpillar host of C. sinensis | Hubei Province, China | 07/08/2014 |
| 4 | Caterpillar host of C. sinensis | AnHui Province, China | 09/23/2014 |
| 5 | Mycelium of C. sinensis | AnHui Province, China | 09/23/2014 |
| 6 | Cordyceps 8:1 extract | Local Market | 140417 04/16 |
| 7 | Reishi Standardized | Local Market | 20027414 |
| 8 | Cordyceps Mushroom | Local Market | 764402 |
| 9 | Cordyceps Sinensis | Local Market | 04/30/2016 |
| 10 | Cordyceps | Local Market | H2372 08/15 |
Samples #6~10 were supplement tablets prepared from C. sinensis or its substitutes.
Sample preparation
C. sinensis samples were cryogenically ground and homogenized to obtain a uniform matrix. The dietary supplements samples were in the form of capsules or tablets. Ten capsules or tablets were weighed for each sample, and the average was taken as the weight of each capsule or tablet of the sample. About 500 mg of C. sinensis samples or equivalent amount for the dietary samples were weighed out and transferred into a centrifuge tube. Methanol/water (8:2, 20 mL) was added to the sample. The mixture was placed on a shaker for 12 hours, and then in an ultrasonic bath for 30 min. After extraction, the mixture was centrifuged at 6000 rpm for 10 min to obtain extract. The extract was diluted with the mobile phase (1:1) and filtered through a 0.22 μm nylon syringe filter before being injected into the HPLC-MS/MS system for analysis.
Standard solutions
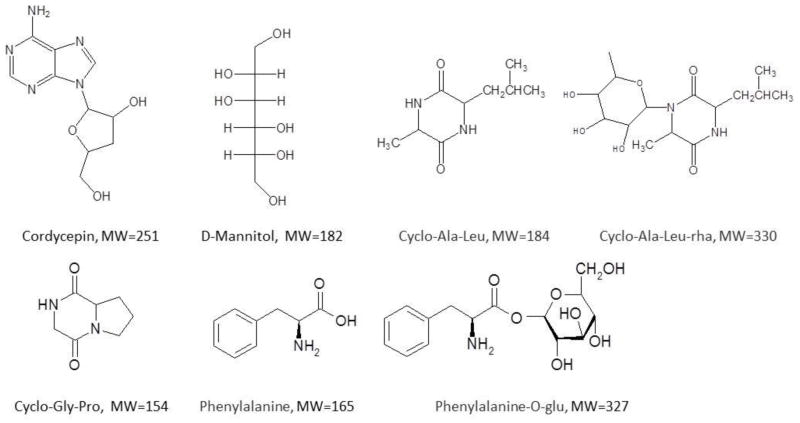
A portion of each compound was weighed out and added to 5.00 mL methanol/water (8:2) to make a stock solution at a concentration of 1.20 mg mL−1 (for cordycepin and D-mannitol), 3.1 mg mL−1 (for Cyclo-Gly-Pro), 2.0 mg mL−1 (for Cyclo-Ala-Leu), and 1.0 mg mL−1 (for phenylalanine). Stock solutions were diluted appropriately to obtain working standard solution with the mobile phase. The compounds involved in the study are shown in Fig. 1.
Figure 1.
Chemical structures of the compounds involved in this work: cordycepin, D-mannitol, cyclo-Ala-Leu, cyclo-Ala-Leu-rha, cyclo-Gly-Pro, phenylalanine, and phenylalanine-o-glu.
HPLC-MS/MS analysis
The system consisted of two pumps (LC-10ADvp, Shimadzu, Toyoto, Japan), an on-line degasser (DGU-12A, Shimadzu), and a triple quadrupole mass spectrometer equipped with a heated ESI source (TSQ Quantum, Thermo Scientific, San Jose, CA, USA). The mass spectrometer was controlled by Xcalibur software (Thermo Finnigan). A C18 reversed-phase column (Ascentis® 3 μm particle size, 10 cm × 2.1 mm, Sigma-Aldrich Chemicals, St. Louise, USA) was used for separation. MeOH/Water mixture (5/95, v/v) with 0.2% formic acid was used as the mobile phase at a flow rate of 0.20 mL/min. Sample injection volume was 5 μL. Data was acquired in full scan and SRM mode. The MS detector was operated in positive ion mode with the following settings: spray voltage 3 KV, vaporization temperature 250 °C, capillary temperature 300 °C, sheath gas pressure 35 (arb), auxiliary gas pressure 10 (arb), tube lens voltage of 150 V, and capillary voltage of 35 V, respectively. SRM parameters for MS detection of the test compounds are summarized in Table 2.
Table 2.
SRM parameters for the test compounds
| Compound | Mol. Weight | Precursor ion (m/z) | Product ion (m/z) | Collision energy |
|---|---|---|---|---|
| Cordycepin | 251 | 252 | 136, 234 | 10 |
| Mannitol | 182 | 183 | 147, 69 | 10 |
| Cyclo-Gly-Pro | 154 | 155 | 127, 70 | 10 |
| Cyclo-Ala-Leu | 184 | 185 | 173, 155 | 10 |
| Cyclo-Ala-Leu-rha | 330 | 331 | 184, 118 | 10 |
| Phenylalanine | 165 | 166 | 120, 103 | 10 |
| Phenylalanine-o-glu | 327 | 328 | 310, 292 | 10 |
Method validation
Validation of method linearity, selectivity, sensitivity, and recovery was performed according to the US Food and Drugs Administration (FDA) guidelines analytical assay [30].
Results and Discussion
Detection of bioactive ingredients in C. sinensis
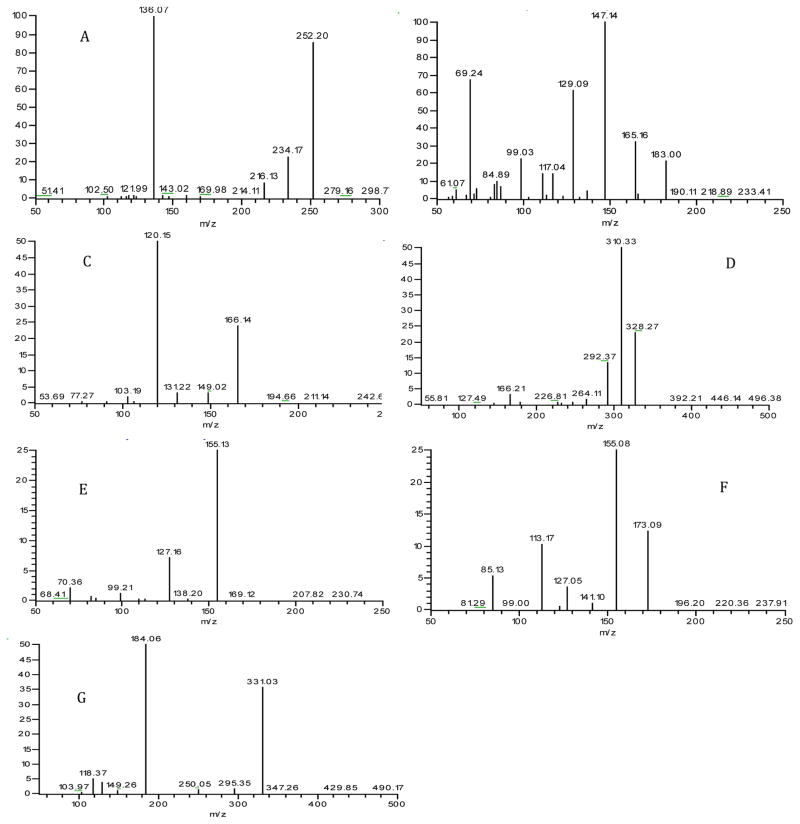
Detection of various bioactive compounds in C. sinensis has been reported [1–5]. These compounds included nucleosides and the bases, polysaccharides, amino acids, cyclic peptides, and sterols, etc. To identify effective biomarkers for authentication and quality control purposes, the present study was focused on detection of cordycepin, mannitol, amino acids, cyclic peptides and glycosides. These compounds were selected either because they were known as the active ingredients for pharmacological effects of C. sinensis or because they specifically occurred to C. sinensis. Authentic C. sinensis samples were carefully screened by HPLC-MS/MS. Chemical structures of the ingredients detected were verified by comparing HPLC retention times and MS2 spectra with authentic chemicals. Cordycepin, D-mannitol, and phenylalanine were detected. D-Mannitol, once being mistaken as “cordycepic acid”, is one of the major bioactive compounds in natural Cordyceps. Studies have shown that it exhibits diuretic, antitussive, and anti-free radical activities. Therefore, mannitol was considered as one of the markers for quality control of Cordyceps [31]. It’s well documented that C. sinensis contains many amino acids at high levels. In this work phenylalanine was included because during the screening its glycoside with a molecule of glucose, i.e. phenylalanine-o-glu, was detected. To our knowledge, this is the first report on the detection of glycosylated amino acids in C. sinensis. Two cyclic peptides, cyclo-Gly-Pro and cyclo-Ala-Leu-rhamnose were detected. Little study has been so far carried out to investigate small cyclic peptides in C. sinensis. Detection of cyclo-Gly-Pro was reported in only one study [32]. There has been no report on the occurrence of cyclo-Ala-Leu-rhamnose in C. sinensis. It’s worth noting that the cyclodipeptide, cyclo-Ala-Leu, was found not detectable. Its concentration in the extract sample was below the detection limit of the assay which was estimated to be at the sub-μg/mL level. Interestingly, several cyclic peptides isolated from cultures of C. sinensis and from C. militaris, i.e. cycloaspeptides A, C, F, and G [33–35] were not detected in C. sinensis. The MS spectra of the compounds detected are shown in Fig. 2. The six compounds, i.e. cordycepin, D-mannitol, phenylalanine, phenylalaine-o-glu, cyclo-Gly-Pro, and cyclo-Ala-Leu-rha were selected as a collection of chemical markers for authentication and quality assessment of C. sinensis. Although they are not necessarily the most abundant components in C. sinensis, they either specifically occur to this natural material or make significant contribution to its pharmacological activity.
Figure 2.
MS spectra of: A) cordycepin, B) D-mannitol, C)Phe, D) Phe-o-glu, E) cyclo-Gly-Pro, F) cyclo-Ala-Leu, and G) cyclo-Ala-Leu-rha.
HPLC-MS/MS quantification of C. sinensis markers selected
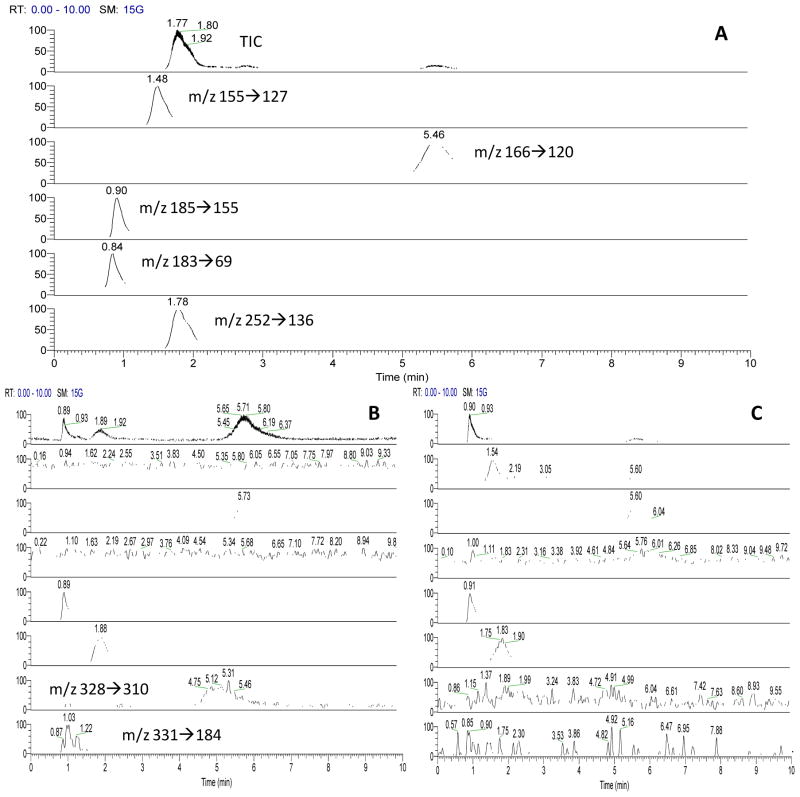
No study on simultaneous determination of the six compounds above selected has been reported. Since this group of compounds represented a broad variation of structural features, HPLC-MS/MS, one of the most powerful analytical techniques, was deployed for their quantification in this work. Several mobile phases, including MeOH/H2O (60:40) containing 5mM ammonium acetate, MeOH/H2O (60:40) containing 0.1% formic acid, ACN/H2O (60:40) containing 5 mM ammonium acetate, MeOH/ACN/H2O (60:20:20) were tested for the separation on a C18 column. It was found that isocratic elution with MeOH/H2O (5:95) containing 0.2% formic acid resulted in the best analytical results in terms of separation efficiency and detection sensitivity. To obtain the analytical figures of merit for the HPLC-MS/MS method, analysis of mixture solutions prepared from authentic cordycepin, D-mannitol, phenylalanine, cyclo-Gly-Pro, and cyclo-Ala-Leu was performed. Under the selected HPLC-MS/MS analytical conditions the six compounds were well separated from each other within 6 min as shown in Figure 3A. Moreover, a characteristic product ion was obtained for each of the compounds involved (see Table 2), which ensured sensitive assays. Following the US Food and Drugs Administration (FDA) guideline for analytical assay [30], validation of linearity, selectivity, sensitivity, and recovery was performed. Peak areas were used for the calculation. The results are summarized in Table 3. Five-point calibration curves for all the compounds involved showed good linearity. In the tested concentration ranges (0.05 – 35 μg mL−1), regression coefficients (R2) were ≥ 0.997. Limits of detection (LODs, signal-to-noise ratio = 3) were in the range from 0.008 μg mL−1 for cordycepin to 0.75 μg mL−1 for cyclo-Gly-Pro. The corresponding limits of quantification (LOQs, signal-to-noise ratio = 10) ranged from 0.0181 μg mL− −1 to 1.55 μg mL−1, respectively. Selectivity and accuracy were evaluated by spiking a pooled sample of C. sinensis extracts with authentic test compounds at three concentrations. Recoveries were found in the range from 96% and 103% in all tests. Relative standard deviation values (RSD) were ≤ 2.0%. Therefore, all compounds were within the acceptable limits for bioanalytical method validation. The data indicated that the proposed HPLC-MS/MS method for simultaneous quantification of the target compounds in C. sinensis and its bio-derivatives products was accurate and selective. It should be pointed out that in the present work good method validation results were obtained without using an internal standard for the quantification. It was because the methanol extract of C. sinensis was relatively clean and showed no matrix effects on the HPLC-MS determination. However, in routine analysis of samples from a variety of sources, use of an internal standard, e.g. stable isotope labeled analogs of mannitol and phenylalanine, will certainly improve the repeatability and reliability of the analytical results.
Figure 3.
TIC and extracted ion chromatograms obtained from analysis of: A) a mixture of authentic compounds, including cordycepin, D-mannitol, Phe, cyclo-Gly-Pro, and cyclo-Ala-Leu; B) a sample of authentic C. sinensis, and C) a sample of supplement tablets claimed as C. sinensis extract. Monitored ion transitions: m/z 155→127 for cyclo-Gly-Pro, m/z 166→120 for Phe, m/z 185→155 for cyclo-Ala-Leu, m/z 183→69 for D-mannitol, m/z 252→136 for cordycepin, m/z 328→310 for Phe-o-glu, and m/z 331→184 for cyclo-Ala-Leu-rha.
Tab. 3.
Linearity and LOD, LOQ of the present HPLC-MS/MS method
| Standard | Linear dynamic range (μg mL−1) | Slope | Intercept | R2 | LOD (μg mL−1) | LOQ (μg mL−1) |
|---|---|---|---|---|---|---|
| Cordycepin | 0.05~10.0 | 8556285.9 | 4148044.3 | 0.998 | 0.008 | 0.018 |
| Mannitol | 0.6~12.0 | 15099.9 | 59059.7 | 0.997 | 0.3 | 0.6 |
| Cyclo-Gly-Pro | 1.55~31.0 | 64396.1 | 174127.5 | 0.998 | 0.75 | 1.55 |
| Cyclo-Ala-Leu | 1.0~24.0 | 97875.9 | 57706.7 | 0.999 | 0.3 | 0.83 |
| Phenylalanine | 0.5~10.0 | 3465243.2 | 3251173.1 | 0.998 | 0.071 | 0.20 |
Analysis of C. sinensis samples
Authentication and quality control of precious natural products are highly significant.36–37 In order to evaluate the effectiveness of the proposed marker collection for authentication and quality assessment of C. sinensis, 5 authentic C. sinensis samples and 5 samples of its substitutes were analyzed by the validated HPLC-MS/MS method. Typical chromatograms obtained from analysis of authentic C. sinensis and its substitute samples are shown in Figure 3B and 3C. Peaks corresponding to the target compounds were well identified. No unknown peaks appeared in the chromatogram, indicating the method was specific for the determination of the markers selected. The analytical results are summarized in Table 5. Cordycepin, D-mannitol, and Phe were detected in all the samples tested. The contents ranged from 0.0076 to 0.0290% (w/w) for cordycepin, from 0.33 to 18.9 % for mannitol, and from 0.0013 to 0.642% for Phe. These results are in consistence with those reported previously [1–3, 18–20, 38–39]. It should be pointed out that samples #6 ~#10 were tablets of C. sinensis extracts sold at local healthy supplements stores and the %(w/w) content values may not be comparable with those for authentic C. sinensis since information on the composition of these tablet samples was not available. Very interestingly, the two glycosides, i.e. cyclo-Ala-Leu-rha and Phe-o-glu, were detected only in the samples of authentic C. sinensis. These results indicated a high level of glycosidases activity in C. sinensis, but not in the cultured or cultivated substitutes. Further study is needed to confirm this point of view. From the results of sample analysis, the collection of C. sinensis markers proposed herein has a good potential in authentication and quality assessment of this precious natural product.
Table 5.
Analytical results of C. sinensis samples
| Sample # | Cordycepin (%, w/w) | D-Mannitol (%, w/w) | Phe (%, w/w) | Phe-O-glu (%, w/w) | Cyclo-Gly-Pro (%, w/w) | Cyclo-Ala-Leu-rha (%, w/w) |
|---|---|---|---|---|---|---|
| 1 | 0.0182 | 11.195 | 0.6423 | 0.0366 | ND | 0.1888 |
| 2 | 0.0290 | 9.782 | 0.1413 | 0.0243 | ND | 0.1105 |
| 3 | 0.0173 | 18.568 | 0.4587 | 0.0138 | ND | 0.0468 |
| 4 | 0.0167 | 18.862 | 0.1884 | 0.0221 | ND | 0.0153 |
| 5 | 0.0217 | 7.291 | 0.0413 | 0.0148 | ND | 0.0063 |
| 6 | 0.0076 | 9.713 | 0.0097 | ND | 0.0016 | ND |
| 7 | 0.0070 | 0.325 | 0.0617 | ND | ND | ND |
| 8 | 0.0068 | 5.372 | 0.1033 | ND | 0.0057 | ND |
| 9 | 0.0074 | 5.212 | 0.1646 | ND | 0.0024 | ND |
| 10 | 0.0155 | 0.967 | 0.0013 | ND | ND | ND |
ND: not detected.
Conclusions
A facile and reliable protocol for authentication and quality assessment of C. sinensis, a precious and pricey medicinal material, was developed. The authentic material was thoroughly screened by HPLC-MS/MS to identify effective markers. Occurrence of cordycepin (i.e. 3′-deoxyadenosine), D-mannitol, phenylalanine, and cyclo-Gly-Pro was confirmed. Two glycosides, i.e. phenylalanine-o-glu and cyclo-Ala-Leu-rha, were detected for the first time. The six compounds were selected in a collection as markers of C. sinensis. A fast and reliable HPLC-MS/MS method was developed for simultaneous quantification of these chemical markers. As demonstrated, the proposed protocol based on HPLC-MS/MS quantification of this marker collection has a great potential in authentication and quality assessment of C. sinensis.
Table 4.
Recovery of target compounds from C. sinensis sample matrix
| Compound | Content (μg) | Spiked (μg) | Observed (μg) | Recovery (%) | Mean (%) | RSD (%, n=9) |
|---|---|---|---|---|---|---|
| Cordycepin | 0.080 | 0.06 | 0.1421 | 103.5% | 101.2 | 1.90 |
| 0.12 | 0.2008 | 100.7% | ||||
| 0.18 | 0.2591 | 99.5% | ||||
|
| ||||||
| Mannitol | 37.3 | 18.0 | 55.20 | 99.4% | 101.4 | 1.87 |
| 36.0 | 73.91 | 101.7% | ||||
| 54.0 | 92.99 | 103.1% | ||||
|
| ||||||
| Cyclo-Gly-Pro | 0.0 | 2.40 | 2.412 | 100.5% | 101.8 | 1.36 |
| 4.80 | 4.876 | 101.6% | ||||
| 7.20 | 7.434 | 103.3% | ||||
|
| ||||||
| Cyclo-Ala-Leu | 0.0 | 1.00 | 1.004 | 100.4% | 101.5 | 1.96 |
| 2.00 | 2.076 | 103.8% | ||||
| 3.00 | 3.009 | 100.3% | ||||
|
| ||||||
| Phenylalanine | 2.14 | 1.00 | 3.105 | 96.4% | 97.8 | 1.33 |
| 2.00 | 4.098 | 97.9% | ||||
| 3.00 | 5.111 | 99.0% | ||||
Acknowledgments
The study was supported partially by a grant from US National Institutes of Health (GM 089557 to YML). LX is a visiting scholar supported by Hubei Provincial Institute for Food and Drug Control.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Li S, Tsim KWK. Oxidative Stress and Disease. 2004;14:657–684. [Google Scholar]
- 2.Ng TB, Wang HX. J Pharm Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Gong Z, Su Y. J Pharm Pharmacol. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 4.Das SK, Masuda M, Sakurai A, Sakakibara M. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Chen PX, Wang SA, Nie SP, Marcone M. J Funct Foods. 2013;5:550–569. doi: 10.1016/j.jff.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji DB, Ye J, Li CL, Wang YH, Zhao J, Cai SQ. Phytother Res. 2009;23:116–122. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- 7.Ohta Y, Lee JB, Hayashi K, Fujita A, Park DK, Hayashi T. J Agri Food Chem. 2007;55:10194–10199. doi: 10.1021/jf0721287. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Cho JY, Rhee MH, Park HJ. Euro J Pharmacol. 2007;558:43–51. doi: 10.1016/j.ejphar.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CH, Sun HL, Sheu JN, Ku MS, Hu CM, Chan Y, Lue KH. Pediatrics & Neonatology. 2008;49:171–178. doi: 10.1016/S1875-9572(09)60004-8. [DOI] [PubMed] [Google Scholar]
- 10.Park DK, Choi WS, Park HJ. J Agri Food Chem. 2012;60:2309–2315. doi: 10.1021/jf205199j. [DOI] [PubMed] [Google Scholar]
- 11.Tsai YJ, Lin LC, Tsai TH. J Agri Food Chem. 2010;58:4638–4643. doi: 10.1021/jf100269g. [DOI] [PubMed] [Google Scholar]
- 12.Smiderle FR, Sassaki GL, Van Griensven LJ, Lacomini M. Carbohydrate Polymers. 2013;97:74–80. doi: 10.1016/j.carbpol.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Jing Y, Zhu J, Liu T, Bi S, Hu X, Chen Z, Song L, Lv W, Yu R. J Agric Food Chem. 2015 doi: 10.1021/jf505915t. [DOI] [PubMed] [Google Scholar]
- 14.Yu HM, Wang BS, Huang SC, Duh PD. J Agri Food Chem. 2006;54:3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 15.Yue GGL, Bik-San Lau C, Fung KP, Leung PC, Ko WH. J Ethnopharmacology. 2008;117:92–101. doi: 10.1016/j.jep.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Dong CH, Yao YJ. LWT-Food Sci Tech. 2008;41:669–677. doi: 10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan J, Zhao J, Feng K, Hu DJ, Li SP. Anal Bioanal Chem. 2011;399:3465–3474. doi: 10.1007/s00216-010-4396-y. [DOI] [PubMed] [Google Scholar]
- 18.Li SP, Yang FQ, Tsim KWK. J Pharm Biomed Analysis. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Zhao J, Li SP, Fan H, Hong M, Wang YT, Zhu Q. J Sep Sci. 2006;29:953–958. doi: 10.1002/jssc.200600007. [DOI] [PubMed] [Google Scholar]
- 20.Huang HY, Zhong JL, Xie QF. China Pharm. 2010;19:88–90. [Google Scholar]
- 21.Zhou X, Luo L, Dressel W, Shadier G, Krumbiegel D, Schmidtke P, Meyer CU. Amer J Chin Med. 2008;36:967–980. doi: 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZB, Li N, Wang M, Wang Y, Du L, Ji XF, Yu AM, Zhang HQ, Qiu FP. J Sep Sci. 2013;36:2348–2357. doi: 10.1002/jssc.201300204. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Dai X, Xu F, Wang F, Gong B, Wei Y. Anal Bioanal Chem. 2012;404:1477–1484. doi: 10.1007/s00216-012-6210-5. [DOI] [PubMed] [Google Scholar]
- 24.Gong YX, Li SP, Li P, Liu JJ, Wang YT. J Chromatogr A. 2004;1055:215–221. doi: 10.1016/j.chroma.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Rao YK, Chou CH, Tzeng YM. Anal Chim Acta. 2006;566:253–258. [Google Scholar]
- 26.Yang FQ, Li DQ, Feng K, Hu DJ, Li SP. J Chromatogr A. 2010;1217:5501–5510. doi: 10.1016/j.chroma.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 27.Fan H, Li SP, Xiang JJ, Lai CM, Yang FQ, Gao JL, Wang YT. Anal Chim Acta. 2006;567:218–228. [Google Scholar]
- 28.Zhao HQ, Wang X, Li HM, Yang B, Yang HJ, Huang L. Molecules. 2013;18:9755–9769. doi: 10.3390/molecules18089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang FQ, Ge LY, Yong JWH, Tan SN, Li SP. J Pharm Biomed Anal. 2009;50:307–314. doi: 10.1016/j.jpba.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Guidance for Industry, Bioanalytical Method Validation. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM); 2001. [Google Scholar]
- 31.Lin BQ, Li SP. Cordyceps as herbal drugs. In: Benzie Iris FF, Wachtel-Galor Sissi., editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2. CRC Press; Boca Raton: 2011. pp. 73–106. [PubMed] [Google Scholar]
- 32.Holliday J, Cleaver M, Wasser SP. Cordyceps, in Encyclopedia of Dietary Supplements. Taylor & Francis; 2005. [DOI] [Google Scholar]
- 33.Jia JM, Ma XC, Wu CF, Wu LJ, Hu GS. Chem Pharm Bull. 2005;53:582–583. doi: 10.1248/cpb.53.582. [DOI] [PubMed] [Google Scholar]
- 34.Rukachaisirikul V, Chantaruk S, Tansakul C, Saithong S, Chaicharernwimonkoon L, Pakawatchai C, Intereya K. J Nat Prod. 2006;69:305–307. doi: 10.1021/np050433l. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu S, Liu H, Liu X, Che Y. J Nat Prod. 2009;72:1364–1367. doi: 10.1021/np900205m. [DOI] [PubMed] [Google Scholar]
- 36.Qin J, Leung FC, Fung Y, Zhu D, Lin B. Anal Bioanal Chem. 2005;381:812–819. doi: 10.1007/s00216-004-2889-2. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Chen P. Anal Bioanal Chem. 2011;401:1577–1584. doi: 10.1007/s00216-011-5246-2. [DOI] [PubMed] [Google Scholar]
- 38.Hsu TH, Shiao LH, Hsieh C, Chang DM. Food Chem. 2002;78:463–469. [Google Scholar]
- 39.Hur H. Mycobiology. 2008;36:233–235. doi: 10.4489/MYCO.2008.36.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]