Abstract
Obsessive–compulsive disorder (OCD), like other illnesses with prominent anxiety, may involve abnormal fear regulation and consolidation of safety memories. Impaired fear extinction memory (extinction recall, ER) has been shown in individuals with current symptoms of OCD [1]. However, contrary to expectations, the only previous study investigating this phenomenon showed a positive correlation between extinction recall abilities and OCD symptomology (i.e., as OCD symptoms worsened, extinction memory improved). The purpose of the current study was to determine if patients with a lifetime diagnosis of OCD (not necessarily currently symptomatic) also demonstrate impairments in extinction memory, and the relationship between OCD symptomology and extinction memory in this type of sample. In addition, we also examined fear renewal, which has never been investigated in an OCD sample. We enrolled 37 patients with OCD, the majority of whom were on serotonin reuptake inhibitors, and 18 healthy control participants in a 2-day paradigm assessing fear conditioning and extinction (Day 1) and extinction retention and renewal (Day 2). Skin conductance responses (SCRs) were the dependent measure. Results, as in the prior study, indicated that the only between-group difference was impaired ER in OCD patients relative to controls. Contrary to our prediction, OCD symptom severity was not correlated with the magnitude of extinction recall. There were no differences in fear renewal between OCD patients and controls.
Keywords: Anxiety/anxiety disorders, OCD/obsessive–compulsive disorder, CBT/cognitive behavior therapy, Biological markers, Cognition
1. Introduction
Fear conditioning and fear extinction are central to disorders of fear and anxiety [2], including obsessive–compulsive disorder (OCD) – a disorder characterized by the presence of intrusive thoughts and ritualistic compulsions aimed at reducing anxiety or discomfort. From a behavioral perspective, OCD is maintained through continued engagement in ritualistic compulsions in order to “prevent” a feared outcome from occurring, despite some degree of knowledge that this fear is unreasonable. The first-line psychosocial treatment for OCD – exposure and response prevention (ERP) – is based on principles of fear extinction. In order to improve OCD symptoms, therapists guide patients to place themselves in increasingly difficult, OCD-triggering situations while refraining from engaging in their compulsions. Over time, patient fears are extinguished.
The ability to retain a memory of fear extinction over time, also called extinction recall or retention (ER), appears to be impaired in individuals with anxiety disorders. Extinction retention has been found to be abnormal in post-traumatic stress disorder (PTSD) [3], and individuals with panic disorder appear to be resistant to extinction [4]. A recent study using Pavlovian conditioning to associate a shock with certain lights found that fear extinction retention is reduced in OCD, compared to healthy controls [1]. This study also found a direct correlation between OCD symptom severity and ER – that is, participants with greater OCD severity also had better recall of fear extinction. While the specific causal mechanism of these results remains unclear, together these findings suggest problems with ER as a potential mechanism in the development and/or maintenance of anxiety-related disorders.
In the current study, given the unexpected finding in Milad et al. [1] showing improved extinction memory with greater OCD severity, we tested the hypothesis that participants with a lifetime diagnosis of OCD would also have impaired extinction recall as compared to healthy controls, and assessed whether there continued to be a relationship between OCD severity and extinction memory. We also examined differences between participants with OCD and healthy control patients during an additional fear renewal phase, hypothesizing impairments in participants with OCD.
2. Materials and methods
2.1. Participants
We enrolled 37 participants with OCD and 18 healthy controls. OCD participants were recruited from the OCD clinic at Butler Hospital in Providence, RI, and met DSM-IV criteria for a lifetime diagnosis of obsessive–compulsive disorder [5]. Exclusion criteria for the OCD group included current or past psychotic disorder and a clinical history of post-traumatic stress disorder (PTSD; excluded because of the known ER deficits in this disorder). Controls, free of current psychiatric disorders or past anxiety or psychotic disorder, were recruited from the community through advertisements at local colleges and in local cafes and stores. Informed consent was obtained for this Butler Hospital IRB-approved study.
2.2. Procedures
2.2.1. Rating scales
Structured Clinical Interview for DSM-IV, SCID-IV [6]. The Structured Clinical Interview for DSM-IV is a semi-structured interview for making major Axis I diagnoses. It is administered by trained evaluators and includes an introductory overview, followed by specific diagnostic modules.
Yale–Brown Obsessive Compulsive Scale, Y-BOCS [7]. The Y-BOCS is an evaluator-administered questionnaire assessing severity of OCD symptoms, separated by obsessions and compulsions.
Yale–Brown Obsessive Compulsive Scale Symptom Checklist, Y-BOCS SC; [7]. The Y-BOCS SC is a questionnaire assessing the presence of current or past OCD symptoms.
2.2.2. Fear conditioning paradigm
The experimental protocol was administered over two separate days. On Day 1, participants underwent three different phases where they were presented with visual stimuli: the habituation, conditioning, and extinction phases (Fig. 1). This day was designed to condition participants to a stimulus and extinguish the conditioned stimulus. On Day 2, approximately 24 h after conditioning and extinction, participants underwent two additional blocks, extinction retention and fear renewal. The goal of these blocks was to show the conditioned and unconditioned stimuli again, to determine if the conditioned stimulus remained extinguished (i.e., extinction recall). During both days of the procedure, participants sat in a comfortable chair in front of a computer monitor. On Day 1, after the electrodes were attached, prior to task initiation, the intensity of the electric shock was set by each participant, and determined by each participant to be “highly annoying but not painful”. The shock was generated by a Coulbourn Transcutaneous Aversive Finger Stimulator, which was isolated from line current and powered by a 9 V dry cell battery attached to an adjustable step-up transformer. Participants were then asked to passively view digital photographs of two rooms containing lamps that appeared on the computer screen (Fig. 1). Photographs of the two rooms (a conference room and an office) constituted the two virtual contexts (CX). During the procedure, one context was associated (CX+) and one was not associated (CX−) with receiving the unconditioned stimulus (US). Each room contained a lamp. Two colors of the lit lampshade (blue or red) constituted the conditioned stimuli (CS). One CS was paired (CS+) and one was not paired (CS−) with presentations of the US. The selection of the CS+ and CS− colors and the CX+ and CX− rooms was counterbalanced across participants. For each trial during the experiment, the CX was presented for 9 s: 3 s alone, followed by 6 s in combination with the CS+ or CS−. Skin conductance was recorded for 5 s before the presentation of the CX, during the 3 s presentation of the CX alone, and during the 6 s presentation of the CX plus the CS. The US occurred during the last 500 ms of the CS+. The US was a 500 ms electric shock delivered through electrodes attached to the second and third fingers of the dominant hand. The average inter-trial interval was 15 s.
Fig. 1.
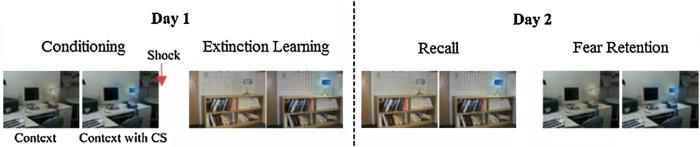
Schematic of experimental protocol. Pictures showing the visual contexts used in the experiment within which conditioned stimuli (CS) were presented. In this example, pictures of an office and a conference room represent conditioning and extinction (E) contexts, respectively, whereas the blue light represents the CS+ that was paired with the shock and later extinguished. Extinction recall and fear renewal were conducted on Day 2.
2.2.3. Psychophysiological measures
See Milad et al. [1] for additional details. A Coulbourn Modular Instruments System (Allentown, PA) was used to record skin conductance levels via a Coulbourn Isolated Skin Conductance Coupler using a constant 0.5 V through 8 mm (sensor diameter) electrodes. Electrodes were filled with isotonic paste and placed on the palm of the participant's non-dominant hand. The skin conductance electrodes were separated by approximately 8 mm, as determined by the width of the adhesive collar. A Coulbourn analog-to-digital converter digitized the analog signals, which were then sampled and stored by a personal computer.
2.3. Day 1
2.3.1. Phase 1 – habituation phase
Prior to the habituation phase, participants were instructed that the purpose of this phase was to show them all of the possible pictures that they would see in the experiment, and that no shock would be delivered. In the habituation phase, four CS+ and four CS− were presented in a counterbalanced manner within the acquisition context (CX+) or the extinction context (CX−).
2.3.2. Phase 2 – conditioning phase
Prior to the conditioning phase, participants were instructed that they “may or may not be shocked” during that phase and the following phases of the experiment. One of the lights (e.g., red or a blue light) was depicted within a photograph and paired with the US (i.e., shock) at a 100% reinforcement rate, within the CX+. Each participant was administered five CS+ and five CS− trials. The US occurred immediately following each CS+ offset. This phase was followed by a 1 min break.
2.3.3. Phase 3 – extinction training phase
Prior to the extinction training phase, participants were reminded that as with the last phase they “may or may not be shocked” during this phase. During this phase the conditioned stimulus was presented (CS+; e.g., room with blue light) in the absence of the US (i.e., shock). The extinction phase was divided into two subphases, early and late, which were separated by an approximately 1 min rest period. Five CS+ and five CS− trials were presented within both the early and late blocks, for a total of 20 trials. CS were presented in the CX−. No shocks were delivered during the extinction phase.
2.4. Day 2
The order of recall and renewal were counterbalanced across participants. The electrodes were once again connected in the same manner as Day 1. Participants were told that the experiment would be similar to the previous day, and that they “may or may not be shocked”.
2.4.1. Phase 1 – recall phase
During the recall phase, as in the extinction phase, the five CS+ pictures (i.e., previously conditioned stimuli that underwent the extinction) were shown along with the five CS− pictures (i.e., stimuli that were never paired with shock) within the CX−. Participants did not receive any shocks during this assessment phase.
2.4.2. Phase 2 – renewal phase
During the renewal phase, as in the extinction phase, the five CS+ pictures were shown along with the five CS− pictures within the CX+. No shocks were delivered in the renewal phase.
2.4.2.1. Skin conductance level scores and data analysis
Consistent with prior studies using this paradigm, SCR for each CS trial was calculated by subtracting the mean skin conductance level during the 2 s before CS onset (during which the context alone was being presented) from the highest skin conductance level during the 6 s CS duration. Thus, SCRs to the CS+ and CS− reflected changes in skin conductance level beyond any change in SC level produced by the context. The magnitude of extinction retention (recall) was quantified as follows: each subject's average SCR to the first two CS+ trials of the extinction recall phase was divided by their largest SCR to a CS+ trial during the conditioning phase and then multiplied by 100, yielding a percentage of maximal conditioned responding. This in turn was subtracted from 100% to yield an “extinction retention index”. The purpose of calculating the extinction retention index was to normalize each subject's SCR during extinction recall to that exhibited during the conditioning phase. The index is designed to adjust the SCR during extinction recall for differences in CR magnitude during acquisition.
3. Results
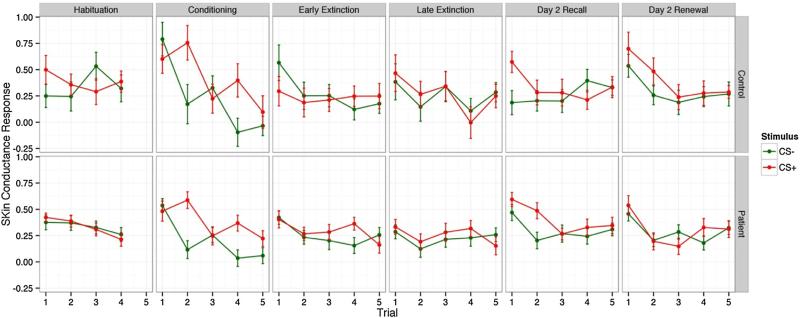
The demographic and comorbid psychiatric characteristics of OCD and healthy controls (HC) are detailed in Table 1. We removed three controls and six OCD patients. Six participants were removed due to poor skin conductance acquisition, resulting in unreliable values, and three participants were removed due to unclear conditioning (i.e., when we did not observe two or more trials where SCR ≥ 0.05 for CS+ on any conditioning trial, due to lack of confidence that conditioning took place). Thus, 31 OCD patients and 15 healthy controls remained for the presented analyses. There were no significant differences between the groups in age or education (see Table 1). Ages of the OCD group ranged from 21 to 64 years (M = 42.52, SD = 11.61); for the control group they were 18–65 (M = 41.20, SD = 13.62). Mean Y-BOCS OCD severity at entry was 20.10 (SD = 8.19; range 2–34). Twenty-four of 31 OCD participants met current criteria for OCD (Y-BOCS ≥ 16). Comorbidities for the OCD group included current major depressive disorder/episode (n = 11), mania (n = 1), hypomania (n = 1), dysthymic disorder (n = 4), alcohol abuse (n = 2), alcohol dependence (n = 1), panic disorder (n = 2), social phobia (n = 9), generalized anxiety disorder (n = 6), specific phobia (n = 4), chronic motor tics (n = 1), and impulse control disorder (n = 2). The OCD group also met criteria for past major depressive disorder/episode (n = 13), mania (n = 1), dysthymic disorder (n = 1), bipolar disorder (n = 1), alcohol abuse (n = 8), alcohol dependence (n = 6), substance abuse (n = 4), substance dependence (n = 3), panic disorder (n = 4), agoraphobia without panic (n = 1), social phobia (n = 3), and PTSD (n = 2). Control participants did not meet criteria for any current clinical disorders, but did meet criteria for past major depressive disorder/episode (n = 3), alcohol dependence (n = 1), and substance abuse (n = 1). Twenty-nine out of 31 OCD participants were prescribed serotonin reuptake inhibitors, and 17 out of 31 OCD participants were also prescribed benzodiazepines. See Fig. 2 for trial by trial data for each block of the paradigm.
Table 1.
Demographic and OCD symptom characteristics for OCD patients and healthy controls.
| Characteristic | OCD patients (n = 31) | Healthy controls (n = 15) | p |
|---|---|---|---|
| Age | 42.52 (11.61) | 41.20 (13.62) | 0.75 |
| Female (%) | 11 (35.48%) | 7 (46.67%) | 0.68 |
| Education (% >high school) | 25 (80.65%) | 13 (86.67%) | 0.70 |
| OCD symptom severity (Y-BOCS) | 20.10 (8.19) | 0.60 (1.45) | 0.00 |
Fig. 2.
Trial by trial skin conductance responses to CS+ and CS− for healthy control and OCD patient groups during each phase.
3.1. Habituation
Linear mixed effects models were used to compare main effects of group, stimulus type, and changes in skin conductance response over repeated trials. There was a significant decrease in SCR over trials (b = −0.02, SE = 0.01, p < 0.03). OCD and HC did not have significantly different SCR (b = −0.027, SE = 0.08, p < 0.74) over trials and SCR were similar across different stimulus types (b = −0.01, SE = 0.03, p = 0.74). There was no interaction between patient group and stimulus type (b = −0.05, SE = 0.07, p < 0.52). The three-way interaction between trial, stimulus type and group also was not significant (all ps < 0.52).
3.2. Fear acquisition
Linear mixed effects models were used to compare main effects of group, stimulus type, and changes in skin conductance response over repeated trials. Likelihood ratio testing of nested unconditional models did not suggest a benefit of including any additional random effects for changes over trials (Likelihood ratio = 0.48, p = 0.79).
Both the OCD and HC displayed stimulus specific conditioning indicative of learning. Skin conductance responses (SCR) were significantly different between the CS+ and CS− trials during fear acquisition in both groups (b = 0.29, SE = 0.05, p < 0.001). The magnitude of SCR decreased over trials (b = −0.04, SE = 0.01, p < 0.001). There were no significant group differences (b = 0.01, SE = 0.07, p < 0.93) or interactions between the stimulus type and group (b = −0.04, SE = 0.10, p < 0.73), indicating no evidence of differences in levels of fear conditioning between the OCD and HC groups. The three-way interaction between trial, stimulus type and group also was not significant (ps < 0.63).
3.3. Extinction training
To assess learning during extinction training, the first three trials (coded 0) were compared to the last two trials (coded 1) using a dummy-coded variable for trials in linear mixed effects models along with terms for stimulus type and group. Level of SCR decreased significantly from the first three to the last two trials (b = −0.07, SE = 0.04, p < 0.04). Level of SCR during extinction training did not significantly differ across stimulus types (b = 0.02, SE = 0.04, p < 0.65) or between the OCD and HC groups (b = 0.02, SE = 0.07, p < 0.78), indicating that the OCD groups extinguished to a level comparable to the healthy controls. There was no significant interaction between stimulus type and group (b = 0.07, SE = 0.07, p < 0.31) or three-way interaction of trial, stimulus type, and group (ps < 0.21).
3.4. Extinction recall
Linear mixed effects models were used to compare main effects of group, stimulus type, and changes in skin conductance response over repeated trials. Likelihood ratio testing of nested unconditional models did not suggest a benefit of including an additional random effect for changes over trials (Likelihood ratio = 0.44, p = 0.80).
Both the OCD and HC displayed stimulus specific SCR during the recall trials. Skin conductance responses (SCR) were significantly different between the CS+ and CS− trials during recall in both groups (b = 0.23, SE = 0.08, p < 0.001). The magnitude of SCR decreased significantly over trials (b = −0.17, SE = 0.01, p < 0.001). As displayed in Fig. 2, the higher SCR for CS+ than CS− was apparent for HC on the first trial pair only. For OCD, there was a strong SCR to both CS+ and CS− on the first trial and a higher SCR for CS+ than CS− during the second trial pair. The three-way interaction between trial, stimulus type and group was significant statistically (b = 0.47, SE = 0.20, p = 0.0199). This interaction reflects the larger difference in SCR for CS+ and CS− among OCD relative to HC during earlier trial pairs relative to later trial pairs.
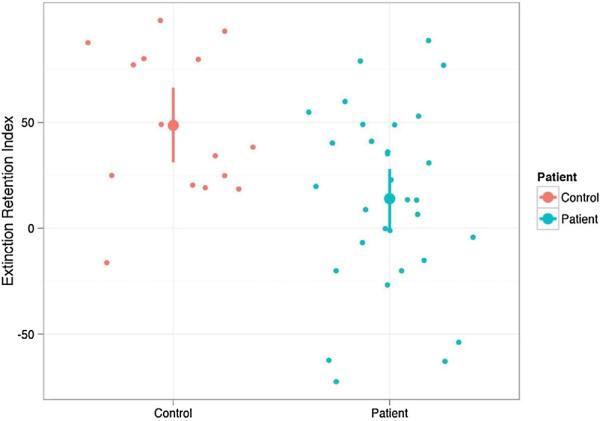
Consistent with previous studies, we also constructed the extinction retention index (ERI). To reflect the degree to which participants continue to react to the previously extinguished (CS+) stimuli, the ERI is constructed using the average SCR from the first two presentations of the CS+ during the Day 2 recall stage relative to the maximum SCR observed during the Day 1 fear acquisition stage. A t-test for unequal variances comparing OCD and HC resulted in a significant group difference (t(33.37) = 2.94, Cohen's d = 0.89, p < 0.006). ERI was 48.56 (SD = 42.50) and 13.89 (SD = 34.82) for HC and OCD, respectively. Fig. 3 displays the ERI scores for OCD and HC. As shown in Fig. 2, HC did not show elevated SCR during presentation of CS−. Although elevated on the first presentation, the SCR for previously extinguished CS+ stimuli returned to levels similar to CS− on trial 2. For OCD patients, SCR were elevated for both CS+ and CS− stimuli. Responses to CS− were reduced by the second trial and responses to CS+ remained elevated. We also examined groups of participants who reported elevated symptoms with Y-BOCS ≥ 16 (i.e., current OCD; n = 24) to other participants (n = 22). Average ERI for participants with elevated symptoms was 18.92 (SD = 46.21) and 32.05 (SD = 39.84) among other participants (t(41.69)= 1.03, Cohen's d = 0.31, p = 0.31).
Fig. 3.
Extinction retention index (ERI) for OCD and healthy controls.
3.5. Fear renewal
We used linear mixed effects regression models to examine SCR during the first two presentations of CS+ and CS− stimuli. There was a significant decrease in SCR over trials (b =−0.11, SE = 0.02, p < 0.001) and a significantly higher SCR to CS+ than CS− (b = 0.23, SE = 0.06, p < 0.001). SCR levels were not significantly different for OCD and HC groups (b = −0.02, SE = 0.20, p < 0.90). Although HC appeared to have differentiated stimulus types more clearly than OCD patients (see Fig. 3, renewal panel), the interaction between group and stimulus type did not exceed traditional levels of significance (b = −0.19, SE = 0.12, p < 0.11). There was no significant three way interaction for trial, stimulus type and group. We also used linear mixed effects models to evaluate whether patients and controls differed in expected increase in SCR when presented with the conditioning context (Day 2 renewal) relative to the extinction context (Day 2 recall). Models included the main effects for trial, stimulus type, patient status and phase (renewal vs. recall). Two-way interactions of stimulus type and patient status with trial and phase were entered as a block after all lower order terms. We observed significant trial by stimulus (b = −0.03 SE = 0.01, p = 0.009) and phase by patient (b = −0.12, SE = 0.06, p = 0.033) interactions. As shown in Fig. 2, we observed SCR decreased over trials and differences in SCR to CS+ when compared to CS− were larger in earlier trials than later trials across both phases. When compared to the Recall phase, increases in SCR during the renewal phase were larger for controls than for patients. Controls demonstrated higher SCR when presented the conditioning context than when presented the extinction context, a pattern that was not observed for the patient sample.
3.6. Extinction retention index (ERI) as a predictor of OCD symptom severity
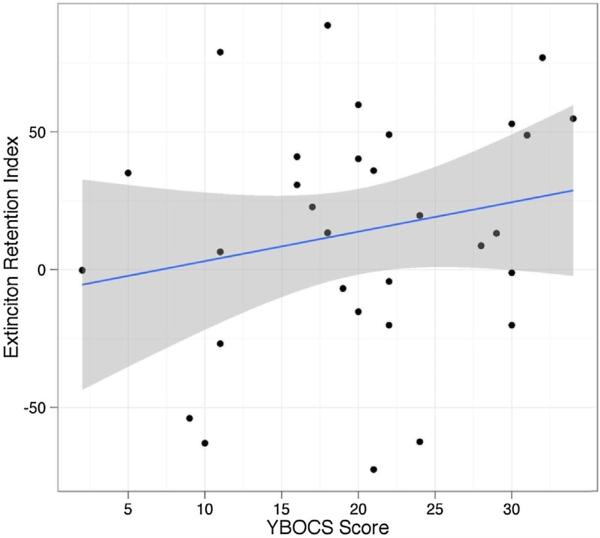
Mean levels of symptoms on the Y-BOCS were 20.10 (SD = 8.19, interquartile range = 16–26) and 0.60 (SD = 1.45, interquartile range = 0–0) in OCD and HC, respectively. To examine relationships between ERI and reports of symptoms, we limited analyses to the OCD group. As displayed in Fig. 4, ERI was not significantly related to OCD symptom severity (r = 0.21, p < 0.27).
Fig. 4.
Relationship between extinction recall index and level of OCD symptom severity among patients with OCD.
4. Discussion
In this study, we demonstrated that participants with OCD exhibit worse extinction recall than healthy controls. There were no significant between-group differences in conditioning, extinction training, or fear renewal. Results were consistent with the study completed by Milad et al. [1]. Milad et al. [1] showed that OCD symptom severity was positively correlated with the magnitude of extinction recall. In the current study, we did not find the same result. There was no correlation with symptom severity, either in the entire sample, or even in those participants who were symptomatic at the time of the assessment. However, both studies showed significant variability in ERI across the OCD samples; this appears to be in contrast to other populations, such as PTSD [3], and may represent the heterogeneity in fear expression that is present in OCD. Given conflicting findings, continued research into the relationship between extinction memory and OC symptomology is warranted.
In contrast to Milad et al. [1], we used participants with a lifetime diagnosis of OCD, and they may not have met diagnostic criteria for OCD (Y-BOCS ≥ 16) at the time of the assessment. As noted above, there was no clear relationship between OCD symptoms and ER. This may indicate that ER represents a trait, rather than a state effect of the disorder. Fear conditioning has been shown to be heritable [9], with genetic effects accounting for 34–43% of the total variance; this may also be the case with recall of extinction memories.
Limitations of this study include the small sample size, though even with the small sample, we were able to achieve significant results. Although behavioral findings were consistent with the study by Milad et al. [1], neuroimaging was not completed in this sample. Future studies should also focus on understanding extinction memory and fear renewal in regard to symptom subtypes and course. This paradigm should be examined across disorders of mood and anxiety with patients of varying clinical characteristics, as we anticipate this effect is not specific to OCD. In addition, the majority of the participants were on serotonin reuptake inhibitors at the time of testing; though this is typical for adults with OCD, this is another limitation to the study. There are reports that chronic antidepressant treatment impacts fear extinction acquisition in rats [10]. Chronic treatment with fluoxetine has also been shown to improve extinction memory in rats [11], though one study has indicated that administration of fluoxetine reduces fear responses during extinction learning and recall only in female rats, with modulation by the estrous cycle [12]. A very recent study in humans demonstrated that short-term (2 weeks) use of escitalopram did not impact fear acquisition but facilitated extinction learning [13]. Our sample was able to extinguish, but it is unclear if there is an impact of SRIs or benzodiazepines on extinction memory in humans as well.
The inability to extinguish fearful responses when they are no longer appropriate is a hallmark feature of OCD. The most effective treatment for OCD, exposure with response/ritual prevention (ERP) is essentially extinction training, in which patients are systematically exposed to OCD triggering cues and encouraged to resist engaging in compulsive rituals. Therapeutic gains are maintained and deepened as individuals recall the extinction learning upon subsequent cue exposures. Impairment in extinction recall may explain the need for this type of treatment approach. That is, where healthy individuals likely extinguish any conditioned irrational fears easily during normal day-to-day activities, patients with OCD may require this highly structured, repetitive treatment approach. However, high degrees of dysfunction in ability to recall extinction may even interfere with the ability to receive therapeutic benefit from ERP.
Understanding the mechanisms behind fear expression in OCD may enable us to use extinction memory or fear renewal as a predictor of outcome in behavior therapy or other therapies for OCD. In addition, novel techniques can be developed to facilitate retention of extinction memories, to improve CBT across anxiety disorders and thus reduce symptoms. The relationship between dimensions of fear expression and OCD has been supported in two studies. Though the relationship with clinical symptomology is unclear, there does seem to be an overlap between neurocircuitry involved in modulation of fear and the neurocircuitry implicated in OCD [1,17]. Further research should focus on investigation of this overlap and the relationship with clinical outcome.
HIGHLIGHTS.
Obsessive–compulsive disorder (OCD) may involve impaired fear extinction retention (ER).
Fear conditioning/extinction and ER was assessed in OCD patients.
OCD patients, relative to controls, showed impaired ER.
OCD symptom severity was not correlated with the magnitude of ER.
There were no differences in fear renewal between OCD patients and controls.
Acknowledgements
This research was supported by NIH grant MH086400 and funding from a private donor to Butler Hospital's OCD Clinic. The authors would like to thank Melissa Warstadt and Jason Kirschner for their help with data collection.
References
- 1.Milad MR, et al. Deficits in conditioned fear extinction in obsessive–compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608–18. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- 2.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michael T, et al. Fear conditioning in panic disorder: enhanced resistance to extinction. J Abnorm Psychol. 2007;116(3):612–7. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- 5.Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 6.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 7.Goodman WK, et al. The Yale–Brown Obsessive Compulsive Scale. I. Development: use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 9.Hettema JM, et al. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60(7):702–8. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 10.Burghardt NS, et al. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73(11):1078–86. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschaux O, et al. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215(2):231–7. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 12.Lebron-Milad K, et al. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res. 2013;253:217–22. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui E, et al. Two weeks of pretreatment with escitalopram facilitates extinction learning in healthy individuals. Hum Psychopharmacol. 2013;28(5):447–456. doi: 10.1002/hup.2330. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109(22):8764–9. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]