Abstract
Capecitabine produces an objective response rate of up to 25 % in anthracycline-treated, taxane-resistant metastatic breast cancer (MBC). The farnesyltransferase inhibitor tipifarnib inhibits Ras signaling and has clinical activity when used alone in MBC. The objective of this study was to determine the efficacy and safety of tipifarnib–capecitabine combination in MBC patients who were previously treated with an anthracycline and progressed on taxane therapy. Eligible patients received oral capecitabine 1,000 mg/m2 twice daily plus oral tipifarnib 300 mg twice daily on days 1–14 every 21 days. The primary endpoint was ORR. The trial was powered to detect an improvement in response rate from 25 to 40 %. Among 63 eligible, partial response occurred in six patients (9.5 %; 90 % CI 4.2–17.9 %), median progression-free survival was 2.6 months (95 % CI 2.1–4.4), and median overall survival was 11.4 months (95 % CI 7.7–14.0). Dose modifications were required for 43 patients (68 %) for either tipifarnib and/or capecitabine. Grades 3 and 4 toxicities were seen in 30 patients (44 %; 90 % CI 44.4–67.0 %) and 11 patients (16 %; 90 % CI 10.8–29.0 %), respectively. The most common grade 3 toxicities included neutropenia, nausea, and vomiting; and the most common grade 4 toxicity was neutropenia (8 out of 11 cases). The tipifarnib–capecitabine combination is not more effective than capecitabine alone in MBC patients who were previously treated with an anthracycline and taxane therapy.
Keywords: Farnesyltransferase inhibitor, Tipifarnib, Capecitabine, Metastatic breast cancer
Introduction
Anthracyclines and taxanes are among the most active chemotherapy agents for the treatment of metastatic breast cancer (MBC). Capecitabine is an oral fluoropyrimidine prodrug that is modified to 5-fluorouracil (5-FU) preferentially in tumor tissues by a three-step enzymatic reaction [1]. It has become a widely used for the treatment for MBC resistant to paclitaxel and pretreated with anthracycline-containing chemotherapy regimen or resistant to paclitaxel, and also as a first-line regimen as an alternative to parenteral cytotoxic therapy. Post-marketing use of capecitabine at the FDA-approved dose [2,500 mg/(m2 day)] leads to unacceptable toxicity in many patients. A retrospective analysis and phase II study support a starting dose of capecitabine at 2,000 mg/(m2 day) improved tolerability without compromising efficacy, and was associated with an objective response rate (ORR) of up to about 25 % in MBC patients with paclitaxel-refractory breast cancer who had progressive disease after two prior chemotherapy regimens [2, 3]. However, patients may still have toxic effects and individualization of dosing is necessary [4, 5].
Ras proteins are guanine nucleotide-binding proteins that play pivotal roles in the control of normal and transformed cell. After stimulation by various growth factors and cytokines, Ras activates several downstream effectors, including the Raf-1/mitogen-activated protein kinase pathway and the Rac/Rho pathway [6]. Ras undergoes several post-translational modifications that facilitate its attachment to the inner surface of the plasma membrane. The first- and most-critical modification is the addition of a farnesyl isoprenoid moiety in a reaction catalyzed by the enzyme protein farnesyltransferase (FTase). Inhibiting FTase would prevent Ras from maturing into its biologically active form. Hyperactivation of Ras/MAPK signal transduction pathway has been implicated as a key resistance mechanism of both endocrine therapy and chemotherapy with anthracycline and taxanes, in breast cancer [7–9]. Although initially developed for tumors with Ras mutations that result in constitutive activation of the Ras pathway, several inhibitors for the Ras signaling molecules, such as farnesyltransferase inhibitors (FTIs), are also active in breast cancer cell lines and xenografts that lack Ras mutations via growth inhibition, induction of apoptosis and cell cycle arrest in the G2/M phase [10, 11]. This is an important consideration given that Ras mutations occur only rarely in breast cancer [12]. Tipifarnib (Zarnestra™, formerly R115777, Johnson and Johnson Pharmaceutical Research and Development, LLC, Ratitan, NJ) is a non-peptidomimetic, orally bioavailable competitive inhibitor of FTPase [13]. Tipifarnib produced a response rate of 10 % and CBR of 23 % in MBC patients resistant to chemotherapy and/or endocrine therapy [14].The intermittent dosing regimen of tipifarnib (i.e., 300 mg bid in a cyclical regimen of 21 days of treatment followed by 7 days of rest) has a significantly improved therapeutic index compared with the continuous dosing regimen (i.e., 400 or 300 mg bid continuously) [14]. In addition, the combination of tipifarnib (200 mg orally administered days 2–7) plus dose-dense doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) resulted in a pCR of 25 % in the breast at surgery when used as neoadjuvant therapy in patients with locally advanced breast cancer, a rate significantly higher than the 10 % historical rate expected in this population [15]. Moreover, tipifarnib-inhibited FTase enzyme activity by at least 90 % in the majority of patients who underwent sequential biopsy before and after treatment [15]. A previous a phase I trial demonstrated the safety and feasibility of combining capecitabine with tipifarnib for 14 days every 3 weeks [16]. We hypothesized that tipifarnib might enhance the clinical efficacy of capecitabine by overcoming underlying resistance mechanisms. The primary objective of this single-arm, phase II study was to determine the response rate and safety of the capecitabine–tipifarnib combination in MBC patients who were pretreated with an anthracycline (in the adjuvant setting or for metastatic disease) and progressed on a taxane (paclitaxel or docetaxel).
Patients and methods
Patient selection
Women with histologically confirmed adenocarcinoma of the breast with metastatic progression and at least one objectively measurable lesion defined by RECIST [17] were eligible. Hormone receptor (HR)-positive disease was defined as being positive for estrogen and/or progesterone receptors by any local institutional laboratory. Prior hormonal therapy in either the metastatic or adjuvant/neoadjuvant setting was allowed, but patients must have had been off such therapy for ≥1 week prior to registration. Patients must have met all of the following criteria with regard to prior cytotoxic therapy: (1) prior treatment with an anthracycline (e.g., doxorubicin and epirubicin) either in the adjuvant/neoadjuvant setting and/or for metastatic disease, (2) prior treatment with taxane (i.e., paclitaxel and docetaxel) for metastatic disease, or relapse while receiving adjuvant taxane therapy, (3) progressive disease while receiving taxane therapy or up to 30 days after receiving the last taxane dose, (4) no more than three prior cytotoxic regimens for metastatic disease, and (5) no prior treatment with capecitabine or 5-FU for metastatic disease.
Concurrent radiation therapy was not permitted. No prior radiotherapy other than to the conserved breast, to the post-mastectomy chest wall or to a limited field involving <25 % of marrow-containing bone. Previously irradiated tumors could have been used to assess a clinical response. Patients would not be eligible for this study if the previously irradiated tumors had constituted the only site of measurable disease. Patients must not have had received previous treatment with cytotoxic drugs, and/or radiotherapy< 4 weeks prior to registration.
Additional key inclusion criteria included: age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; adequate organ and marrow function (granulocyte count >1,500/µL, platelet count ≥100,000/µL, total bilirubin ≤1.5 mg/dL, SGOT (AST), and/or SGPT (ALT) ≤3.0 × institutional upper limit of normal (unless liver is involved by tumor, in which case SGOT (AST) and SGPT (ALT) can be ≤5 × upper limit of normal), serum creatinine ≤1.5 mg/dL or measured (or calculated) creatinine clearance ≥60 mL/min). Exclusion criteria included prior treatment with any FTI, prior other malignancies ≤5 years with the exception of curatively treated basal or squamous cell carcinoma of the skin or carcinoma in situ of the cervix, patients with prior organ allograft or received immunosuppressive therapy, pregnant or breastfeeding patients, major surgery or radiation therapy within the last 4 weeks, patients with NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 grade 2–4 neuropathy, patients with current or previously treated brain metastases; patients who were taking enzyme inducing anticonvulsant medications (e.g., phenobarbital and phenytoin). Patients must not have had any uncontrolled intercurrent illness including, but not limited to, chronic nausea/vomiting, complete or partial bowel obstruction, dysphagia/odynophagia with inability to swallow pills, ongoing or active infection, symptomatic cardiovascular disease, or other chronic medical or psychiatric conditions that would impair compliance or would substantially increase the risk of participating in this study. Because of the potential for a drug interaction between warfarin and both tipifarnib and capecitabine, patients taking warfarin adjusted to an elevated INR were not eligible. Patients taking prophylactic low-dose warfarin (i.e., 1 mg daily) were eligible, but a PT and INR were required within 2 weeks of registration and must have been normal. Women of childbearing potential were strongly advised to use an accepted and effective method of contraception.
The study was coordinated and conducted by the ECOG, and the North Central Cancer Treatment Group (NCCTG) also participated. The trial was reviewed, approved, and sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute (ClinicalTrials.gov, identifier NCT00077363, E1103). The local institutional review board at each participating institution approved the protocol. All patients gave written, informed consent.
Treatment
Tipifarnib (NSC # 702818, IND # 58359; supplied by the NCI) (300 mg) was taken with food (e.g., snacks, breakfast, and dinner) twice daily. Capecitabine (1,000 mg/m2) was taken twice daily with at least 8 oz of water and without fruit juice, at the same time as tipifarnib or within 30 min after a meal. Both drugs were taken for 14 consecutive days, followed by 7 days of rest, every 21 days (i.e., 1 cycle). At each pre-cycle visit, patients underwent a history, physical exam, complete blood count, serum cre-atinine, electrolytes, liver function tests, and assessment of performance status, adverse events, and drug adherence (using history, a pill diary, and return of unused drug). Treatment was continued without interruption until disease progression, severe or intolerable toxicity, or 4 cycles beyond achieving a complete response (CR), or withdrawal of consent. Concurrent bisphosphonate therapy was permitted for patients with bone metastases.
For patients who experienced grades 3–4 toxicity (or grade 2 neuropathy), the tipifarnib was held until resolution to grades 0–1, then resumed in the same cycle (if before day 21) or next cycle with a one-dose level reduction (to 200 mg BID for the first reduction, 100 mg BID for the second reduction). Grade 3 neurotoxicity lasting more than 5 days or grade 4 non-hematological toxicity required permanent discontinuation of tipifarnib. Capecitabine was not held if tipifarnib was held for toxicity. Patients who stopped tipifarnib due to severe or intolerable toxicity continued capecitabine alone until disease progression.
Evaluation of response and toxicity
All patients underwent computed tomography (CT) of the chest and abdomen and a bone scan within 4 weeks of registration. Tumor response was assessed every 3 cycles by CT using RECIST [17], and bone scans were repeated if the original bone scan was positive or progressive bony metastatic disease was suspected. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (CTC) Version 3.0.
Statistical design
The primary endpoint of the study was ORR, which was defined as CR plus PR. The regimen would be considered promising if at least 21 responses (CR or PR) are observed in 64 eligible patients. This corresponds to 90.5 % power to detect an improvement in ORR from 25 to 40 % with a one-sided type I error rate of 9.9 %. To allow for 9 % ineligibility rate, 70 patients were to be recruited.
Secondary endpoints include toxicities, progression-free survival (PFS), time to treatment failure (TTF) and overall survival (OS). The Kaplan–Meier method was used to estimate the distribution of these endpoints. With a follow-up duration of 7 months, this study would give a 93 % power to detect an improvement in the median PFS from 3 to 4.5 months, with a one-sided type I error rate of 8 %. With a follow-up duration of 2 years, there would be 86 % power to detect an improvement in the median OS from 12.5 to 18 months, with a one-sided type I error rate of 10 %. Assuming that 40 % of the patients would achieve a response and would be followed up for a minimum of 17 months after achieving a response, there would be 70 % power to detect an improvement in the median duration of response from 8 to 12 months, with a one-sided type I error rate of 12 %. We assumed an exponential distribution for the failure time and uniform distribution for patients’ entry to the study. PFS failure was defined as the time from registration to disease progression or death, whichever occurred first. TTF was defined as the time from registration to disease progression, permanent discontinuation of treatment due to toxicity or death, whichever occurs first.
Another endpoint of the study was to document toxicities. Assuming the true probability of a rare toxicity is 1 %, the probability of observing one or more toxicities was 51 % for 70 patients. Assuming the true probability of a toxicity was 3 %, the probability of observing one or more toxicities was 88 %. Assuming the true probability of a toxicity was 5 %, the probability of observing one or more toxicities was 97 %. The 90 % confidence interval (CI) for any grade 3 or higher toxicity would be no wider than 21 %.
Statistical analysis
Efficacy analysis (ORR, OS, PFS, and TTF) includes the eligible patients who started treatment (N = 63) and the safety analysis (toxicity) includes all the patients who started treatment (N = 68). Descriptive statistics were used to characterize patients at baseline. Best response rate was reported and its CI was computed by the method of exact binomial CI [18]. The Kaplan–Meier method was used to characterize OS and PFS distributions, estimate OS and PFS rates [19]. Standard error of the estimates was calculated using Greenwood’s formula [20]. The CI of the median OS and PFS was constructed using the method by Brookmeyer and Crowley [18].
Results
Patient characteristics
Seventy-one patients were enrolled from ECOG and NCCTG institutions between May 19, 2004 and April 17, 2006. Seven patients were ineligible because of disease evaluation after registration (N = 2), second primary cancer (N = 2), brain metastases (N = 1), and use of either a prohibited anticonvulsant (N = 1) or another investigational drug (N = 1). Sixty-four patients were classified as eligible, among which one patient did not start treatment because of disease progression, leaving 63 patients in the efficacy analysis. Baseline characteristics of all eligible patients are shown in Table 1.
Table 1.
Baseline clinical characteristics of eligible patients
| Baseline characteristics | No. | % |
|---|---|---|
| Patients eligible/enrolled | 63/68 | 93 |
| Age at enrollment, years | ||
| Median | 52.0 | |
| Range | 35–79 | |
| Race/ethnicity | ||
| White and hispanic | 1 | 2 |
| White and non-hispanic | 50 | 79 |
| Black | 9 | 14 |
| Asian | 1 | 2 |
| Unknown | 2 | 3 |
| ECOG performance status | ||
| 0 | 24 | 38 |
| 1 | 38 | 60 |
| 2 | 1 | 2 |
| Menopausal status | ||
| Premenopausal | 10 | 16 |
| Postmenopausal | 35 | 56 |
| Unkonwn | 18 | 29 |
| ER/PR status at initial diagnosis | ||
| Positive (ER+ and/or PgR+) | 27 | 43 |
| Negative (ER−/PR−) | 36 | 57 |
| ER/PR status at recurrence or metastatsis | ||
| Positive (ER+ and/or PgR+) | 16 | 25 |
| Negative (ER−/PR−) | 15 | 24 |
| Unknown | 32 | 51 |
| Sites of metastatic disease | ||
| Non-visceral only | 12 | 19 |
| Visceral (liver, lung, adrenal) only | 14 | 22 |
| Both | 37 | 59 |
| No. of metastatic sites | ||
| 1 | 6 | 10 |
| 2 | 12 | 19 |
| 3 | 24 | 38 |
| ≥4 | 21 | 33 |
| Prior adjuvant therapy | ||
| Adjuvant endocrine therapy (ET) | 24 | 38 |
| Adjuvant/neoadjuvant chemotherapy | 54 | 86 |
| Surgery | 50 | 79 |
| Radiation | 36 | 57 |
| Prior metastatic therapy | ||
| ET | 21 | 33 |
| Radiation | 14 | 22 |
| Chemotherapy regimena | ||
| 0 | 5 | 8 |
| 1 | 29 | 46 |
| 2 | 24 | 38 |
| 3 | 5 | 8 |
Note: no Her2/neu status was recorded in the CRFs, but 10 patients received prior Herceptin treatment. In addition, 3 patients received prior bevacizumab treatment
One hundred percentage is calculated for each subgroup
Best response
Of the 63 eligible patients, a PR was observed in six patients (9.5 %; 90 % CI 4.2–17.9 %), and no patient had a complete response. Fourteen additional patients (22.2 %; 90 % CI 14.0–32.6 %) had stable disease (SD) for at least 6 months, and 37 patients (59 %; 90 % CI 47.6–69.2 %) had disease progression as their best response to therapy (Table 2).
Table 2.
Efficacy
| Best response | No. of patients (%) |
|---|---|
| Partial response | 6 (9.5 %) |
| Stable disease | 14 (22.2 %) |
| Progression disease | 37 (58.7 %) |
| Nonevaluable | 6 (9.5 %) |
Progression-free survival, time to treatment failure and overall survival
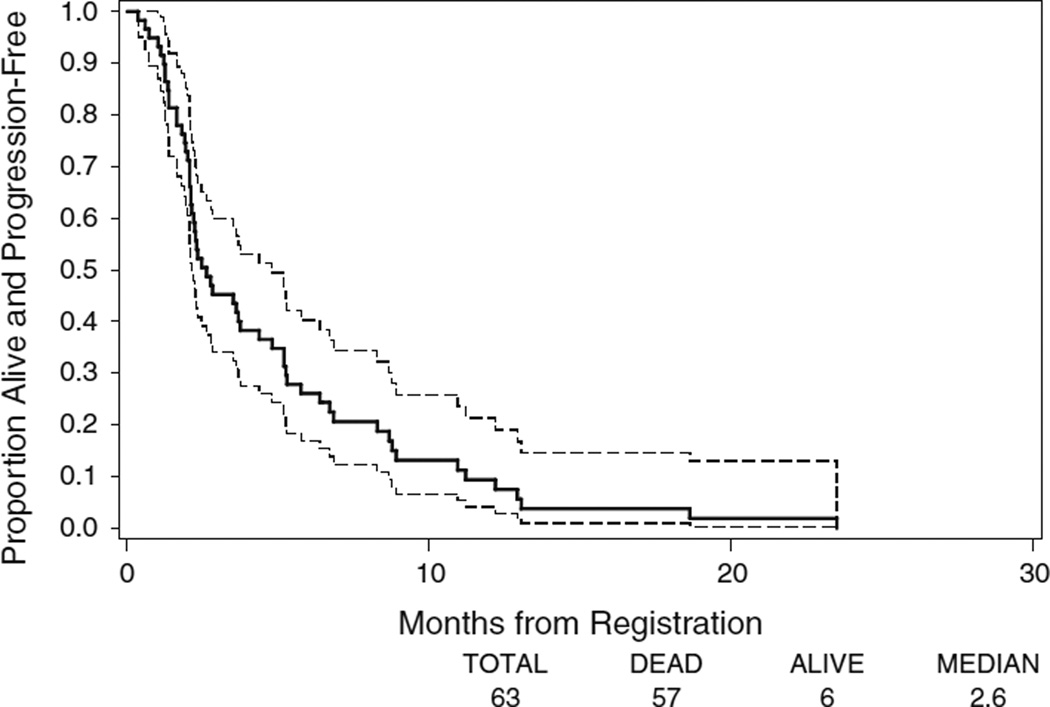
At the median follow-up of ~28 months, 56 (89 %) patients had disease progression, two patients died without documented disease progression, one patient was still alive and progression-free, and four patients died or withdrew before any follow-up evaluation. The median PFS was 2.6 months (95 % CI 2.1–4.4) (Fig. 1). The 1-year PFS rate was 9 % (95 % CI 2–17 %).
Fig. 1.
Kaplan–Meier plots of PFS in all eligible patients. The ranges of 95 % CI for the median TTP are included in the graphs
The median TTF was 2.1 months (95 % CI 1.8–2.8). Fifty-three (84.1 %) patients discontinued treatment because of disease progression or death on study. Other reasons for discontinuing therapy included symptomatic deterioration in one (10 %) patient, >2 weeks delay in treatment in one patient, physician’s decision of progression disease not confirmed by RECIST, and severe adverse events (neuropathy, skin changes, and unable to swallow pills) in one patient.
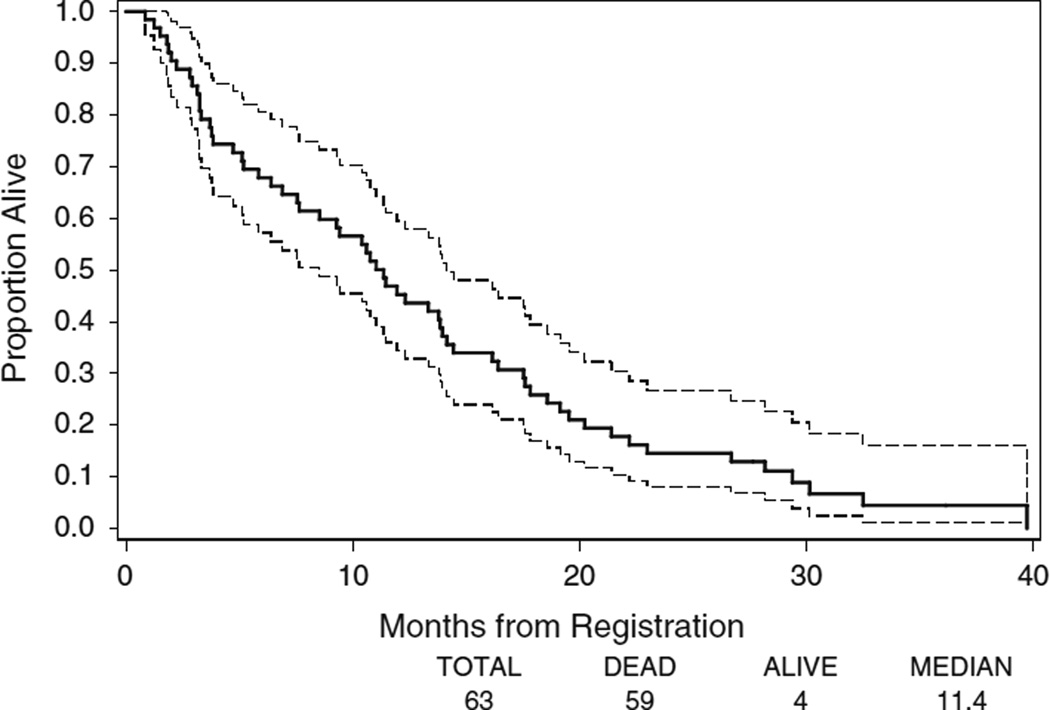
Among the 63 patients, 59 (94 %) had died. The median follow-up time for the four patients who were still alive on the date of data extraction was 28 months. Among the four surviving patients, two patients withdrew after 3 cycles of protocol therapy, one patient had progression disease after 2 cycles of protocol therapy, and one patient had progression disease after 18 cycles of therapy. The median OS was 11.4 months (95 % CI 7.7–14.0) (Fig. 2). One-year survival rate was 45.2 % (95 % CI 32.9–57.6 %) and 2-year survival rate was 14.5 % (95 % CI 5.8–23.3 %), respectively.
Fig. 2.
Kaplan–Meier plots of OS in all eligible patients. The ranges of 95 % CI for the median OS are included in the graphs
Treatment administered and adverse effects
All 68 registered patients who started treatment were included used in the safety analysis. The median number of cycles on treatment was 3 per patient (range, 0–20 cycles). Fourteen (22 %) patients received more than 9 cycles of treatment. Dose modifications were required for 43 patients (68 %) for either tipifarnib and/or capecitabine.
Table 3 summarizes the adverse events that were possibly, probably, or definitely treatment related. The most common adverse events (all grades) were anemia (76 %), nausea (65 %), fatigue (63 %), neutropenia (53 %), thrombocytopenia (53 %), hand–food reaction (47 %), diarrhea (47 %), anorexia (46 %), AST elevation (40 %), hypokalemia (39 %), and neuropathy (33 %). Thirty patients (44 %; 90 % CI 44.4–67.0 %) experienced grade 3 toxicity as the worst grade, and 11 patients (16 %; 90 % CI 10.8–29.0 %) experienced grade 4 toxicity as the worst grade. The most common grade 3 toxicities included neutropenia (19 %), nausea (12 %), and vomiting (12 %). The most common grade 4 toxicity was neutropenia (12 %) and dyspnea (1 %). One patient received 12 days of treatment on cycle 1 and died of disease progression on day 26 of cycle 1.
Table 3.
Adverse events that were at least possibly due to treatment in at least 10 % of patients
| Adverse event (N = 68) | Grades 1 or 2 | Grade 3 | Grade 4 | Any grade | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | |
| Hematologic | ||||||||
| Neutropenia | 15 | 22 | 13 | 19 | 8 | 12 | 36 | 53 |
| Anemia | 47 | 69 | 5 | 7 | 0 | 0 | 52 | 76 |
| Thrombocytopenia | 30 | 44 | 4 | 6 | 2 | 3 | 36 | 53 |
| Infection | 2 | 3 | 0 | 0 | 0 | 0 | 2 | 3 |
| Gastrointestinal | ||||||||
| Nausea | 36 | 53 | 8 | 12 | 0 | 0 | 44 | 65 |
| Vomiting | 15 | 22 | 8 | 12 | 0 | 0 | 23 | 34 |
| Diarrhea | 26 | 38 | 6 | 9 | 0 | 0 | 32 | 47 |
| Dehydration | 10 | 15 | 1 | 1 | 0 | 0 | 11 | 16 |
| Constipation | 10 | 15 | 1 | 1 | 0 | 0 | 11 | 16 |
| Abdominal pain | 10 | 15 | 2 | 3 | 0 | 0 | 12 | 18 |
| Constitutional | ||||||||
| Anorexia | 25 | 37 | 6 | 9 | 0 | 0 | 31 | 46 |
| Fatigue | 38 | 56 | 5 | 7 | 0 | 0 | 43 | 63 |
| Weight loss | 11 | 16 | 0 | 0 | 0 | 0 | 11 | 16 |
| Metabolic | ||||||||
| Hypokalemia | 24 | 35 | 3 | 4 | 0 | 0 | 27 | 39 |
| Hyponatremia | 6 | 9 | 2 | 3 | 0 | 0 | 8 | 12 |
| ALT, SGPT | 19 | 28 | 0 | 0 | 0 | 0 | 19 | 28 |
| AST, SGOT | 27 | 40 | 0 | 0 | 0 | 0 | 27 | 40 |
| Bilirubin | 12 | 18 | 1 | 1 | 0 | 0 | 13 | 19 |
| Neurologic | ||||||||
| Neuropathy, sensory | 20 | 29 | 3 | 4 | 0 | 0 | 23 | 33 |
| Dizziness | 10 | 15 | 0 | 0 | 0 | 0 | 10 | 15 |
| Insomnia | 7 | 10 | 0 | 0 | 0 | 0 | 7 | 10 |
| Pulmonary | ||||||||
| Dyspnea | 10 | 15 | 0 | 0 | 1 | 1 | 11 | 16 |
| Skin | ||||||||
| Hand–foot reaction | 27 | 40 | 5 | 7 | 0 | 0 | 32 | 47 |
| Rash/desquamation | 15 | 22 | 1 | 1 | 0 | 0 | 16 | 23 |
Discussion
We performed this phase II trial of tipifarnib–capecitabine combination in 63 eligible MBC patients who were previously treated with an anthracycline and progressed on taxane therapy. Treatment included capecitabine given at a commonly used dose and schedule [2,000 mg/(m2 day) for 14 of 21 days] plus concurrent tipifarnib (300 mg BID × 14 of 21 days). The adverse event profile for the combination was comparable to prior studies of capecitabine alone, although 68 % required dose modification because of toxicity, which may be somewhat higher that previous studies with capecitabine alone at this dose and schedule. We did not observe the target 40 % response rate or median PFS of 4.5 months which the trial was designed to detect. The observed response rate of about 10 %, median PFS of 2.6 months, and median OS of 11.4 months is very similar to the results observed in large phase III trials using a higher capecitabine dose (2,500 mg/m2 × 14 of 21 days) [21–24].
There are several possible explanations for the low clinical activity observed in this study. First, capecitabine might not be a suitable agent to combine with tipifarnib. About two-thirds of patients treated with the combination required a dose reduction, which may have compromised the efficacy of capecitabine. Secondly, due to lack of a predictive factor for response to tipifarnib, there was no enrichment of patients most likely to respond to the addition of FTI. FTase is one of three prenyltransferases used by normal and cancer cells to catalyze covalent attachment of prenyl groups to some 300 polypeptides in the human proteome [12]. Many of these peptides could serve as correlative biomarkers. In addition, low serum fibrinogen alpha peptide level detected by the proteomic analysis by SELDI-TOF and LTQ-FT-Orbitrap is associated with clinical benefit from tipifarnib–tamoxifen combination in tamoxifen-resistant MBC after 8 weeks of treatment [25]. Recently, the RASness gene signature has been identified as a promising biomarker for identifying tumor subsets potentially sensitive to Ras targeting therapy in acute leukemia [26, 27]. Its utility in predicting clinical response to tipifarnib and capecitabine merits further evaluation.
In conclusion, this phase II study suggests that the tipifarnib–capecitabine combination is not more effective than capecitabine alone in MBC patients who were previously treated with an anthracycline and progressed on taxane therapy. In order to determine whether FTIs, such as tipifarnib, will have a role in the treatment of breast cancer, novel trial designs will be required with correlative laboratory components to determine whether FTase inhibition is attained and to what degree this effect contributes to clinical benefit. Further preclinical and clinical studies are needed to identify predictive biomarkers and optimal strategies to incorporate tipifarnib in the treatment of MBC.
Acknowledgments
This study was conducted by the ECOG (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants CA23318 (to the EGOG statistical center), CA66636 (to the ECOG data management center), CA21115 (to the ECOG coordinating center), CA14958 (to Albert Einstein College of Medicine), CA17145 (to Northwestern University Medical Center), CA49883 (to Indiana University Medical Center), CA25224 (to NCCTG) and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. TL is also supported by UL1 RR024146 from the National Center for Research Resources.
Footnotes
Conflict of interest All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence their study.
Contributor Information
Tianhong Li, Montefiore Medical Center, Albert Einstein Cancer Center, Bronx, NY, USA.
Mengye Guo, Dana-Farber Cancer, Boston, MA, USA.
William J. Gradishar, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL, USA
Joseph A. Sparano, Montefiore Medical Center, Albert Einstein Cancer Center, Bronx, NY, USA
Edith A. Perez, Mayo Clinic, Jacksonville, FL, USA
Molin Wang, Dana-Farber Cancer, Boston, MA, USA.
George W. Sledge, Indiana University Cancer Center, Indianapolis, IN, USA
References
- 1.Ishikawa T, Sekiguchi F, Fukase Y, et al. Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res. 1998;58:685–690. [PubMed] [Google Scholar]
- 2.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 3.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–1768. doi: 10.1002/1097-0142(20011001)92:7<1759::aid-cncr1691>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Hennessy BT, Gauthier AM, Michaud LB, et al. Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at MD Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol. 2005;16:1289–1296. doi: 10.1093/annonc/mdi253. [DOI] [PubMed] [Google Scholar]
- 5.Leonard R, Hennessy BT, Blum JL, O’Shaughnessy J. Dose-adjusting capecitabine minimizes adverse effects while maintaining efficacy: a retrospective review of capecitabine for metastatic breast cancer. Clin Breast Cancer. 2011;11:349–356. doi: 10.1016/j.clbc.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Downward J. Signal transduction. Prelude to an anniversary for the RAS oncogene. Science. 2006;314:433–434. doi: 10.1126/science.1134727. [DOI] [PubMed] [Google Scholar]
- 7.Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berstein LM, Zheng H, Yue W, et al. New approaches to the understanding of tamoxifen action and resistance. Endocr Relat Cancer. 2003;10:267–277. doi: 10.1677/erc.0.0100267. [DOI] [PubMed] [Google Scholar]
- 9.Eralp Y, Derin D, Ozluk Y, et al. MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol. 2008;19:669–674. doi: 10.1093/annonc/mdm522. [DOI] [PubMed] [Google Scholar]
- 10.Kelland LR, Smith V, Valenti M, et al. Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res. 2001;7:3544–3550. [PubMed] [Google Scholar]
- 11.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Sparano JA. Inhibiting ras signaling in the therapy of breast cancer. Clin Breast Cancer. 2003;3:405–416. doi: 10.3816/CBC.2003.n.005. [DOI] [PubMed] [Google Scholar]
- 13.Appels NM, Beijnen JH, Schellens JH. Development of farnesyl transferase inhibitors: a review. Oncologist. 2005;10:565–578. doi: 10.1634/theoncologist.10-8-565. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SR, Hickish T, Ellis P, et al. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol. 2003;21:2492–2499. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 15.Sparano JA, Moulder S, Kazi A, et al. Targeted inhibition of farnesyltransferase in locally advanced breast cancer: a phase I and II trial of tipifarnib plus dose-dense doxorubicin and cyclophosphamide. J Clin Oncol. 2006;24:3013–3018. doi: 10.1200/JCO.2005.04.9114. [DOI] [PubMed] [Google Scholar]
- 16.Gore L, Holden SN, Cohen RB, et al. A phase I safety, pharmacological and biological study of the farnesyl protein transferase inhibitor, tipifarnib and capecitabine in advanced solid tumors. Ann Oncol. 2006;17:1709–1717. doi: 10.1093/annonc/mdl282. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Crowley J. A confidence interval for the medial survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 19.Kaplan ELaMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Greenwood M. Reports on Public Health and Medical Subjects. Vol. 22. London: Her Majesty’s Stationery Office; 1926. The natural duration of cancer; pp. 1–26. [Google Scholar]
- 21.Hortobagyi GN, Gomez HL, Li RK, et al. Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes. Breast Cancer Res Treat. 2010;122:409–418. doi: 10.1007/s10549-010-0901-4. [DOI] [PubMed] [Google Scholar]
- 22.Jassem J, Fein L, Karwal M, Campone M, Peck R, Poulart V, Vahdat L. Ixabepilone plus capecitabine in advanced breast cancer patients with early relapse after adjuvant anthracyclines and taxanes: a pooled subset analysis of two phase III studies. Breast. 2012;21(1):89–94. doi: 10.1016/j.breast.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SR, Semiglazov VF, Manikhas GM, Spaeth D, Romieu G, Dodwell DJ, Wardley AM, Neven P, Bessems A, Park YC, De Porre PM, Perez Ruixo JJ, Howes AJ. A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat. 2008;110(2):327–335. doi: 10.1007/s10549-007-9726-1. [DOI] [PubMed] [Google Scholar]
- 26.Raponi M, Belly RT, Karp JE, et al. Microarray analysis reveals genetic pathways modulated by tipifarnib in acute myeloid leukemia. BMC Cancer. 2004;4:56. doi: 10.1186/1471-2407-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raponi M, Harousseau JL, Lancet JE, et al. Identification of molecular predictors of response in a study of tipifarnib treatment in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2007;13:2254–2260. doi: 10.1158/1078-0432.CCR-06-2609. [DOI] [PubMed] [Google Scholar]