Abstract
Behavioral traits are likely to influence species vulnerability to anthropogenic threats and in consequence, their risk of extinction. Several studies have addressed this question and have highlighted a correlation between reproductive strategies and different viability proxies, such as introduction success and local extinction risk. Yet, very few studies have investigated the effective impact of social behaviour, and evidence regarding global extinction risk remains scant. Here we examined the effects of three main behavioral factors: the group size, the social and reproductive system, and the strength of sexual selection on global extinction risk. Using Primates as biological model, we performed comparative analysis on 93 species. The conservation status as described by the IUCN Red List was considered as a proxy for extinction risk. In addition, we added previously identified intrinsic factors of vulnerability to extinction, and a measure of the strength of the human impact for each species, described by the human footprint. Our analysis highlighted a significant effect of two of the three studied behavioral traits, group size and social and reproductive system. Extinction risk is negatively correlated with mean group size, which may be due to an Allee effect resulting from the difficulties for solitary and monogamous species to find a partner at low densities. Our results also indicate that species with a flexible mating system are less vulnerable. Taking into account these behavioral variables is thus of high importance when establishing conservation plans, particularly when assessing species relative vulnerability.
Introduction
There is increasing evidence that we may be facing a sixth mass extinction in the near future [1,2]. Species extinction rates are very high, and the survival of many other species is threatened [3]. The causes of population decline and present vulnerability to extinction are mainly anthropogenic, and can be classified into six categories including habitat degradation, overexploitation, invasion of non-native species, climate change, pollution, and spread of disease [2,4,5]. These threats constitute the extrinsic factors of vulnerability. Because they are not distributed homogeneously across the world, the extrinsic factors of vulnerability do not concern all the species. The degree of exposure to extrinsic factors is very likely the primary factor leading to species extinction risk. Nevertheless, species living in the same area, and experiencing the same anthropogenic pressure are not necessarily at the same risk of extinction and will not share the same conservation status as measured by the IUCN Red List [6]. This is because several species-specific characteristics can modulate species vulnerability to extinction threats and to extinction. These characteristics are known as “intrinsic factors of vulnerability” and include life history traits and ecological characteristics. Two life history traits often identified as intrinsic factors of vulnerability are gestation length and body mass. The impact of gestation length on species vulnerability is well documented: species with longer gestation time are more prone to extinction because of their longer recovery time [7–9]. On the contrary, the impact of body mass on extinction risk is more controversial. While many studies highlighted that the higher the body mass the more species are at risk of extinction [7,10–13], several other studies did not find any body mass effect [14–16]. The higher vulnerability of large-bodied species is usually explained by lower densities of large-bodied species [17], higher requirements (for diet and habitat [18,19]), and longer recovery times (due to a correlation between gestation length and body mass [8]). Concerning the ecological variables impacting the extinction risk, geographic range, population density, and trophic level may affect species vulnerability to extinction risk [8,20]. Species with small geographic range and low population density have less ability to recover from any potential perturbation, and are thus more prone to extinction [8]. High trophic level species would be more at risk because of their dependence on lowered-chain species to survive [8].
While many studies have investigated the impact of life history and ecological characteristics on the extinction risk, very few have focused on the potential influence of behavioral variables. Yet, the idea that behavior could have an important role in the extinction risks of many species is quite old, and has often been raised [21–24]. Social system and sexual selection are among the most cited behaviour variables to potentially affect extinction risk. Social system may impact species vulnerability through group size, social organisation or mating system. In group-living species, several behavioral components are likely to require a threshold number of conspecifics, like raising youngs [25], reducing predation risk (either by dilution [26,27], or by active watching [28,29]), or foraging [30,31]. Group living as long been hypothesised as a possible vulnerability factors [25], but evidence remains scant. Nevertheless, when examining mammals extinction risk as evaluated bu the UICN, Davidson and colleagues [32] highlighted a negative correlation between group size and extinction risk, for species with relatively slow life cycle and restricted distribution range (for more details, see Davidson and colleagues [32]). Species extinction risk may also depend on social organisation, since solitary species are less likely to find a mate when population density becomes too low [32]. Another social system component proposed to contribute to extinction risk is the mating system, but conclusions about its impact are diverse. A theoretical study regarding the effect of the mating system on extinction risk showed that all mating systems are likely to experience a strong Allee effect when the operational sex ratio (OSR) is balanced [33]. Hence, the demographic structure is paramount to understand the impact of mating systems. Monogamy may be more likely to approach a balanced OSR than polygyny, but anthropogenic disturbance such as selective hunting may render polygynous species more extinction prone [33]. Moreover, polygyny has the potential to result in higher demographic variance [34]. One study in birds found no effect of mating system on the extinction risk [33], whereas a study based on Mammals assessed that monogamous and polygynous species living in small groups were more at risk [35]. Several studies have also assumed an influence of sexual selection in species extinction risk [36,37], but its real impact remains hard to demonstrate. A comparative analyse using an extensive database on bird introductions concluded that sexual selection was negatively correlated to introduction success [38]. This result was supported by ulterior analysis of local extinction rate [39] and conservation status of North American birds [40], but not by the comparative analysis of the conservation status of European birds and Mammals [41]. Despite non-concordant results, group size, socio-reproductive system and sexual dimorphism are often mentioned as potentially intrinsic factors of vulnerability in studies on the causes of extinction risks.
The main aim of this study is to examine the role of three major behavioral components in the extinction risk in Primate species: group size, social and mating system, and sexual dimorphism. The choice of Primates as study model was made for three reasons. First, they present a large variety of social systems [42] and a wide range of sexual selection intensity [43]. Second, as Primates constitute one of the best-studied taxa, the behavioral data available is numerous compared to many other species groups. Finally, more than 50% of the Primate species are currently threatened by extinction [6], which renders the study of their vulnerability particularly relevant. Because Primates have been the objects of previous studies concerning intrinsic vulnerability factors, we included in our study previously identified factors relevant to the explanation of extinction risk, in order to prevent bias [43,44]. In addition, as Primates are also submitted to anthropogenic pressures, it was important to take it into account in the analysis. If not, we may have lost an important part of the variance explaining the extinction risk. In other words, because we suspected the extrinsic factors of vulnerability to be responsible in a high proportion for species conservation status, we had to include a proxy of human impact in our models, the human footprint index [45].
Materials and Methods
Data collection
Conservation status
We chose the IUCN status from the IUCN Red List (2013) as a proxy of extinction risk (downloaded from the IUCN Red List website, http://www.iucnredlist.org/). The IUCN status of threat downloaded from the IUCN Red List are part of a qualitative nominal variable with five modalities: “Least Concern”, “Near Threatened”, “Vulnerable”, “Endangered” and “Critical” (LC, NT, VU, EN and CR). For our analysis we transformed the IUCN status into an ordinal variable (0, 1, 2, 3, 4, respectively). This type of transformation is commonly used in other studies using the IUCN status of threat as a proxy of species extinction risk [8,16,35,44].
Extrinsic factors of vulnerability
We chose the human footprint index (version 2: 1995–2004) as an indicator of the strength of the extrinsic factors of vulnerability. The human footprint index measures the impact of human societies at the surface of Earth [45]. It combines four variables: human population density, land transformation, electrical power infrastructures and accessibility (for more details, see [45]). The use of the human footprint index as a proxy of human impact on terrestrial ecosystems presents at least three advantages. First, the data contained in this index provide good information of the main threats that affect Primate species. Primates are mainly threatened by habitat degradation and hunting [46], which we believe should be well described by the human population density/land transformation variables of human footprint and the human population density/accessibility variables, respectively. The link between land transformation and habitat degradation seems obvious, and several studies have found a correlation between human density and hunting intensity [47,48] (however it might not be the case at a very local scale [49]), or for habitat loss [50,51]. Additionally, accessibility to Primates by roads may lead to an increase of hunting intensity [46]. Second, the human footprint index is available for the distribution range of all Primate species. Finally, to our knowledge, the influence of human footprint on Primate extinction risk has never been studied at a global scale, despite its potential important effect on Primates population dynamics.
In this study, we focused on the mean human footprint in species distribution range. Data on the worldwide human footprint was downloaded from the NASA SocioEconomic Data and Applications Center (SEDAC) website (http://sedac.ciesin.columbia.edu/). Polygons representing Primate species distribution range were downloaded from the IUCN website, as ESRI shapefiles format. Polygons result from the liaison of occurrence points of the species, expended to take into account species habitat preference, where unsuitable habitats are removed. Thus, polygons provide the information that species probably occurs within it, but do not mean that it is distributed equally or occurs everywhere within that polygon.
Intrinsic factors of vulnerability
We examined three behavioral variables: social and mating system, average group size, and sexual dimorphism. The social organisation and the mating system were combined into a single qualitative variable because both are strongly correlated (species living in pairs are always monogamous, species living in group are either polygynous or promiscuous, and solitary species are polygynous). This variable will be referred as the Social and Mating System. The modalities were defined as follow: solitary, monogamous, polygynous, promiscuous, and polygynous-promiscuous species (i.e., species for which the mating system varies among groups). Those categories are adapted from Eisenberg and colleagues work on Primate social systems [52]. The average group size was chosen in order to assess species vulnerability to threats according to their group size. As a surrogate of sexual selection, we chose the weight sexual dimorphism, which is often used to indicate the strength of sexual selection [53].
Species life history traits included in our analysis were gestation length and body mass. As ecological variables, we selected the percentage of frugivory (i.e. the percentage of fruits in the diet of a species) and the mean home range (i.e. the mean living area of an individual/pair/group). Three climatic variables indicating the ecological flexibility of a species were also added in the models: the annual temperature range, the mean annual precipitations, and the annual precipitation range. Data concerning these climatic variables were collated on the NASA website. The annual temperature and precipitation ranges were calculated as the difference between the mean highest annual temperatures/precipitations over the distribution area with the mean lowest annual temperatures/precipitations among this same area. The mean annual precipitations were measured as the mean of all mean annual precipitation pixels among the distribution area.
Data were collated from peer-reviewed articles and online scientific databases. In order to minimise the bias due to multiple sources of information, data for each variable was collected from comparative analysis published in scientific articles and scientific databases (All the world’s Primates [54]; panTHERIA [55]. Final dataset comprised 93 Primate species for each we disposed of information on all factors.
Data transformations
Prior to the analysis, some explicative variables were transformed to ensure a normal distribution. A log-transformation was applied to the explanatory variables that did not have zero values (female body mass, male body mass, average group size, home range). Because the gestation length and the female body mass were highly correlated (Spearman’s correlation test: rho = 0.73, p-v = 2.1e-15), we performed a linear regression between those two variables. Instead of the log(body mass), we used the residuals of this regression in our models, which correspond to the part of the female body mass that is not explained by the gestation. The same method was used to obtain a variable indicating the weight sexual dimorphism (i.e. the mean weight difference between female body mass and male body mass): we took the residuals corresponding to the part of the male body mass not explained by the female body mass [53].
Tests of phylogenetic signal
A common issue in comparative analysis is that compared species are not phylogenetically independent. This can introduce an error in the analysis when the response variable is not explained by the explicative variables, but by species proximity in the phylogeny. To prevent this error, it is necessary to test for the presence of a significant phylogenetic signal in the response variable, before starting the analysis. The presence of a phylogenetic signal in the status of threat was tested by measuring Pagel’s lambda [56], one of the most used indicator of phylogenetic signal. Even if this metric was originally designed for continuous variables, the function FitDiscrete in package Geiger in R allows its calculation for discrete traits [57,58]. We found a lambda of 0.78, indicating a positive phylogenetic signal (i.e. close related species are more probable to have the same status than species taken randomly in the phylogeny, see also Fig 1).
Fig 1. The phylogenetic inertia of the conservation status in Primate species.
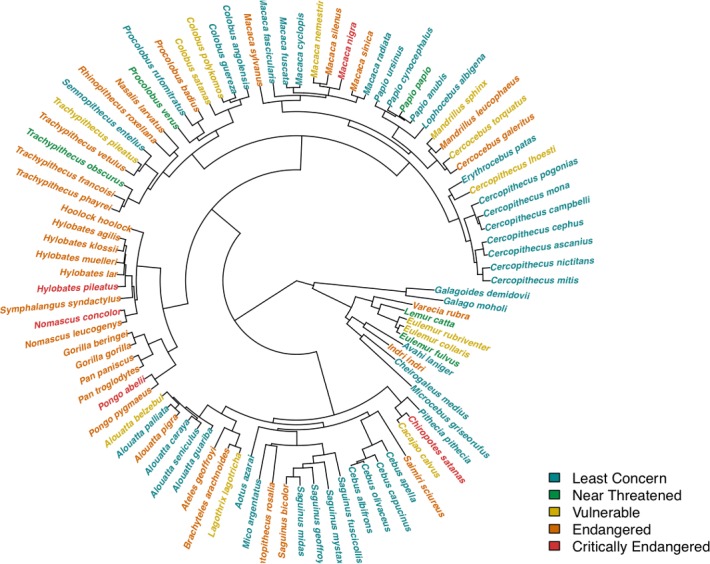
Species names are written in different colours according to their conservation status.
Statistical models
As we measured a significant positive phylogenetic signal in the response variable (i.e. transformed extinction risk), we performed models correcting for the phylogenetic inertia [59–61]. We chose to use “Phylogenetic generalized Least Squares” (PGLSs), one of the most used methods of correction for phylogenetic inertia. PGLSs are particularly appropriate in our case, since they allow the consideration of categorical variable, which is the case of the conservation status we used as response variable [62–64]. The lambda value of 0.78 was incorporated into the models to correct for the phylogenetic non-independence of the conservation status. We began by a PGLS model containing all the explicative variables, and we adopted a top-down approach, removing at each step the less significant variable (among the insignificant ones). For each model performed, we measured the AICc (a measure of AIC for small datasets) and the ΔAICc (i.e. the difference between the AICc of the model, and the smallest AICc of all models). All models with ΔAICc<2 can be considered as the best models (Burnham & Anderson, 2002). Statistical analyses and SIG manipulations were conducted using R software (Development-Core Team 2011, packages geiger, maptools, raster and rgdal).
Results
Several models can be considered as the best model, since five models had a ΔAICc < 2. The five models were relatively consistent, and most variables were significant in all the best models (Table 1 and S1 File). All models highlighted a significant impact of the extrinsic factor of vulnerability to extinction risk, with higher vulnerabilities of species experiencing high mean human footprint values in their distribution areas. Many intrinsic factors were also consistently present in all models and significant. Ecological factors such as the ecological flexibility, home range size and percentage of frugivory were significant in each of the 5 models, with higher extinction risk for low flexible, large home range and highly frugivorous species, respectively. On the contrary, life history traits were not present in all models, and even when present they where not consistently significant.
Table 1. Best explanatory models according to the AICc (ΔAICc ≤ 2 in relation the smaller AICc).
| AICc | 288.20 | 288.93 | 289.44 | 289.44 | 289.77 | ||
|---|---|---|---|---|---|---|---|
| AICc | - | 0.73 | 1.24 | 1.24 | 1.57 | ||
| Extrinsinc factors | Mean human footprint | +++ | +++ | +++ | +++ | +++ | |
| Intrinsic Factors: Ecology | Annual range of precipitations | −−− | −−− | −−− | −−− | −−− | |
| Mean temperature range | −−− | −− | −− | −−− | −−− | ||
| Frugivory | +++ | +++ | +++ | +++ | +++ | ||
| Home range (log) | +++ | +++ | +++ | +++ | +++ | ||
| Life history | Gestation | +++ | + | +++ | +++ | ||
| Female body mass (res) | + | + | + | ||||
| Behaviour | Mean group size (log) | −− | −−− | −− | −− | −−− | |
| Socio-reproductive system: | |||||||
| polygynous and promiscuous | − | − | |||||
| polygynous | + | + | |||||
| promiscuous | + | + | |||||
| solitary | − | − | |||||
| Sexual dimorphism | − | ||||||
Signs indicate the sense of the effect of the variables included in the model. The number of signs inform on the significance level of the estimates (***: p ≤ 0.05, **: 0.05 < p ≤ 0.1, and * p > 0.1).
Concerning the behavioural traits, all models pointed out to a significant impact of the mean group size in the vulnerability to extinction. According to our results, species with a higher mean group size presented a lower extinction risk. Interestingly, two of the models selected incorporated the social and mating system as an explicative variable, but this variable was not significant. Finally, the model with the lowest AICc among our 5 best models also included sexual dimorphism, even though this variable was not significant.
Discussion
This study is one of the first to highlight the existence of a behavioural dimension in species’ intrinsic vulnerability factors, and this while controlling for the intensity of extrinsic factors of vulnerability. More precisely, the average group size of Primate species has an impact on the conservation status, with species living in small groups being more vulnerable to extinction than those living in large groups. This result is consistent with the pattern highlighted by Davidson and colleagues [32], when considering mammals extinction risk. This pattern could be explained by the occurrence of an Allee effect in species living in small groups, since a well-known source of Allee effect is the difficulty to find a partner [25,65], which is supposed to be more difficult for solitary and monogamous species, (for monogamous versus polygynous species [66]). Although we did not find a significant effect of the “social and mating system” variable in this study, the model with the smallest AICc was the one including this variable in the explicative parameters. In other words, species conservation status is better explained when incorporating the “social and mating system” variable in the model, despite the absence of a significant effect. Although hardly interpretable without supplementary investigations, this might be due to a hidden real effect of the social and mating system variable. Indeed, this variable is a qualitative variable with 5 modalities, which requires more statistical power than the other variables, and it is possible we did not use enough data to detect effects in this variable
We also identified various additional intrinsic factors of vulnerability, and that included previously identified ecological and life-history traits [8,35,67]. As intrinsic factors of vulnerability other than behaviour, we found in every model a negative correlation between ecological flexibility and extinction risk (two variables indicating ecological flexibility were significant in each case). This indicates that more ecologically flexible species are more tolerant to environmental changes and disturbances, and able to better adapt to disturbed habitats. This is consistent with previous studies in Primates where increased vulnerability to logging is related to low ecological flexibility [68]. Two other ecological variables were significant in all models: species with large home range and high degree of frugivory are more at risk. The effect of home range may be due to higher ecological requirements of species with large home range to survive. If these species necessitate a high quantity of food or a wider range of habitats, they may be less tolerant toward anthropogenic perturbations. Additionally, the impact of home range size found here might also be a masked effect of the mean group size, since a large home range is quite correlated with a high mean group size (Pearson’s correlation test, cor = 0.67). The higher vulnerability of highly frugivore species is concordant with most of previous studies, and could be attributed to frugivore species dependence on scarce and patchy food resource [66], which makes them more vulnerable to habitat disturbance ([69], cited by [18]) and possibly to hunting [68].
In some of the 5 selected models, gestation length had a significant impact on the extinction risk, while the part of female body mass unexplained by gestation length had a trend to be higher with higher extinction risks. The higher vulnerability to extinction of species with higher gestation length is well-known and often highlighted in field studies (in Birds [70], in Carnivores and Primates [8], and in Mammals [44]). This is due to a longer recovery rate of species with long gestation length ([71] cited by [8]). In our study, we were able to disentangle the impact of gestation length and female body mass, which are highly correlated, by a linear regression approach. Because of this transformation, we can affirm the trend obtained for the body mass is a proper effect of the body mass and not a masked effect of gestation length (due to the high correlation of those two variables). Thus, we cannot input the increase of extinction risk with body mass to the lower recovery time of large body mass species, because the recovery time depends primarily on gestation length. The increase of extinction risk with body mass could be due to higher requirements of large body mass species (for example, large area requirements, greater food intake or high habitat specificity [72,73], or to an inverse correlation between body mass and population size [17]. An alternative explanation, less common but potentially relevant in Primates, would be that species with higher body mass are more targeted by hunters [46].
Our results confirm the low tolerance of Primate species to anthropogenic pressures [46,68], since species with higher mean human footprints were significantly more at risk of extinction, whatever the model. In our study, we only focused on the mean human footprint, which provides good bases to investigate the impact of anthropogenic pressures on the extinction risk. However, the human footprint is never homogeneous in species distribution areas. In reality, some regions among the distribution area of a species can be highly impacted by human activities, whereas some others are much more preserved [6], with the intensity of the heterogeneity depending on species. Thus, species that can take refuge in low human footprint regions in their distribution area may be less vulnerable to extinction than species that cannot benefit of from refuge zones, for a similar mean human footprint. A future important point to investigate will be to take into account the heterogeneity of the human footprint.
Another point to consider with caution is the use of IUCN conservation status as proxies for extinction risk. The conversion of IUCN status into a quantitative variable ranging from 0 to 4 could induce an error because it leads to a quantification of the extinction risk that did not exist in the raw IUCN status. Thus, it is at high interest that future studies investigate the way we could transform IUCN status into a variable that does not introduce errors.
Our work is one of the first to highlight the impact of behavior in the extinction risk in Primates, by disentangling the effects of different factors of vulnerability, both extrinsic and intrinsic. By doing so, we provide new elements to answer the old and hard-to-investigate question of the impact of behaviour in the extinction risk. In addition, identifying the factors responsible for the extinction of Primates, by which more than 50% of species are threatened of extinction, is critical to establish successful conservation plans. Indeed, previous works [74,75] highlighted the importance of identifying priority areas for conservation and integrating the information on intrinsic factors of vulnerability when establishing conservation plans is particularly relevant. Thus, knowing some behavioural aspects render species more prone to extinction can lead to specific measures in conservation plans, to counter the negative impacts of some behaviors. By doing so, the preservation of populations threatened by extinction should be more efficient.
Supporting Information
Models presented here are the one with the smallest AICc, and other models which have ΔAICc <2.
(PDF)
Acknowledgments
We would like to acknowledge the biodiversity research group at ESE for all their comments and enriching stimulus. The comments and support of Luis Verde Arregotia, Céline Bellard, Gloria Luque and Elsa Bonnaud were invaluable, as was the help of Adèle de Baudouin regarding data collection. A particular thanks goes to Franck Courchamp and Nathalie Frascaria-Lacoste, without whom this study would not had been possible. The authors were supported by a grant from the “Agence Nationale pour la Recherche” (ANR), for the project “Research on Allee and Rarity Effects” (RARE; 2009PEXT01001). http://www.agence-nationale-recherche.fr/
Data Availability
All data can be found as supplementary material.
Funding Statement
The authors were supported by a grant from the "Agence Nationale pour la Reherche" (ANR), for the project "Research on Allee and Rarity Effects" (RARE; 2009PEXT01001). AL’s salary was funded by ANR-RARE. http://www.agence-nationale-recherche.fr/.
References
- 1. Wake DB, Vredenburg VT (2008) Colloquium paper: are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci U S A 105 Suppl: 11466–11473. 10.1073/pnas.0801921105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, et al. (2011) Has the Earth’s sixth mass extinction already arrived? Nature 471: 51–57. 10.1038/nature09678 [DOI] [PubMed] [Google Scholar]
- 3. Pimm SL, Russell GJ, Gittleman JL, Brooks TM (1995) The future of biodiversity. Science 269: 347–350. [DOI] [PubMed] [Google Scholar]
- 4. Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying Threats to Imperiled Species in the United States. Bioscience 48: 607–615. [Google Scholar]
- 5. Daszak P (2000) Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science (80-) 287: 443–449. [DOI] [PubMed] [Google Scholar]
- 6.IUCN (2012) No Title. IUCN RedList Threantened Species Version 20121.
- 7. Bennett P, Owens I (1997) Variation in extinction risk among birds: Chance or evolutionary predisposition? Proc R Soc B- Biological Sci 264: 401–408. [Google Scholar]
- 8. Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc B-Biological Sci 267: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dulvy NK, Sadovy Y, Reynolds JD (2003) Extinction vulnerability in marine populations. Fish Fish 4: 25–64. [Google Scholar]
- 10. Gaston KJ, Blackburn TM, Goldewijk KK (2003) Habitat conversion and global avian biodiversity loss. Proc R Soc London Ser B-Biological Sci 270: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foufopoulos J, Ives AR (1999) Reptile Extinctions on Land‐Bridge Islands: Life‐History Attributes and Vulnerability to Extinction. Am Nat 153: 1–25. [DOI] [PubMed] [Google Scholar]
- 12. Cardillo M, Bromham L (2001) Body Size and Risk of Extinction in Australian Mammals. Conserv Biol 15: 1435–1440. [Google Scholar]
- 13. Lips KR, Reeve JD, Witters LR (2003) Ecological Traits Predicting Amphibian Population Declines in Central America. Conserv Biol 17: 1078–1088. [Google Scholar]
- 14. Gotelli NJ, Graves GR (1990) Body Size and the Occurrence of Avian Species on Land-Bridge Islands. J Biogeogr 17: 315. [Google Scholar]
- 15. Williams S, Hero J (1998) Rainforest frogs of the Australian Wet Tropics: guild classification and the ecological similarity of declining species. Proc R Soc B- Biological Sci 265: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones KE, Purvis A, Gittleman JL (2003) Biological correlates of extinction risk in bats. Am Nat 161: 601–614. [DOI] [PubMed] [Google Scholar]
- 17. Pimm SL, Jones HL, Diamond J (1988) On the risk of extinction. Am Nat 132: 757–785. [Google Scholar]
- 18. Sodhi NS, Lee TM, Koh LP, Prawiradilaga DM (2006) Long-term avifaunal impoverishment in an isolated tropical woodlot. Conserv Biol 20: 772–779. [DOI] [PubMed] [Google Scholar]
- 19. Peh KS-H, Jong J de, Sodhi NS, Lim SL-H, Yap CA-M (2005) Lowland rainforest avifauna and human disturbance: persistence of primary forest birds in selectively logged forests and mixed-rural habitats of southern Peninsular Malaysia. Biol Conserv 123: 489–505. [Google Scholar]
- 20. Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM, et al. (2004) Human population density and extinction risk in the world’s carnivores. PLoS Biol 2: E197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allee WC (1931) Animal aggregations. University of Chicago Press, editor. [Google Scholar]
- 22. Caro TM (1998) Behavioral ecology and conservation biology New York, Oxford: Oxford University Press. [Google Scholar]
- 23. Gosling L, Sutherland W (2000) Behaviour and conservation Conservati. Cambridge: Cambridge University Press. [Google Scholar]
- 24. Danchin E, Giraldeau L-A, Cézilly F (2008) Behavioural Ecology. Oxford University Press, editor. [Google Scholar]
- 25. Courchamp F, Clutton-Brock T, Grenfell B (1999) Inverse density dependence and the Allee effect. Trends Ecol Evol 14: 405–410. [DOI] [PubMed] [Google Scholar]
- 26.Estes RD (1974) Social organization of the African Bovidae. In: Geist V, Walther F, editors. The behaviour of ungulates and its relation to management. pp. 166–205.
- 27.Bertram BCR (1978) Living in groups: predators and prey. In: Krebs JR, Davies NB, editors. Behavioral ecology. Oxford. pp. 64–96.
- 28. Pulliam HR, Pyke GH, Caraco T (1982) The scanning behavior of juncos—a game-theoretical approach. J Theor Biol 95: 89–103. [Google Scholar]
- 29. McNamara JM, Houston AI (1992) Evolutionarily stable levels of vigilance as a function of group-size. Anim Behav 43: 641–658. [Google Scholar]
- 30. Fryxell JM (1991) Forage Quality and Aggregation by Large Herbivores. Am Nat 138: 478. [Google Scholar]
- 31. Snaith T V, Chapman CA (2008) Red colobus monkeys display alternative behavioral responses to the costs of scramble competition. Behav Ecol 19: 1289–1296. [Google Scholar]
- 32. Davidson A, Hamilton M, Boyer AG, Brown JH, Ceballos G (2009) Multiple ecological pathways to extinction in mammals. PNAS 106: 10702–10705. 10.1073/pnas.0901956106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jager HI, Carr EA, Efroymson RA (2006) Simulated effects of habitat loss and fragmentation on a solitary mustelid predator. Ecol Modell 191: 416–430. [Google Scholar]
- 34. Bessa-Gomes C, Legendre S, Clobert J (2004) Allee effects, mating systems and the extinction risk in populations with two sexes. Ecol Lett 7: 802–812. [Google Scholar]
- 35. Lee AM, Saether BE, Engen S (2011) Demographic Stochasticity, Allee Effects, and Extinction: The Influence of Mating System and Sex Ratio. Am Nat 177: 301–313. 10.1086/658344 [DOI] [PubMed] [Google Scholar]
- 36. Brashares JS (2003) Ecological, behavioral, and life-history correlates of mammal extinctions in West Africa. Conserv Biol 17: 733–743. [Google Scholar]
- 37. Kokko H, Brooks R (2003) Sexy to die for? Sexual selection and the risk of extinction. Ann Zool Fennici 40: 207–219. [Google Scholar]
- 38. Moller AP (1999) Asymmetry as a predictor of growth, fecundity and survival. Ecol Lett 2: 149–156. [Google Scholar]
- 39. Bessa-Gomes C, Danek-Gontard M, Cassey P, Moller AP, Legendre S, Clobert J (2003) Mating behaviour influences extinction risk: insights from demographic modelling and comparative analysis of avian extinction risk. Ann Zool Fennici 40: 231–245. [Google Scholar]
- 40. Doherty PF, Sorci G, Royle JA, Hines JE, Nichols JD, Boulinier T (2003) Sexual selection affects local extinction and turnover in bird communities. Proc Natl Acad Sci U S A 100: 5858–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Candolin U, Heuschele J (2008) Is sexual selection beneficial during adaptation to environmental change? Trends Ecol Evol [DOI] [PubMed] [Google Scholar]
- 42. Morrow EH, Fricke C (2004) Sexual selection and the risk of extinction in mammals. Proc R Soc B-Biological Sci 271: 2395–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kappeler PM, van Schaik CP (2002) Evolution of primate social systems. Int J Primatol 23: 707–740. [Google Scholar]
- 44. Rowe N (1996) The pictorial guide of living primates Myers L, Sheffield R, editors Charleston, Rhode Island: Pogonias Press. [Google Scholar]
- 45. Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds ORP, et al. (2005) Multiple causes of high extinction risk in large mammal species. Science (80-) 309: 1239–1241. [DOI] [PubMed] [Google Scholar]
- 46. Sanderson EW, Jaiteh M, Levy MA, Redford KH, Wannebo AV, Woolmer G, et al. (2002) The Human Footprint and the Last of the Wild. Bioscience 52: 891. [Google Scholar]
- 47. Cowlishaw G, Dunbar R (2000) Primate conservation biology Chicago: The University of Chicago Press. [Google Scholar]
- 48. Peres CA (2000) Effects of Subsistence Hunting on Vertebrate Community Structure in Amazonian Forests. Conserv Biol 14: 240–253. [Google Scholar]
- 49. Woodroffe R (2000) Predators and people: using human densities to interpret declines of large carnivores. Anim Conserv 3: 165–173. [Google Scholar]
- 50. Urquiza-Haas T, Peres CA, Dolman PM (2009) Regional scale effects of human density and forest disturbance on large-bodied vertebrates throughout the Yucatán Peninsula, Mexico. Biol Conserv 142: 134–148. [Google Scholar]
- 51. Cortés Y, Fernández-Salvador R, García F, Virgós E, Llorente M (1998) Changes in otter Lutra lutra distribution in Central Spain in the 1964–1995 period. Biol Conserv 86: 179–183. [Google Scholar]
- 52. O’Brien T, Kinnaird M, Wibisono H (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6: 131–139. [Google Scholar]
- 53. Eisenberg JF, Muckenhirn NA, Rundran R (1972) The Relation between Ecology a Social Structure in Primates. Science 176: 863–874. [DOI] [PubMed] [Google Scholar]
- 54. Mitani JC, GrosLouis J, Richards AF (1996) Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am Nat 147: 966–980. [Google Scholar]
- 55.Rowe N, Myers M (n.d.) All the World’s Primates. Available: http://www.alltheworldsprimates.org Primate Conserv Inc, Charlest RI.
- 56. Jones K, Bielby J, Cardillo M, Fritz S, O’Dell J, Orme CDL, et al. (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90: 26–48. [Google Scholar]
- 57. Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- 58. Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24: 129–131. [DOI] [PubMed] [Google Scholar]
- 59. Engler R, Guisan A, Rechsteiner L (2004) An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J Appl Ecol 41: 263–274. [Google Scholar]
- 60. Cheverud JM, Dow MM, Leutenegger W (1985) The quantitative assessment of phylogenetic constraints in comparative analyses—sexual dimorphism in body-weight among Primates. Evolution (N Y) 39: 1335–1351. [DOI] [PubMed] [Google Scholar]
- 61. Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125: 1–15. [Google Scholar]
- 62. Purvis A (2008) Phylogenetic Approaches to the Study of Extinction. Annual Review of Ecology Evolution and Systematics. Vol. 39 pp. 301–319. [Google Scholar]
- 63. Putland D (2005) Problems of studying extinction risks. Science (80-) 310: 1277. [PubMed] [Google Scholar]
- 64. Rohlf FJ (2006) A comment on phylogenetic correction. Evolution (N Y) 60: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 65. Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63: 495–502. [Google Scholar]
- 66. Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14: 401–405. [DOI] [PubMed] [Google Scholar]
- 67. Legendre S, Clobert J, Møller AP, Sorci G (1999) Demographic Stochasticity and Social Mating System in the Process of Extinction of Small Populations: The Case of Passerines Introduced to New Zealand. Am Nat 153: 449–463. [DOI] [PubMed] [Google Scholar]
- 68. Cowlishaw G, Pettifor RA, Isaac NJB (2009) High variability in patterns of population decline: the importance of local processes in species extinctions. Proc R Soc B Biol Sci 276: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Isaac NJB, Cowlishaw G (2004) How species respond to multiple extinction threats. Proc R Soc B-Biological Sci 271: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gittleman J (1993) Carnivore life histories: a re-analysis in the light of new models. In: Dunstone N, Gorman M, editors. Mammals as predators. pp. 65–86.
- 71. Leck C (1979) Avian extinctions in an isolated tropical wet-forest preserve, Ecuador. AUK 96: 343–352. [Google Scholar]
- 72. Owens IPF, Bennett PM (2000) Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. Proc Natl Acad Sci U S A 97: 12144–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. KATTAN GH (1992) Rarity and Vulnerability: The Birds of the Cordillera Central of Colombia. Conserv Biol 6: 64–70. [Google Scholar]
- 74. Loyola RD, Oliveira-Santos LGR, Almeida-Neto M, Nogueira DM, Kubota U, Diniz-Filho JAF, et al. (2009) Integrating economic costs and biological traits into global conservation priorities for carnivores. PLoS One 4: e6807 10.1371/journal.pone.0006807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Becker CG, Loyola RD, Haddad CFB, Zamudio KR (2010) Integrating species life history traits and patterns of deforestation in amphibian conservation planning. Diversity and Distributions 16: 10–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Models presented here are the one with the smallest AICc, and other models which have ΔAICc <2.
(PDF)
Data Availability Statement
All data can be found as supplementary material.


