Abstract
Purpose
Given the long-term, although potentially fatal, nature of prostate cancer, there is increasing observational evidence for the reduction in disease progression and mortality through changes in lifestyle factors.
Methods
We systematically reviewed dietary, nutritional, and physical activity randomized interventions aimed at modifying prostate cancer progression and disease-specific mortality, including a detailed assessment of risk of bias and methodological quality.
Results
Forty-four randomized controlled trials of lifestyle interventions, with prostate cancer progression or mortality outcomes, were identified. Substantial heterogeneity of the data prevented a meta-analysis. The included trials involved 3,418 prostate cancer patients, median 64 men per trial, from 13 countries. A trial of a nutritional supplement of pomegranate seed, green tea, broccoli, and turmeric; a trial comparing flaxseed, low-fat diet, flaxseed, and low-fat diet versus usual diet; and a trial supplementing soy, lycopene, selenium, and coenzyme Q10, all demonstrated beneficial effects. These trials were also assessed as having low risk of bias and high methodological quality (as were seven other trials with no evidence of benefit). The remaining trials were either underpowered, at high or unclear risk of bias, inadequately reported, of short duration or measured surrogate outcomes of unproven relationship to mortality or disease progression, which precluded any benefits reported being reliable.
Conclusion
Large, well-designed randomized trials with clinical endpoints are recommended for lifestyle modification interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s10552-015-0659-4) contains supplementary material, which is available to authorized users.
Keywords: Physical activity, Diet, Nutrition, Randomized controlled trials, Prostate cancer, Systematic review
Introduction
Prostate cancer is the most common cancer in men in the Western world [1]. In the UK, for example, it accounts for a quarter of newly diagnosed cancers [2] and one in eight men will receive a prostate cancer diagnosis [3]. Prostate cancer is often localized and grows slowly, so men may live for many years with the disease. However, prostate cancer may behave more aggressively and is an important cause of morbidity and mortality [4]. Given the long-term chronic, but potentially fatal, nature of the disease, there is growing interest in low-toxicity interventions in the tertiary prevention of morbidity and mortality due to prostate cancer. This is of particular importance as noninvasive active surveillance, as treatment for localized disease, becomes more widely implemented and increases in popularity as a strategy for reducing potential overtreatment [5]. As the number of cancer survivors in the USA increases beyond 13 million [6], the American Society of Clinical Oncology highlights the need for clinician and survivor to understand secondary prevention and lifestyle modifications that could benefit their prostate, as well as overall, health [7]. Observationally, poor diet, low levels of physical activity, and obesity are thought to play an important role in cancer, including the progression of prostate cancer [8–13]. Higher levels of physical activity have been associated with reduced rates of overall, and prostate cancer-specific, mortality [14]. World Cancer Research Fund International guidelines for cancer prevention include being physically active for at least 30 min every day, limiting consumption of energy-dense foods, eating a variety of vegetables, fruits, wholegrains, and pulses, and limiting consumption of red and processed meats [9]. Published systematic reviews in the field have tended to examine only one specific nutritional element, such as soy isoflavones [15], or have not always focused specifically on prostate cancer [16, 17]. Those that explored the implications of diet and nutrition more broadly often looked at risk of disease development, not progression and mortality [18], or did not include physical activity interventions [18, 19]. Systematic reviews with a focus on physical activity failed to include diet and nutrition interventions or restrict the population to prostate cancer patients [20]. Where diet, nutrition, and physical activity interventions have been reviewed, primary outcomes were not progression or mortality, but measures such as body weight [21], or all cancers and pre-invasive lesions were included [22]. Additionally, some reviews have not focused purely on randomized controlled trials (RCTs), which introduces further potential for bias [18], and study methodology and risk of bias were not always assessed [16, 19].
We therefore conducted a systematic review of dietary, nutritional, and physical activity interventions aimed at modifying prostate cancer progression and mortality in men with prostate cancer. We update and broaden the scope of previous systematic reviews [15–22] and undertake detailed assessment of risk of bias and methodological quality.
Materials and methods
Search strategy
Studies were identified through a systematic search of the following bibliographic databases from inception to July 2014: AMED, CINCH, the Cochrane library, Embase, MEDLINE, and Web of Science. The search strategy specified terms for RCTs, prostate cancer, dietary, nutritional, or physical activity interventions, and surrogate or clinical measures of prostate cancer progression or mortality (see Supplemental Data for Medline search strategy, Online Resource 1). Reference lists of all eligible full-text articles and all relevant systematic reviews that were identified were hand searched for additional studies.
Inclusion and exclusion criteria
To be eligible, studies had to be RCTs in men with prostate cancer who were randomized to dietary, nutritional, or physical activity interventions, which reported on surrogate or clinical measures of prostate cancer progression or mortality. Dietary or nutritional interventions were considered to be those that altered the intake of foods or dietary constituents either directly (e.g., by giving vitamin supplements) or indirectly (e.g., through nutrition education). Physical activity interventions were those involving any movement using skeletal muscles. RCTs that involved a combination of dietary, nutritional, and physical activity interventions were included. Outcomes were post-intervention effects on recognized surrogate measures of prostate cancer progression [Gleason score; prostate-specific antigen (PSA)] and clinical measures of prostate cancer progression (metastases, recurrence, disease-free survival, or prostate cancer mortality). An additional outcome was circulating insulin-like growth factor (IGF). We extracted data on any adverse events that were reported. There were no language restrictions. Commentaries and other related documents were excluded unless they provided additional data.
Data screening
All titles and abstracts were independently screened by two of three reviewers (LHM, RP, and VL) using pre-defined inclusion and exclusion criteria. Exact duplicates were removed. Any abstracts meeting the inclusion criteria were retrieved as a full article. These were then independently considered for inclusion by two of the three reviewers (LHM, RP, and VL). Any disagreements were resolved through discussion, and if necessary, the third reviewer was consulted. An additional 5 % of titles and abstracts were triple-screened for accuracy (LHM, VL, and SQ).
Data extraction
All data were extracted by one reviewer (VL) and double-extracted by a second (LHM or RP) using a specifically designed data extraction form. Any disagreement was resolved by consulting the third reviewer. All extracted data were then checked for a final time by the third reviewer (LHM or RP). We extracted data on study characteristics, methodological quality (based on seven design and implementation questions), variables required for a Cochrane risk of bias assessment [23], and our pre-specified primary and secondary outcomes. The quality criteria assessed were: similarity of baseline characteristics and prognostic indicators between randomized arms; reporting of a power calculation and whether this sample size was achieved; reporting of withdrawal numbers and reasons by group; description of equal therapeutic time between groups. The risks of bias criteria assessed were: reporting of sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; completeness of outcome data; and selective outcome reporting. Descriptions of what classifies as high and low risk can be found in Supplementary Table 1 (Online resource 2). Published protocols and trial registries were additionally searched, where available and when necessary, for further methodological detail related to methodological quality and risk of bias assessment. Authors were contacted if further data were required. Authors of non-peer-reviewed documents (such as conference abstracts) were contacted for published peer reviewed data; where none were provided, these were not included in the main analysis, but the description of the study included at the end of Tables 1 and 4.
Table 1.
Characteristics of included papers
| Author, country of data collection | Intervention and duration (weeks) | Total n intervention: control at randomization (number analyzed, if different) | Population age mean (SD) | Clinical characteristics PSA, mean (SD) Gleason (where reported) | Attrition rate (%) and recruitment rate (%) | Compliance or adherence | Total n of withdrawals Intervention: control |
|---|---|---|---|---|---|---|---|
| Nutritional or dietary intervention (single factor) | |||||||
| Chen et al. [69] China |
Qilan (astragalus, fenugreek, gynostremma pentaphyllan, smilaz glabra) 4 |
72 34:31 (72 randomized, but only data on 65) |
I: 74.22 (5.94) C: 73.71 (5.25) |
PSA, I: 15.76 (11.22), C: 14.98 (11.66) | 10 NR |
NR | 7 |
| Stenner-Liewen et al. [78] Switzerland |
Pomegranate juice 4 |
97 49:48 (48:46) |
I: 49 (8.6) C: 48 (8.4) |
PSA, I: 60 (82), C: 90 (222) (based on 48 intervention group and 46 controls) Gleason,% <8, I: 46, C: 57, ≥8, I: 54, C: 43 |
9.3 95.1 |
94 (96 %) completed days 1–28 | 3 1:2 |
| Freedland et al. [25] USA |
Pomegranate extract 4 |
69 33:36 |
I: 60.03 (7.935) C: 57.09 (6.254) |
PSA, I: 6.89 (3.884), C: 6.83 (4.274) p = 0.878 Gleason, % 6, I: 39.4, C: 55.6, 7, I: 54.5, C: 38.9, 8, I: 6.1, C: 2.8, 9, I: 0, C: 2.8 p = 0.363 |
NR | 80 % compliance of pill. One had approved protocol deviation: 75 % compliance | NR |
| Paller et al. [26] USA |
Pomegranate extract Up to 78 |
101 51:50 (45:47) |
I: 71.8 (51–89) C: 73.5 (54–92) |
PSADT, I: 15.1 (12.9), C: 14.4 (9.5) Gleason, % ≤6 or 3&4, I: 24, C: 74.5, 4&3, or ≥8, I: 24, C: 25.5 |
42 NR |
58 % | 9 5:4 |
| Gee et al. [27] USA |
Vitamin D 4 |
31 15:16 (Unclear) |
I: 59.9 (5.8) C: 57.9 (6.2) |
PSA, I: 11.7 (12.4), C: 6.8 (5.3) Gleason, mean (SD), I: 6.2 (1.32), C: 6.56 (0.88) |
NR | 15/16 took ≥95 % of study drug 11/15 took ≥99 % of study drug |
NR |
| Wagner et al. [28] USA |
Vitamin D3 3–8 |
66 21:22:23 (Unclear) |
Total: 57.4 (6.8) I1: 58.9 (6.2) I2: 55.9 (7.3) I3: 57.6 (6.7) |
PSA, Total: 6.99 (4.56), I1: 7.08 (4.55), I2: 7.02 (4.75), I3: 6.87 (4.59) Gleason, % 6, Total: 52, I1: 50, I2: 42.9, I3: 60.9, 7, Total: 48, I1: 50, I2: 57.1, I3: 39.1 |
4.6 70.2 |
97 % compliance to vitamin D3 treatment | 4 I1, 2: I2, 1: I3, 1 |
| Margalit et al. [31] USA |
Beta carotene NR |
383 192:191 |
Median (IQR) I: 73 (68–76) C: 73 (69–76) |
PSA, median (IQR), I: 7.1 (5.6–13), C: 8.2 (5.5–15.8) Gleason, % 2–6, I: 67, C: 55, 7, I: 22, C: 27, 8–10, I: 11, C: 16, Missing, I: 1, C: 2 |
NR | I1: 80 %, C: 80 % | NR |
| Nguyen et al. [32] USA |
Polyphenon E 3–6 |
50 25:25 (24:22) |
I: 63.4 (5.9) C: 61.3. (5.7) |
PSA, I: 6.71, (4.04), C: 7.90 (5.54) Gleason, % 6 (3&3), I: 70.8, C: 70.8, 7 (3&4), I: 12.5, C: 12.5, 7 (4&3), I: 4.2, C: 8.3, ≥8, I: 12.5, C: 8.3 |
4 NR |
NR | 2 1:1 |
| Lazarevic et al. [75] Norway |
Genistein 3–6 |
47 | NR | NR | NR | NR | NR |
| Lazarevic et al. [76] Norway |
Genistein 3–6 |
47 23:24 (23:17) |
Median (range) I: 60 (53–68) C: 59 (47–70) |
PSA (CI), I: 8.9 (7.0–10.8), C: 8.2 (6.4–9.9) Gleason, % 6, I: 47.8, C: 52.9, 7, I: 47.8, C: 41.2, 8, I: 4.3, C: 5.9 |
14.8 87 |
Returned pills, mean (95 % CI) I: 98.3 (97.2–99.4) C: 96.6 (93.8–99.4) p = 0.200 |
7 0:7 |
| Paller et al. [34] (Abstract only) USA |
Pomegranate extract Up to 78 |
104 | Median, Total: 74.5 | NR | NR | NR | NR |
| Higashihara et al. [74] Japan |
Eicosapentanenoic acid 104 |
68 34: 34 (32:32) |
I: 68 (5) C: 68 (7) |
PSA, I: 7.8 (4.3), C: 10.2 (6.6) Gleason, mean (SD), I: 6.3 (0.9), C: 6.2 (1.4) |
NR 84 |
NR | 6 2:4 |
| Stratton et al. [37] USA |
Selenium Up to 260 |
140 47 (200 g): 47 (800 g): 46 (control) |
I1: 73.6 (6.0) I2: 72.0 (7.5) C: 72.9 (6.5) |
PSA, I1: 8.0 (7.0), I2: 8.3 (6.2), C: 7.4 (5.6) Gleason, % >4, I1: 87.23, I2: 91.49, C: 91.11 |
27.9 70.3 |
Mean % after 5 years. I1: 90, I2: 89, C: 90 p = 0.695 |
39 13:16:10 |
| Vidlar et al. [70] Czech Republic |
Selenomethionine (570 mg silymarin, 240 µg selenium) 26 |
37 19:18 |
I: 62.4 (6.4) C: 65.0 (3.9) |
NR | NR | NR | 0 |
| Kumar et al. [41] USA |
Lycopene 4–6 |
45 10:10:14:11 |
I1: 60.94 (7.05) I2: 57.54 (7.29) I3: 59.98 (6.46) C: 60.30 (6.54) |
PSA, I1: 6.46 (2.74), I2: 5.86 (2.45), I3: 5.97 (4.0), C: 5.48 (3.38) | 6.7 NR |
NR | 3 2:1 |
| Kumar et al. [50] USA |
Soy protein (genistein) 12 |
76 39:37 (29:30) |
I: 72.5 (5.0) C: 37 (70.9) |
PSA, I: 7.38 (5.62), C: 7.45 (5.36) Gleason, % >6, I: 100, C: 100 |
22.3 63.3 |
NR | 17 8:9 |
| Beer et al. [49] USA |
Calcitriol (vitamin D) 4 |
37 17:20 (Unclear) |
Median (range) I: 63 (54–71) C: 58 (46–72) |
PSA, median (range), I: 6 (2.3–51.5); C: 5.8 (1.7–36) Gleason, % 5–6 I:64.7, C:50; 7 I: 23.5, C:30; 8–10 I:11.8, C:15 |
NR NR |
NR | 5 |
| Ansari et al. [71] (Methods only) India |
Lycopene 13 |
20 | Total: median (range) 72 (56–90) |
PSA, I: 50.10 Gleason, % 2–4, I: 60, 5–7, I: 25, 8–10, I: 15 |
NR | NR | NR |
| Ansari et al. [73] (Letter) India |
Lycopene NR |
NR | NR | NR | NR | NR | NR |
| Ansari et al. [72] India |
Lycopene NR |
54 27:27 |
NR | PSA, I: 250.7 (857.3), C: 259.7 (860.5) | NR | NR | NR |
| Stratton et al. [52] USA |
Selenium Up to 260 |
157 43 (200 g):42 (800 g):45 (control) |
I1: 73.27 (5.79) I2: 73.8 (6.44) C: 74.01 (5.9) |
PSA, I1: 8.33 (5.67), I2: 8.48 (5.56), C: 8.14 (6.75) Gleason, 5–7, I1: 86, I2: 85.7, C: 84.4 |
33.1 82.2 |
NR | 52 |
| Bylund et al. [77] Sweden |
Rye bran bread 11 |
23 12:11 (10:8) |
I: 69.9 (5.3) C: 69.7 (5.4) |
PSA, I: 15.0 (9.5), C: 13.5 (11.4) | 21.7 NR |
Mean grams of rye bread consumption. I: 267, C: 264 | 5 2:3 |
| Kucuk et al. [53] USA |
Lycopene 3 |
26 15:11 |
NR | NR | 25.7 NR |
NR | 2 |
| Kucuk et al. [54] USA |
Lycopene 3 |
26 15:11 |
NR | NR | 25.7 NR |
NR | 2 |
| Kucuk et al. [55] USA |
Lycopene 3 |
26 15:11 |
NR | NR | 3.4 NR |
NR | 9 |
| Kucuk et al. [56] USA |
Lycopene 3 |
35 (26) 15:11 (35 randomized, data only presented for 26) |
I: 62.3 (1.9) C: 62.0 (1.8) |
PSA, I: 6.89 (0.81), C: 6.74 (0.88) Gleason, % ≤6, I: 73.3, C: 45.4, >6, I: 26.6, C: 54.5 |
2.6 NR |
3.8 % had 93 % pill count 19.2 % had 55–79 % |
9 Unclear by group |
| Nutritional or dietary intervention (multiple factor) | |||||||
| Thomas et al. [64] UK |
Supplement of pomegranate seed, green tea, broccoli and turmeric 26 |
203 136:67 (total 199) |
Mean (range) Total: 74 (53–89) I: 71.8 C: 76.4 |
PSA, I: 6.5, C: 6.5 Gleason, % ≤7, I: 95, C: 88, >7, I: 5, C: 12 Mean, I: 6.5, C: 6.2 |
1.97 97.5 |
NR | 4 2:2 |
| Wright et al. [29] USA |
Calorie reduced diet and <30 % energy from fat and nutritional teaching 6 |
19 10:9 (Unclear) |
Median (range) I: 55 (40–66) C: 60 (47–73) |
PSA, median (range), I: 4.4 (0.9–9.5), C: 4.8 (3.3–19) Gleason, 3 + 3, I: 80, C: 66.6, 3 + 4, I: 20, C: 33.3 |
NR | % change calories consumed, I: −46.6, C: −11.3 p = 0.03 between groups |
NR |
| Bosland et al. [30] USA |
Isoflavones (genistein, daidzein, glycetin) 104 |
177 87:90 (78:73) |
I: 61.3 (7.2) C: 60.7 (6.6) |
PSA, I: 7.13 (3.87), C: 7.71 (4.20) Gleason, mean (SD) 5, I: 1 (1), C: 0, 6, I: 19 (23), C: 18 (23), 7, I: 45 (56), C: 47 (60), 8, I: 11 (14), C: 7 (9), 9, I: 5 (6), C: 6 (8) p = 0.78 |
8.2 51.5 |
96 % consumed 90 % of pills supplied. Seven reported non-adherence; <50 % of packet, I: 3, C: 4 | 26 9:17 |
| Aronson et al. [35] USA |
Low-fat diet and fish oil supplementation Approx. 4 |
55 29:26 (27:21) |
I: 60.5 (6.3) C: 60.4 (6.7) |
PSA, I: 6.9 (4.9), C: 7.6 (5.6) Gleason, % 6, I: 59.3, C: 40, 7, I: 33.3, C: 55, 3&4, I: 25.9, C: 35, 4&3, I: 7.4, C: 25, 8–9, I: 7.4, C: 5 |
12.7 96.5 |
I: 89.5 %, C: 94.8 % | 7 2:5 |
| Aronson et al. [36] USA |
Low-fat diet and soy 4 |
19 9:10 (9:9) |
I: 63.8 (2.3) C: 64.7 (2.7) |
PSA, I: 9.2 (2.7), C: 7.28 (1.5) Gleason, mean (SD), I: 6.2 (0.2), C: 6.2 (0.2) |
10 95 |
% food consumed I1: 98.9, I2: 96.5 |
1 0:1 |
| DeVere White et al. [38] USA |
Supplement of genistein, daidzein and other isoflavones 26 |
66 36:30 (28:25) |
I: 70.5 (9.3) C: 68.6 (7.3) |
PSA, range (SD), I: 0.7–16.2 (3.7), C: 1.1–22.6 (4.7) Gleason, % 2–4, I: 0, C: 8, 5–6, I: 89.3, C: 8.4, 7, I: 7.1, C: 8, 8–10, I: 3.6, C: 0 |
19.7 NR |
NR | 13 |
| Kumar et al. [39] USA |
Isoflavones (genistein, daidzein, glycetin) 4 (±3 days) |
44 12:11:10:11 |
I1: 59.9 (7.14) I2: 58.96 (6.41) I3: 58.66 (7.14) C: 60.30 (6.54) |
PSA, I1: 4.88 (2.9), I2: 6.12 (2.6), I3: 5.08 (2.58), C: 5.48 (3.38) | 2.3 NR |
NR | 1 1:0 |
| Carmody et al. [40] USA |
Weekly cooking classes 11 |
36 17:19 (Unclear) |
Total: 69.1 (9) | PSA, Total: 2.96 (4.51) | 33.3 NR |
NR | 5 (3:2) |
| Demark-Wahnefried et al. [42] USA |
Low-fat and/or flaxseed-supplemented diets Average 4 |
161 40:40:40:41 |
I1: 60.2 (7.0) I2: 59.2 (8.0) I3: 59.3 (7.6) C: 58.2 (6.8) |
PSA, I1: 5.2 (2.4), I2: 5.6 (5.0), I3: 6.8 (4.3), C: 5.2 (2.7) Gleason, % ≤5, I1: 5, I2: 0, I3: 5, C: 0, 6 (3,3), I1: 60, I2: 67, I3: 60, C: 66, 7 (3, 4), I1: 27, I2: 27, I3: 20, C: 22, 7 (4, 3), I1: 5, I2: 3, I3: 10, C: 7, ≥8, I1: 3, I2: 3, I3: 5, C: 5 |
7.5 25.8 |
Flaxseed, I1: 97.5 %, I3: 100 p = 0.001 (flaxseed) p = 0.905 (low-fat) |
12 1:5:4:2 |
| Parsons et al. [43] USA |
Increased vegetables, wholegrains, beans/legumes and dietary education 26 |
43 30:13 (29:13) |
Total: 64 (7.5) range 50–80 | PSA, I: 7.21 (4.14), C: 6.94 (6.55), PSA median (range), I: 5.47 (3.00–17.2), C: 4.85 (1.77–23.0) | NR | NR | 1 1:0 |
| Li et al. [44] USA |
Low-fat diet supplemented with soy protein 208 |
40 26:14 |
I: 60.2 (1.3) C: 63.3 (2.2) |
PSA, I: 8 (0.71), C: 8.63 (1.35) Gleason, 5–6, I: 23.1, C: 21.4, 7, I: 53.8, C: 71.4, 8–9, I: 23.1, C: 7.1 |
37.5 NR |
NR | 15 7:8 |
| Grainger et al. [45] USA |
Lycopene and soy protein 4 |
41 20:21 |
Total: 70 (7) | NR | NR | Lycopene, mg/day, week 0–4, I1: 43, I2: 0 Week 4–8, I1: 40, I2: 36 Soy, g/day of soy Week 0–4, I1: 0, I2: 39 week 4–8, I1: 36, I2:39 |
0 |
| Vaishampayan et al. [47] USA |
Lycopene and soy Maximum 26 |
71 38:33 |
Median (range) I1: 73 (57–89) I2: 76 (50–91) |
PSA, median (range), I1: 6.1 (1.1–147), I2: 6.9 (0.8–60.9) | 1.41 NR |
% Completed study I1: 60, I2: 48 |
1 1:0 |
| Hoenjet et al. [61] The Netherlands |
Supplement containing vitamins E and C, selenium, coenzyme C10 21 |
80 (Total 70) |
Total mean (range): 73.9 (54–85) | PSA median (range), I: 11.3 (9.0–14.2), C: 12.2 (9.9–15.1) | 12.5 NR |
>90 % in 70 who completed study | 10 |
| Kranse et al. [62] The Netherlands |
Verum supplement containing carotenoids, selenium, isoflavones 6 |
37 19:18 (15:17) |
Total median (range): 70 (54–81) |
PSA, median (range), Total: 3.24 (0.13–87.3) | 16.2 44.6 |
Mean, ng/ml. Daidzein, I: 534, C: 8.0 Genestein, I: 1,589, C: 17 | 6 4:2 |
| Schroder et al. [63] The Netherlands |
Dietary supplement (including soy, lycopene, selenium, Co Q10) 10 |
49 24:25 (22:20) |
Total: 69.8 (7.1) | PSA, Total: 3.29 (4.12) | 14.2 NR |
90 % | 7 2:5 |
| Oh et al. [51] USA |
PC-SPES and DES NK |
90 46:44 (43:42) |
Median (range) I1:71.7 (48.5–91.3) I2:74.4 (43.6–90.3) |
PSA, Median (range), I1: 46.7 (5.6–486.9), I2: 29.4 (8.0–2,548.8) | 5.5 NR |
NR | 5 3:2 |
| Dalais et al. [60] Australia |
Heat-treated soy (genistein, daidzein, glycitein) and Flaxseed NK |
29 (28) (Unclear) |
I1: 61.7 (5.1) I2: 58.4 (4.9) C: 60.5 (5.2) |
PSA, I1: 7.16 (3.23), I2: 6.31 (4.02), C: 5.81 (3.70) Gleason, mean (SD), I1: 6.5 (0.85), I2: 5.75 (0.9), C: 5.71 (1.38) |
3.4 90.63 |
NR | 1 group NR |
| Physical activity interventions | |||||||
| Galvao et al. [58] Australia and New Zealand |
Resistance training and aerobic training 52 |
100 50:50 |
I: 71.9 (5.6) C: 71.5 (7.2) |
Gleason, <7, I: 4, C: 6, 7, I: 48, C: 54, >7, I: 48, C: 40 | 9 37.2 |
Mean (SD) number of sessions: 40 (11.9) 77 % attendance | 22 14:8 |
| Cormie et al. [59] Australia |
Resistance training and aerobic training 13 |
63 32:31 |
I: 69.6 (6.5) C: 67.1 (7.5) |
Gleason, mean (SD), I: 7.3 (0.8), C: 7.7 (1.2) | 12.7 50 |
Mean (SD) number of sessions: 23.1 (2.7) | 8 1:7 |
| Segal et al. [67] Canada |
Resistance training and aerobic training 26 |
121 40:40:41 |
I1: 66.4 (7.6) I2: 66.2 (6.8) C: 65.3 (7.6) |
PSA, I1: 3.0 (3.3), I2: 2.5 (3.8), C: 3.9 (5.5) Gleason, mean (SD), I1: 6.7 (1.1), I2: 6.9 (0.8), C: 6.7 (0.9) |
9 37.2 |
NR | 11 7:3:1 |
| Segal et al. [68] Canada |
Resistance training 52 |
155 82:73 (81:73) |
I: 68.2 (7.9) C: 67.7 (7.5) |
PSA, I: 14.3 (9.0), C: 11.0 (11.2) | 12.9 30.6 |
79 % attended 28 out of 36 sessions | 20 8:12 |
| Nutritional and physical activity combined interventions | |||||||
| Bourke et al. [65] UK |
Aerobic and resistance training and nutrition advice 26 |
100 (42:44) |
I: 71 (6) C: 71 (8) Range 53–87 |
PSA, I: 2.7 (5.9), C: 3.3 (7.6) | 32 73.5 |
94 % attended supervised exercise 82 % completed independent exercise |
32 15:17 |
| Hérbert et al. [33] USA |
Healthy diet and aerobic exercise 26 |
54 29:25 (26:21) |
I: 69.7 (8.8) C: 71.1 (8.1) |
PSA, median (range), I: 0.87 (0.43–1.74), C: 0.71 (0.33–1.54) Gleason, % Missing, I: 19.2, C: 28.6, <5, I: 3.9, C: 0, 5–6, I: 23.1, C: 28.6, ≥7, I: 53.9, C: 42.9 |
13 96.4 |
NR | 7 3:4 |
| Bourke et al. [66] UK |
Aerobic and resistance training and nutrition advice 13 |
50 25:25 |
I: 71.3 (6.4) C: 72.2 (7.7) |
PSA, I: 3.3 (6.8), C: 5.0 (10.2) Gleason, mean (SD), I: 7 (1.3), C: 7 (1.1) |
10 64.1 |
95 % attended exercise sessions 87 % completed self-directed exercise |
22 20:12 |
| Frattaroli et al. [46] USA |
Vegan diet (supplemented with soy, fish oil, vitamin E, selenium, vitamin C) and aerobic exercise 104 |
93 44:49 (43:49) |
Total: 66 (8) | PSA, % 4–10, 100 Gleason, % <7, 100 |
NR 51.4 |
I: 74 %, C: 78 % completed QoL and adhered p > 0.05 |
1 Group NR |
| Ornish et al. [48] USA |
Vegan diet (supplemented with fish oil, selenium, soy, vitamins C and E) and aerobic exercise 52 |
93 44:49 (Unclear) |
I: 65 (7) C: 67 (8) |
PSA, I: 6.32 (1.72), C: 6.28 (1.66) Gleason, mean (SD), I: 5.7 (0.5), C: 5.7 (0.7) |
9.7 51.3 |
I: 95 %, C: 45 % compliant after 12 months | 9 3:6 |
| Ornish et al. [57] USA |
Low-fat, soy-supplemented vegan diet and exercise programs 52 |
93 46:47 (Unclear) |
NR | NR | 7.5 NR |
83 % compliant at 12 months | 7 1:6 |
| Unpublished data (not included in analysis) | |||||||
| Cipolla et al. [80] (Poster only) France |
Sulphoraphane 32 |
81 40:41 (Unclear) |
I: 68.8 (6.4) C: 70.4 (6.8) |
PSA, I: 0.74 (0.64), C: 0.78 (0.68) Gleason, % <6, I: 13.1, C: 12.5, 6 (3 + 3), I: 10.5, C: 22.5, 7 (3 + 4), I: 44.7, C: 45, 7 (4 + 3), I: 26.3, C: 20, 8 (4 + 4), I: 2.6, C: 0, 8 (3 + 5), I: 2.6, C: 0 |
21 NR |
NR | 17 11:6 |
| Nayan et al. [79] (Abstract only) NK |
Lycopene 156 average |
78 40:38 |
NR | NR | NR | NR | NR |
C control, CI confidence interval, I intervention, n number, NR not reported, PSADT prostate-specific antigen doubling time, SD standard deviation
Table 4.
Primary outcomes and summaries of included papers
| Author—related publications | Intervention type and intervention duration | Prostate cancer stage (where reported) and treatment received | Systematic review outcomes (intervention vs. control only) | Outcome in original paper |
|---|---|---|---|---|
| Nutritional or dietary intervention (single factor) | ||||
| Chen et al. [69] | QiIan (astragalus, fenugreek, gynostremma, pentaphyllan, smilaz glabra) supplement, four capsules, three times a day versus P (starch) | Hormone therapy/ADT: 100 % Orchiectomy: 100 % |
PSA: Change, baseline to 4 weeks; mean (SD) I: 15.76 (11.22)–3.44 (3.9), C: 14.98 (11.66)–4.16 (3.88); p > 0.05 between groupsd | Unclear |
| Stenner-Liewen et al. [78] | Pomegranate juice, 500 ml/day, 2,294 mg/l polyphenol gallic acid versus P juice | %. T0, I: 0, C: 4. T1, I: 17, C: 17. T2, I: 17, C: 41. T3, I: 51, C: 31. T4, I: 15, C: 7, No, I: 55, C: 78. N1, I: 45, C: 22. M1, I: 44, C: 18. Watchful waiting: 28.7 % Radiotherapy: 20.2 % Prostatectomy: 15.9 % Hormone therapy/ADT: 42.5 % Radiation and prostatectomy: 15.9 % Chemotherapy: 12.7 % |
PSA: Progression (phase 1 day 1–28); mean (%) I: 18 (38 %), C: 19 (41 %); p = 0.83d | Primary |
| PSA: Stabilization (phase 1 day 1–28); mean (%) I: 27 (56 %), C: 26 (57 %); NSd | Primary | |||
| PSA: Response (phase 1 day 1–28); >50 % mean I: 0 (0 %), C: 0 (0 %); ≥30 % mean I: 3 (6 %), C: 1 (2 %); NSd | Primary | |||
| Freedland et al. [25] | 2,000 mg of POMx powder, including 1,200 mg polyphenol daily versus P matching pill with same administration schedule | Due to undergo prostatectomy: 100 % | PSA: Change in ratio of baseline to pre-surgery; p = 0.443 | Secondary |
| Cell proliferation; ki67 mean (SD), I: 0.60 (0.89), C: 0.76 (0.90) p = 0.164; | Secondary | |||
| Nf-KB mean (SD); I: 44.44 (35.47), C: 44.85 (37.88); p = 0.887d | ||||
| Cell development progression; ps6 kinase mean (SD); I: 46.10 (24.85), | ||||
| C: 39.53 (26.50); p = 0.245 | ||||
| Paller et al. [26, 34] | Pomegranate extract 1 g versus Pomegranate extract 3 g daily | Radiotherapy: 53.45 % Surgery: 51.55 % Brachytherapy: 75.2 % Hormone therapy/ADT: 27.65 % Radiation and Prostatectomy: 11.9 % Cryotherapy: 1.95 % |
PSA: Doubling time, median difference; I1: 6.9 months, I2: 5.3 months; p = 0.554d | Primary |
| PSA: Objective response rates; % patients I1: 2 %, I2: 2 %; p = NRd | Secondary | |||
| Progression-free survival (stable disease); % patients I1: 78 %, I2: 82 %; p = NRd | Secondary | |||
| Progressive disease rates; % patients I1: 20 %, I2: 16 %; p = NRd | Secondary | |||
| PSA: Declining levels; “declining PSA seen in 13 % patients” p = NR Paller et al. [34] | Unclear | |||
| Gee et al. [27] | Vitamin D analog 10 µg daily versus observation | Localized: 100 % Due to undergo radical retropublic prostatectomy: 100 % |
PSA: Change in total; diff between groups; Day 15; I: 8.9, C: 10; p = 0.397; Day 21; I: 8.3, C: 10.3; p = 0.024; Off study; I: 11, C: 10.5; p = 0.077; ITT; I: 9.9, C: 9.2; p = 0.156e | Primary |
| IGF-I: Change, µg/10E6 platelets between groups; Day 15; I: 0.433, C: 0.426; p = 0.599; Day 21; I: 0.458, C: 0.435; p = 0.413; Study end; I: 0.4, C: 0.419; p = 0.682; ITT; I: 0.4, C: 0.418; p = 0.743e | Primary | |||
| Change in level of intervention element, ng/ml, within intervention group; Day 8 p = 0.219; Day 28 p = 0.148e | Secondary | |||
| Wagner et al. [28] | Vitamin D3 doses of either 400, 10,000, or 40,000 IU/day | Localized: 100 % Due to undergo radical prostatectomy: 100 % |
PSA: Change in serum, between groups; p = 0.60; NB. PSA was lower in the combined higher dose groups than 400 IU; p < 0.02e | Secondary |
| Cell proliferation; Ki67, between groups; p = 0.46e | Primary | |||
| Margalit et al. [31] | Beta carotene (50 mg on alternate days) versus control (P) | T1/T2, I: 88, C: 85. T4/N1, I: 3, C: 3. T3, I: 7, C: 8. Missing, I: 2, C: 4 Radiotherapy: 100 %, Brachytherapy: 30.3 % External beam radiation: 78 % |
Prostate cancer mortality—median FU of 10.5 years; hazard ratio = 0.85, 95 % CI (0.49–1.50) following adjustment |
Primary |
| Nguyen et al. [32] | Polyphenon E (800 mg daily) versus P | Due to undergo surgery: 100 % | PSA: taken at 3–6 weeks; Absolute change in PSA mean (SD), ng/ml; I: −0.66 (SD 2.56), C: −0.08 (SD 1.28); p = 0.26; % decrease I: 58.3 %, C: 36.4 %; p = 0.15d | Secondary |
| IGF-I; Absolute change mean (SD), ng/ml; I: −6.89 (20.97), C: −1.20 (21.82); p = 0.53 decrease %; I: 54.2 %, C: 36.4 %; p = 0.25d | Secondary | |||
| Cell proliferation, % Ki67 mean (SD); I: 5.65 (9.47), C: 4.37 (6.11); p = 0.68d | Secondary | |||
| Cell apoptosis, % cleaved caspase-3 mean (SD); I: 0.39 (0.57), C: 0.46 (0.64); p = 0.29d | Secondary | |||
| Angiogenesis, n of microvessels mean (SD); I: 22.43 (9.93), C: 23.04 (10.40); p = 0.89d | Secondary | |||
| Decrease in Gleason score; I: 20.8 %, C: 8.3 %; p = 0.22e | Secondary | |||
| Lazarevic et al. [75, 76] | Genistein (30 mg daily, 3–6 weeks prior to surgery) versus Control (P) | 1c—I: 52.2 %, C: 76.5 % 2a—I: 47.8 %, C: 23.5 % Lazarevic et al. [76] Localized: 100 % Awaiting radical prostatectomy: 100 % |
PSA; % change, mean (CI); I: −7.8 (−16.1 to 0.6); C: 4.4 (−5.0 to 13.9); p = 0.051; Change in mean (CI), I: 7.9 (6.6–9.2), C: 8.3 (6.5–10.2); p = 0.655 Lazarevic et al. [76]d | Primary |
| Tumor response: androgen related biomarkers (KLK4); Difference in mean; p = 0.033 | Primary | |||
| Cell cycle regulation G3 cells; difference in expression (p27); p = 0.016 | Primary | |||
| Cell proliferation (Ki67) G3 cells; difference in expression; p < 0.001 | Primary | |||
| Cell apoptosis (BAX, BCL-2) G3 cells difference in expression; BAX p = 0.011; BCL-2 p = 0.125 | Primary | |||
| Neuroendocrine tumor response (CgA); difference in expression difference; p < 0.001 | Primary | |||
| Higashihara et al. [74] | EPA (2.4 g/day) versus control (no intervention) | %. PT1, I: 9.38, C: 6.6. PT2, I: 68.75, C: 70. PT3>, I: 21.88, C: 23.3 Awaiting to undergo surgery—100 % |
PSA: failurea, end of study; n (%) participants; I: 4 (12.5), C: 8 (26.7) p = 0.16d | Primary |
| Stratton et al. [37, 52] | 3 arms: 200 µg/day selenium versus 800 µg/day selenium versus control (P) | Localized: 100 % Active monitoring/surveillance: 100 % (2010) Watchful waiting: 100 % (2003) |
PSA: doubling time; median years; I: 6.98, I2: 8.45, C: 6.24 I1 versus control, p = 0.613 I2 versus control, p = 0.328 |
Primary |
| Vidlar et al. [70] | Selenomethionine (570 mg silymarin, 240 µg selenium) v P—Isomalt (250 mg), microcrystalline cellulose (250 mg), hydroxypropyl cellulose (10 mg) |
Prostatectomy/surgery: 100 % | PSA: Median change; After 6 months PSA was unchanged in both groups | Secondary |
| Median change in intervention element, selenium µmol/l; Change within both groups from baseline to 6 months p < 0.05 | Secondary | |||
| Kumar et al. [41] | 15 mg/day lycopene versus 30 mg/day lycopene versus 45 mg/day lycopene versus control (no supplement) | Localized: 100 % Due to undergo prostatectomy: 100 % |
PSA: total, difference in mean; no evidence of any difference in mean p = 0.28 (all I arms versus C) | Primary |
| Cell proliferation: Ki-67; Mean % (SD) post-intervention; I1: 2.63 (1.41), I2: 3.51 (1.43), I3: 3.64 (1.9), C: 4.22 (1.86); p = NRd | Primary | |||
| Beer et al. [49] | Calcitriol (0.5 µg/kg/day) versus control (starch) | %. T1c, I: 58.8, C: 45. T2, I: 0, C: 5. T2a, I: 29.4, C: 30. T2b, I: 5.9, C: 15. T2c, I: 0, C: 5. T3a, I: 5.9, C: 0. Due to undergo prostatectomy: 100 % |
Cell apoptosis (BCL-2 and C-Myc); No BCL-2 staining in cancer cells was detected; 14 % of adenocarcinoma stained positive for C-Myc; p = NRe | Primary |
| % PSA undetectable post surgically; I: 100 %; C: 84 %; p = NR | Secondary | |||
| Kumar et al. [50] | Soy protein (60 mg genistein daily) versus Control (standard American diet with isocaloric) | Watchful waiting: 100 % | Total PSA: Change, difference in mean (SD), baseline to 12 weeks; no evidence of any difference in mean; p = 0.96d | Primary |
| Free PSA: Change, difference in mean (SD), baseline to 12 weeks; no evidence of any difference in mean; p = 0.13d | Primary | |||
| Total testosterone: change in mean (SD); baseline to 12 weeks; no evidence of any difference in mean; p = 0.11d | Primary | |||
| Free Testosterone: Change in mean (SD); baseline to 12 weeks; no evidence of any difference in mean; p = 0.15 | Primary | |||
| Ansari et al. [71–73] | Orchidectomy plus lycopene (2 mg twice daily) versus orchidectomy | Advanced/metastatic: 100 % Bilateral orchiectomy and anti-androgen: 100 % |
PSA: change in mean baseline, 6 and 24 months; Unclear reporting of between-group differences. Change within intervention group at 24 months; p < 0.001 | Primary |
| PSA: clinical response; % with progression; I: 7, C: 25; p < 0.05 | Primary | |||
| Bone metastasis; n (%) progression; I: 2 (7), C: 4 (15); p < 0.02 | Primary | |||
| Prostate cancer mortality; total death n (%); I: 7 (13), C: 12 22); p < 0.001 | Primary | |||
| Bylund et al. [77] | Rye bran bread (295 g/day) versus control (wheat bread) | T2%, I: 70, C: 87.5, T3%, I: 30, C: 12.5 No active treatment: 100 % |
PSA: change in mean baseline and 3 weeks; Unclear reporting of between-group differences. No changes in plasma levels of PSA were observed for total or free forms of PSA | Secondary |
| IGF-I, ng/ml. Change in mean; baseline and 3 weeks; Unclear reporting of between-group differences. IGF-I remained essentially unaltered in both groups | Secondary | |||
| Cell proliferation (Ki67, P27); Mean rate, baseline and 3 weeks; Unclear reporting of between-group differences. Within-groups Ki67 increased (p < 0.05) and p27 decreased (p < 0.05)d | Primary | |||
| Cell apoptosis: TUNEL; Difference in Mean % (SD), baseline to 3 weeks; No between-group differences reported. Significant increase in intervention group I: 1.5 (1.3)–5.6 (3.1); p < 0.05d | Primary | |||
| Kucuk et al. [53–56] | Lycopene (15 mg twice daily) versus control (usual care) | Due to undergo prostatectomy: 100 % | PSA: Change, difference in mean (SE); pre to post-intervention; no evidence of any difference in mean; p = 0.25 | Primary |
| Cell apoptosis, (Bax), expression levels, mean (SE); Malignant Bax; I: 1.05 (0.29), C: 0.68 (0.18); p = 0.33; Benign Bax; I: 0.62 (0.1), C: 0.79 (0.11); p = 0.28 | Primary | |||
| Cell apoptosis (BCL-2), mean (SE); malignant BCL-2; I: 0.54 (0.01), C: 0.51 (0.06); p = 0.59; Benign BCL-2; I: 0.63 (0.04), C: 0.58 (0.04); p = 0.31 | Primary | |||
| IGF-I, difference in mean; pre to post-intervention; % change (SE); I: 28.8 (5.5), C: 29.9 (5.3) p = 0.88 Kucuk et al. [56]d. Plasma levels decreased in both groups, I: p = 0.0002, C: p = 0.0003 Kucuk et al. [53–56]d | Primary | |||
| Nutritional or dietary intervention (multiple factor) | ||||
| Thomas et al. [64] | Oral capsule containing pomegranate seed, green tea, broccoli, and turmeric versus identical P | Watchful waiting: 40 % Primary active surveillance: 60 % |
PSA: Change; % rise, median (CI); I: 14.7 (3.4–36.7), C: 78.5 (48.1–115.5); p = 0.0008d | Primary |
| PSA: stable % participants; After 6 months; I: 46, C: 14; p = 0.00001d | Secondary | |||
| Wright et al. [29] | Calorie reduced diet of 1,200–2,000 kcal/day and <30 % daily energy from fat. Nutritional and behavioral teaching versus continued normal diet | T1%, I: 90, C: 66.6. T2%, I: 10, C: 33.3 Active monitoring/surveillance—47 % Due to undergo prostatectomy—53 % |
IGF-I: % change; baseline to 6 weeks; geometric mean. I: 17.0, C: 20.9; p = 0.84 between groupse | Primary |
| Change in intervention element; Calories consumed, % change; I: −46.6, C: −11.3; p = 0.03 between groupse | Secondary | |||
| Bosland et al. [30] | Beverage powder of soy protein isolate, 19.2 g, containing, 1.24 mg genistein, 0.78 mg daidzein, 0.11 mg glycitein versus calcium caseinate, 19.8 g | T1c or T2: 100 % Prostatectomy/surgery: 100 % |
Recurrence-free survival; Median time to recurrence; I: 31.5, C: 44; p = 0.62; % recurrence; I: 27.2, C: 29.5; HR, coefficient, 0.96 (0.53–1.72) p = 0.89d |
Primary |
| Aronson et al. [35] | Low-fat diet and fish oil supplement (200 mg eicosapentaenoic acid and 367 mg docosahaxaenoic acid daily) versus Western diet (40 % fat, 15 % protein, 45 % carbs) (control) | Localized: 100 % Due to undergo surgery: 100 % |
IGF-I: Change, mean difference; pre- and post-intervention; mean (SD), I: 8.8 (6.2), C: −0.4 (4.3); p = 0.25d | Primary |
| PSA: Change, mean difference; pre- and post-intervention; mean (SD), I: 0.08 (0.4), C: −0.09 (0.3); p = 0.53d | Secondary | |||
| Cell proliferation, % decrease of Ki67; I: 32.2 %, C: NR; p = 0.026d | Secondary | |||
| Aronson et al. [36] | Low-fat diet, 15 % kcal from fat, 30 % kcal from protein, including 35 g soy, 55 % kcal from carbohydrates, including 35 g fiber per day versus Western diet | Active monitoring: 100 % | PSA: Change at 4 weeks, mean (SD); I: 9.2 (2.7)–11.4 (5), C: 7.8 (1.5)–6.3 (3.6); p = 0.23d | Primary |
| IGF-I: Change at 4 weeks, mean (SD); I: 58 (16.4), C: 24 (9); p = 0.09d | Primary | |||
| DeVere White et al. [38] | 450 mg genistein, 300 mg daidzein and other isoflavones daily versus 5 g/day of inert cellulose (P) | Active monitoring/surveillance: 100 % | PSA: % change, n (%); Increased, I: 14 (50 %), C: 17 (68 %); >20 % increase, I: 6 (21.4 %), C: 7 (28 %); Stable/reduced, I: 14 (50 %), C: 8 (32 %); >20 % reduction, I: 3 (10.7 %), C: 1 (4 %); p = 0.29d | Primary |
| Relationship of isoflavones to PSA levels. Intercept value (SE); Genistein: 0.0021 (0.0171); Daidzein: −0.0020 (0.0017); Equol: 0.01388 (0.0435); p = 0.25d | Secondary | |||
| Kumar et al. [39] | Isoflavones, 40, 60, 80 mg versus control (usual care) | Localized: 100 % Due to undergo prostatectomy: 100 % |
PSA: change, difference in mean; pre to post treatment, mean (SD); I1: 4.88 (2.9)–5.52 (2.92), I2: 6.12 (2.6)–6.73 (NR), I3: 5.08 (2.58)–5.16 (8.66), C: 5.48 (3.38)–5.12 (1.86); Between-group p value = NR | Secondary |
| Cell proliferation. Mean Ki67%, mean (SD); I1: 3.2 (2.25), I2: 4.11 (3.53), I3: 4.63 (2.67), C: 4.22 (1.86); p > 0.05, NS | Primary | |||
| Carmody et al. [40] | Dietary advice (reduced meat, dairy, increased veg, plant based diet) and cooking classes versus control (wait-list control) | Radiotherapy: 30.6 % Surgery: 55.6 % Seed implantation: 13.9 % |
PSA: Kinetics, Log PSA, mean difference baseline to 11 weeks, mean (CI); I: 0.032 (0.013–0.054) to 0.011 (−0.023 to 0.047), C: 0.038 (0.018–0.057) to 0.037 (0.009–0.065); p = 0.28e | Secondary |
| PSA: doubling time, mean difference in months; baseline to 3 months, mean (CI); I: 21.5 (12.8–66.8) to 58.5 (14.7–∞), C: 18.4 (12.1–39.2) to 18.7 (10.6–81); p = NRe | Secondary | |||
| Denmark-Wahnefried et al. [42] | Flaxseed-supplemented diet versus low-fat diet versus flaxseed-supplemented low-fat diet versus control (usual diet) | Due to undergo prostatectomy: 100 % | Proliferation rate: Mean Ki67; mean (CI); I1: 1.66 (1.13–2.64), I2: 2.56 (2.00–3.69), I3: 1.50 (1.05–2.65), C: 3.23 (2.42–3.92); I1 versus C: p = 0.0013; I2 versus C: p = 0.661 | Primary |
| Tumor apoptotic rate, n (%); 0 %: I1: 29 (74 %), I2: 26 (74 %), I3: 32 (89 %), C: 33 (84 %); >0–1 %: I1: 6 (16 %), I2: 5 (14 %), I3: 1 (3 %), C: 5 (13 %); >1–2 %: I1: 4 (10 %), I2: 4 (12 %), I3: 3 (8 %), C: 1 (3 %); I1 versus C: p = 0.880; I2 versus C: p = 0.730 | Secondary | |||
| PSA: Change, median difference baseline to follow up; median (CI); I1: 6.2 (4.8–7.7) to 6.4 (5–7), I2: 5.5 (4.6–6.7) to 5.6 (3.9–6.7), I3: 5.9 (4.9–9.4) to 5.7 (4.9–8.6), C: 5.3 (3.7–5.8) to 4.9 (3.5–6.2); I1 versus C: p = 0.286; I2 versus C: p = 0.764 | Secondary | |||
| IGF-I: Change, difference in median baseline to follow up, median (CI); I1: 124 (115–148) to 119 (107–133), I2: 133 (109–150) to 123 (100–141), I3: 129 (110–148) to 125 (113–139), C: 128 (106–133) to 112 (98–128); I1 versus C: p = 0.174; I2 versus C: p = 0.370 | Secondary | |||
| Parsons et al. [43] | Structured dietary education and telephone based counseling. Targeted increasing intake of vegetables, wholegrains, beans/legumes versus Control, printed material with standard guidelines recommending five servings of fruit and vegetables a day | Active monitoring/surveillance: 100 % | PSA: Change, mean difference; baseline to 6 months, mean (SD); I: 7.21 (4.14) to 9.94 (12.9), C: 6.94 (6.55) to 6.88 (6.98); p = 0.29; PSA: change, median difference baseline to 6 months, median (range); I: 5.47 (3–17.2) to 6.39 (2.56–72.5), C: 4.85 (1.77–23) to 4.09 (1.58–24.5); p = 0.21d | Secondary |
| Li et al. [44] | Low-fat (15 % fat), high-fiber (18 g/1,000 kcal) with 40 g soy protein daily versus control (USDA recommender diet) | %. T1c, I: 3.8, C: 7.1. T2a, I: 7.7, C: 14.3. T2b, I: 7.7, C: 0. T2c, I: 50, C: 57.1. T3a, I: 11.5, C: 14.3. T3b, I: 7.7, C: 0. T3c, I: 11.5, C: 7.1 Surgery: 100 % |
PSA: change; three participants had a raised PSA; 0.5 at 12 months; 0.7 at 18 months; 0.4 at 4 years; All other participants had PSA remaining at <0.2; p = NR | Secondary |
| IGF: change, mean difference; baseline to 6 months, mean (SD); I: 260.4 (8.6) to 220.5 (7.9), C: 262.9 (8.6) to 259.5 (14.3); p = 0.04 | Secondary | |||
| Grainger et al. [45] | Lycopene 25 mg/day for 4 weeks versus soy 40 g daily, versus lycopene and soy, 25 and 40 g daily | Radiotherapy (NR) Surgery (NR) Brachytherapy (NR) |
PSA: Change, % with change Prolongation compared with pre-enrollment, n (%); I1: 13 (65 %), I2: 10 (50 %); p = NR; lower PSA at end of study than at enrollment, n (%); I1: n (25 %), I2: 9 (43 %); p = NR; NB. Outcome data given for 8 week intervention but needs to be handled with caution as intervention at 8 weeks is difficult to interpret due to being a cross over design | Secondary |
| Prior to enrollment 12 men (30 %) who were in the slowest doubling time; by end of study this number had increased to 19 men (48 %); p = 0.08 | Secondary | |||
| IGF-I: Change; No significant changes during the course of the study for either group; p = NR | Secondary | |||
| Vaishampayan et al. [47] | Lycopene 15 mg twice daily versus lycopene 15 mg twice daily and soy isoflavone 40 mg twice daily | %. absence of metastases, I1: 79, I2: 70 presence of metastases, I1: 21, I2: 30 PSA progression without hormone therapy: 64.7 % Hormone therapy/ADT: 35.2 % |
PSA: rate of PSA riseb, difference in mean. No between-group analysis reported, only reported results by treatment stratification | Primary |
| PSA stabilizationc; n (%) reaching stabilization; I1: 35 (95 %), I2: 22 (67 %)d p = NR | Primary | |||
| Hoenjet et al. [61] | Supplement, vitamin C (750 mg/day), selenium (200 µg/day), vitamin E (250 mg/day), coenzyme Q10 (2 × 100 mg/day) versus control (P) | Either: CT1-4Nx Mo (with no curative treatment) or CT1-4 N + Mo Watchful waiting, radiotherapy, prostatectomy: 62.5 % No curative treatment: 37.5 % |
PSA: Change, difference in mean (CI); pre to post-intervention; I: 1.3 (1.2–1.4), C: 1.1 (0.9–1.4); p = 0.67; NB. Geometric means are reported from nonparametric data. The outcome is presented on change in log PSA scoresd | Primary |
| Kranse et al. [62] | Verum, selenium (0.6 mg daily), genestein (180 mg daily), daidzein (120 mg daily), lycopene (30 mg daily), margarine (20 mg daily) versus control (P) | % total. T1 or T2, 83, Grades 1 or 2, 60. Watchful waiting: 13.5 % Radiotherapy: 16.2 % Surgery: 70.3 % |
PSA: total PSA slope; mean response; 0.024; p < 0.001; Treatment effect; −0.0018; p = 0.84d | Primary |
| PSA: doubling time, weeks, median (CI); I: 44 (32–71), C: 41 (30–63); p = 0.84d | Primary | |||
| Schroder et al. [63] | Dietary supplement (soy (62.5 mg), lycopene (15 mg), selenium (128 mg), Co Q10 (4 mg) daily versus Control (P) | Radiotherapy: 31 % Prostatectomy/surgery: 69.4 % |
PSA: slope, log2 serum total difference in median (range); I1: 0.0009 (−0.008 to 0.014); I2: 0.0022 (−0.004 to 0.014); p = 0.041; PSA: slope, non-transformed difference in median (range); I1: 0.0010 (−0.041 to 0.279), I2: 0.0025 (−0.003 to 0.110); p = 0.030d | Primary |
| PSA: doubling time, days; I: 1,150, C: 445; Doubling time changed by factor of 2.6d | Primary | |||
| Total PSA: Change concentration; Median (range); I: 0.10 (−2 to 17), C: 0.1 (−0.1 to −8); p = 0.076d | Primary | |||
| Free PSA: I: 0 (−0.1 to 4.5), C: 0 (0–1.4); p = 0.988d | Primary | |||
| Oh et al. [51] | PC-SPES (3 capsules daily/960 mg) or DES (3 mg daily) | %. Rising PSA only, I1: 22, I2: 14. Bone metastases, I1: 41, I2: 57. Soft tissue metastases, I1: 9, I2: 11. Bone and soft tissue metastases, I1: 28, I2: 18. Radiotherapy: 18 % Surgery: 29 % Radiation and Prostatectomy: 14.5 % None: 38.9 % |
PSA: decrease after 1st round of treatment, % mean (range); I1: 80 (59.3–99.4), I2: 72 (63.3–78.2); p = NRe NB. As reported in paper | Secondary |
| PSA: Time to progression; median n of months; I1: 5.5, I2: 2.9d; p = NR | Secondary | |||
| PSA: Nadir after initial treatment, median (range); I1: 3.0 (0.2–16.8), I2: 22.1 (2.5–907); p = NRd | Secondary | |||
| Dalais et al. [60] | 50 g HT soy or 50 g HT soy and 20 g linseed daily versus P (pearled wheat bread) | Due to undergo prostatectomy: 100 % | Total PSA: Change, difference in mean baseline to follow up, mean (SD); I1: 7.16 (3.23)–6.34 (3.05), I2: 6.31 (4.02)–6.99 (3.24), C: 5.81 (3.7)–7.11 (4.23); % change in Total PSA; I1: −12.7 %, C: 40 %; p = 0.02f; p for I2 versus C = NR | Primary |
| Free PSA: Change, difference in mean baseline to follow up, mean (SD); I1: 0.69 (0.28)–0.74 (0.36), I2: 0.62 (0.26)–0.65 (0.42), C: 0.64 (0.54)–0.63 (0.48); p = NRe | Primary | |||
| PSA: Free/total ratio; Change in total ratio; I1: 27.4 %, I2: −10 %, C: −15.6 %; I1 versus C p = 0.01; I1 versus I2 p = 0.007e | Primary | |||
| Physical activity interventions | ||||
| Galvao et al. [58] | Resistance and aerobic training versus control (education booklet) | %, T2—I: 62, C: 62, T3/T4—I: 38, C: 38 Radiotherapy/Hormone therapy/ADT: 100 % |
PSA: Change, adjusted group difference in mean change at 6–12 months, mean(CI).; 6 months, 0.1 (−0.71 to 1.1); p = 0.687; 12 months, −0.3 (−1.4 to 0.8); p = 0.584 | Secondary |
| Cormie et al. [59] | Resistance and aerobic training versus usual care | Hormone therapy/ADT: 100 % | PSA: change, adjusted group differences in mean change over 3 months, mean (CI); 0.18 (−0.25 to 0.60); p = 0.410 | Secondary |
| Segal et al. [67] | Resistance training versus aerobic training versus control (usual care) | %. stage 1, I1: 0, I2: 2.5, C: 0. Stage 2, I1: 77.5, I2: 72.5, C: 85.4. Stage 3, I1: 20, I2: 22.5, C: 9.8. Stage 4, I1: 0, I2: 0, C: 2.4. Unassignable, I1: 2.5, I2: 2.5, C: 2.4 Hormone therapy/ADT: 61.2 % Radiotherapy: 100 % |
PSA: Change, mean difference (CI); baseline to 24 weeks; I1: −1.75 (−3.01 to −0.51), I2: −2.14 (−3.34 to −0.94), C: −3.29 (−4.46 to −2.11); I1 versus C: 1.53 (−0.18 to 3.25); p = 0.09 I2 versus C: 1.14 (−0.53 to 2.82); p = 0.181 | Secondary |
| Segal et al. [68] | Resistance training, 60–70 % max, 3 × per week versus waiting list | % Stage I, I: 0, C: 0. Stage II, I: 48.8, C: 47.9, Stage III, I: 13.4, C: 18.1, Stage IV, I: 20.7, C: 13.9. Unassigned, I: 17.1, C: 20.8 Scheduled to receive ADT—100 % |
PSA: change; I: 1.78 decrease, C: 5.40 increase; p = 0.31d | Secondary |
| Nutritional and physical activity combined interventions | ||||
| Bourke et al. [65] | Aerobic and resistance training combined with healthy eating advice versus usual care | Locally advanced: 80 %, Advanced: 20 % Hormone therapy/ADT—100 % |
PSA: change, mean at 12 weeks; I: 3.5, C: 4.6; Mean difference, unadjusted (CI): O.6 (−0.6 to 1.8); p = 0.35; Mean difference, adjusted (CI): 0.5 (−0.7 to 1.7); p = 0.41d | Secondary |
| Hébert et al. [33] | Healthy diet (decrease meat and dairy, increased veg and soy) and aerobic exercise versus control (usual care) | Radiotherapy: 36.2 % Surgery: 14.9 % Radiation and Prostatectomy: 48.9 % |
PSA: Change, difference in mean baseline to 6 months, mean (CI); I: 0.87 (0.43–1.74) to 0.84 (0.42–1.68), C: 0.71 (0.33–1.54) to 0.78 (0.36–1.7); p = 0.45d | Primary |
| Bourke et al. [66] | Aerobic and resistance training combined with healthy diet advice versus control (usual care) | %. Advanced/metastatic, I: 24, C: 28 ADT—100 % |
PSA: change, difference; baseline to 12 weeks, mean (SD); I: 3.32 (6.83)–4.55 (8.74), C: 5.02 (10.2)–6.24 (13.6); Group mean difference (CI); 0.01 (−2.2 to 2.2); greater increase in intervention group, p = 0.61 | Secondary |
| IGF-I: Change, difference; baseline to 12 weeks, mean (SD); I: 74.5 (21.5)–78.3 (22.6), C: 77.6 (25.8)–79.4 (27.2); Group mean difference (CI); 1.9 (−6.9 to 10.8); greater increase in intervention group, p = 0.72 | Secondary | |||
| Ornish et al. [48, 57] Frattaroli et al. [46] |
Aerobic exercise and vegan diet supplemented with soy (58 g), vitamin E (400 IU), selenium (200 mcg), fish oil (3 g), vitamin C (2 g) daily versus Control (usual care) | T1 or T2: 100 % Active monitoring/surveillance: 100 % Watchful waiting: 100 % |
PSA: change, difference in mean baseline to 12 months, mean (SD); I: −0.25 (1.2), C: 0.38 (1.3); p = 0.016d | Primary |
| PSA: Change, mean increase over 24 months, mean (SD); I: 0.88 (1.88), C: 0.99 (2.09); p > 0.05 Frattaroli et al. [46]d | Secondary | |||
| PSA: change in velocity, ng/ml/years; I: 0.58, C: 0.50; p > 0.05 Frattaroli et al. [46]d | Secondary | |||
| Prostate cancer treatment undergone, n; 0–12 months, I: 0, C: 6; 13–24 months; I: 2, C: 7; p = 0.005; Effect size (CI): 0.255 (0.053–0.437) Frattaroli et al. [46]d | Primary | |||
| Unpublished data (not included in analysis) | ||||
| Cipolla et al. [80] (Poster only) |
Sulforaphane, 60 mg daily for 6 months, followed by a 2 month wash out period versus P (not stated) | No metastasis: 100 % Prostatectomy: 37 % Prostatectomy and RTE: 50 % RTE and Hormone therapy: 12.8 % |
PSA: Change, ng/ml, mean (SD); I: 0.099 (0.341), C: 0.620 (1.417); p = 0.03e | Primary |
| Nayan et al. [79] (Abstract only) |
Lycopene (8 mg daily) versus follow-up care only | Advanced/metastatic: 100 % Hormone therapy/ADT: 100 % |
Disease progression; progression to hormone resistant cancer n (%); I: 4 (10 %), C: 18 (47.3 %); p = NR | Unknown |
C control, CI 95 % confidence interval, HR hazard ratio, I intervention, NR not reported, NS not significant, P placebo, PSA prostate-specific antigen, SD standard deviation, SE Standard error
aPSA failure: PSA values were more than 0.2 ng/ml on two consecutive measurements Higashihara et al. [74]
bRate of PSA rise: PSA velocity. Rate of PSA change over a period of time (http://www.upmccancercenter.com/cancer/prostate/psaelevated.cfm)
cPSA stabilization: for minimum of 3 months
dNumber analyzed different to number randomized
eNumber analyzed unclear
Data analysis
Due to substantial heterogeneity across the studies in relation to intervention design, delivery mode and outcomes reported, formal pooling of the data by meta-analysis was not appropriate or possible. Therefore, a qualitative synthesis of all studies in a narrative format was undertaken.
The PRISMA statement was followed and adhered to [24]. The protocol was registered with PROSPERO International Prospective Register of systematic reviews, Ref: CRD42014008701.
Results
Descriptions of studies
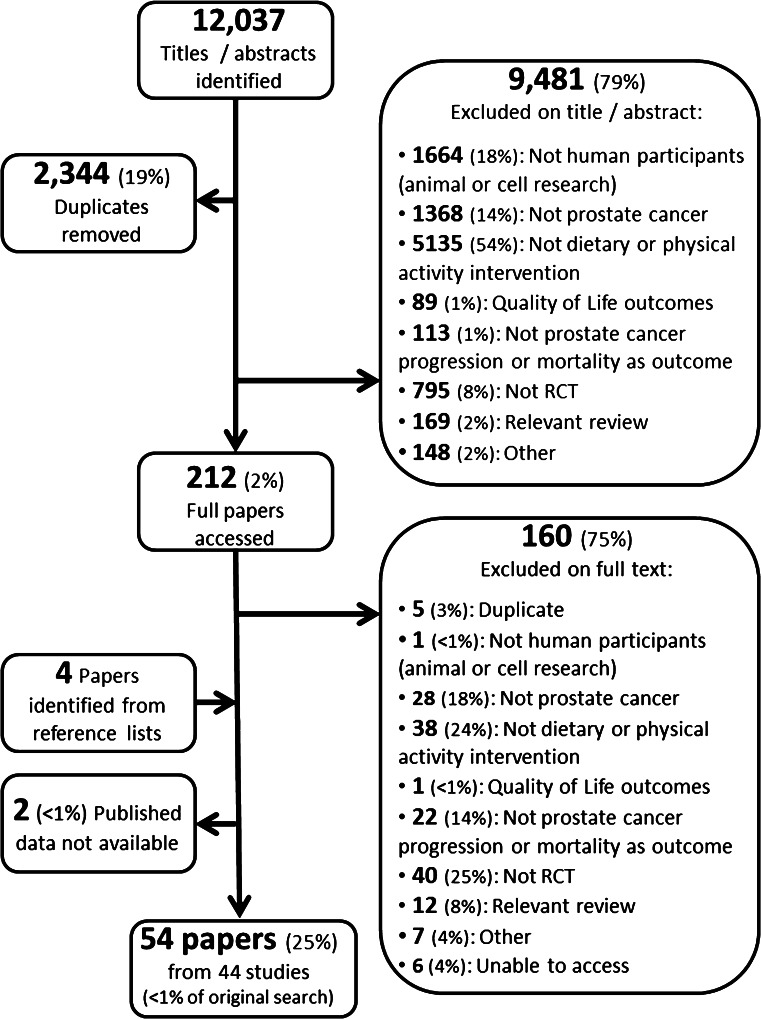
The search identified 12,037 titles and abstracts, of which 9,481 (79 %) papers that did not meet our inclusion criteria and 2,344 (19 %) exact duplicates were removed. The remaining full texts of 212 (2 %) papers were retrieved and read in full; 44 RCTs reported in 54 papers met the inclusion criteria (Fig. 1).
Fig. 1.
PRISMA flow diagram
The characteristics of the included studies are summarized in Table 1. The 44 RCTs that were eligible for inclusion in our review were published between 2001 and 2014 and involved 3,418 participants from 13 countries: 26 from the USA [25–57], three in Australia [58–60], the Netherlands [61–63], and the UK [64–66], two in Canada [67, 68], and one in each of China [69], Czech Republic [70], India [71–73], Japan [74], New Zealand [58], Norway [75, 76], Sweden [77], and Switzerland [78]. Where multiple papers were identified for the same RCT, all references are reported; however, data reported in multiple publications was only extracted once. The median size of the trials was 64 men (interquartile range 42–98, range 19–383).
The men had undergone a variety of treatments: radical prostatectomy followed by implementation of the intervention (n = 13 [26, 30, 33, 34, 40, 44, 45, 51, 61–63, 70, 78]) or the commencement of the intervention in men prior to undergoing radical prostatectomy (n = 13 [25, 27–29, 32, 35, 39, 41, 42, 49, 53–56, 60, 74–76]); active surveillance, active monitoring, or watchful waiting (n = 13 [29, 36–38, 43, 46, 48, 50–52, 57, 61, 62, 64, 77, 78]); hormone therapy or androgen deprivation therapy (ADT) (n = 11 [26, 34, 45, 47, 58, 59, 65–69, 78–80]); external beam radiotherapy or brachytherapy (n = 12 [26, 31, 33, 34, 40, 45, 51, 58, 61–63, 67, 78]); orchiectomy (n = 2 [69, 71–73]); chemotherapy (n = 1 [78]); and cryotherapy (n = 1 [26, 34]). The majority of studies were parallel group RCTs, with one (n = 31 [25, 27, 29–33, 35, 36, 38, 40, 43, 44, 46, 48–50, 53–59, 61, 64–66, 68–70, 72–78]), two (n = 3 [37, 52, 60, 67]), or three intervention arms (n = 3 [39, 41, 42]) versus usual care or some other control group. There were two dual arm parallel group RCTs without a usual care or control group comparator [26, 34, 47] and one three arm parallel group RCT without a usual care or control group comparator [28]. Four studies had a crossover design, two with one intervention arm and a usual care or control group arm [62, 63], one with two intervention arms and no usual care or control group arm [51], and one with three intervention arms and no usual care or control group arm [45].
Excluded studies
Of the 212 texts read in full, 160 (75 %) were excluded. Thirty-eight did not involve a diet, nutrition, or physical activity intervention, and these included Ernst et al. [81], Peng et al. [82], and Sternberg et al. [83]. Twenty-two did not include prostate cancer progression or mortality as an outcome, for example James et al. [84], Zhang et al. [85], and Lee et al. [86]. Trials that only included a small proportion of prostate cancer patients within their total sample and had analyzed the data as a whole were excluded, for example Hamilton-Reeves et al. [87] (8.6 % of the study sample had prostate cancer) and Hernáandez et al. [88] (“patients with a biopsy negative for prostate cancer comprised the principal study sample” p520). Figure 1 highlights all reasons for exclusion.
Quality of the evidence
Risk of bias
Overall, most of the included papers demonstrated high risk of bias on the majority of criteria or failed to adequately report how they had conducted the study on these essential criteria (Table 2). For sequence generation, half of the 44 trials were assessed as being unclear risk of bias and 22 had low risk of bias. The corresponding figures were, respectively: 30 unclear, 14 low, for allocation concealment; 22 high, five unclear, 17 low, for blinding of participants; nine high, 28 unclear, seven low, for blinding of personnel; and four high, 29 unclear, 11 low, for blinding of outcome assessor. In contrast, for completeness of outcome data 14 demonstrated high, three unclear, and 27 low risk of bias and for selective outcome reporting four demonstrated high but 40 had low risk of bias. Of note, Bosland et al. [30] and Stenner-Liewen et al. [78] were assessed to have low overall risk of bias, and Kucuk et al. [53–56], Beer et al. [49], Kumar et al. [50], Segal et al. [68], Demark-Wahnefried et al. [42], Schroder et al. [63], Thomas et al. [64], and Bourke et al. [66] were assessed to have relatively low overall risk of bias.
Table 2.
Assessment of risk of bias
| Sequence generation | Allocation concealment | Blinding of participants | Blinding of personnel | Blinding of outcome assessor | Completeness of outcome data | Selective outcome reporting | |
|---|---|---|---|---|---|---|---|
| Nutritional interventions | |||||||
| Kucuk et al. [53–56] | + | + | − | ? | + | +a | + |
| Bylund et al. [77] | ? | ? | + | ? | + | − | + |
| Ansari and Gupta [71–73] | ? | ? | − | ? | ? | ? | + |
| Beer% et al. [49] | ? | ? | + | + | + | + | + |
| Kumar et al. [50] | + | + | + | + | ? | − | + |
| Kumar et al. [41] | + | + | − | ? | ? | +a | + |
| Higashihara et al. [74] | ? | ? | − | ? | ? | + | + |
| Stratton et al. [37, 52] | + | ? | + | ? | ? | + | − |
| Vidlar et al. [70] | ? | ? | + | ? | ? | + | + |
| Margalit et al. [31] | + | ? | ? | ? | ? | + | + |
| Lazarevic% et al. [75, 76] | + | ? | + | ? | ? | − | +a,b |
| Nguyen% et al. [32] | ? | ? | + | ? | ? | − | −c |
| Stenner-Liewen et al. [78] | + | + | + | + | + | + | + |
| Chen et al. [69] | ? | ? | + | ? | ? | + | + |
| Wagner% et al. [28] | + | ? | + | ? | ? | +a | + |
| Gee et al. [27] | ? | ? | − | − | − | ? | + |
| Freedland% et al. [25] | + | ? | + | ? | ? | + | + |
| Paller et al. [26, 34] | ? | ? | ? | ? | ? | − | + |
| Complex nutritional interventions | |||||||
| Demark-Wahnefried% et al. [42] | + | + | − | − | + | + | + |
| Dalais et al. [60] | ? | ? | + | ? | ? | + | + |
| Vaishampayan et al. [47] | ? | ? | ? | ? | ? | − | + |
| Kranse et al. [62] | ? | ? | + | ? | ? | − | + |
| Schroder et al. [63] | ? | + | + | + | ? | +a | + |
| Hoenjet et al. [61] | ? | ? | ? | ? | ? | − | + |
| Grainger et al. [45] | ? | ? | −* | − | − | + | + |
| DeVere White% et al. [38] | ? | ? | + | ? | ? | − | + |
| Li et al. [44] | + | ? | −* | ? | +a | ? | + |
| Oh et al. [51] | ? | ? | ? | ? | ? | + | + |
| Aronson% et al. [36] | ? | ? | −* | ? | ? | + | + |
| Kumar% et al. [50] | + | + | − | ? | ? | + | + |
| Carmody et al. [40] | ? | ? | −* | ? | ? | − | + |
| Parsons et al. [43] | ? | ? | −* | ? | ? | + | + |
| Aronson et al. [35] | + | ? | −* | ? | ? | − | + |
| Wright et al. [29] | + | ? | −* | − | ? | + | + |
| Thomas% et al. [64] | + | + | + | + | ? | + | − |
| Bosland et al. [30] | + | + | + | + | + | − | + |
| Nutritional and physical activity interventions | |||||||
| Ornish et al. [48, 51], Frattaroli et al. [46] | ? | ? | −* | ? | + | +a | + |
| Hébert [33] | ? | ? | −* | ? | ? | − | + |
| Bourke et al. [66] | + | + | −* | − | + | + | + |
| Bourke% et al. [65] | + | + | −* | − | + | − | +a |
| Physical activity interventions | |||||||
| Segal et al. [68] | + | + | −* | − | + | + | +c |
| Segal% et al. [67] | + | + | −* | + | ? | + | − |
| Galvao% et al. [58] | + | ? | −* | − | − | + | +c |
| Cormie% et al. [59] | + | + | −* | − | − | + | +c |
Due to the nature of the interventions, we modified the Cochrane guidelines to separate blinding of participants and blinding of personnel; this addressed the difficulty in blinding participants in some of the interventions considered. Each trial was given a low (+), high (−), or unclear (?) risk of bias score for each dimension. Where a full paper was not available risk of bias was not assessed, as it was felt this would be a biased assessment without full data available
Key: + low risk of bias; − high risk of bias; ? unclear; * impossible to blind
% papers with protocols or trial registration (protocols were not accessed for the majority of studies)
aNot on all outcomes
bInformation from protocol
cPSA reported but not pre-specified in protocol or trial registration
Methodological quality
The methodological quality of the trials was variable; although it was generally acceptable in the majority of the RCTs, some scored very low (Table 3). In particular, only 15 RCTs reported that they had reached an adequately powered sample size and reasons for withdrawals were described for only 20 RCTs.
Table 3.
Methodological quality
| Similar baseline characteristics | Similar prognostic indicators | Power calculation conducted | Power sample size reached | Withdrawal numbers by gp | Withdrawal reasons by gp | Equal therapeutic time by gp | |
|---|---|---|---|---|---|---|---|
| Nutritional interventions | |||||||
| Kucuk et al. [53–56] | ? | ? | ? | ? | ? | − | + |
| Bylund et al. [77] | − | + | − | ? | + | − | + |
| Ansari and Gupta [71–73] | + | + | ? | ? | ? | ? | + |
| Beer et al. [49] | ? | ? | + | + | − | − | + |
| Kumar et al. [50] | ? | ? | + | − | + | − | + |
| Kumar et al. [41] | + | + | − | − | + | + | + |
| Higashihara et al. [74] | + | + | − | ? | − | − | + |
| Stratton et al. [37, 52] | − | + | + | + | + | + | + |
| Vidlar et al. [70] | + | + | − | ? | na | na | + |
| Margalit et al. [31] | + | − | ? | ? | − | − | + |
| Lazarevic et al. [75, 76] | ? | ? | ? | ? | − | − | + |
| Nguyen et al. [32] | + | + | − | − | + | + | + |
| Stenner-Liewen et al. [78] | + | + | + | + | + | − | + |
| Chen et al. [69] | + | + | ? | ? | − | − | + |
| Wagner et al. [28] | + | + | − | ? | + | + | + |
| Gee et al. [27] | ? | ? | + | − | − | − | − |
| Freedland et al. [25] | + | + | + | −a | − | − | + |
| Paller et al. [26, 34] | + | + | + | + | − | − | + |
| Complex nutritional interventions | |||||||
| Demark-Wahnefried et al. [42] | + | + | + | + | + | + | + |
| Dalais et al. [60] | + | + | − | − | − | − | + |
| Vaishampayan et al. [47] | ? | ? | ? | ? | + | + | + |
| Kranse et al. [62] | ? | ? | + | − | + | − | + |
| Schroder et al. [63] | + | + | + | − | + | + | + |
| Hoenjet et al. [61] | ? | ? | + | + | − | − | + |
| Grainger et al. [45] | ? | ? | − | ? | na | na | + |
| DeVere White et al. [38] | + | + | + | + | − | − | + |
| Li et al. [44] | + | + | + | ? | + | + | − |
| Oh et al. [51] | + | + | + | − | ? | ? | + |
| Aronson et al. [36] | + | + | + | − | + | + | + |
| Kumar et al. [50] | + | + | na | + | + | + | + |
| Carmody et al. [40] | + | + | ? | na | + | + | − |
| Parsons et al. [43] | ? | ? | − | ? | + | + | − |
| Aronson et al. [35] | ? | ? | − | + | + | + | + |
| Wright et al. [29] | − | + | − | ? | − | − | − |
| Thomas et al. [64] | −b | + | ? | ? | + | − | + |
| Bosland et al. [30] | + | + | + | − | + | + | + |
| Nutritional and physical activity interventions | |||||||
| Ornish et al. [48, 51], Frattaroli et al. [46] | + | + | − | − | + | + | − |
| Hébert [33] | + | + | + | + | + | + | − |
| Bourke et al. [66] | + | + | − | na | + | + | + |
| Bourke et al. [65] | + | + | + | + | + | + | − |
| Physical activity interventions | |||||||
| Segal et al. [68] | + | + | + | + | + | − | − |
| Segal et al. [67] | + | + | + | + | + | − | − |
| Galvao et al. [58] | + | + | + | + | + | + | − |
| Cormie et al. [59] | + | + | + | + | + | + | − |
Seven design and implementation questions were posed; RCTs were scored as yes (+), no (−), or unclear (?) for each question. Where a full paper was not available methodological quality was not assessed, as it was felt this would be a biased assessment without full data available
Key: + yes; − no; ? unclear
aSample size not reached by n = 1
bNot similar on baseline age
Interventions
The median intervention duration was 12 weeks [interquartile range 4–26 weeks (6 months), range 3–260 weeks (65 months)].
Single-factor dietary interventions
Calcitriol (vitamin D3)
The effect of calcitriol supplementation up to 2 months prior to radical prostatectomy was reported in three RCTs [27, 28, 49]. Men were randomized in the three trials, respectively, to doses of 10 µg vitamin D daily versus no supplement; 400 versus 10,000 versus 40,000 IU vitamin D3 daily; and 0.5 µg/kg calcitriol daily versus placebo. In all three trials, there was little evidence of an effect of vitamin D3 on change in total PSA, IGF-I, cell apoptosis, or proliferation.
Lycopene
Lycopene supplementation up to 6 weeks prior to radical prostatectomy was investigated in two RCTs reported in five publications [41, 53–56]. Men were randomized in the two trials, respectively, to doses of 15, 30, or 45 mg lycopene daily versus no supplementation and 15 mg lycopene versus usual care. No between-group differences in PSA change [41, 53–56], IGF-I change [53–56], or cellular response [41, 53–56] were observed. In a trial assessed as having high or unclear risk of bias on six of the seven criteria, where low risk of bias was only attributed to selective outcome reporting, Ansari and Gupta [71–73] randomized men, with advanced or metastatic disease, to orchiectomy alone versus orchiectomy plus 2 mg lycopene supplementation twice daily. A difference in change in PSA between the groups at 24 months was observed (p < 0.001); fewer intervention men had a clinically raised PSA indicating progression than the control arm (p < 0.05); there were fewer bone metastasis in the intervention group (p < 0.02), and prostate cancer mortality was lower in the intervention group (p < 0.001).
Pomegranate
Three trials [25, 26, 34, 78] randomized men to pomegranate extract supplements, one in men for four weeks prior to radical prostatectomy [25]; one for up to 18 months following radiotherapy, prostatectomy, hormone therapy or ADT, or cryotherapy [26, 34]; and one following radiotherapy, prostatectomy, hormone therapy or ADT, chemotherapy, or watchful waiting for 28 days [78]. In men randomized to 2 g of pomegranate extract daily (including 1.2 g of polyphenol), there was no difference in measures of cell proliferation, progression, or change in PSA [25]. In the studies that randomized men at a variety of TNM classification of malignant tumors stages to pomegranate extract following definitive treatment, no differences were observed in median PSA doubling time or PSA change between experimental versus control groups [26, 34]. The third trial, which was assessed to have low risk of bias, found no between-group differences in PSA change [78].
Genistein (soy)
The effect of genistein supplementation was investigated in two studies [50, 75, 76]. In men randomized to 30 mg genistein daily for three to six weeks prior to prostatectomy, differences in favor of the experimental, versus control, group were reported for percentage change in PSA (p = 0.051), cellular response (p = 0.033), and cell proliferation (p < 0.001). However, the trial was assessed as having high or unclear risk of bias on four of the seven criteria, and it was assessed to have low risk of bias for sequence generation and blinding of participants, as well as selective outcome reporting [75, 76]. Comparably, in a trial with relatively low risk of bias, in men undergoing watchful waiting, randomization to 60 mg genistein daily versus an isocaloric placebo for 12 weeks had no impact on mean change in PSA [50].
Selenium
Two RCTs investigated the effects of selenium [37, 52] or selenium and silymarin [70] supplementation: one in men with localized disease on active monitoring, active surveillance, or watchful waiting supplemented with 200 or 800 µg selenium versus placebo for up to 60 months; and the other in men following prostatectomy who were supplemented with selenomethionine (240 µg selenium and 570 mg silymarin) or placebo for 6 months. There were no between trial group differences in measures of PSA in either trial.
Other nutritional interventions
There was no evidence of any effect of any of the following interventions: Qilan capsules (consisting of astragalus, fenugreek, gynostremma, pentaphyllan, and smilaz glabra) given for 4 weeks versus placebo in men who had undergone hormone therapy or ADT and orchiectomy on PSA outcomes [69]; 50 mg of beta carotene on alternate days (for an unreported duration) versus placebo in men undergoing radiotherapy on prostate cancer mortality [31]; 800 mg of polyphenol E versus placebo daily for 3–6 weeks prior to undergoing prostatectomy on changes in PSA, IGF-I, or Gleason score or on tissue measures of cell proliferation, cell apoptosis, angiogenesis [32]; or 2.4 g eicosapentaenoic acid daily versus no intervention for 24 months in men who had undergone radical prostatectomy on PSA failure [74]. However, in men not undergoing active treatment, 295 g of rye bread versus a wheat bread control for 11 weeks resulted in increased cell apoptosis (p < 0.05) in the intervention group, although no effect on change in PSA or IGF-I were reported. This trial was assessed to have high or unclear risk of bias on four of seven criteria, and low risk of bias was found for blinding of participants and outcome assessors, as well as selective outcome reporting [77].
Multiple factor dietary interventions
Isoflavones
Three studies explored the effect of combinations of isoflavones within individual supplements [30, 38, 39]. In a study assessed as being of low risk of bias, Bosland et al. [30] found no effect on recurrence-free survival of powdered soy protein (combining genistein, daidzein, and glycitein) compared with calcium caseinate given for 24 months in a population of men who had undergone radical prostatectomy. Others found no effect of combinations of isoflavones on PSA measures in men due to undergo radical prostatectomy [39] or men undergoing active surveillance for a period of 6 months [38]. However, a trial assessed to have high to unclear risk of bias on four of seven criteria, where only blinding of participants, completeness of outcome data and selective outcome reporting were assessed as low risk of bias, randomising men awaiting radical prostatectomy to either 50 g of heat-treated soy, or 50 g of heat-treated soy plus 20 g of linseed, or placebo, found a difference in change in PSA between the soy-only and the placebo group (p = 0.02) and a difference in change in free–total PSA ratio between the two intervention groups (p = 0.007) and the soy-only and placebo group (p = 0.01) [60].
Other complex nutritional supplement interventions
In an RCT of men undergoing active surveillance or watchful waiting, a capsule containing pomegranate seed, green tea, broccoli and turmeric in a capsule versus placebo given for 6 months was associated with a reduced rise in PSA (p = 0.0008) and an increase in the percentage of participants with stable PSA at 6 months (p = 0.00001). This trial was reported to have relatively low risk of bias [64]. Schroder et al. [63] randomized men undergoing radiotherapy or radical prostatectomy to a supplement consisting of soy, lycopene, selenium and coenzyme Q10. The intervention was associated with improved measures of PSA during follow-up, and was assessed to have low risk of bias on five of seven criteria, however, unclear risk of bias for sequence generation and blinding of outcome assessors. Further to this, several trials of various combinations of nutrients in a variety of populations of men with prostate cancer observed no evidence for any differences [45, 47, 51, 61, 62].
Low-fat diet combined with other nutritional elements
Three studies combined low-fat diet with another nutritional element [35, 42, 44]. Aronson et al. [35] randomized men due to undergo radical prostatectomy to 4 weeks of a daily low-fat and fish oil diet versus a Western diet, the trial was assessed to have high or unclear risk of bias for five of seven criteria, and low risk of bias was only awarded for sequence generation and selective outcome reporting. No differences were noted in change in mean PSA or change in mean IGF-I, but there was a reduction in cell proliferation (p = 0.026). Li et al. [44] compared a daily low-fat, high-fiber and soy protein (40 g) diet with a standard recommended control diet given for 48 months in men who had undergone radical prostatectomy. A difference was observed in IGF-I change between the groups (p = 0.04); however, none was seen for change in PSA. This RCT was reported to have unclear or high risk of bias on four of seven criteria, and low risk of bias was only awarded for sequence generation, blinding of outcome assessors and selective outcome reporting. Demark-Wahnefried et al. [42] randomized men due to undergo radical prostatectomy to flaxseed, low-fat diet, or flaxseed and low-fat diet versus usual diet, over an average of 31 days. The trial was assessed to have low risk of bias for five of seven criteria, and blinding of participants and of personnel were assessed to show high risk of bias; there was a change in proliferation rate between the flaxseed only and control groups (p = 0.0013) but no difference between apoptotic rate, median change in PSA, or median change in IGF-I. Aronson et al. [36] randomized men undergoing active monitoring to a low-fat diet, which included 35 mg of soy protein per day for 1 month, versus a Western diet but observed no differences in mean change in PSA or mean change in IGF-I between experimental and control groups.
Three RCTs included an educational element within their complex nutritional intervention [29, 40, 43] but found no consistent effects in men awaiting radical prostatectomy or undergoing radiotherapy, active monitoring, or active surveillance on PSA or IGF-I outcomes.
Physical activity
Four RCTs reported a physical activity intervention, resistance and/or aerobic training [58, 59, 67, 68], but found no consistent effects in men who had undergone hormone therapy, androgen deprivation therapy, or radiotherapy on PSA-based measures of progression. One of these four trials was reported to have relatively low overall risk of bias [68].
Combination interventions
Four RCTS combined both a nutritional and a physical activity element in their intervention [33, 46, 48, 57, 65, 66]. All of these implemented an aerobic or aerobic and resistance training program in combination with a nutritional element. There was no consistent effect in men who had undergone radiotherapy, radical prostatectomy, active surveillance, or were on ADT. Only one of these trials had relatively low overall risk of bias [66]. Further information about all studies can be found in Table 4.
Adverse events
A variety of adverse events was reported in the included RCTs, and these most often included gastrointestinal events, such as mild abdominal pain, constipation, diarrhea and nausea; also reported were myalgia, including aches and pains and fever like symptoms, such as chills.
Discussion
Among 54 papers reporting the results of 44 RCTs that explored dietary, nutritional, and physical activity interventions in men with prostate cancer, there was a large degree of heterogeneity with regard to intervention aims, methods of implementation and outcomes, with the quality of the research often being poor. Only three of ten studies with the lowest risk of bias and highest methodological rigor found a possible beneficial effect; a study in men undergoing watchful waiting or primary active surveillance suggested that a capsule containing pomegranate seed, green tea, broccoli and turmeric improved PSA kinetics in the intervention group compared to the control arm [64]. A study randomising men due to undergo radical prostatectomy to flaxseed, low-fat diet, or flaxseed and low-fat diet versus usual diet, over an average of 31 days, demonstrated a change in proliferation rate between the flaxseed only and control groups; however, no difference between apoptotic rate, median change in PSA, or median change in IGF-I were noted [42]. Finally, in a trial that randomized men undergoing radiotherapy or radical prostatectomy to a supplement consisting of soy, lycopene, selenium and coenzyme Q10, the intervention was associated with improved measures of PSA during follow-up. It should be noted that despite PSA being the most widely available, and cited, biomarker for prostate cancer, taken alone it may not be an appropriate surrogate marker of long-term therapeutic benefit in prostate cancer trials, which has not been proven to be a suitable replacement for a final survival endpoint [89].
Of the remaining studies with low risk of bias; an RCT randomising men, who were due to undergo radical prostatectomy, to calcitriol or control reported no difference in cell apoptosis or rise in PSA [49]. A trial where patients were randomized to 15 mg lycopene versus usual care reported no between-group differences in PSA change, IGF-I change, or cellular response. A study which scored low for all risk of bias, and also had excellent methodological quality, randomized participants to take pomegranate extract supplements following radiotherapy, prostatectomy, hormone therapy or ADT, chemotherapy, or watchful waiting for 28 days, and found no between-group differences in PSA change [78]. A complex isoflavone intervention, with strong methodological vigor and low risk of bias, resulted in no change in recurrence-free survival between groups [30], similarly, a trial, with relatively low risk of bias, assessing the effect of genistein supplementation on men undergoing watchful waiting were randomized to 60 mg genistein daily versus an isocaloric placebo for 12 weeks, no impact on mean change in PSA was reported [50]. It should be noted that these previous studies support World Cancer Research Fund International guidelines which state “Don’t use supplements to protect against cancer” [9]. One study, identified as being relatively low risk of bias, which combined aerobic and resistance training with diet advice [66], reported no difference in PSA or IGF-I outcomes. Finally, a resistance training intervention in men due to undergo ADT concluded no change in PSA [68], and this RCT was reported to have relatively low risk of bias. Most of the other studies reviewed were assessed as having high or unclear risk of bias, often with poor methodological vigor, so it is not possible to draw any conclusions from those studies.
This is the first systematic review, to our knowledge, which has combined interventions of modifiable lifestyle risk factors, with a primary outcome of prostate cancer progression or mortality. This is clinically relevant as it is unlikely that patients would make changes to a singular lifestyle behavior [90]; instead, for example, a clinician may recommend changes to diet alongside an increase in physical activity. As the number of cancer survivors living for longer increases [7], particularly for those with prostate cancer who are turning to active surveillance [5], further understanding of diet, nutritional, and physical activity interventions is of great importance.
The review was systematic and comprehensive and had no language restrictions. All papers were at least double-extracted. Risk of bias and methodological quality were assessed by at least two independent reviewers. This review does have some limitations. The primary outcomes of the review were not always reported as primary outcomes in the papers. Thus, we relied on reported secondary outcomes and the RCTs may not have been powered to detect differences in these outcomes. Meta-analysis was not possible due to heterogeneity of trial design, outcomes and statistical presentation; however, a qualitative synthesis was conducted. The limited quality of most of the RCTs, and the possibility of publication bias (which we were unable to formally assess in the absence of a meta-analysis), restricted the definitive conclusions that could be drawn.
Conclusion
The complex nature of dietary, nutritional, and physical activity interventions, along with the slow-growing nature of prostate cancer that causes difficulties in measuring long-term clinically relevant change, makes research in this area difficult. Poor quality, variability in methodology, inconsistency of results, and a variety of proxies for prostate cancer progression make firm conclusions hard to draw. The RCTs identified in our review were generally likely to be underpowered, appeared to be at high or unclear risk of bias and were often inadequately reported, intervened for only short durations and followed-up men for surrogate outcomes of questionable relationship to clinical outcomes. Such trials are unlikely to have any clinical impact and should be abandoned in favor of large, well-designed trials with endpoints that will impact on clinical practice. These findings are in line with previous systematic reviews which concluded that the impact of interventions could not be reliably estimated due to limited, and low-quality, RCTs [18, 22].
Electronic supplementary material
Acknowledgments
We would like to acknowledge Vanessa Er for translation.
Financial support
The research was funded by the National Institute for Health Research (NIHR) Bristol Nutritional Biomedical Research Unit based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. RMM and JAL are supported by a Cancer Research UK (C18281/A19169) Programme Grant (the Integrative Cancer Epidemiology Programme).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Rachel E. Perry and Verity A. Leach have contributed equally to this work.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51(1):15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK (2013) Prostate cancer key facts. http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/prostate-cancer/
- 3.Cancer Research UK (2012) Prostate cancer incidence statistics. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/incidence/
- 4.Prostate Cancer UK (2012) Prostate Cancer: a guide for newly diagnosed men. http://prostatecanceruk.org/media/41578/newly_diagnosed_booklet.pdf
- 5.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67(1):44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 7.McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS, Hudson MM, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WCRF/AIRC . Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: AIRC; 2007. [Google Scholar]
- 9.WCRF, AIRC . Diet, nutrition, physical activity and prostate cancer. Washington, DC: AIRC; 2014. [Google Scholar]
- 10.American Cancer Society . Cancer facts and figures. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 11.Chan JM, Holick CN, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Giovannucci EL. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Cause Control. 2006;17(2):199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 12.Kenfield SADN, Richman EL, Stampfer MJ, Chan JM, Giovannucci EL. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur Urol. 2014;65(5):887–894. doi: 10.1016/j.eururo.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012;131(1):201–210. doi: 10.1002/ijc.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonn SE, Sjolander A, Lagerros YT, Wiklund F, Stattin P, Holmberg E, Grönberg H, et al. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidem Biomar. 2015;24(1):57–64. doi: 10.1158/1055-9965.EPI-14-0707. [DOI] [PubMed] [Google Scholar]
- 15.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113(5b):E119–E130. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
- 16.Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol. 2010;21:116–179. doi: 10.1093/annonc/mdp286. [DOI] [PubMed] [Google Scholar]
- 17.Alkhenizan A, Hafez K. The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials. Ann Saudi Med. 2007;27(6):409–414. doi: 10.4103/0256-4947.51459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements: a systematic review. Nutr Metab. 2014;11:30. doi: 10.1186/1743-7075-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22(3):187–199. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SSK, Cerin E, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamad H, McNeill G, Haseen F, N’Dow J, Craig LC, Heys SD. The effect of dietary and exercise interventions on body weight in prostate cancer patients: a systematic review. Nutr Cancer. 2015;67(1):43–60. doi: 10.1080/01635581.2015.976313. [DOI] [PubMed] [Google Scholar]
- 22.Davies AA, Davey Smith G, Harbord R, Bekkering GE, Sterne JA, Beynon R, Thomas S. Nutritional interventions and outcome in patients with cancer or preinvasive lesions: systematic review. J Natl Cancer I. 2006;94(14):961–973. doi: 10.1093/jnci/djj263. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 [PMC free article] [PubMed] [Google Scholar]
- 25.Freedland SJ, Carducci M, Kroeger N, Partin A, Rao JY, Jin Y, Kerkoutian S, et al. A double-blind, randomized, neoadjuvant study of the tissue effects of POMx pills in men with prostate cancer before radical prostatectomy. Cancer Prev Res. 2013;6(10):1120–1127. doi: 10.1158/1940-6207.CAPR-12-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(1):50–55. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gee J, Bailey H, Kim K, Kolesar J, Havighurst T, Tutsch KD, See W, et al. Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. Prostate. 2013;73(9):970–978. doi: 10.1002/pros.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner D, Trudel D, Van der Kwast T, Nonn L, Giangreco AA, Li D, Dias A, et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J Clin Endocr Metab. 2013;98(4):1498–1507. doi: 10.1210/jc.2012-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright JL, Plymate S, D’Oria-Cameron A, Bain C, Haugk K, Xiao L, Lin DW, et al. A study of caloric restriction versus standard diet in overweight men with newly diagnosed prostate cancer: a randomized controlled trial. Prostate. 2013;73(12):1345–1351. doi: 10.1002/pros.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, Kong MX, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310(2):170–178. doi: 10.1001/jama.2013.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margalit DN, Kasperzyk JL, Martin NE, Sesso HD, Gaziano JM, Ma J, Stampfer MJ, et al. Beta-carotene antioxidant use during radiation therapy and prostate cancer outcome in the physicians’ health study. Int J Radiat Oncol. 2012;83(1):28–32. doi: 10.1016/j.ijrobp.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen M, Ahmann F, Nagle R, Hsu CH, Tangrea JA, Parnes HL, Sokoloff MH, et al. Randomized, double-blind, placebo controlled trial of polyphenon E in prostate cancer patients before radical prostatectomy: evaluation of potential chemopreventive activities. J Urol. 2012;5(2):290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert JR, Hurley TG, Harmon BE, Heiney S, Hebert CJ, Steck SE. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012;36(2):e128–e136. doi: 10.1016/j.canep.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paller CJ, Ye X, Wozniak P, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, et al. A phase II study of pomegranate extract for men with rising prostate-specific antigen following primary therapy. J Clin Oncol. 2011;16(1):50–55. doi: 10.1007/s10147-010-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, Liu B, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res. 2011;4(12):2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, Cohen P, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183(1):345–350. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stratton MS, Algotar AM, Ranger-Moore J, Stratton SP, Slate EH, Hsu CH, Thompson PA, et al. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev Res. 2010;3(8):1035–1043. doi: 10.1158/1940-6207.CAPR-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62(8):1036–1043. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar NB, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, Besterman-Dahan K, et al. Results of a randomized phase I dose-finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. J Soc Integr Oncol. 2010;8(1):3–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Carmody J, Olendzki B, Reed G, Andersen V, Rosenzweig P. A dietary intervention for recurrent prostate cancer after definitive primary treatment: results of a randomized pilot trial. Urology. 2008;72(6):1324–1328. doi: 10.1016/j.urology.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Kumar NBB-DK, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, Krischer JP. Results of a randomized clinical trial of the action of several doses of lycopene in localized prostate cancer: administration prior to radical prostatectomy. Clin Med Urol. 2008;16(1):1–14. doi: 10.4137/cmu.s718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MT, Snyder DC, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidem Biomark. 2008;17(12):3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101(10):1227–1231. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Aronson WJ, Arteaga JR, Hong K, Thames G, Henning SM, Liu W, et al. Feasibility of a low-fat/high-fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. Eur J Clin Nutr. 2008;62(4):526–536. doi: 10.1038/sj.ejcn.1602743. [DOI] [PubMed] [Google Scholar]
- 45.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, Monk JP, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–154. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 46.Frattaroli J, Weidner G, Dnistrian AM, Kemp C, Daubenmier JJ, Marlin RO, Crutchfield L, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319–1323. doi: 10.1016/j.urology.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 47.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 48.Ornish D, Weidner G, Fair WR, Marlin R, Pettengill EB, Raisin CJ, Dunn-Emke S, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–1069. doi: 10.1097/01.ju.0000169487.49018.73. [DOI] [PubMed] [Google Scholar]
- 49.Beer TM, Myrthue A, Garzotto M, O’hara MF, Chin R, Lowe BA, Montalto MA, et al. Randomized study of high-dose pulse calcitriol or placebo prior to radical prostatectomy. Cancer Epidem Biomark. 2004;13(12):2225–2232. [PubMed] [Google Scholar]
- 50.Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, Seigne J, Helal M, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59(2):141–147. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 51.Oh WK, Kantoff PW, Weinberg V, Jones G, Rini BI, Derynck MK, Bok R, et al. Prospective, multicenter, randomized phase II trial of the herbal supplement, PC-SPES, and diethylstilbestrol in patients with androgen-independent prostate cancer. J Clin Oncol. 2004;22(18):3705–3712. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 52.Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, Marshall JR, et al. Selenium and inhibition of disease progression in men diagnosed with prostate carcinoma: study design and baseline characteristics of the ‘Watchful Waiting’ Study. Anti Cancer Drug. 2003;14(8):595–600. doi: 10.1097/00001813-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med. 2002;227(10):881–885. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]
- 54.Kucuk O, Sarkar FH, Sakr W, Khachik F, Djuric Z, Banerjee M, Pollak MN, et al. Lycopene in the treatment of prostate cancer. Pure Appl Chem. 2002;74(8):1443–1450. doi: 10.1351/pac200274081443. [DOI] [Google Scholar]
- 55.Kucuk O. Chemoprevention of prostate cancer. Cancer Metast Rev. 2002;21(2):111–124. doi: 10.1023/A:1020809806121. [DOI] [PubMed] [Google Scholar]
- 56.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, Li YW, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidem Biomark. 2001;10(8):861–868. [PubMed] [Google Scholar]
- 57.Ornish DM, Lee KL, Fair WR, Pettengill EB, Carroll PR. Dietary trial in prostate cancer: early experience and implications for clinical trial design. Urology. 2001;57(4A):200–201. doi: 10.1016/S0090-4295(00)00974-2. [DOI] [PubMed] [Google Scholar]
- 58.Galvao DA, Spry N, Denham J, Taaffe DR, Cormie P, Joseph D, Lamb DS, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856–864. doi: 10.1016/j.eururo.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Cormie PGD, Spry N, Joseph D, Chee R, Taaffe DR, Chambers SK, Newton RU. Can supervised exercise prevent treatment toxicity in prostate cancer patients initiating androgen deprivation therapy: a randomised controlled trial. Br J Urol. 2014;115(2):256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 60.Dalais FS, Meliala A, Wattanapenpaiboon N, Frydenberg M, Suter DA, Thomson WK, Wahlqvist ML. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64(3):510–515. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Hoenjet K, Dagnelie PC, Delaere KPJ, Wijckmans NEG, Zambon JV, Oosterhof GON. Effect of a nutritional supplement containing vitamin E, selenium, vitamin C and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: a randomised placebo-controlled study. Euro Urol. 2005;47(4):433–440. doi: 10.1016/j.eururo.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Kranse R, Dagnelie PC, Kemenade MC, de Jong FH, Blom JH, Tijburg LB, Weststrate JA, et al. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int J Cancer. 2005;113(5):835–840. doi: 10.1002/ijc.20653. [DOI] [PubMed] [Google Scholar]
- 63.Schroder FH, Roobol MJ, Boeve ER, de Mutsert R, Zuijdgeest-van Leeuwen SD, Kersten I, Wildhagen MF, et al. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol. 2005;48(6):922–930. doi: 10.1016/j.eururo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer-the UK NCRN Pomi-T study. Prostate Cancer Prostatic Dis. 2014;17(2):180–186. doi: 10.1038/pcan.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bourke L, Gilbert S, Hooper R, Steed LA, Joshi M, Catto JW, Saxton JM, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Euro Urol. 2014;65:865–872. doi: 10.1016/j.eururo.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 66.Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidem Biomark. 2011;20(4):647–657. doi: 10.1158/1055-9965.EPI-10-1143. [DOI] [PubMed] [Google Scholar]
- 67.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 68.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, Venner PM, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 69.Chen D, Zhang P, Wu T, Yu X, Chang D (2013) Clinical effects of qilan capsule combined with androgen deprivation therapy on the treatment of prostate cancer (Deficiency of Qi and blood stasis type), 6 edn. Shanghai Jiaotong University School of Medicine (639 Zhizaoju Rd., Shanghai 200011, China), China, pp 19–22
- 70.Vidlar A, Vostalova J, Ulrichova J, Student V, Krajicek M, Vrbkova J, Simanek V. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy—a six month placebo-controlled double-blind clinical trial. Biomed Pap Med Fac Palacky Univ Olomouc Czech Repub. 2010;154(3):239–244. doi: 10.5507/bp.2010.036. [DOI] [PubMed] [Google Scholar]
- 71.Ansari MS, Gupta NP. Lycopene: a novel drug therapy in hormone refractory metastatic prostate cancer. Urol Oncol. 2004;22(5):415–420. doi: 10.1016/S1078-1439(04)00122-X. [DOI] [PubMed] [Google Scholar]
- 72.Ansari MS, Gupta NP. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003;92(4):375–378. doi: 10.1046/j.1464-410X.2003.04370.x. [DOI] [PubMed] [Google Scholar]
- 73.Ansari MS, Gupta NP. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer [3] BJU Int. 2004;94(4):678. doi: 10.1111/j.1464-410x.2003.05067_4.x. [DOI] [PubMed] [Google Scholar]
- 74.Higashihara E, Itomura M, Terachi T, Matsuda T, Kawakita M, Kameyama S, Fuse H, et al. Effects of eicosapentaenoic acid on biochemical failure after radical prostatectomy for prostate cancer. Vivo. 2010;24(4):561–565. [PubMed] [Google Scholar]
- 75.Lazarevic B, Hammarstrom C, Yang J, Ramberg H, Diep LM, Karlsen SJ, Kucuk O, et al. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012;108(12):2138–2147. doi: 10.1017/S0007114512000384. [DOI] [PubMed] [Google Scholar]
- 76.Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, Moen A, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer. 2011;63(6):889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 77.Bylund A, Lundin E, Zhang JX, Nordin A, Kaaks R, Stenman UH, Aman P, et al. Randomised controlled short-term intervention pilot study on rye bran bread in prostate cancer. Eur J Cancer Prev. 2003;12(5):407–415. doi: 10.1097/00008469-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 78.Stenner-Liewen LH, Cathomas R, Renner C, Petrausch U, Sulser T, Spanaus K, Seifert HH, et al. Daily pomegranate intake has no impact on PSA levels in patients with advanced prostate cancer—results of a phase IIb randomized controlled trial. J Cancer. 2013;29(4):7. doi: 10.7150/jca.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nayan M, Anup K, Pawan V, Arora RP. Intermittent therapy with lycopene delays disease progression in hormone sensitive cancer prostate. Int J Urol. 2012;19:155. doi: 10.1111/j.1442-2042.2011.02905.x. [DOI] [PubMed] [Google Scholar]
- 80.Cipolla B, Mottet N, Efstathiou T (2014) First double-blind placebo controlled, multi-centre, randomized trial of sulforaphane in men with rising PSA following radical prostatectomy. In: 2014 annual meeting of the american urological association, p. e809
- 81.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, Chi K, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 82.Peng Y, Yao Q, Hu X, Li X, Zhou G. (2010) Treatment of prostate cancer with Qi-enriching and Yin-nourishing therapy combined with androgen deprivation [Chinese]. Chin J Androl 24(6): 44–6 + 53
- 83.Sternberg CN, Dumez H, Van Poppel H, Skoneczna I, Sella A, Daugaard G, Gil T, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20(7):1264–1269. doi: 10.1093/annonc/mdn784. [DOI] [PubMed] [Google Scholar]
- 84.James E, Boyes A, Courbeya K, Lubans D, Stacey F, Morgan P, Brookes T, et al. A home-based resistance training program for survivors of prostate cancer: a pilot randomized controlled trial. J Sci Med Sport. 2012;15:S333. doi: 10.1016/j.jsams.2012.11.809. [DOI] [Google Scholar]
- 85.Zhang AY, Strauss GJ, Siminoff LA. Effects of combined pelvic floor muscle exercise and a support group on urinary incontinence and quality of life of postprostatectomy patients. Oncol Nurs Forum. 2007;34(1):47–53. doi: 10.1188/07.ONF.47-53. [DOI] [PubMed] [Google Scholar]
- 86.Lee CE, Kilgour A, Lau YKJ. Efficacy of walking exercise in promoting cognitive-psychosocial functions in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer. 2012;12:324. doi: 10.1186/1471-2407-12-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60(1):7–13. doi: 10.1080/01635580701586770. [DOI] [PubMed] [Google Scholar]
- 88.Hernáandez J, Syed S, Weiss G, Fernandes G, von Merveldt D, Troyer DA, Basler JW, et al. The modulation of prostate cancer risk with alpha-tocopherol: a pilot randomized, controlled clinical trial. J Urol. 2005;174(2):519–522. doi: 10.1097/01.ju.0000165151.08560.6a. [DOI] [PubMed] [Google Scholar]
- 89.Collette L, Burzykowski T, Schroder FH. Prostate-specific antigen (PSA) alone is not an appropriate surrogate marker of long-term therapeutic benefit in prostate cancer trials. Eur J Cancer. 2006;42(10):1344–1350. doi: 10.1016/j.ejca.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 90.Horwood JP, Avery KN, Metcalfe C, Donovan JL, Hamdy F, Neal D, Lane JA. Men’s knowledge and attitudes towards dietary prevention of a prostate cancer diagnosis: a qualitative study. BMC Cancer. 2014;14:812. doi: 10.1186/1471-2407-14-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.